Abstract
Atlantic killifish (Fundulus heteroclitus) from the Atlantic Wood Superfund site on the Elizabeth River (ER), VA are dramatically resistant to the acute toxicity and teratogenesis caused by polycyclic aromatic hydrocarbons (PAHs). To understand the consequences of adaptation to chronic PAH pollution, we have attempted to further define the chemical tolerance associated with this resistance. An important component of the PAH adaptation of ER fish is the dramatic down-regulation of the aryl hydrocarbon receptor (AHR) pathway, resulting in decreased cytochrome p450 (CYP) 1 activity. Herein, we compared the susceptibility to several insecticides of ER fish to that of reference site fish; use of these chemicals as probes of the resistance will help to demonstrate if the contaminant adaptation exhibited by ER fish is broad or narrow and AHR-focused. We hypothesized that ER fish would be less susceptible to the organophosphate chlorpyrifos (activated by CYP) and more susceptible to the pyrethroid permethrin (detoxified by CYP). Comparison of acute toxicity in 5-day-old larvae supported this hypothesis for chlorpyrifos. As expected in KC, chemical up-regulation of CYP by co-exposure to β-naphthoflavone (BNF) enhanced the susceptibility of KC but it did not affect ER larvae. Unexpectedly, ER larvae were much less susceptible to permethrin than KC larvae. However, co-exposure to BNF greatly decreased the susceptibility of KC larvae, indicating that metabolism of permethrin by CYP was protective. Additionally, fish from each population were compared for susceptibility to the carbamate carbaryl, an acute neurotoxicant and weak AHR agonist that induces teratogenesis similar to that caused by PAHs. ER embryos and larvae were less susceptible than KC fish. These results suggest that the adaptive phenotype of ER fish is multi-faceted and that aspects other than CYP response are likely to greatly affect their response to contaminants.
Keywords: Fundulus heteroclitus, adaptation, polycyclic aromatic hydrocarbon, pesticides, Elizabeth River
Introduction
The population of Fundulus heteroclitus (the Atlantic killifish or mummichog; hereafter referred to as killifish) inhabiting the Atlantic Wood Industries Superfund site on the Elizabeth River, Virginia, USA are resident in an area heavily contaminated with a complex mixture of polycyclic aromatic hydrocarbons (PAHs) from former creosote operations. Total PAH concentrations of 100-500 μg/g dry sediment are commonly reported for the site (Mulvey et al. 2002; Vogelbein and Unger 2003; Walker et al. 2004), placing it among the more heavily PAH-polluted sites in the world (Walker et al. 2004). However, Elizabeth River killifish have developed resistance to the acute toxicity and severe cardiac teratogenesis caused by Elizabeth River sediments, some PAHs, and PCB-126 (3,3′,4,4′,5-pentachlorobiphenyl) (Meyer and Di Giulio 2002; Meyer et al. 2002; Ownby et al. 2002).
Although Elizabeth River killifish have developed resistance to some of the acute effects of PAHs, they are not wholly unaffected by the contaminants. They exhibit hepatic neoplasms (Vogelbein et al. 1990), have altered immune function and elevated disease susceptibility (Faisal et al. 1991; Frederick et al. 2007; Weeks et al. 1988), are more sensitive to hypoxia and fluoranthene-mediated phototoxicity (Meyer and Di Giulio 2003), and have reduced growth and survivorship compared to reference fish (Meyer and Di Giulio 2003).
A striking difference between the Elizabeth River killifish and naïve killifish is their dramatic and heritable recalcitrance to induction of multiple cytochrome P450 (CYP) metabolic enzymes by aryl hydrocarbon receptor (AHR) agonists (Meyer et al. 2002; Van Veld and Westbrook 1995; Wills et al. 2010). In the Elizabeth River killifish and other fish populations exposed to dioxin-like compound (DLC) pollution, recalcitrance to CYP induction is correlated with marked resistance to the toxic effects of the contaminants (Bello et al. 2001; Nacci et al. 1999; Prince and Cooper 1995; Roy et al. 2002). Lack of CYP induction is generally considered to be a marker of down-regulation of the AHR pathway. Elizabeth River killifish also demonstrate recalcitrance to induction of another AHR-responsive gene, the AHR repressor (AHRR) (Meyer et al. 2003). Recalcitrance to induction of multiple components of the AHR pathway (CYP1A, CYP1B1, CYP1C1, and AHRR) strongly suggests that the alteration occurs at a shared, upstream regulator, such as the AHR itself. Furthermore, morpholino knockdown of the AHR in zebrafish (Billiard et al. 2006; Prasch et al. 2003) and killifish (Clark et al. 2010) has demonstrated that blockade of the AHR can provide protection from cardiac teratogenesis caused by aryl hydrocarbons such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), PCB-126, and a variety of PAHs. However, it is noteworthy that in some cases this protection is incomplete.
The incomplete protection provided by AHR knockdown may indicate a role for additional adaptive changes in Elizabeth River killifish. They exhibit alterations in several xenobiotic metabolism and excretion pathways that may contribute to their survival in a contaminated habitat, including elevated levels of glutathione S-transferase (Armknecht et al. 1998; Van Veld et al. 1991), hepatic P-glycoprotein (Cooper et al. 1999), UDP-glucuronosyl transferase (UGT), and sulfotransferase (Gaworecki et al. 2004). Although clearly important, the relative roles of the AHR pathway and other adaptive genes in the toxicity of PAH mixtures and the resistance of Elizabeth River killifish remains uncertain.
Other than the aforementioned studies, limited data are available concerning the cost of contaminant resistance with respect to sensitivity to other environmental pollutants and stressors. This is potentially very important, as estuarine fish are faced with a wide variety of chemical stressors. Furthermore, the biochemical and physiological changes that engender or are coincident with resistance to PAHs are likely to alter toxic responses to other contaminants; these alterations may be either costly or beneficial. For example, compounds activated by CYP1s, such as the organophosphate insecticide chlorpyrifos, might be expected to be more toxic to reference killifish than Elizabeth River killifish. Conversely, the refractory CYP1 phenotype may lead to reduced breakdown of a toxic parent compound, increasing its effect. This might be expected for compounds such as pyrethroid insecticides, which are highly toxic to fish, but are much more so as parent compounds than metabolites resulting from CYP1 activity (Coats et al. 1989).
The goal of the current study was to further define the chemical tolerance exhibited by Elizabeth River killifish and to investigate the consequences of PAH-adaptation for response to contaminants other than aryl hydrocarbons. Our hypothesis was that the adaptive changes necessitated by long-term exposure to a complex mixture of PAHs would alter the response of Elizabeth River killifish when faced with other contaminants. In particular, compounds activated by CYP1s, such as chlorpyrifos, would be less toxic to Elizabeth River killifish and compounds that are generally inactivated by CYP1s, such as permethrin and carbaryl, would be more toxic to Elizabeth River killifish. To investigate this hypothesis, we challenged offspring of the PAH-adapted Elizabeth River killifish and a reference population with acutely neurotoxic insecticides – the organophosphate chlorpyrifos, the pyrethroid permethrin, and the carbamate carbaryl. Because of unexpected results with exposure to permethrin, we added a second pyrethroid, fenvalerate, which varies slightly in structure. To further illustrate the role of CYP1s in the toxicity, larvae were co-exposed to β-naphthoflavone, a known CYP1 inducer, with the individual insecticides. In addition, because there is evidence that carbaryl can act as an AHR agonist (Bohonowych and Denison 2007; Denison et al. 1998), we compared the in ovo susceptibility of the two populations to cardiac teratogenesis and CYP induction mediated by carbaryl. Use of these insecticides as probes of the resistance will help to demonstrate if the contaminant adaptation exhibited by Elizabeth River killifish is broad or narrow and AHR-focused.
Materials and methods
Fish
Adult killifish from the PAH-adapted population were collected with wire mesh minnow traps at the Atlantic Wood Industries Superfund Site (36°48′ 27.2″ N, 76°17′38.1″ W). Adult killifish from a reference population were collected on King's Creek, a relatively uncontaminated tributary of the Severn River, Virginia, USA (37°18′16.2″N, 76° 24′58.9″W). After transport to the laboratory, fish were maintained in 20‰ artificial sea water (ASW; Instant Ocean, Foster & Smith, Rhinelander, WI, USA), at 23-25 °C, on a 14:10 light:dark cycle. They were fed pelleted fish feed ad libitum (Aquamax ® Fingerling Starter 300, PMI Nutritional International, LLC, Brentwood, MO, USA). All experiments were conducted with F1 offspring of wild-caught Elizabeth River and King's Creek adults, obtained by manual spawning as described previously (Clark et al. 2010). For experiments utilizing larvae, embryos were maintained in Petri dishes (VWR International, West Chester, PA, USA) lined with absorbent filter paper (No. 3MM chromatography paper, Whatman International Ltd., Maidstone, England). Enough ASW was added to the dishes to keep the embryos moist but not completely submerged. Embryos were maintained in an incubator for 12-14 days at 27 °C. For hatching, more ASW was added to the dishes, the absorbent paper was removed, and the dishes were gently rocked in a shaker. After hatching, larvae were maintained in ASW in the incubator at 27 °C and fed Artemia nauplii. All larval experiments were initiated at five days post hatch (dph). All adult care, reproductive, and rearing techniques were non-invasive and approved by the Duke University Institutional Animal Care & Use Committee (A234-07-08).
Chemicals and dosing
β-naphthoflavone (BNF), ethoxyresorufin, dimethyl sulfoxide (DMSO), chlorpyrifos, permethrin, carbaryl, and fenvalerate were purchased from Sigma-Aldrich (St. Louis, MO, USA). Stocks were prepared by dissolving chlorpyrifos, permethrin, fenvalerate, carbaryl, or BNF in DMSO. For both larval and in ovo experiments, dosing solutions were prepared in 20‰ ASW. Larval exposures were conducted in 5 mL of dosing solution (embryo exposures in 10 mL), with final concentrations of 5 μg/L and 10 μg/L of chlorpyrifos, 400 μg/L and 600 μg/L permethrin, 1 mg/L and 10 mg/L carbaryl, and 10 μg/L and 25 μg/L fenvalerate. In treatments with BNF co-exposure, the final concentration of BNF was 1 μg/L. These challenge doses were based on values from the literature and range-finding experiments with small numbers of individuals (data not shown). Control groups were exposed to DMSO at a concentration (v/v) equal to that in the appropriate dosed groups for a given experiment; in all cases DMSO concentrations were held at <0.03% across all treatments.
Larval susceptibility – chlorpyrifos, permethrin, fenvalerate, and carbaryl with and without co-exposure to BNF
To determine the acute response to neurotoxic pesticides, larvae (5 dph) from each population were placed in 20-mL glass scintillation vials (VWR, West Chester, PA, USA) in groups of five larvae per vial with approximately 5 mL of ASW. Larvae were allowed to acclimate for approximately 30 minutes; all individuals that died due to handling or appeared to be in distress were replaced. For dosing, the original ASW was removed from the scintillation vial using a transfer pipet (VWR, West Chester, PA, USA) and replaced with 5 mL of the appropriate dosing solution. Following dosing, the larvae were again observed briefly for stress or mortality due to handling and were replaced if necessary. Larvae were maintained in an incubator at 27 °C for 24 hours, after which percent mortality in each vial was recorded. Initial experiments investigated the response to chlorpyrifos, permethrin, and carbaryl. Additional experiments were added with fenvalerate because of unexpected results observed with permethrin. Each experiment consisted of n ≥ 15 vials per treatment group (5 larvae per vial). Each experiment was performed three times.
In ovo response to carbaryl
To investigate the ability of carbaryl to induce CYP1 activity and generate cardiac deformities, embryos from each population were dosed individually in 10 mL of solution in 20-mL glass scintillation vials. Embryos were exposed to DMSO or carbaryl beginning at 24 hours post fertilization (hpf) through 144 hpf. In addition, all dosing solutions contained 21 μg/L ethoxyresorufin, a substrate for the ethoxyresorufin-o-deethylase (EROD) assay. EROD activity was assessed at 96 hpf and embryos were screened for cardiac deformity at 144 hpf. All experiments consisted of n ≥ 10 individuals per treatment group (5 larvae per vial). Each experiment was performed three times.
EROD assay
CYP1 activity was measured via the in ovo EROD assay modified from Nacci et al. (1998). In brief, embryos were exposed to ethoxyresorufin with dosing solutions, as described in section 4.2.4. Cleavage of the ethoxy group by CYP1s yielded resorufin, a fluorescent product, which collected in the urinary bladder of the embryo. At 96 hpf, the fluorescence due to resorufin in the urinary bladder was visualized using fluorescent microscopy (50× magnification, rhodamine red filter set; Axioskop, Zeiss, Thornwood, NY, USA). EROD activity was measured as intensity of fluorescence within the bladder normalized to intensity in a region outside the bladder and quantified using IPLab software (BD Biosciences, Rockville, MD, USA).
Deformity assessment
Embryos dosed with carbaryl at 24 hpf were scored for cardiac deformities at 144 hpf. Deformity assessment was performed blind using a scale shown in detail previously (Matson et al. 2008). The scale consists of three scores categorized as normal (0), mild deformities (1), and severe deformities (2).
Determination of pesticides in field site sediments
Sediment was collected at each field site concurrent with fish capture. Sediments were transported in glass jars and stored at 4 °C. The sediments were analyzed following the methods of Hintzen et al. (2009) for a suite of common use pesticides, including multiple pyrethroids, organophosphates, and the compounds used in the current study. Briefly, samples (20 g) were extracted by Soxhlet extractors using methylene chloride. Extracts were evaporated to 5 ml and passed through 1 g silica gel cartridges. The cartridges were eluted with 1:1 ethyl ether:hexane to recover pesticides while leaving some interfering compounds behind. The samples were then evaporated to near dryness and re-eluted in 1.0 ml of hexane. Analysis was conducted by GC/MS using electron ionization and 3-ion sim. Calibration was performed using internal standards. Mean extraction recovery for all analytes was between 70-120% for all analytes.
Statistical analyses
All analyses were performed using JMP 8.0 (SAS Institute Inc, Cary, NC, USA). For all analyses, the individual vial was the unit of replication. Data were analyzed by non-parametric analysis of variance (ANOVA), followed by least square means (LSMeans) procedures. As stated previously, the experiments were repeated a minimum of three times. Experiment was included as a factor in the initial analysis to determine if the experimental replicates could be combined. No statistical differences between the experimental replicates were observed for any of the assays, so the data presented are from the combination of all experimental replicates. For post hoc comparisons, Tukey-adjusted pairwise comparisons were conducted to determine differences between all groups. For in ovo carbaryl exposure, Dunnett's adjustment was used to make post hoc comparisons to the appropriate control group. Statistical significance was accepted at p≤0.05 for all tests.
Results
Larval susceptibility – chlorpyrifos, permethrin, and carbaryl with BNF co-exposure
As hypothesized, chlorpyrifos was significantly more toxic to King's Creek than Elizabeth River larvae (10 μg/L, p<0.0001), and co-exposure to BNF to induce CYP significantly increased mortality only in King's Creek larvae (Figure 1). Interestingly, co-exposure to BNF did not cause increased mortality at 5 μg/L chlorpyrifos in King's Creek larvae, even though it demonstrated the ability to increase mortality at the higher dose of chlorpyrifos. As with chlorpyrifos, permethrin was significantly more toxic to King's Creek larvae than to Elizabeth River larvae (p<0.0001, both doses) (Figure 2). In contrast to chlorpyrifos but as predicted, BNF co-exposure significantly decreased the toxicity of permethrin in King's Creek larvae (p<0.0001, both doses). However, co-exposure to BNF had no effect on the mortality of Elizabeth River larvae exposed to either dose of permethrin. Similarly, carbaryl was more toxic to King's Creek larvae than to Elizabeth River larvae (10 mg/L, p<0.0001) (Figure 3). Unlike in the other challenges, co-exposure to BNF only resulted in a slight increase in the toxicity of 10 mg/L carbaryl to Elizabeth River larvae (p=0.0337).
Fig. 1.
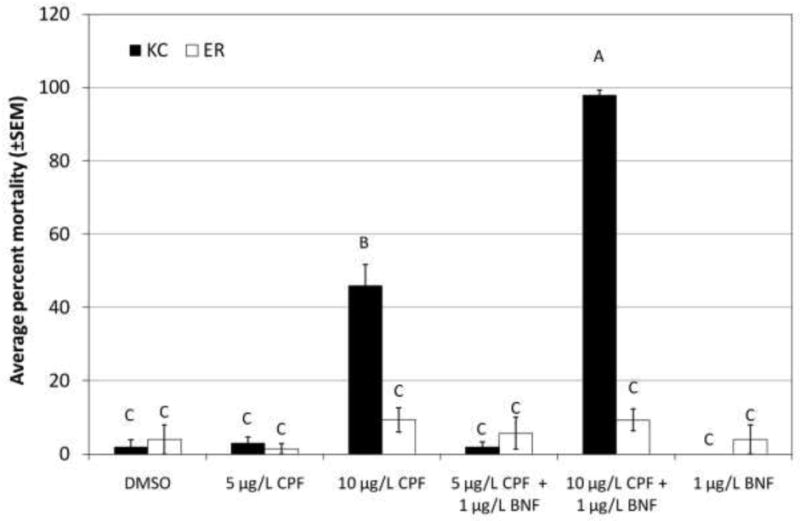
Response of King's Creek and Elizabeth River larvae exposed to chlorpyrifos.
Average percent mortality (±SEM) of King's Creek (black bars) and Elizabeth River (white bars) 5 dph larvae exposed for 24 h to 5 μg/L chlorpyrifos (CPF) or 10 μg/L chlorpyrifos, alone or in combination with 1 μg/L β-naphthoflavone (BNF). Bars not marked by the same letter are significantly different at p <0.05 (ANOVA, Tukey-adjusted LSMeans). n≥45 per treatment group.
Fig. 2.
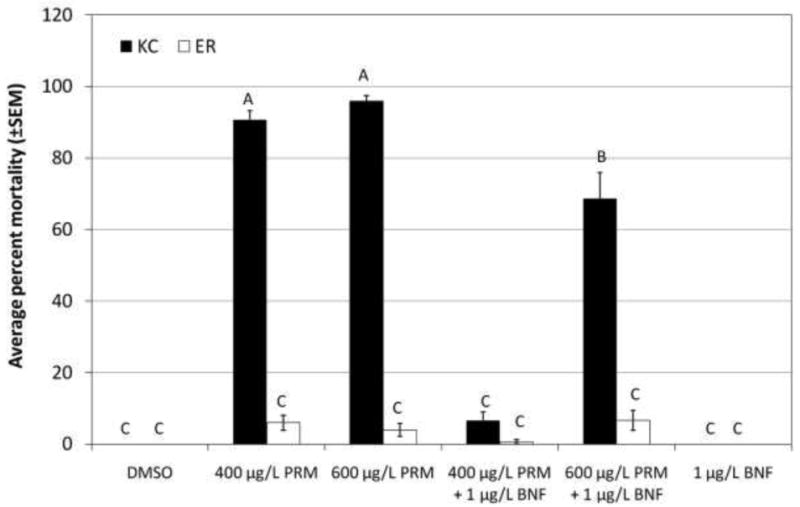
Response of King's Creek and Elizabeth River larvae exposed to permethrin.
Average percent mortality (±SEM) of King's Creek (black bars) and Elizabeth River (white bars) 5 dph larvae exposed for 24 h to 400 μg/L permethrin (PRM) or 10 μg/L permethrin, alone or in combination with 1 μg/L β-naphthoflavone (BNF). Bars not marked by the same letter are significantly different at p <0.05 (ANOVA, Tukey-adjusted LSMeans). n≥45 per treatment group.
Fig. 3.
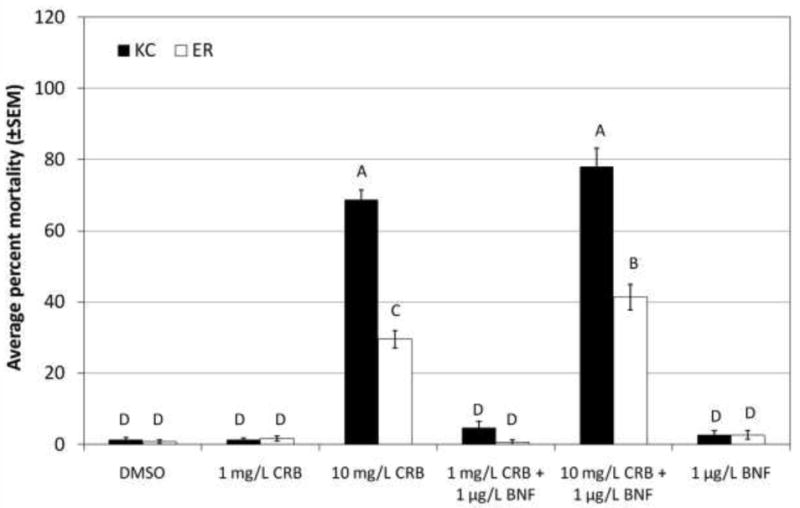
Response of King's Creek and Elizabeth River larvae exposed to carbaryl.
Average percent mortality (±SEM) of King's Creek (black bars) and Elizabeth River (white bars) 5 dph larvae exposed for 24 h to 1 mg/L carbaryl (CRB) or 10 mg/L carbaryl, alone or in combination with 1 μg/L β-naphthoflavone (BNF). Bars not marked by the same letter are significantly different at p <0.05 (ANOVA, Tukey-adjusted LSMeans). n≥45 per treatment group.
In ovo response to carbaryl
As in the larval experiments, carbaryl was more toxic to King's Creek embryos than Elizabeth River embryos (p<0.05, both doses) (Figure 4). In addition, the CYP1 response as measured by EROD activity was greatly elevated in King's Creek embryos at both 1 mg/L and 10 mg/L (p=0.0327 and p<0.0001, respectively), but only elevated in Elizabeth River embryos exposed to 10 mg/L carbaryl (p<0.0001).
Fig. 4.
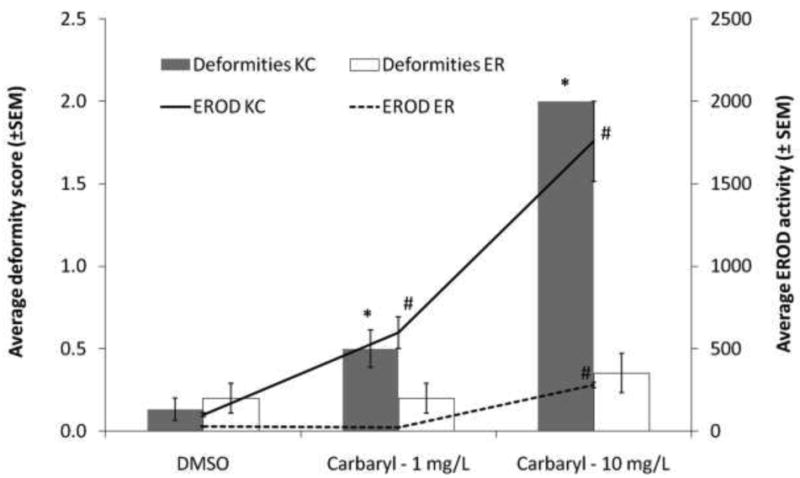
Ethoxyresorufin-o-deethylase (EROD) and deformity response of King's Creek and Elizabeth River embryos exposed to carbaryl.
Response of King's Creek (black bars and solid line) and Elizabeth River (white bars and dashed line) embryos exposed to 1 mg/L carbaryl or 10 mg/L carbaryl from 24 to 144 hours post fertilization (hpf). CYP1 activity was measured by the in ovo ethoxyresorufin-o-deethylase (EROD) assay at 96 hpf; lines show average EROD activity (±SEM) expressed as percent of King's Creek DMSO-dosed control EROD activity. Cardiac deformities were scored blind on a three point scale (0 = normal heart, 1 = mild deformity, 2 = severe deformity) at 144 hpf; bars show average deformity score (±SEM). Bars marked by * are significantly different from the corresponding DMSO-dosed control at p<0.05 (ANOVA, Dunnett's adjustment). EROD points marked by # are significantly different at p<0.05 (ANOVA, Dunnett's adjustment). n=30 per treatment group.
Determination of pesticides in field site sediments
The concentrations of all of the pesticides in the sediments were below reporting limits, indicating that prior exposure to these compounds of parental killifish from either population was unlikely.
Discussion
In these experiments, we further defined the chemical tolerance exhibited by Elizabeth River killifish. As probes of the adaptation, we used insecticides for which the primary modes of action differed greatly from those of the PAHs, but that were likely to interact with one of the major aspects of the PAH adaptation (recalcitrance to CYP induction). We expected that these challenges would increase our understanding of the mechanisms underlying the adaptation itself and provide an example of potential consequence of PAH-adaptation for response to contaminants other than the aryl hydrocarbons that drive the adaptation.
Larval susceptibility to insecticides
Due to the dramatic suppression of CYP inducibility and activity observed in Elizabeth River killifish (Meyer et al. 2002; Nacci et al. 2010; Van Veld and Westbrook 1995; Wills et al. 2010), we hypothesized that some contaminants would be more or less toxic to Elizabeth River killifish depending on their relative activation or inactivation by CYP. However, challenge of Elizabeth River and King's Creek killifish demonstrated that PAH-adapted Elizabeth River offspring were consistently less susceptible than reference fish to all of the insecticides tested except fenvalerate. At the challenge doses used, King's Creek killifish suffered approximately 3-20 fold higher mortality.
When challenged with the organophosphate insecticide chlorpyrifos, the two populations responded as predicted. Conversion of chlorpyrifos to chlorpyrifos oxon, the active metabolite, is catalyzed by multiple CYP enzymes and the greater mortality of King's Creek larvae was likely due to their greater CYP metabolic activity. This conclusion is supported by the observed ability of BNF co-exposure to potentiate chlorpyrifos toxicity in King's Creek larvae, but not in the non-inducible Elizabeth River larvae. However, CYP2B6 and CYP3A4 are primarily responsible for production of the oxon in humans (Hodgson and Rose 2008). Genes similar to these mammalian P450s have been identified in fish (Goldstone et al. 2010; Jones et al. 2010), but have not been studied in the Elizabeth River killifish population.
Overall, killifish larvae were not as susceptible to permethrin as expected based on literature values for fish toxicity (reviewed in Baser et al. 2003; Coats et al. 1989). Additionally, the carbaryl challenge doses used were notably higher than those used for the other insecticides, but this was consistent with literature reports (Lin et al. 2007; Shea and Berry 1983; Solomon and Weis 1979). In contrast to chlorpyrifos, permethrin and carbaryl are more active in their parent form and can be detoxified by oxidative metabolism, so it was expected that Elizabeth River larvae would exhibit greater mortality upon exposure to those insecticides. However, the relative susceptibility of Elizabeth River and King's Creek larvae did not confirm the original hypothesis. As stated previously, the Elizabeth River larvae exhibited a markedly lower mortality than did King's Creek larvae in response to permethrin and carbaryl. With permethrin, co-exposure to BNF caused a decrease in mortality of King's Creek larvae, demonstrating that CYP induction could play a role in its detoxification. Permethrin is metabolized by oxidative metabolism in fish and rodents (Bradbury and Coats 1989). Additionally, enhanced oxidative metabolism by CYP has contributed to resistance to pyrethroids, including permethrin and fenvalerate, in a variety of insects (Chen et al. 2005; Hardstone et al. 2009; Soderlund et al. 1987; Yang et al. 2004). While previous data have shown that many vertebrate CYP isoforms, including CYP1A, can metabolize carbaryl (Schmidt et al. 2006), co-exposure to BNF did not decrease the toxicity of carbaryl. CYPs have also been implicated in the resistance of insects to carbaryl (Scharf et al. 1999; Zhao et al. 1996). It is worth noting that many of the cases of insecticide resistance involving increased oxidative metabolism have been linked to the CYP6 gene family (Hemingway et al. 2004). The CYP6 family is found exclusively in insects but is phylogenetically related to the CYP3 family found in mammals and in fish, including killifish (McArthur et al. 2003). The activity of CYP3s in Elizabeth River killifish has not been investigated.
Because fenvalerate has similar properties to permethrin, it is interesting that fenvalerate is more toxic and does not exhibit differential toxicity in the two populations (data not shown). Biotransformation of both pyrethroids can occur via hydrolysis at ester bonds and oxidative metabolism in multiple organisms (Ohkawa et al. 1979; Soderlund et al. 1987). However, fenvalerate has a cyano group near the ester linkage. Therefore, one hypothesis is that the ester linkage of permethrin might be the target of differential metabolism in Elizabeth River killifish. Future studies could compare the toxicity of permethrin and cypermethrin, which is structurally identical to permethrin except for the addition of a cyano group at the ester linkage.
The greater tolerance of Elizabeth River larvae to insecticides both activated and detoxified by CYPs indicates that their xenobiotic resistance is not simply based in suppression of CYP activity via AHR pathway down-regulation. As stated previously, a number of other protective mechanisms are elevated in Elizabeth River killifish compared to reference populations and the current work suggests that these factors play an important role in xenobiotic resistance in Elizabeth River offspring.
In addition to the dramatic down-regulation of the Phase I metabolizing enzyme CYP, a number of Phase II enzymes are elevated in Elizabeth River killifish. Van Veld et al. (1991) noted that hepatic and intestinal GST activity was elevated 3-4 fold in intestine, liver, and liver lesions of adult Elizabeth River killifish compared to reference killifish. Along with increased GST activity, elevated levels of GST protein have been observed (Armknecht et al. 1998). In addition, Elizabeth River killifish exhibited elevation of UDP-glucuronosyltransferase (UGT) activity and a slight elevation in sulfotransferase (SULT) activity (Gaworecki et al. 2004).
It is possible that upregulation of Phase II enzymes could play a significant role in the observed pesticide resistance of Elizabeth River killifish larvae. GSTs have been shown to participate in O-dealkylation and O-dearylation of organophosphates (Chiang and Sun 1993; Oppenoorth et al. 1979). GSTs have not been shown to have as direct a role in metabolism of pyrethroids, but they may play a role in the conjugation of the products of ester cleavage. GST has been implicated in invertebrate resistance to a number of insecticide classes, including organophosphates, carbamates, and pyrethroids (Hemingway et al. 1991; Kostaropoulos et al. 2001; Wu et al. 1998; Yu and McCord 2007).
Another protective factor that may contribute to insecticide resistance in Elizabeth River killifish is the efflux transporter P-glycoprotein (Pgp), also known as ATP-binding cassette transporter B1 (ABCB1). Although they have been identified in a variety of normal tissues, they are frequently elevated in multidrug-resistant cell lines and chemotherapy-resistant tumors (Litman et al. 2001). Cooper et al. (1999) demonstrated that Pgp was expressed 2-3 fold higher in liver and liver tumors of adult Atlantic Wood fish than in reference killifish.
It is possible that the elevated Pgp observed in PAH-resistant killifish could be protective against a number of pesticides. Pgp has been shown to transport a wide variety of pesticides, including the pyrethroids cypermethrin and fenvalerate, the organophosphate methyl parathion, and the carbamate thiodicarb (reviewed in Buss and Callaghan 2008). Furthermore, Pgp is suspected to play a role in pesticide resistance of a number of insect populations (Buss et al. 2002; Lanning et al. 1996; Srinivas et al. 2004) Overall, the literature suggests that the potential contribution of Pgp to pesticide resistance in Elizabeth River killifish deserves further investigation. However, the heritability of increased Pgp expression has not been investigated in Elizabeth River killifish, so it is unclear if Pgp could play a role in the observed resistance of F1 larvae in the current study.
Ideally, we could begin to differentiate between the potential mechanisms underlying resistance to the pesticides by measuring the presence of the parent compounds and metabolites in tissues. For example, Elizabeth River fish might be expected to accumulate greater burdens of parent chlorpyrifos versus the oxon or demonstrate a greater rate of permethrin metabolism. In the current study, measurement of metabolism was not practical, due to the size of the fish (a single 5-day-old killifish larva weighs less than one milligram) and the reported detection limits for the parent pesticides in tissues are in the ng/g range (e.g., Mekebri et al. 2008; Sapozhnikova et al. 2004). Future work could use older, larger fish to better address this issue.
In ovo susceptibility to carbaryl
We performed additional embryonic exposures with carbaryl because there was some evidence that carbaryl was a weak AHR agonist and could cause cardiac teratogenesis similar to that observed with some aryl hydrocarbons. The developmental effects due to carbaryl observed in the current study are only somewhat consistent with other studies. Previous work demonstrated an inconsistent effect of carbaryl on cardiac development in killifish (Weis and Weis 1974). Effects on circulatory development were also observed in exposure of Japanese medaka (Oryzias latipes) to carbaryl (Solomon and Weis 1979). Recent attempts to investigate the effect of carbaryl on development in zebrafish (Danio rerio) did not replicate the gross cardiac teratogenesis, but did demonstrate some pericardial edema, tail malformations, and bradycardia (Lin et al. 2007).
It is unclear if carbaryl is truly an AHR ligand. Ledirac et al. (1997) reported that carbaryl could induce CYP1A1 gene expression in vitro, but did not appear to bind to the AHR. However, Denison and co-workers showed that carbaryl could drive in vitro AHR-dependent luciferase expression, induce AHR to bind to DNA, and compete with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) for AHR binding (Bohonowych and Denison 2007; Denison et al. 1998). The authors estimated that carbaryl was about 300,000-fold less potent than TCDD, a prototypical AHR ligand. The overall weak interaction of carbaryl with the AHR may explain the inconsistent results seen in previous studies and the high doses required to induce EROD activity and cardiac teratogenesis in the current study.
Although effects were only observed at mg/L doses in the current study, carbaryl was clearly able to induce cardiac malformations similar to those previously observed in fish exposed to a variety of AHR agonists (Clark et al. 2010; Prasch et al. 2003; Wassenberg and Di Giulio 2004). In addition, the aryl hydrocarbon-resistant F1 Elizabeth River embryos were resistant to the cardiac teratogenesis caused by carbaryl. Interestingly, 10 mg/L carbaryl induced a roughly 10-fold increase in EROD activity in Elizabeth River embryos. Except for a slight induction of EROD activity by Elizabeth River pore water (Meyer et al. 2002), induction of EROD activity in F1 Elizabeth River embryos has rarely been observed. It is possible that this indicates that carbaryl is able to induce EROD activity through AHR-independent means, or that the mechanism of EROD suppression in the PAH-adapted Elizabeth River killifish is not suited to blocking the effects of high concentrations of carbaryl. However, if these effects are mediated through the AHR, the previously described suppression of the AHR pathway in Elizabeth River killifish probably contributes to the protection from carbaryl observed in Elizabeth River larvae.
Cross-resistance to insecticides associated with PAH-resistance
Very few studies in vertebrates have investigated the effect of contaminant adaptation on response to other chemical stressors, especially classes of contaminants with divergent modes of action. Lab-reared offspring of Elizabeth River killifish demonstrated increased sensitivity to PAH-mediated phototoxicity and hypoxia (Meyer and Di Giulio 2003). In addition, there is some evidence that mosquitofish adapted to exposure to toxaphene and endrin were also resistant to other organochlorine pesticides and to some herbicides (Culley and Ferguson 1969; Fabacher and Chambers 1974). Increased sensitivity to other stressors may be evidence of the fitness costs of adaptation (see Kinnison and Hairston 2007; Wirgin and Waldman 2004). Alternately, differential sensitivity may be tied directly to the mechanism of adaptation and be detrimental or beneficial (e.g., cross-resistance in insects to multiple insecticides). In the current study, no cost in contaminant sensitivity due to development of PAH-resistance was apparent. Instead, adaptation to PAHs appears to have enabled the Elizabeth River population to resist unrelated contaminants. However, we only investigated a few compounds under specific conditions. Further work is necessary to understand the breadth of resistance and the mechanisms underlying the cross-resistance.
Conclusion
In the current study, we demonstrated that a population of fish adapted to chronic PAH pollution is cross-resistant to some of the effects of contaminants for which the primary modes of action differ from those of the PAHs. We used insecticides as probes of the role of CYP suppression in the contaminant resistance of Elizabeth River killifish, and the results suggested that factors other than a lack of CYP activity can play an important role in the population's contaminant resistance. The ability of adaptation to chronic anthropogenic contamination to drive changes in response to xenobiotics with different modes of action demonstrates the ability of complex mixtures of contaminants to alter population response in a relatively broad manner.
Acknowledgments
We gratefully acknowledge the laboratory of Dr. Jason Belden for performing pesticide analyses. We also thank Susannah Butters, Dr. Cole Matson, Lindsey Van Tiem, and Dr. Dawoon Jung for their assistance with exposure and analysis. This work was supported by the National Institute of Environmental Health supported Superfund Research Program (P42ES10356) and Integrated Toxicology Program (T32ES007031).
References
- Armknecht SL, Kaattari SL, Van Veld PA. An elevated glutathione S-transferase in creosote-resistant mummichog (Fundulus heteroclitus) Aquat Toxicol. 1998;41(1-2):1–16. [Google Scholar]
- Baser S, Erkoc F, Selvi M, Kocak O. Investigation of acute toxicity of permethrin on guppies Poecilia reticulata. Chemosphere. 2003;51(6):469–474. doi: 10.1016/S0045-6535(03)00033-X. [DOI] [PubMed] [Google Scholar]
- Bello SM, Franks DG, Stegeman JJ, Hahn ME. Acquired resistance to Ah receptor agonists in a population of Atlantic killifish (Fundulus heteroclitus) inhabiting a marine superfund site: In vivo and in vitro studies on the inducibility of xenobiotic metabolizing enzymes. Toxicol Sci. 2001;60(1):77–91. doi: 10.1093/toxsci/60.1.77. [DOI] [PubMed] [Google Scholar]
- Billiard SM, Timme-Laragy AR, Wassenberg DM, Cockman C, Di Giulio RT. The role of the aryl hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons to zebrafish. Toxicol Sci. 2006;92(2):526–536. doi: 10.1093/toxsci/kfl011. [DOI] [PubMed] [Google Scholar]
- Bohonowych JE, Denison MS. Persistent binding of ligands to the aryl hydrocarbon receptor. Toxicol Sci. 2007;98(1):99–109. doi: 10.1093/toxsci/kfm085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury SP, Coats JR. Toxicokinetics and toxicodynamics of pyrethroid insecticides in fish. Environ Toxicol Chem. 1989;8(5):373–380. [Google Scholar]
- Buss DS, Callaghan A. Interaction of pesticides with p-glycoprotein and other ABC proteins: A survey of the possible importance to insecticide, herbicide and fungicide resistance. Pestic Biochem Physiol. 2008;90(3):141–153. [Google Scholar]
- Buss DS, McCaffery AR, Callaghan A. Evidence for p-glycoprotein modification of insecticide toxicity in mosquitoes of the Culex pipiens complex. Med Vet Entomol. 2002;16(2):218–222. doi: 10.1046/j.1365-2915.2002.00365.x. [DOI] [PubMed] [Google Scholar]
- Chen S, Yang YH, Wu YD. Correlation between fenvalerate resistance and cytochrome P450-mediated O-demethylation activity in Helicoverpa armigera (Lepidoptera : Noctuidae) J Econ Entomol. 2005;98(3):943–946. doi: 10.1603/0022-0493-98.3.943. [DOI] [PubMed] [Google Scholar]
- Chiang FM, Sun CN. Glutathione transferase isozymes of diamondback moth larvae and their role in the degradation of some organophosphorous insecticides. Pestic Biochem Physiol. 1993;45(1):7–14. [Google Scholar]
- Clark BW, Matson CW, Jung D, Di Giulio RT. AHR2 mediates cardiac teratogenesis of polycyclic aromatic hydrocarbons and PCB-126 in Atlantic killifish (Fundulus heteroclitus) Aquat Toxicol. 2010;99:232–240. doi: 10.1016/j.aquatox.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats JR, Symonik DM, Bradbury SP, Dyer SD, Timson LK, Atchison GJ. Toxicology of synthetic pyrethroids in aquatic organisms - an overview. Environ Toxicol Chem. 1989;8(8):671–679. [Google Scholar]
- Cooper PS, Vogelbein WK, Van Veld PA. Altered expression of the xenobiotic transporter P-glycoprotein in liver and liver tumours of mummichog (Fundulus heteroclitus) from a creosote-contaminated environment. Biomarkers. 1999;4(1):48–58. doi: 10.1080/135475099230994. [DOI] [PubMed] [Google Scholar]
- Culley DD, Ferguson DE. Patterns of insecticide resistance in mosquitofish, Gambusia affinis. Journal of the Fisheries Research Board of Canada. 1969;26(9):2395–&. [Google Scholar]
- Denison MS, Phelan D, Winter GM, Ziccardi MH. Carbaryl, a carbamate insecticide, is a ligand for the hepatic Ah (dioxin) receptor. Toxicol Appl Pharmacol. 1998;152(2):406–414. doi: 10.1006/taap.1998.9999. [DOI] [PubMed] [Google Scholar]
- Fabacher DL, Chambers H. Resistance to herbicides in insecticide-resistant mosquitofish, Gambusia affinis. Environ Lett. 1974;7(1):15–20. [Google Scholar]
- Faisal M, Weeks BA, Vogelbein WK, Huggett RJ. Evidence of Aberration of the Natural Cytotoxic-Cell Activity in Fundulus-Heteroclitus (Pisces, Cyprinodontidae) from the Elizabeth River, Virginia. Veterinary Immunology and Immunopathology. 1991;29(3-4):339–351. doi: 10.1016/0165-2427(91)90024-7. [DOI] [PubMed] [Google Scholar]
- Frederick LA, Van Veld PA, Rice CD. Bioindicators of immune function in creosote-adapted estuarine killifish, Fundulus heteroclitus. Journal of Toxicology and Environmental Health-Part a-Current Issues. 2007;70(17):1433–1442. doi: 10.1080/15287390701382910. [DOI] [PubMed] [Google Scholar]
- Gaworecki KM, Rice CD, van den Hurk P. Induction of phenol-type sulfotransferase and glucuronosyltransferase in channel catfish and mummichog. Mar Environ Res. 2004;58(2-5):525–528. doi: 10.1016/j.marenvres.2004.03.041. [DOI] [PubMed] [Google Scholar]
- Goldstone JV, McArthur AG, Kubota A, Zanette J, Parente T, Jonsson ME, et al. Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish. BMC Genomics. 2010;11:21. doi: 10.1186/1471-2164-11-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardstone MC, Leichter CA, Scott JG. Multiplicative interaction between the two major mechanisms of permethrin resistance, kdr and cytochrome P450-monooxygenase detoxification, in mosquitoes. J Evol Biol. 2009;22(2):416–423. doi: 10.1111/j.1420-9101.2008.01661.x. [DOI] [PubMed] [Google Scholar]
- Hemingway J, Hawkes NJ, McCarroll L, Ranson H. The molecular basis of insecticide resistance in mosquitoes. Insect Biochem Mol Biol. 2004;34(7):653–665. doi: 10.1016/j.ibmb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Hemingway J, Miyamoto J, Herath PRJ. A possible novel link between organophosphorous and DDT insecticide resistance genes in Anopheles - supporting evidence from fenitrothion metabolsim studies. Pestic Biochem Physiol. 1991;39(1):49–56. [Google Scholar]
- Hintzen EP, Lydy MJ, Belden JB. Occurrence and potential toxicity of pyrethroids and other insecticides in bed sediments of urban streams in central Texas. Environ Pollut. 2009;157(1):110–116. doi: 10.1016/j.envpol.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Hodgson E, Rose RL. Metabolic interactions of agrochemicals in humans. Pest Manag Sci. 2008;64(6):617–621. doi: 10.1002/ps.1563. [DOI] [PubMed] [Google Scholar]
- Jones HS, Panter GH, Hutchinson TH, Chipman JK. Oxidative and Conjugative Xenobiotic Metabolism in Zebrafish Larvae In Vivo. Zebrafish. 2010;7(1):23–30. doi: 10.1089/zeb.2009.0630. [DOI] [PubMed] [Google Scholar]
- Kinnison MT, Hairston NG. Eco-evolutionary conservation biology: contemporary evolution and the dynamics of persistence. Funct Ecol. 2007;21(3):444–454. [Google Scholar]
- Kostaropoulos I, Papadopoulos AI, Metaxakis A, Boukouvala E, Papadopoulou-Mourkidou E. Glutathione S-transferase in the defence against pyrethroids in insects. Insect Biochem Mol Biol. 2001;31(4-5):313–319. doi: 10.1016/s0965-1748(00)00123-5. [DOI] [PubMed] [Google Scholar]
- Lanning CL, Ayad HM, AbouDonia MB. P-glycoprotein involvement in cuticular penetration of [C-14]thiodicarb in resistant tobacco budworms. Toxicol Lett. 1996;85(3):127–133. doi: 10.1016/0378-4274(96)03654-5. [DOI] [PubMed] [Google Scholar]
- Ledirac N, Delescluse C, deSousa G, Pralavorio M, Lesca P, Amichot M, et al. Carbaryl induces CYP1A1 gene expression in HepG2 and HaCaT cells but is not a ligand of the human hepatic Ah receptor. Toxicol Appl Pharmacol. 1997;144(1):177–182. doi: 10.1006/taap.1997.8120. [DOI] [PubMed] [Google Scholar]
- Lin CC, Hui MNY, Cheng SH. Toxicity and cardiac effects of carbaryl in early developing zebrafish (Danio rerio) embryos. Toxicol Appl Pharmacol. 2007;222(2):159–168. doi: 10.1016/j.taap.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Litman T, Druley TE, Stein WD, Bates SE. From MDR to MXR: new understanding of multidrug resistance systems, their properties and clinical significance. Cell Mol Life Sci. 2001;58(7):931–959. doi: 10.1007/PL00000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson CW, Clark BW, Jenny MJ, Fleming CR, Hahn ME, Di Giulio RT. Development of the morpholino gene knockdown technique in Fundulus heteroclitus: A tool for studying molecular mechanisms in an established environmental model. Aquat Toxicol. 2008;87(4):289–295. doi: 10.1016/j.aquatox.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur A, Hegelund T, Cox R, Stegeman J, Liljenberg M, Olsson U, et al. Phylogenetic analysis of the cytochrome P450 3 (CYP3) gene family. J Mol Evol. 2003;57(2):200–211. doi: 10.1007/s00239-003-2466-x. [DOI] [PubMed] [Google Scholar]
- Mekebri A, Crane DB, Blondina GJ, Oros DR, Rocca JL. Extraction and analysis methods for the determination of pyrethroid insecticides in surface water, sediments and biological tissues at environmentally relevant concentrations. Bull Environ Contam Toxicol. 2008;80(5):455–460. doi: 10.1007/s00128-008-9382-0. [DOI] [PubMed] [Google Scholar]
- Meyer J, Di Giulio R. Patterns of heritability of decreased EROD activity and resistance to PCB 126-induced teratogenesis in laboratory-reared offspring of killifish (Fundulus heteroclitus) from a creosote-contaminated site in the Elizabeth River, VA, USA. Mar Environ Res. 2002;54(3-5):621–626. doi: 10.1016/s0141-1136(02)00170-8. [DOI] [PubMed] [Google Scholar]
- Meyer JN, Di Giulio RT. Heritable adaptation and fitness costs in killifish (Fundulus beteroclitus) inhabiting a polluted estuary. Ecol Appl. 2003;13(2):490–503. [Google Scholar]
- Meyer JN, Nacci DE, Di Giulio RT. Cytochrome P4501A (CYP1A) in killifish (Fundulus heteroclitus): Heritability of altered expression and relationship to survival in contaminated sediments. Toxicol Sci. 2002;68(1):69–81. doi: 10.1093/toxsci/68.1.69. [DOI] [PubMed] [Google Scholar]
- Meyer JN, Wassenberg DM, Karchner SI, Hahn ME, Di Giulio RT. Expression and inducibility of aryl hydrocarbon receptor pathway genes in wild-caught killifish (Fundulus heteroclitus) with different contaminant-exposure histories. Environ Toxicol Chem. 2003;22(10):2337–2343. doi: 10.1897/02-495. [DOI] [PubMed] [Google Scholar]
- Mulvey M, Newman MC, Vogelbein W, Unger MA. Genetic structure of Fundulus heteroclitus from PAH-contaminated and neighboring sites in the Elizabeth and York Rivers. Aquat Toxicol. 2002;61(3-4):195–209. doi: 10.1016/s0166-445x(02)00055-3. [DOI] [PubMed] [Google Scholar]
- Nacci D, Champlin D, Jayaraman S. Adaptation of the Estuarine Fish Fundulus heteroclitus (Atlantic Killifish) to Polychlorinated Biphenyls (PCBs) Estuar Coasts. 2010;33(4):853–864. [Google Scholar]
- Nacci D, Coiro L, Champlin D, Jayaraman S, McKinney R, Gleason TR, et al. Adaptations of wild populations of the estuarine fish Fundulus heteroclitus to persistent environmental contaminants. Mar Biol. 1999;134(1):9–17. [Google Scholar]
- Nacci D, Coiro L, Kuhn A, Champlin D, Munns W, Specker J, et al. Nondestructive indicator of ethoxyresorufin-O-deethylase activity in embryonic fish. Environ Toxicol Chem. 1998;17(12):2481–2486. [Google Scholar]
- Ohkawa H, Kaneko H, Tsuji H, Miyamoto J. Metabolsim of fenvalerate (Sumicidin) in rats. J Pestic Sci. 1979;4(2):143–155. [Google Scholar]
- Oppenoorth FJ, Vanderpas LJT, Houx NWH. Glutathione-s-transferase and hydrolytic activity in a tetrachlorvinphos-resistant strain of housefly and their influence on resistance. Pestic Biochem Physiol. 1979;11(1-3):176–188. [Google Scholar]
- Ownby DR, Newman MC, Mulvey M, Vogelbein WK, Unger MA, Arzayus LF. Fish (Fundulus heteroclitus) populations with different exposure histories differ in tolerance of creosote-contaminated sediments. Environ Toxicol Chem. 2002;21(9):1897–1902. [PubMed] [Google Scholar]
- Prasch AL, Teraoka H, Carney SA, Dong W, Hiraga T, Stegeman JJ, et al. Aryl hydrocarbon receptor 2 mediates 2,3,7,8-tetrachlorodibenzo-p-dioxin developmental toxicity in zebrafish. Toxicol Sci. 2003;76(1):138–150. doi: 10.1093/toxsci/kfg202. [DOI] [PubMed] [Google Scholar]
- Prince R, Cooper KR. Comparisons of the effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on chemically impacted and nonimnpacted subpopulations of Fundulus heteroclitus 2. Metabolic considerations. Environ Toxicol Chem. 1995;14(4):589–595. [Google Scholar]
- Roy NK, Courtenay S, Maxwell G, Yuan ZP, Chambers RC, Wirgin I. Cytochrome P4501A1 is induced by PCB 77 and benzo[a] pyrene treatment but not by exposure to the Hudson River environment in Atlantic tomcod (Microgadus tomcod) post-yolk sac larvae. Biomarkers. 2002;7(2):162–173. doi: 10.1080/13547500110113981. [DOI] [PubMed] [Google Scholar]
- Sapozhnikova Y, Bawardi O, Schlenk D. Pesticides and PCBs in sediments and fish from the Salton Sea, California, USA. Chemosphere. 2004;55(6):797–809. doi: 10.1016/j.chemosphere.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Scharf ME, Meinke LJ, Wright RJ, Chandler LD, Siegfried BD. Metabolism of carbaryl by insecticide-resistant and -susceptible western corn rootworm populations (Coleoptera : Chrysomelidae) Pestic Biochem Physiol. 1999;63(2):85–96. [Google Scholar]
- Schmidt B, Faymonville T, Gembe E, Joussen N, Schuphan I. Comparison of the biotransformation of the C-14-labelled insecticide carbaryl by non-transformed and human CYP1A1-, CYP1A2-, and CYP3A4-transgenic cell cultures of Nicotiana tabacum. Chem Biodivers. 2006;3(8):878–896. doi: 10.1002/cbdv.200690091. [DOI] [PubMed] [Google Scholar]
- Shea TB, Berry ES. Toxicity of carbaryl and 1-naphthol to goldfish (Carassius auratus) and killifish (Fundulus heteroclitus) Bull Environ Contam Toxicol. 1983;31(5):526–529. doi: 10.1007/BF01605469. [DOI] [PubMed] [Google Scholar]
- Soderlund DM, Hessney CW, Jiang M. Metabolism of fenvalerate by resistant Colorado potato beetles. J Agric Food Chem. 1987;35(1):100–105. [Google Scholar]
- Solomon HM, Weis JS. Abnormal circulatory development in medaka caused by the insecticides carbaryl, malathion and parathion. Teratology. 1979;19(1):51–62. doi: 10.1002/tera.1420190109. [DOI] [PubMed] [Google Scholar]
- Srinivas R, Udikeri SS, Jayalakshmi SK, Sreeramulu K. Identification of factors responsible for insecticide resistance in Helicoverpa armigera. Comparative Biochemistry and Physiology C-Toxicology & Pharmacology. 2004;137(3):261–269. doi: 10.1016/j.cca.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Van Veld PA, Ko UC, Vogelbein WK, Westbrook DJ. Glutathione-s-transferase in intestine, liver and hepatic lesions of mummichog (Fundulus heteroclitus) from a creosote-contaminated environment. Fish Physiol Biochem. 1991;9(4):369–376. doi: 10.1007/BF02265157. [DOI] [PubMed] [Google Scholar]
- Van Veld PA, Westbrook DJ. Evidence for depression of cytochrome P4501A in a population of chemically resistant mummichog (Fundulus heteroclitus) Environ Sci. 1995;3:221–234. [Google Scholar]
- Vogelbein W, Unger MA. The Elizabeth River Monitoring Program 2001-2002: Association Between Mummichog Liver Histopathology and Sediment Chemical Contamination. 2003 Final Report. [Google Scholar]
- Vogelbein WK, Fournie JW, Vanveld PA, Huggett RJ. Hepatic neoplasms in the mummichog Fundulus heteroclitus from a creosote-contaminated site. Cancer Res. 1990;50(18):5978–5986. [PubMed] [Google Scholar]
- Walker SE, Dickhut RM, Chisholm-Brause C. Polycyclic aromatic hydrocarbons in a highly industrialized urban estuary: Inventories and trends. Environ Toxicol Chem. 2004;23(11):2655–2664. doi: 10.1897/03-628. [DOI] [PubMed] [Google Scholar]
- Wassenberg DM, Di Giulio RT. Synergistic embryotoxicity of polycyclic aromatic hydrocarbon aryl hydrocarbon receptor agonists with cytochrome P4501A inhibitors in Fundulus heteroclitus. Environ Health Perspect. 2004;112(17):1658–1664. doi: 10.1289/ehp.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks BA, Warinner JE, Mathews ES. Influence of toxicants on phagocytosis, pinocytosis and melanin accumulation by fish macrophages. Aquat Toxicol. 1988;11(3-4):424–424. [Google Scholar]
- Wills LP, Matson CW, Landon CD, Di Giulio RT. Characterization of the recalcitrant CYP1 phenotype found in Atlantic killifish (Fundulus heteroclitus) inhabiting a Superfund site on the Elizabeth River, VA. Aquat Toxicol. 2010;99(1):33–41. doi: 10.1016/j.aquatox.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirgin I, Waldman JR. Resistance to contaminants in North American fish populations. Mutat Res-Fundam Mol Mech Mutag. 2004;552(1-2):73–100. doi: 10.1016/j.mrfmmm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Wu DX, Scharf ME, Neal JJ, Suiter DR, Bennett GW. Mechanisms of fenvalerate resistance in the German cockroach, Blattella germanica (L.) Pestic Biochem Physiol. 1998;61(1):53–62. [Google Scholar]
- Yang Y, Wu Y, Chen S, Devine GJ, Denholm I, Jewess P, et al. The involvement of microsomal oxidases in pyrethroid resistance in Helicoverpa armigera from Asia. Insect Biochem Mol Biol. 2004;34(8):763–773. doi: 10.1016/j.ibmb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Yu SJ, McCord E. Lack of cross-resistance to indoxacarb in insecticide-resistant Spodoptera frugiperda (Lepidoptera : Noctuidae) and Plutella xylostella (Lepidoptera : Yponomeutidaie) Pest Manag Sci. 2007;63(1):63–67. doi: 10.1002/ps.1309. [DOI] [PubMed] [Google Scholar]
- Zhao GY, Rose RL, Hodgson E, Roe RM. Biochemical mechanisms and diagnostic microassays for pyrethroid, carbamate, and organophosphate insecticide resistance/cross-resistance in the tobacco budworm, Heliothis virescens. Pestic Biochem Physiol. 1996;56(3):183–195. [Google Scholar]


