Abstract
Canine malignant histiocytosis (MH) is an aggressive neoplasm of macrophages and dendritic cells. It carries a poor prognosis due to the development of widespread metastasis and poor sensitivity to chemotherapy. Thus, there is a large need for new treatments for MH. We hypothesized that bisphosphonates might be useful to increase the effectiveness of cytotoxic chemotherapy against MH. To address this question, we conducted in vitro screening studies using MH cell lines and a panel of 6 chemotherapy and 5 bisphosphonate drugs. The combination of clodronate with vincristine was found to elicit synergistic killing which was associated with a significant increase in cell cycle arrest. Second, zoledronate combined with doxorubicin also significantly increased cell killing. Zoledronate significantly increased the uptake of doxorubicin by MH cells. Based on these findings, we conclude that certain bisphosphonate drugs may increase the overall effectiveness of chemotherapy for MH in dogs.
Keywords: cancer, immune, dog, macrophage, chemotherapy
Introduction
Malignant histiocytosis in dogs, also known as histiocytic sarcoma, is a tumor that arises from cells of the histiocytic lineage, including monocytes and dendritic cells 1-6. The disease is more common in certain breeds of dogs, including Bernese Mountain Dogs, Flat Coated Retrievers, and Rottweilers, suggesting a genetic component to disease susceptibility3, 7, 8. However, the disease also occurs sporadically in other breeds of dogs as well as in mixed breed animals. Malignant histiocytosis may develop as either a localized tumor, or may instead present as widely disseminated disease4. However, even animals with initially localized disease often develop distant metastases 7, 9. Therefore, in many cases traditional tumor treatment modalities such as surgery or radiation therapy are often ineffective for long term control of this neoplasm. Chemotherapy is usually administered to dogs with MH to help prevent local or systemic recurrence of tumor10. The use of single agent chemotherapy has been largely unrewarding for treatment of MH, as treatment responses are typically incomplete and/or short-lived 7, 11, 12. Consequently, combined therapy is usually implemented for the initial treatment of MH, including various combinations of prednisone, doxorubicin, lomustine (CCNU), and carboplatin10, 11, 13. Unfortunately, even with aggressive treatment the disease is often fatal. In cases with disseminated disease the median reported survival time is 2-4 months2, 4, 7, 8, 12.
The disseminated canine disease has its primary origins in the bone marrow, spleen, and lung4, 7-9, 14. This clinical syndrome most closely resembles the human disease Langerhans cell histiocytosis which can also be multisystemic and refractory to single agent chemotherapy15-17. These patients are most often treated using vinca alkaloids in combination with multiple immunosuppressive agents16, 17, 18. Zoledronate, an aminobisphosphonate, has also been used for palliation in cases with bone involvement19.
Bisphosphonates have been studied extensively for their ability to deplete macrophages20-23. Clodronate is a first generation, non aminobisphosphonate that is metabolized by osteoclasts and macrophages into a non-hydrolysable ATP analogue24-26. The lack of ATP leads to mitochondrial dysfunction and subsequent apoptosis of the cell26-28. Liposomal clodronate has been used for efficient, systemic macrophage depletion in multiple rodent models29-33. More recently it has been applied in multiple tumor models where it has been shown to be very effective in depleting tumor associated macrophages34-38. Our laboratory has shown that liposomal clodronate is able to kill MH cells in vitro and is a safe treatment that may be efficacious for treatment of MH in dogs39, 40.
Newer generation bisphosphonates incorporate nitrogen into their structure and subsequently work via a different mechanism of action41. These drugs inhibit the enzyme farnesyl diphosphate synthase, which inhibits macrophages and tumor cells from protein prenylation. This stops the cells from being able to activate signaling GTPases such as Ras, which leads to subsequent apoptosis of the cell41-43. The amine ring containing bisphosphonate zoledronate has been used extensively in the palliative treatment of bone metastases in humans44-46. Recent work has shown that in addition to its effects on osteoclasts, zoledronate can work synergistically with doxorubicin to kill tumor cells in vitro and decrease tumor growth in vivo in multiple tumor types47-52. This drug has also been shown to be very effective at killing tumor associated macrophages in mouse tumor models38. Zoledronate has also been shown to be safe for administration in dogs53.
As MH is a tumor of macrophages and dendritic cells, we sought to determine if combining bisphosphonates, drugs specific for macrophage killing, with traditional cytotoxic chemotherapy would demonstrate synergistic killing of MH tumor cells.
Materials and Methods
Cell lines
The canine MH tumor cell line DH82 was obtained from the American Type Tissue Collection (Gaithersburg, MD). The other two MH cell lines (designated MH-1 and MH-2) were established from primary cultures of biopsies obtained from dogs with MH and were a kind gift of Dr. Peter Moore (College of Veterinary Medicine, University of California-Davis). In order to validate these cell lines, we have recently performed karyotypes and stained them with canine specific antibodies to confirm their species origin. We have also stained with antibodies such as CD45, CD11c, and CD18 in order to confirm that they are most likely histiocytic in origin. All cell lines were maintained in MEM (minimal essential medium, Invitrogen, Grand Island, NY USA) supplemented with 10% heat inactivated fetal bovine serum (Hyclone, Logan, UT), non-essential amino acids, L-glutamine, sodium bicarbonate, penicillin and streptomycin (Sigma-Aldrich, St. Louis, MO). The cell lines were maintained at 37° C in a humidified atmosphere containing 5% CO2.
Drug preparation, storage and initial screening
Stock solutions of all drugs were stored at -20°C. Working concentrations of each drug were made by diluting stock solutions in sterile water. New working concentrations of each drug were made prior to each analysis. The following drugs were initially screened alone, using the concentration ranges given in parentheses, for their effects on DH82 cell viability using the MTT assay as described below; dexamethasone (0.15-15 μg/mL), doxorubicin (0.002-2 μg/mL), chlorambucil (0.35-35 μg/mL), carboplatin (0.5-0.005 μg/mL), CCNU (0.15-1.5 μg/mL), vincristine (0.25-25 μg/mL), clodronate (0.5-50 μg/mL), zoledronate (0.02-2 μg/mL), pamidronate (0.02-2 μg/mL), alendronate (0.02-1 μg/mL), and etidronate (0.02-2 μg/mL). The dosages of these drugs that elicited 5-20% killing were used in subsequent experiments. All bisphosphonates were tested with all chemotherapy drugs using the optimized doses of each drug.
Cell viability assays
The cells previously diluted in MEM were pipetted into a 96 well flat bottomed plate using a final volume of 100 μL/well to give a final number of four thousand cells/well. The cell number plated per well was consistent for all cell lines used. The cells were allowed to adhere for 24 hours. After this time, the cells were treated with chemotherapeutics alone, bisphosphonates alone, or both in combination. Control cells were treated with sterile water at the same volume used for the diluted drugs. The cells were incubated with the drugs for 72 hours prior to MTT analysis.
Cell viability was assessed using the MTT assay, as described previously54. Briefly, MTT (Thiazolyl Blue Tetrazolium Bromide; Sigma-Aldrich) was added to wells containing live cells and incubated for 2 hours at 37C. The cells and resultant tetrazolium bromide crystals were then dissolved in a 0.1N HCl solution in isopropanol and the absorbance was determined using an ELISA plate reader (Thermo Lab Systems, Salem NH) at 570 nm. Cell viability was then calculated as the percent absorbance of the treated wells as compared to the average absorbance of the untreated control wells, with the inverse of this value representing the percent killing. To confirm the MTT results, a second set of cells was treated exactly as described previously, then trypsinized, stained with trypan blue to exclude dead cells, and counted using an electronic cell counter (Cellometer Nexcelcom Bioscience, Lawrence MA). All drugs were obtained from Sigma-Aldrich with the exception of zoledronate which was a kind gift from Novartis.
Apoptosis assays
Induction of apoptosis was quantitated using Annexin V and propidium iodide (PI) staining and flow cytometry, as previously described55. Briefly, cells in triplicate wells were treated with the indicated concentrations of drugs, alone or in combination, for 48 hours. The negative control consisted of a population of untreated cells. A positive control included cells incubated for 6 hours with 50 μg/mL camptothecin (Sigma-Aldrich, St. Louis MO). Cells were then detached and washed prior to analysis of phosphatidylserine expression with Annexin V. Cells were incubated with FITC-conjugated Annexin V, according to manufacturer's directions (BD Biosciences, San Jose CA). Immediately prior to analysis by flow cytometry, PI was also added to the cells to assess cell membrane integrity. Cells were assessed using flow cytometry and data were analyzed using Summit software. The percentage of cells in early or late apoptosis or necrosis was calculated as noted previously55
As a second measure of apoptosis, after cells were treated with drug combinations for the indicated periods of time they were then treated using the SensoLyte® Homogenous AMC Caspase-3/7 Assay Kit (AnaSpec, San Jose, CA), which was performed according to manufacturers' recommendations. Briefly, MH cells were incubated in 0.2 μg/mL doxorubicin and 0.2 μg/mL zoledronate or 0.25 μg/mL vincristine and 5 μg/mL clodronate for 48h. Cells were then removed from the incubator, and 50 μL of a dual caspase 3/7 substrate and lysis solution was added to each well (AnaSpec). Reagents were mixed by shaking on a plate shaker for 180min at 200rpm. Fluorescence emission was determined at a wavelength of 360/460nm, using an optical density reader (BioTek, Winooski VT). Assay results were reported in relative fluorescence units. All reported results are representative of at least three independent experiments. Similar results were obtained between the DH82, MH-1, and MH-2 cell lines.
Cell Cycle Analysis and Doxorubicin Uptake
For determination of intracellular doxorubicin accumulation, cells were treated for 24 hours with doxorubicin at a concentration of 0.2 μg/mL, zoledronate at a concentration of 0.2 μg/mL, or with both doxorubicin and zoledronate at the above concentrations. The cells were trypsinized and analyzed via flow cytometry to determine the innate mean fluorescence intensity (MFI) of doxorubicin fluorescence, using flow cytometry.
For determination of cell cycle arrest, cells were grown in serum-free medium for 24 hours to initiate cell cycle synchronization. The cells were then switched to complete cell culture medium with 10% FBS and then either untreated or treated with vincristine at a concentration of 0.25 μg/mL, with clodronate at 5 μg/mL, or with both drugs in combination, for 48 hours. The cells were detached and washed twice and then resuspended in 70% ice cold ETOH and frozen overnight. The cells were then washed and resuspended in 250 μL extraction buffer and 100 μL PI-RNAse reagent (Sigma-Aldrich). The cells were then analyzed via flow cytometry and the data were analyzed using Summit software (Dako Colorado, Inc. Ft. Collins, CO) to determine cell cycle parameters. All reported results are representative of at least three independent experiments. Similar results were obtained between the DH82, MH-1, and MH-2 cell lines.
Statistical analyses
In experiments where the mean of more than two groups was compared, one way ANOVA was used, followed by Tukey's multiple means comparison test. For comparison between two groups, the Student's t-test was used. For synergy calculations treatment groups were compared using a 2-way ANOVA, as described previously56. Bliss analysis was also used in synergy calculations, as described previously57. For example, to determine whether the addition of bisphosphonates to chemotherapy drugs synergistically enhanced cell killing, the Bliss independence model was utilized. Briefly, Bliss synergy is derived by the following equation: E (x,y) = E(x) + E(y) – E(x) × E(y)
For these comparisons E(x) is the fractional inhibition of bisphosphonates (clodronate (5 μg/mL) or zoledronate (0.2 μg/mL) respectively) (between 0 and 1), E(y) is the fractional inhibition of concentration y of vincristine (0.25 μg/mL) or doxorubicin(0.2 μg/mL) respectively, and E(x,y) is the combined inhibition. Theoretical growth inhibition curves were derived utilizing this equation, and standard deviations were estimated by error propagation of experimental SD. Differences between treatment groups (Bliss theoretical vs. experimental) were assessed using two-way ANOVA and Tukey's post-test. Statistical analyses were performed using Prism5 software (GraphPad, San Diego, CA). Differences were considered statistically significant for p values less than 0.05.
Results
Bisphosphonates synergize with cytotoxic chemotherapy to kill MH cells in vitro
We conducted in vitro screens to determine whether aminobisphosphonates or non-aminobisphosphonate drugs increased the activity of 6 commonly used chemotherapy drugs against 3 different canine MH cell lines. The chemotherapy drugs were administered in vitro at concentrations that elicited only 5-20% cell killing in order to allow the detection of synergistic activity of the bisphosphonate-chemotherapy drug combinations. We found the following drugs had activity against canine MH cells at the following drug concentrations: dexamethasone (dex) (15 μg/mL), doxorubicin (dox) (0.2 μg/mL), lomustine (CCNU) (1.5 μg/mL) and vincristine (vinc) (0.25 μg/mL) (Fig. 1).
Figure 1. Certain bisphosphonate and chemotherapy combinations elicit significantly increased killing of canine MH cells in vitro.
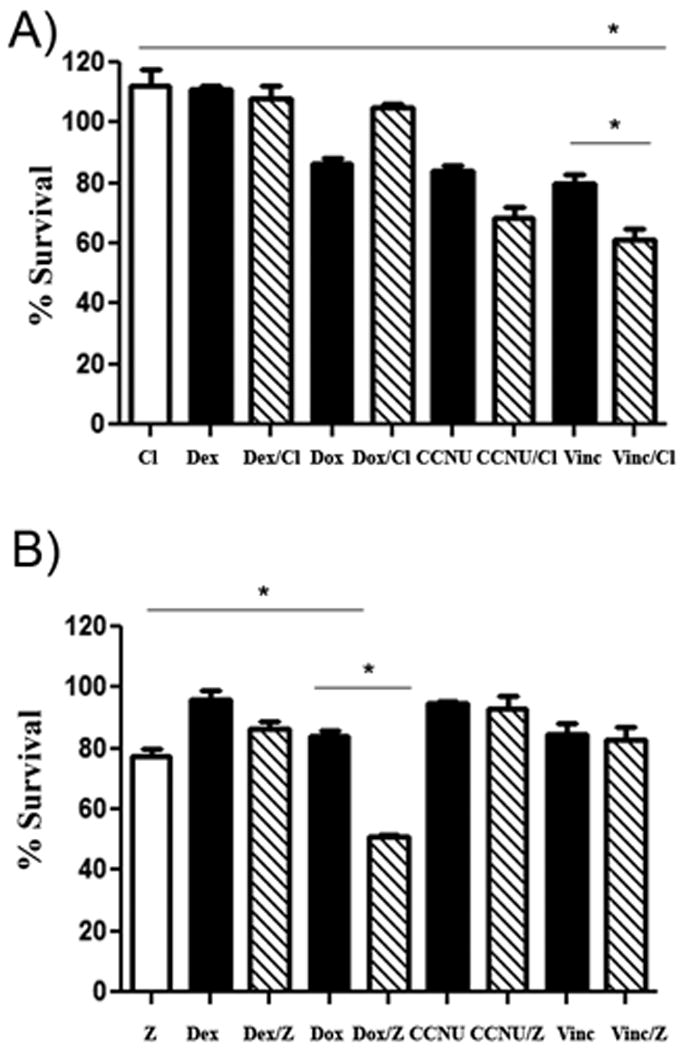
In (A), the effects of clodronate on chemotherapy-induced killing of canine DH82 MH cells was assessed, using an MTT assay to assess tumor cell viability. Cells were treated with chemotherapy drug alone (black), clodronate alone (white), or with the combination of clodronate and chemotherapy drug (cross-hatch). In these assays, only the combination of clodronate and vincristine demonstrated a significant positive interaction (* = p < 0.05), as assessed by 1 way ANOVA with Tukey's post test. In (B), the effects of zoledronate on DH82 MH cell sensitivity to killing with chemotherapy drugs were assessed, using a similar approach as for (A). Cells were treated with chemotherapy drugs alone (black), zoledronate alone (white), or the two in combination (cross-hatch). The combination of zoledronate with doxorubicin showed a significant positive interaction (* = p < 0.05), as assessed by 1 way ANOVA with Tukey's post test. Cl = clodronate (5 μg/mL), Dex = dexamethasone (15 μg/mL), Dox = doxorubicin (0.2 μg/mL), CCNU = lomustine (1.5 μg/mL), vinc = vincristine (0.25 μg/mL), z = zoledronate (0.2 μg/mL).
Next, these 4 chemotherapy drugs were evaluated for enhanced activity when combined with the following concentrations of clodronate (5 μg/mL) and zoledronate (0.2 μg/mL)41, 58, 59. The bisphosphonate drugs were also screened for activity alone against the MH cell lines (Figs. 1 and 2). After incubation for 72 hours, the cells were analyzed for viability using the MTT assay. With clodronate, we detected a significant (p < 0.0001) interaction in terms of increased cell killing when clodronate and vincristine were combined, while an interaction was not observed between clodronate and dexamethasone, doxorubicin, or lomustine (Fig 1). A significant interaction (p <0.0001) in terms of increased MH cell killing was also noted between zoledronate and doxorubicin, while no interaction between zoledronate and dexamethasone, vincristine, or lomustine was observed (Fig 1). Similar results were obtained using all three MH cell lines.
Figure 2. Synergistic enhancement of MH cell killing by combinations of bisphosphonates with vincristine or doxorubicin.
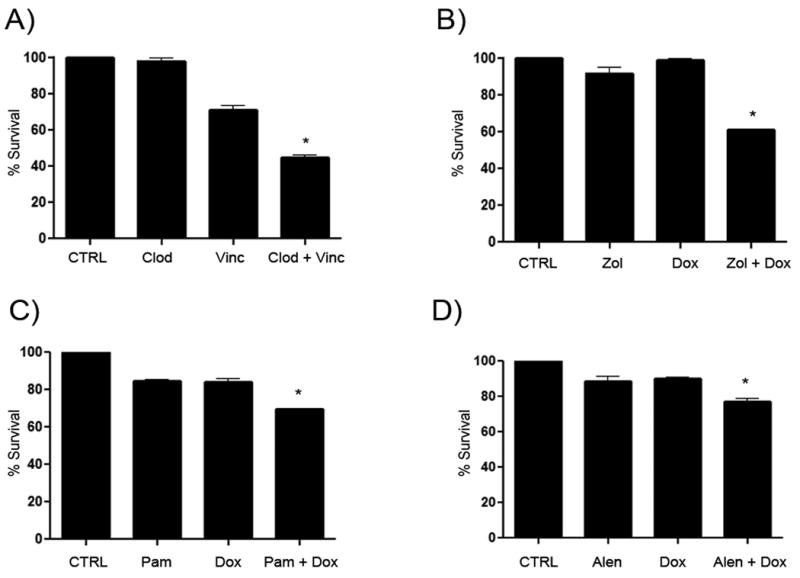
Significant killing of MH cells was seen in A-D when bisphosphonates were combined with chemotherapy as determined via MTT assay (A), DH82 MH cells were either untreated (CTRL) or treated with clodronate (Clod) or vincristine (Vinc) or both in combination (Clod + Vinc) for 72 hours. In (B), the interaction between zoledronate (Zol) and doxorubicin (Dox) was assessed. Similar experiments were done in (C) for the combination of pamidronate (Pam) with doxorubicin and in (D) for the combination of alendronate (Alen) and doxorubicin. (* = p < 0.05).
Two additional aminobisphosphonates (alendronate and pamidronate) were screened for activity with doxorubicin and each showed a significant interaction (p <0.05) (Fig. 2). These experiments were also repeated using two additional canine MH cell lines, designated MH-1 and MH-2. In all experiments, similar results were obtained with all three MH cell lines evaluated (data not shown). In addition, since the MTT assay does not differentiate between decreased metabolic activity and decreased cell number, we also assessed the effects of the bisphosphonate and chemotherapy drug combinations on cell numbers by direct counting of cells and confirmed that the results obtained using the MTT assay were indeed due to decreased cell numbers, with control and single agent treated cells having cell counts greater than 400,000 cells/mL and combination treated cells showing counts less than 200,000 cells /mL which was significantly (p = 0.04) reduced.
We next sought to determine whether the interactions between bisphosphonates and cytotoxic chemotherapy drugs reflected truly synergistic interactions. To determine synergy, two different statistical analyses were used. First, the effects on MH cell viability of increasing concentrations of doxorubicin, with or without the addition of zoledronate (0.2 μg/mL), were evaluated. The results of the first analysis demonstrated a significant reduction (p < 0.05) in the IC50 concentration of doxorubicin when combined with zoledronate (Fig. 3A). In addition, the combination of drugs induced synergistic killing as described below. Similar experiments were done using increasing concentrations of vincristine with clodronate (5 μg/mL). This combination also demonstrated a synergistic interaction (p < 0.05) (Fig. 3B).
Figure 3. Dose response curves for determination of drug interactions between zoledronate and doxorubicin or between clodronate and vincristine.
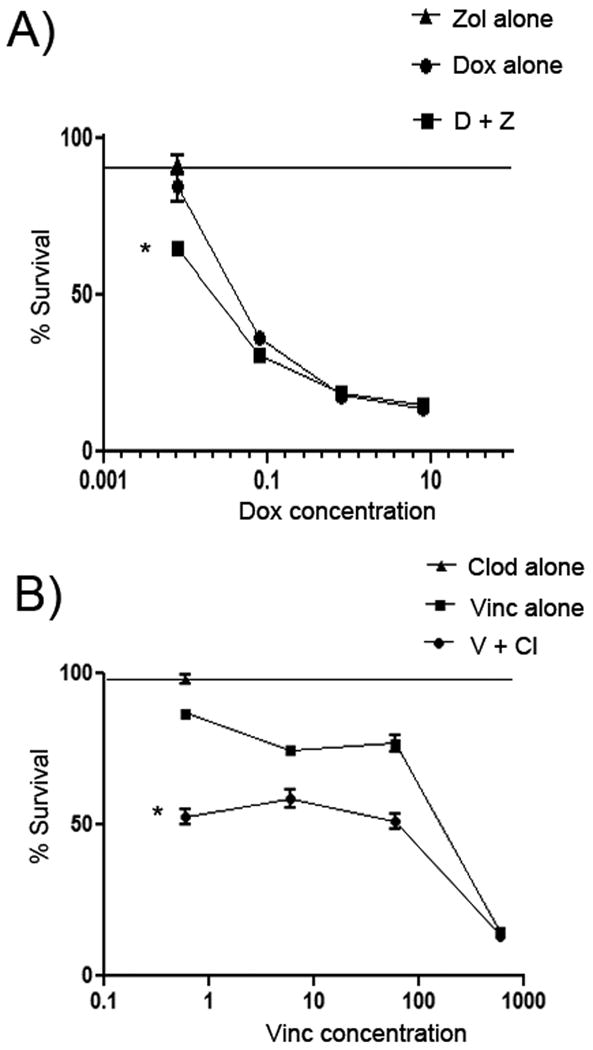
In (A), dose response curves were generated for DH82 cells treated with zoledronate, doxorubicin, or zoledronate plus doxorubicin in order to compare drug interactions via Bliss analysis. Cell viability was significantly reduced in cells treated with zoledronate (0.2 μg/mL) and increasing doses of doxorubicin, compared to cells treated with zoledronate alone (0.2 μg/mL) or increasing doses of doxorubicin alone, as assessed by Bliss analysis. In (B), a similar analysis was conducted using DH82 cells treated with clodronate (5 μg/mL) alone, with clodronate (5 μg/mL) plus increasing doses of vincristine, or with increasing doses of vincristine alone. The viability of MH cells was found using Bliss analysis to be significantly reduced in cells treated with the combination of clodronate and vincristine. (* = p < 0.05)
As a second measure of synergistic interactions between bisphosphonates and chemotherapy drugs, Bliss analysis was conducted as described in Materials and Methods. This analysis also revealed a synergistic interaction between doxorubicin and zoledronate in combination (p < 0.05), as well as between clodronate and vincristine in combination (p < 0.05). The Bliss analysis however did not however support a synergistic interaction between pamidronate and doxorubicin or between alendronate and doxorubicin, despite the fact that these drugs exhibited significant interaction via one way ANOVA. To further validate the Bliss analysis, the data was also subjected to synergy calculations as described by Slinker, using two-way ANOVA (Fig. 2)56. This analysis also revealed a significant interaction between doxorubicin and zoledronate (p < 0.001) and between vincristine and clodronate (p < 0.001). In contrast, an interaction between pamidronate and doxorubicin or between alendronate and doxorubicin was not identified using two-way ANOVA. Therefore, we concluded that based on multiple modeling approaches, there was strong evidence of synergistic interactions between these drugs.
Combined bisphosphonate and vincristine or doxorubicin treatment increases MH apoptosis
Experiments were conducted next to elucidate the mechanism(s) by which bisphosphonate drugs increased killing of MH cells when combined with vincristine or doxorubicin. Canine DH82 MH cells were treated with clodronate alone (5 μg/mL) or vincristine alone (0.25 μg/mL), or both drugs together, and the effects on induction of apoptosis were assessed using Annexin V and propidium iodine staining and flow cytometry. Treatment with the combination of vincristine and clodronate induced a significant increase (p < 0.05) in the percentage of apoptotic cells (Fig. 4). Similarly, a significant increase in MH cell apoptosis was also obtained following treatment with combined doxorubicin (0.2 μg/mL) and zoledronate (0.2 μg/mL) (p < 0.05) (Fig. 4).
Figure 4. Combined treatment with zoledronate and doxorubicin and clodronate and vincristine results in increased MH cell apoptosis.
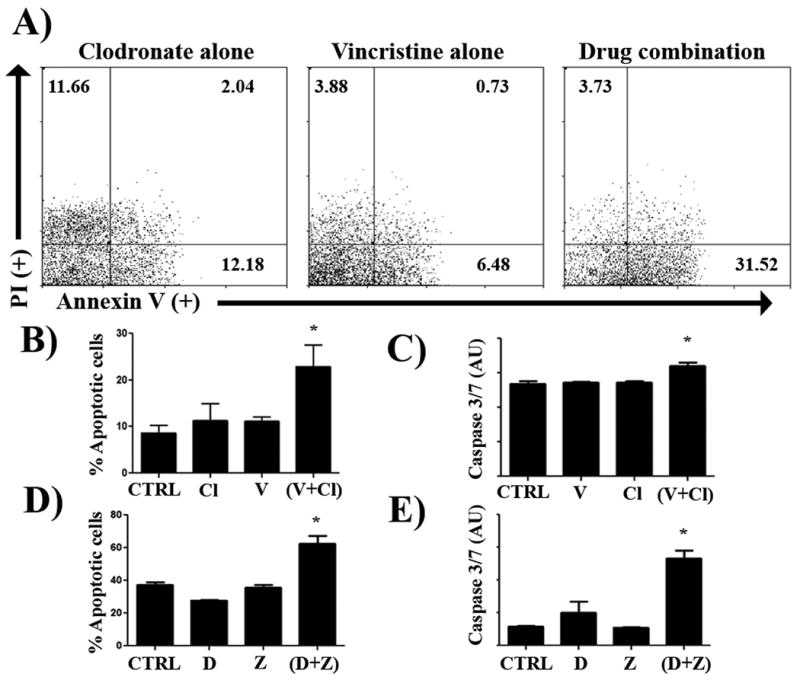
DH82 cells were treated with clodronate (5 μg/mL) and vincristine (0.25 μg/mL), alone or in combination, for 48 hours and cell apoptosis was assessed using Annexin V and propidium iodide staining, as described in Methods. In (A), an increased percentage of Annexin V+ cells were noted in cells treated with the combination of two drugs, as assessed by flow cytometry and depicted in these representative FACS plots. In (B), the mean percentage of apoptotic cells was compared between MH cells treated with clodronate or vincristine alone or in combination. There was a significant increase (* = p < 0.05) in the percentage of apoptotic cells in the combination treated group, as assessed by ANOVA and Tukey's multiple means comparison test. In (C), induction of apoptosis was also assessed by measuring induction of caspase 3 and 7 activity, as described in Methods. Treatment of DH82 cells with the combination of clodronate (5 μg/mL), vincristine (0.25 μg/mL), or both induced a significant increase (*= p < 0.05) in caspase 3/7 fluorescence (AU, arbitrary fluorescence units) as compared to untreated control cells or cells treated with one drug only, as assessed by ANOVA. In (D), combined treatment with zoledronate (0.2 μg/mL) and doxorubicin (0.2 μg/mL) induced a significant increase in the number of apoptotic MH cells, as assessed by Annexin V and propidium iodide staining. In (E), the combined treatment with zoledronate (0.2 μg/mL) and doxorubicin (0.2 μg/mL) resulted in a significant (p < 0.05) increase in caspase 3 and 7 activity in MH cells, compared to cells treated with either drug alone, as assessed by ANOVA. Each of the experiments depicted were repeated independently two additional times and similar results were obtained.
The effects of combined treatment on induction of activated caspase 3/7 activity (a measure of late apoptosis induction) was also assessed. When DH82 cells were treated with the above mentioned concentrations of vincristine and clodronate in combination for 48 hours, there was a significant increase in caspase 3/7 activity, compared to treatment with either drug alone (p < 0.001) (Fig 4). The combination of zoledronate with doxorubicin at the same concentrations as used in the Annexin V assay also generated a significant increase in caspase 3/7 activity when compared to single drug treatment in DH82 cells (p < 0.001) (Fig. 4). Statistically significant results were also obtained using the MH-1 and MH-2 cell lines (data not shown).
Treatment with clodronate enhances G2 cell cycle arrest induced by vincristine
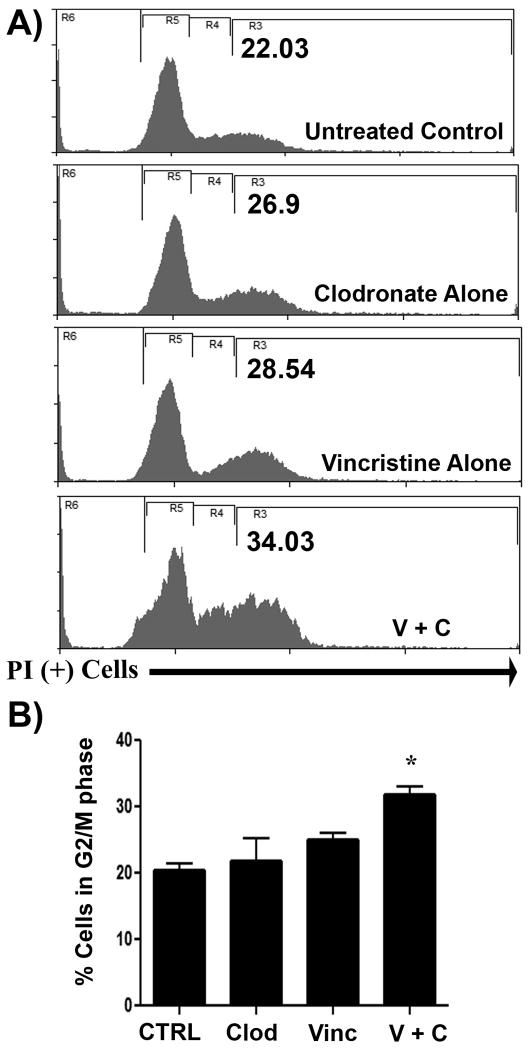
We hypothesized that clodronate may potentiate vincristine's effect on the cell cycle, leading to the observed synergistic interaction. Therefore, we assessed the effects of clodronate treatment on induction of cell cycle arrest by vincristine. We found that addition of clodronate (5 μg/mL) induced a significant increase in sub G1 and G2/M arrest in MH cells treated with vincristine (0.25 μg/mL) (p = 0.015) (Fig. 5).
Figure 5. Addition of clodronate to vincristine treatment increased cell cycle arrest in canine MH cells.
The effect of the addition of clodronate to vincristine-treated DH82 cells was assessed using flow cytometry and cell cycle analysis. In (A), representative flow cytometry histograms are displayed for cells treated for 48 hours with clodronate (5 μg/mL), vincristine (0.2 μg/mL), or with both in combination. In cells treated with the combination of both drugs, there as a notable increase in the percentage of DH82 cells exhibiting G2/M arrest (R3 = G2/M). In (B), the mean percentages of cells in the G2/M stage of cell cycle progression were calculated for untreated cells or cells treated with clodronate or vincristine, alone or together. A significant increase (* = p < 0.05) in cells in the G2/M stage was observed in cells treated with clodronate plus vincristine, as assessed by ANOVA.
Treatment with zoledronate leads to an increase in intracellular doxorubicin accumulation
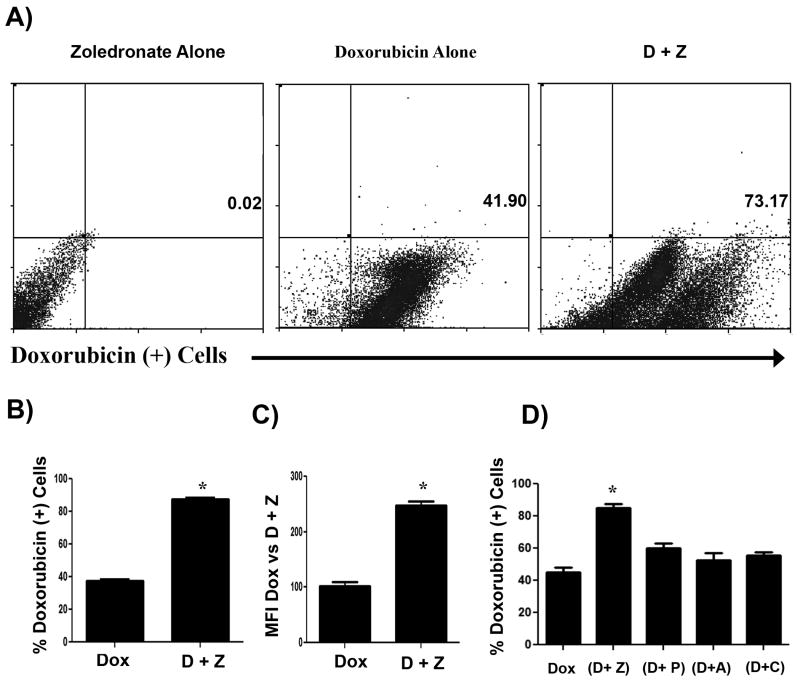
We did not see changes in the cell cycle when cells were treated with zoledronate in addition to doxorubicin. Therefore, we assessed the effects of zoledronate treatment on the permeability of MH cells to doxorubicin, using a fluorescence assay and flow cytometry. Cells were treated for 24 hours with doxorubicin at a concentration of 0.2 μg/mL, with zoledronate at a concentration of 0.2 μg/mL, or with both doxorubicin and zoledronate at the above concentrations. Intracellular doxorubicin was then evaluated via flow cytometry. We found that treatment with zoledronate resulted in a significant increase in doxorubicin uptake by MH cells (p = 0.003), whereas treatment with other bisphosphonates did not. (Fig. 6). These results suggest that increased doxorubicin accumulation might account for the increase in MH cytotoxicity observed following treatment with both zoledronate and doxorubicin.
Figure 6. Treatment with zoledronate increases doxorubicin uptake by DH82 cells.
DH82 cells were treated with doxorubicin alone (0.2 μg/mL), or with zoledronate (0.2 μg/mL) plus doxorubicin for 24 hours. The cells were then analyzed for intracellular concentrations of doxorubicin using flow cytometry. In (A), representative dot plots of doxorubicin fluorescence intensity for cells treated with zoledronate alone, doxorubicin alone, or zoledronate plus doxorubicin are depicted. In (B), the mean percentage of doxorubicin+ MH cells from triplicate wells treated with doxorubicin alone or doxorubicin plus zoledronate was plotted. The percentage of doxorubicin+ cells was significantly increased (* = p < 0.05) in the combination treated cells. In (C), the mean of the mean fluorescence intensities (MFI) of doxorubicin expression by cells treated with doxorubicin alone or doxorubicin plus zoledronate was plotted. The mean MFI of doxorubicin expression was significantly increased (* = p < 0.05) in the combination treated cells. In (D), MH cells in triplicate wells were treated doxorubicin alone or with zoledronate (Z), pamidronate (P), alendronate (A), or clodronate (C) plus doxorubicin for 24 hours and the mean percentage of doxorubicin+ cells was determined by flow cytometry. Only treatment with zoledronate produced a significant increase (* = p < 0.05) in the percentage of doxorubicin+ cells, as assessed by ANOVA.
Discussion
Malignant histiocytosis (MH) is a devastating disease in dogs, with short survival times and poor response rates to treatment7, 8, 11, 12. These tumors often progress very rapidly in dogs and MH is often highly resistant to chemotherapy, a phenomenon that is also observed in humans with aggressive forms of a similar neoplasm known as Langerhans cell histiocytosis16-18. Treatment with vinca alkaloids can be effective against Langerhans cell histiocytosis in humans15, 16. These tumors can originate from the bone marrow in dogs and humans and the bone marrow may also serve as a site for tumor recrudescence4, 7, 9, 14, 16. Bisphosphonate drugs reach their highest concentrations in bone, which may allow this class of drugs to reach effective concentrations against MH tumors involving bone marrow 41, 53, 59, 60.
Our current study revealed that there were two novel drug combinations that might be expected to have significant in vivo activity against canine MH. The first effective combination was clodronate combined with vincristine, which induced a synergistic increase in apoptosis of MH cells, presumably by increasing cell cycle arrest. Such a combination might be particularly effective in dogs with bony involvement with MH. The concentrations of clodronate and vincristine that were used in vitro are relevant to the concentrations of drug achieved in vivo60, 61. The combination of clodronate with vincristine has the additional advantage of being relatively inexpensive to use, although clodronate is not licensed for use in the United States and would thus have to be obtained from foreign sources for treatment of animals here.
While the exact mechanism of this synergistic interaction is not yet fully defined, we have been able to show that the addition of clodronate to vincristine potentiates the effects of vincristine on the cell cycle. We observed a higher percentage of cells in G2/M arrest when treated with the combination of the drugs than with either drug alone. This indicates that clodronate has a direct potentiating effect on the effects of vincristine, as this is the primary anti-tumor mechanism of vincristine via effects on microtubules. As the primary effects of clodronate inhibit ATP usage by the cell, our hypothesis is that clodronate disrupts formation of actin filaments which are essential for successful cytokinesis. The combination of vincristine and clodronate may affectively block both microtubules and actin filaments, thus leading to an increase in G2/M arrest. Further studies are needed to confirm this proposed mechanism.
We also found that zoledronate increased the activity of doxorubicin against canine MH cells. The effects of zoledronate appeared to be mediated at least in part by increasing tumor cell permeability to doxorubicin. Studies in non myeloid tumor cell lines in humans and rodents have previously demonstrated a synergistic interaction between zoledronate and doxorubicin, though the effects of zoledronate on doxorubicin uptake were not examined in those studies 48-50. The mechanism for this increased uptake remains uncertain. The primary effects of zoledronate on the cell are due to decreases in protein prenylation and subsequent inactivation of small GTPases such as Ras. Therefore, the increased drug accumulation could be due to decreased ability of the cells to metabolize doxorubicin secondary to inhibition of these GTPases. Many tumors have upregulated Ras expression, and this increase has been shown to directly protect tumor cells from doxorubicin induced apoptosis62. Further studies will be needed to support this hypothesis.
More recent studies have shown that zoledronate has potent immunomodulatory effects in addition to direct effects on tumor cells38, 51. In particular, zoledronate partially depletes tumor associated macrophages (TAM), which in turn which leads to decreased tumor angiogenesis and increased activation of anti-tumor immunity38, 51. Since TAM have also been shown to decrease the sensitivity of tumor cells to chemotherapy, depletion of TAM using zoledronate could potentially augment the effectiveness of cytotoxic chemotherapeutics in vivo by a mechanism independent of direct drug-drug interactions63.
Zoledronate has been administered previously to dogs with osteosarcoma for relief of malignant osteolysis53, 58. In addition, the combination of zoledronate and doxorubicin has been administered without apparent increased toxicity to dogs with advanced osteosarcoma metastases (Fan, TM; personal communication). The levels of these drugs used in vitro are relevant to the levels that can be achieved in the serum in vivo58, 64. Thus, combined treatment with zoledronate and doxorubicin is feasible in dogs and should be investigated further in dogs with MH.
The results of our in vitro studies reported here indicate that combined treatment with selected bisphosphonates may increase the effectiveness of either vincristine or doxorubicin for treatment of MH in dogs. In particular, the combination of clodronate with vincristine may be indicated for animals with MH bony involvement, as clodronate reaches high concentrations in bone, while zoledronate combined with doxorubicin may be beneficial for treatment of visceral tumor metastases due to greater non-osseous tissue concentrations achieved with zoledronate. In summary, our results provide the rationale behind additional clinical evaluation of combined bisphosphonate and vincristine or doxorubicin chemotherapy for treatment of dogs with advanced MH disease.
Acknowledgments
This work was supported by grants from the Canine Health Foundation and National Institutes of Health NCRR T32-RR-007072-08.
References
- 1.Affolter VK, Moore PF. Canine cutaneous and systemic histiocytosis: reactive histiocytosis of dermal dendritic cells. The American Journal of dermatopathology. 2000;22(1):40–8. doi: 10.1097/00000372-200002000-00009. [DOI] [PubMed] [Google Scholar]
- 2.MacEwen EG, Withrow SJ. Small Animal Clinical Oncology. Philadelphia PA: Saunders Co; 1996. [Google Scholar]
- 3.Fulmer AK, Mauldin GE. Canine histiocytic neoplasia: an overview. Can Vet J. 2007;8:1041–1043. 1046–1050. [PMC free article] [PubMed] [Google Scholar]
- 4.Affolter VK, Moore PF. Localized and disseminated histiocytic sarcoma of dendritic cell origin in dogs. Veterinary pathology. 2002;39(1):74–83. doi: 10.1354/vp.39-1-74. [DOI] [PubMed] [Google Scholar]
- 5.Moore PF, Affolter VK, Vernau W. Canine hemophagocytic histiocytic sarcoma: a proliferative disorder of CD11d+ macrophages. Veterinary pathology. 2006;43(5):632–45. doi: 10.1354/vp.43-5-632. [DOI] [PubMed] [Google Scholar]
- 6.Wellman ML, Krakowka S, Jacobs RM, Kociba GJ. A macrophage-monocyte cell line from a dog with malignant histiocytosis. In vitro cellular & developmental biology : journal of the Tissue Culture Association. 1988;24(3):223–9. doi: 10.1007/BF02623551. [DOI] [PubMed] [Google Scholar]
- 7.Fidel J, S I, Hauser B, Jausi Y, Rohrer-Bley C, Roos M, Kaser-Hotz B. Histiocytic sarcomas in flat-coated retrievers: a summary of 37 cases (November 1998–March 2005) Vet Comp Oncol. 2006;4(2):63–74. doi: 10.1111/j.1476-5810.2006.00090.x. [DOI] [PubMed] [Google Scholar]
- 8.Moore PF, Rosin A. Malignant histiocytosis of Bernese mountain dogs. Veterinary pathology. 1986;23:1–10. doi: 10.1177/030098588602300101. [DOI] [PubMed] [Google Scholar]
- 9.Rossi S, Gelain ME, Comazzi S. Disseminated histiocytic sarcoma with peripheral blood involvement in a Bernese Mountain dog. Vet Clin Pathol. 2009;38(1):126–30. doi: 10.1111/j.1939-165X.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- 10.Skorupski KA, Rodriguez CO, Krick EL, Clifford CA, Ward R, Kent MS. Long-term survival in dogs with localized histiocytic sarcoma treated with CCNU as an adjuvant to local therapy. Vet Comp Oncol. 2009;7(2):139–44. doi: 10.1111/j.1476-5829.2009.00186.x. [DOI] [PubMed] [Google Scholar]
- 11.Skorupski KA, Clifford CA, Paoloni MC, Lara-Garcia A, Barber L, Kent MS, LeBlanc AK, Sabhlok A, Mauldin EA, Shofer FS, Couto CG, Sorenmo KU. CCNU for the treatment of dogs with histiocytic sarcoma. Journal of veterinary internal medicine / American College of Veterinary Internal Medicine. 2007;21(1):121–6. doi: 10.1892/0891-6640(2007)21[121:cfttod]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Zavodovskaya R, Liao AT, Jones CL, Yip B, Chien MB, Moore PF, London CA. Evaluation of dysregulation of the receptor tyrosine kinases Kit, Flt3, and Met in histiocytic sarcomas of dogs. American journal of veterinary research. 2006;67(4):633–41. doi: 10.2460/ajvr.67.4.633. [DOI] [PubMed] [Google Scholar]
- 13.Rosin A, Moore P, Dubielzig R. Malignant histiocytosis in Bernese Mountain dogs. J Am Vet Med Assoc. 1986;188(9):1041–5. [PubMed] [Google Scholar]
- 14.Schultz RM, Puchalski SM, Kent M, Moore PF. Skeletal lesions of histiocytic sarcoma in nineteen dogs. Veterinary Radiology & Ultrasound. 2007;48(6):539–43. doi: 10.1111/j.1740-8261.2007.00292.x. [DOI] [PubMed] [Google Scholar]
- 15.Bucsky P, Egeler RM. Malignant histiocytic disorders in children. Clinical and therapeutic approaches with a nosologic discussion. Hematology/oncology clinics of North America. 1998;12(2):465–71. doi: 10.1016/s0889-8588(05)70523-2. [DOI] [PubMed] [Google Scholar]
- 16.Howarth DM, Gilchrist GS, Mullan BP, Wiseman GA, Edmonson JH, Schomberg PJ. Langerhans cell histiocytosis: diagnosis, natural history, management, and outcome. Cancer. 1999;85(10):2278–90. doi: 10.1002/(sici)1097-0142(19990515)85:10<2278::aid-cncr25>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 17.Ladisch S, G H. Treatment of Langerhans Cell Histiocytosis - Evolution and Current Approaches. Br J Cancer. 1994;70(Suppl. XXIII):s41–s46. [PMC free article] [PubMed] [Google Scholar]
- 18.Egeler R, K J, Voǔte PA. Cytosine-arabinoside, vincristine, and prednisolone in the treatment of children with disseminated langerhans cell histiocytosis with organ dysfunction: Experience at a single institution. Med Pediatr Oncol. 2006;21(4):265–270. doi: 10.1002/mpo.2950210406. [DOI] [PubMed] [Google Scholar]
- 19.Montella L, M C, Merola G, Petillo L, Palmieri G. Zoledronic acid in treatment of bone lesions by Langerhans cell histiocytosis. J Bone Miner Metab. 2009;27:110–113. doi: 10.1007/s00774-008-0001-2. [DOI] [PubMed] [Google Scholar]
- 20.Cecchini MG, Felix R, Fleisch H, Cooper PH. Effect of bisphosphonates on proliferation and viability of mouse bone marrow-derived macrophages. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1987;2(2):135–42. doi: 10.1002/jbmr.5650020209. [DOI] [PubMed] [Google Scholar]
- 21.Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, Mundy GR, Boyce BF. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1995;10(10):1478–87. doi: 10.1002/jbmr.5650101008. [DOI] [PubMed] [Google Scholar]
- 22.Monkkonen J, Heath TD. The effects of liposome-encapsulated and free clodronate on the growth of macrophage-like cells in vitro: the role of calcium and iron. Calcified Tissue International. 1993;53(2):139–46. doi: 10.1007/BF01321893. [DOI] [PubMed] [Google Scholar]
- 23.Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211(6-8):609–18. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 24.Frith JC, Monkkonen J, Blackburn GM, Russell RG, Rogers MJ. Clodronate and liposome-encapsulated clodronate are metabolized to a toxic ATP analog, adenosine 5′-(beta, gamma-dichloromethylene) triphosphate, by mammalian cells in vitro. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1997;12(9):1358–67. doi: 10.1359/jbmr.1997.12.9.1358. [DOI] [PubMed] [Google Scholar]
- 25.Frith JC, Monkkonen J, Auriola S, Monkkonen H, Rogers MJ. The molecular mechanism of action of the antiresorptive and antiinflammatory drug clodronate: evidence for the formation in vivo of a metabolite that inhibits bone resorption and causes osteoclast and macrophage apoptosis. Arthritis and rheumatism. 2001;44(9):2201–10. doi: 10.1002/1529-0131(200109)44:9<2201::aid-art374>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 26.Lehenkari PP, Kellinsalmi M, Napankangas JP, Ylitalo KV, Monkkonen J, Rogers MJ, Azhayev A, Vaananen HK, Hassinen IE. Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Molecular pharmacology. 2002;61(5):1255–62. doi: 10.1124/mol.61.5.1255. [DOI] [PubMed] [Google Scholar]
- 27.Selander KS, Monkkonen J, Karhukorpi EK, Harkonen P, Hannuniemi R, Vaananen HK. Characteristics of clodronate-induced apoptosis in osteoclasts and macrophages. Molecular pharmacology. 1996;50(5):1127–38. [PubMed] [Google Scholar]
- 28.van Rooijen N, Sanders A, van den Berg TK. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. Journal of immunological methods. 1996;193(1):93–9. doi: 10.1016/0022-1759(96)00056-7. [DOI] [PubMed] [Google Scholar]
- 29.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. Journal of immunological methods. 1994;174(1-2):83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 30.Richards PJ, Williams AS, Goodfellow RM, Williams BD. Rheumatology (Oxford) 9. Vol. 38. 1999. Liposomal clodronate eliminates synovial macrophages, reduces inflammation and ameliorates joint destruction in antigen-induced arthritis; pp. 818–25. [DOI] [PubMed] [Google Scholar]
- 31.Jordan MB, van Rooijen N, Izui S, Kappler J, Marrack P. Liposomal clodronate as a novel agent for treating autoimmune hemolytic anemia in a mouse model. Blood. 2003;101(2):594–601. doi: 10.1182/blood-2001-11-0061. [DOI] [PubMed] [Google Scholar]
- 32.Everhart MB, Han W, Parman KS, Polosukhin VV, Zeng H, Sadikot RT, Li B, Yull FE, Christman JW, Blackwell TS. Intratracheal administration of liposomal clodronate accelerates alveolar macrophage reconstitution following fetal liver transplantation. Journal of leukocyte biology. 2005;77(2):173–80. doi: 10.1189/jlb.1203647. [DOI] [PubMed] [Google Scholar]
- 33.Alves-Rosa F, Stanganelli C, Cabrera J, van Rooijen N, Palermo MS, Isturiz MA. Treatment with liposome-encapsulated clodronate as a new strategic approach in the management of immune thrombocytopenic purpura in a mouse model. Blood. 2000;96(8):2834–40. [PubMed] [Google Scholar]
- 34.Halin S, Rudolfsson SH, Van Rooijen N, Bergh A. Extratumoral macrophages promote tumor and vascular growth in an orthotopic rat prostate tumor model. Neoplasia. 2009;11(2):177–86. doi: 10.1593/neo.81338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miselis NR, Wu ZJ, Van Rooijen N, Kane AB. Targeting tumor-associated macrophages in an orthotopic murine model of diffuse malignant mesothelioma. Mol Cancer Ther. 2008;7(4):788–99. doi: 10.1158/1535-7163.MCT-07-0579. [DOI] [PubMed] [Google Scholar]
- 36.Robinson-Smith TM, Isaacsohn I, Mercer CA, Zhou M, Van Rooijen N, Husseinzadeh N, McFarland-Mancini MM, Drew AF. Macrophages mediate inflammation-enhanced metastasis of ovarian tumors in mice. Cancer Research. 2007;67(12):5708–16. doi: 10.1158/0008-5472.CAN-06-4375. [DOI] [PubMed] [Google Scholar]
- 37.Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. British Journal of Cancer. 2006;95(3):272–81. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang W, Z X, Sun H, Xiong Y, Zhuang P, Xu H, Kong L, Wang L, Wu W, Tang Z. Depletion of Tumor-Associated Macrophages Enhances the Effect of Sorafenib in Metastatic Liver Cancer Models by Antimetastatic and Antiangiogenic Effects. Clin Cancer Res. 2010;16(13):3420–3430. doi: 10.1158/1078-0432.CCR-09-2904. [DOI] [PubMed] [Google Scholar]
- 39.Mathes M, Jordan M, Dow S. Evaluation of liposomal clodronate in experimental spontaneous autoimmune hemolytic anemia in dogs. Experimental Hematology. 2006;34(10):1393–402. doi: 10.1016/j.exphem.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 40.Hafeman SD, London CA, Elmslie R, Dow SW. Evaluation of liposomal clodronate for treatment of malignant histiocytosis in dogs. Cancer Immunology and Immunotherapy. 2010;59:441–452. doi: 10.1007/s00262-009-0763-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin JH. Bisphosphonates: a review of their pharmacokinetic properties. Bone. 1996;18(2):75–85. doi: 10.1016/8756-3282(95)00445-9. [DOI] [PubMed] [Google Scholar]
- 42.Roelofs AJ, Thompson K, Gordon S, Rogers MJ. Molecular mechanisms of action of bisphosphonates: current status. Clinical Cancer Research. 2006;12(20 Pt 2):6222s–6230s. doi: 10.1158/1078-0432.CCR-06-0843. [DOI] [PubMed] [Google Scholar]
- 43.Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, Frith JC. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88(12 Suppl):2961–78. doi: 10.1002/1097-0142(20000615)88:12+<2961::aid-cncr12>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- 44.Berenson J, V R, Henick K, Nishikubo C, Rettig M, Swift R, Conde F, Von Teichert J. A Phase I, Open Label, Dose Ranging Trial of Intravenous Bolus Zoledronic Acid, a Novel Bisphosphonate, in Cancer Patients with Metastatic Bone Disease. Cancer. 2001;91(1):144–154. doi: 10.1002/1097-0142(20010101)91:1<144::aid-cncr19>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 45.Green JR. Antitumor effects of bisphosphonates. Cancer. 2003;97(3 Suppl):840–7. doi: 10.1002/cncr.11128. [DOI] [PubMed] [Google Scholar]
- 46.Selander K, Lehenkari P, Vaananen HK. The effects of bisphosphonates on the resorption cycle of isolated osteoclasts. Calcified Tissue International. 1994;55(5):368–75. doi: 10.1007/BF00299317. [DOI] [PubMed] [Google Scholar]
- 47.Clezardin P, Fournier P, Boissier S, Peyruchaud O. In vitro and in vivo antitumor effects of bisphosphonates. Current Medical Chemistry. 2003;10(2):173–80. doi: 10.2174/0929867033368529. [DOI] [PubMed] [Google Scholar]
- 48.Clyburn R, R P, Evans C, LeXey D, Holen I. Increased anti-tumour effects of doxorubicin and zoledronic acid in prostate cancer cells in vitro: supporting the benefits of combination therapy. Cancer Chemother Pharmacol. 2010;65:969–978. doi: 10.1007/s00280-009-1106-6. [DOI] [PubMed] [Google Scholar]
- 49.Ottewell P, D B, Monkkonen H, Cross S, Coleman R, Clezardin P, Holen I. Differential Effect of Doxorubicin and Zoledronic Acid on Intraosseous versus Extraosseous Breast Tumor Growth In vivo. Clin Cancer Res. 2008;14(14):4658–4666. doi: 10.1158/1078-0432.CCR-07-1545. [DOI] [PubMed] [Google Scholar]
- 50.Peng H, S Y, Moats R, Nelson M, Groshen S, Ye W, Reynolds P, DeClerck Y. The Activity of Zoledronic Acid on Neuroblastoma Bone Metastasis Involves Inhibition of Osteoclasts andTumor Cell Survival and Proliferation. Cancer Res. 2007;67(19):9346–9355. doi: 10.1158/0008-5472.CAN-06-4508. [DOI] [PubMed] [Google Scholar]
- 51.Tsagozis P, E F, Pisa P. Zoledronic acid modulates antitumoral responses of prostate cancer-tumor associated macrophages. Cancer Immunology and Immunotherapy. 2008;57:1451–1459. doi: 10.1007/s00262-008-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Webbe N, E C, Coleman R, Holen I. Mechanisms of the synergistic interaction of the bisphosphonate zoledronic acid and paclitaxel in breast cancer cells in vitro. Tumor Biol. 2006;27:92–103. doi: 10.1159/000092489. [DOI] [PubMed] [Google Scholar]
- 53.Lorimier L, F T. Bone Metabolic Effects of Single-Dose Zoledronate in Healthy Dogs. Journal of Veterinary Internal Medicine. 2005;19:924–927. doi: 10.1892/0891-6640(2005)19[924:bmeosz]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 54.Twentyman PR, Luscombe M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. British Journal of Cancer. 1987;56(3):279–85. doi: 10.1038/bjc.1987.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31(1):1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 56.Slinker B. The Statistics of Synergism. Journal of Molecular and Cellular Cardiology. 1998;30:723–731. doi: 10.1006/jmcc.1998.0655. [DOI] [PubMed] [Google Scholar]
- 57.Wittenburg LA, B L, Rose BJ, Korch C, Thamm DH. The histone deacetylase inhibitor valproic acid sensitizes human and canine osteosarcoma to doxorubicin. Cancer Chemotherapy and Pharmacology. 2010;55:210–220. doi: 10.1007/s00280-010-1287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin-Jimenez T, DL L, Fan TM, Freise KJ. Pharmacokinetics and pharmacodynamics of a single dose of zoledronate in healthy dogs. Journal of Veterinary Pharmacology and Therapeutics. 2007;30:492–495. doi: 10.1111/j.1365-2885.2007.00883.x. [DOI] [PubMed] [Google Scholar]
- 59.Lauren L, Osterman T, Karhi T. Pharmacokinetics of clodronate after single intravenous, intramuscular and subcutaneous injections in rats. Pharmacology & toxicology. 1991;69(5):365–8. doi: 10.1111/j.1600-0773.1991.tb01312.x. [DOI] [PubMed] [Google Scholar]
- 60.Villikka K, Perttunen K, Rosnell J, Ikavalko H, Vaho H, Pylkkanen L. The absolute bioavailability of clodronate from two different oral doses. Bone. 2002;31(3):418–21. doi: 10.1016/s8756-3282(02)00841-4. [DOI] [PubMed] [Google Scholar]
- 61.Castle MC, Margileth DA, Oliverio VT. Distribution and excretion of (3H)vincristine in the rat and the dog. Cancer Res. 1976;36(10):3684–9. [PubMed] [Google Scholar]
- 62.Nooter K, B A, Oostruml RG, Burger H, Jochemsen AG, Stoterl G. Constitutive expression of the c-H-ras oncogene inhibits doxorubicin-induced apoptosis and promotes cell survival in a rhabdomyosarcoma cell line. British Journal of Cancer. 1995;71:556–561. doi: 10.1038/bjc.1995.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zheng Y, C Z, Wang Siqing, Zhang Xiang, Qian Jianfei, Hong Sungyoul, Li Haiyan, et al. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug–induced apoptosis. Blood. 2009;114:3625–3628. doi: 10.1182/blood-2009-05-220285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gustafson DL, Thamm DH. Pharmacokinetic modeling of doxorubicin pharmacokinetics in dogs deficient in ABCB1 drug transporters. J Vet Intern Med. 24(3):579–86. doi: 10.1111/j.1939-1676.2010.0496.x. [DOI] [PubMed] [Google Scholar]