Abstract
In many species, including Syrian hamsters, the generation of male reproductive behavior depends critically on the perception of female odor cues from conspecifics in the environment. The behavioral response to these odors is mediated by a network of steroid-sensitive ventral forebrain nuclei including the medial amygdala (MA), posterior bed nucleus of the stria terminalis (BNST) and medial preoptic area (MPOA). Previous studies have demonstrated that each of these three nuclei is required for appropriate sexual behavior and that MA preferentially sends female odor information directly to BNST and MPOA. It is unknown, however, how the functional connections between MA and BNST and/or MPOA are organized to generate different aspects of reproductive behavior. Therefore, the following experiments used the asymmetrical pathway lesion technique to test the role of the functional connections between MA and BNST and/or MPOA in odor preference and copulatory behaviors. Lesions that functionally disconnected MA from MPOA eliminated copulatory behavior but did not affect odor preference. In contrast, lesions that functionally disconnected MA from BNST eliminated preference for volatile female odors but did not affect preference for directly contacted odors or copulatory behavior. These results therefore demonstrate a double dissociation in the functional connections required for attraction to volatile sexual odors and copulation and, more broadly, suggest appetitive and consummatory reproductive behaviors are mediated by distinct neural pathways.
Keywords: Medial amygdala, Bed nucleus of the stria terminalis, Medial preoptic area, Odor Preference, Copulation, Pheromone, Sex
Introduction
In most animals, appropriate reproductive behavior depends critically on the perception of chemical cues from conspecifics in the environment (Baum and Kelliher, 2009; Johnston, 1990). Syrian hamsters (Mesocricetus auratus) are a prominent model species for studying the neural regulation of chemosensory-guided reproductive behaviors because their reproductive behavior is well characterized, stereotyped, and depends critically on the perception of a limited set of chemosensory cues (Landauer et al., 1977; Murphy and Schneider, 1970). Specifically, the correct detection, identification, and valuation of sex-specific odors are needed for both appetitive and consummatory aspects of reproductive behavior in male Syrian hamsters (Murphy and Schneider, 1970; Powers et al., 1979; Powers and Winans, 1975), as well as in other mammals (Rowe and Edwards, 1972; Sachs et al., 1994; Wysocki et al., 1982). Sex-specific odors required for reproductive behaviors are processed by the main (MOB) and accessory (AOB) olfactory bulbs and subsequently integrated in the medial amygdala (MA) (Keller et al., 2009; Scalia and Winans, 1975). MA plays a critical role in odor-guided reproductive behaviors, as neurons in MA are activated during exposure to female odors and copulatory behavior (Fernandez-Fewell and Meredith, 1994), and lesions of MA impair sexual behavior in many rodent species (Heeb and Yahr, 2000; Kondo, 1992; Kondo et al., 1997; Newman, 1999; Petrulis and Johnston, 1999; Stark et al., 1998). Specifically, in male hamsters, large lesions of MA eliminate copulatory behavior and greatly reduce anogenital investigation of a receptive female (Lehman et al., 1980), and more targeted lesions of either the anterior or posterodorsal subnuclei of MA eliminate preference for volatile female odors (Maras and Petrulis, 2006).
From MA, sexual odor information is conveyed to the posterior bed nucleus of the stria terminalis (BNST) and/or the medial preoptic area (MPOA) (Wood, 1997). Specifically, MOB and AOB input that converges on MA (Davis et al., 1978) can be directly sent to BNST and/or MPOA (Coolen and Wood, 1998; Dong et al., 2001; Gomez and Newman, 1992; Simerly and Swanson, 1986), while chemosensory information received by BNST (either directly from AOB or indirectly from MA) can also terminate at MPOA (Davis et al., 1978; Dong and Swanson, 2004; Gomez and Newman, 1992; Wood and Swann, 2005). We have demonstrated that MA preferentially sends female odor information directly to BNST and MPOA, whereas BNST relays male and female odor information to MPOA without sexual specificity (Been and Petrulis, 2011), suggesting that direct MA connections to MPOA and BNST may be the most critical for odor-guided sexual behaviors. Like MA, BNST and MPOA are also critically involved in generating appropriate odor-guided reproductive behaviors. Lesions of MPOA eliminate preference for volatile opposite-sex odors (Been and Petrulis, 2010b) and severely impair or eliminate copulatory behavior in male hamsters (Been and Petrulis, 2010b) and nearly every vertebrate species studied to date (Hull et al., 2002). In comparison, lesions of BNST eliminate male hamsters’ preference for volatile opposite-sex odors and decrease investigation of directly contacted female odors (Been and Petrulis, 2010a), without causing dramatic changes in copulatory behavior in male hamsters and other rodents (Been and Petrulis, 2010a; Claro et al., 1995; Liu et al., 1997). MA, BNST, and MPOA each contain dense populations of steroid-sensitive neurons (Wood et al., 1992; Wood and Newman, 1995a) and site-specific implants of testosterone into either MA or BNST/MPOA restore reproductive behavior in castrated males (Wood and Newman, 1995c). Furthermore, removal of the olfactory bulb ipsilateral to this testosterone implant prevents this restoration of copulatory behavior, suggesting that chemosensory and hormonal cues are integrated within MA (Wood and Coolen, 1997) and BNST/MPOA (Wood and Newman, 1995b), and that this integration is required for the expression of reproductive behavior.
We therefore hypothesized that the functional connections between MA and MPOA are required for both sexual odor preference and copulatory behavior, whereas the functional connections between MA and BNST are only required for sexual odor preference and not copulation. To test these hypotheses, we used the asymmetrical pathway lesion technique to functionally disconnect MA from either MPOA (MAMPOA-X) or BNST (MA-BNST-X). This technique takes advantage of the primarily ipsilateral connections between MA, BNST and MPOA (Kevetter and Winans, 1981; Simerly and Swanson, 1986; Wang and Swann, 2006; Wood and Swann, 2005) and the finding that unilateral lesions of MA (Maras and Petrulis, 2006), BNST (Been and Petrulis, 2010a), or MPOA (Been and Petrulis, 2010b) do not disrupt odor preference or copulation in male Syrian hamsters. Thus, unilateral lesions of two of these nuclei in contralateral brain hemispheres (CONTRA) result in a functional disconnection, in which two nuclei within a hemisphere are prevented from communicating with each other, but a sufficient amount of each nucleus to generate behavior remains. In contrast, unilateral lesions of the two nuclei within the same hemisphere (IPSI) remove the same total volume of nuclei as in CONTRA males, but leave the functional connections intact in one hemisphere. We found that lesions that functionally disconnect MA from MPOA virtually eliminate copulation but have no effect on odor preference; in contrast, lesions that functionally disconnect MA from BNST eliminate volatile odor preference, but do not impact copulatory behavior. These results therefore demonstrate that the neural pathways mediating sexual odor preference and copulatory behavior are functionally and anatomically dissociated.
Materials and Methods
Animals
Adult male Syrian hamsters (n = 120) were purchased from Harlan Laboratories (Prattville, AL, USA) at 110–120 g and singly housed. We have previously demonstrated that bilateral lesions of BNST decrease attraction to female odors and delay copulation in sexually-naïve, but not sexually experienced, male hamsters (Been and Petrulis, 2010a). Similarly, bilateral lesions of MPOA eliminate volatile odor preference and copulatory behavior in sexually-naïve males only (Been and Petrulis, 2010b). Therefore, to maximize the likelihood of observing post-lesion behavioral deficits in the current study, all subjects were sexually naïve.
A separate set of group-housed (3–4 same-sex animals per cage), gonadally intact, adult male and female hamsters (Harlan Laboratories; n = 80) were used to provide odor stimuli. A third group of sexually experienced adult female hamsters (Harlan Laboratories; n = 20) were used as stimulus females for copulatory behavior tests. Animals were maintained on a reversed 16-h light/8-h dark photoperiod (lights off/on at 10 AM/6 PM). Food and water were available ad libitum. All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23; revised 1996) and were approved by the Georgia State University Institutional Animal Care and Use Committee. All efforts were made to minimize the number of animals used and their suffering.
Surgery
All surgeries were performed under 2% isoflurane gas anesthesia vaporized in 100% oxygen (gonadectomy) or a 70:30% oxygen/nitrous oxide mixture (stereotaxic surgery). To minimize post-operative pain, ketoprofen (5 mg/kg subcutaneously, Henry Schein, Melville, NY, USA) was administered intra-operatively.
Gonadectomy and Hormone Implant
Exposure to female odors causes an increase in circulating testosterone levels in male hamsters (Macrides et al., 1974; Pfeiffer and Johnston, 1992) and it is possible that lesions of MA, BNST, and/or MPOA may interfere with this surge. Therefore, to equalize steroid hormone levels across experimental groups, all subjects were gonadectomized and maintained on physiological levels of testosterone during the experiment (Been and Petrulis, 2010a; Been and Petrulis, 2010b; Maras and Petrulis, 2006; Maras and Petrulis, 2008). Briefly, subjects’ testes were bilaterally removed via a midline abdominal incision and cauterization of the ductus deferens and blood vessels. Silastic capsules (i.d. 1.57 mm, o.d. 2.41 mm, Dow Corning, Midland, MI, USA) packed with 20 mm length of crystalline testosterone (Sigma, St. Louis, MO, USA) were implanted subcutaneously between the scapulae immediately following gonadectomy.
Copulatory stimulus females’ ovaries were removed via bilateral flank incisions and cauterization of the uterine horn and blood vessels. Silastic capsules (i.d. 1.57 mm, o.d. 2.41 mm) packed with 5 mm length of crystalline estradiol (Sigma, St. Louis, MO, USA) were implanted subcutaneously between the scapulae immediately following gonadectomy (Been and Petrulis, 2010a; Been and Petrulis, 2010b). To induce behavioral receptivity, stimulus females were injected subcutaneously with 0.15 ml of progesterone dissolved in sesame oil (2.5 mg/ml, Sigma, St. Louis, MO, USA) 4 hours prior to copulatory behavior tests.
Asymmetrical Pathway Lesions
Subjects in MA-MPOA-X and MA-BNST-X experimental groups were randomly assigned to CONTRA, IPSI, or SHAM lesion groups, resulting in six lesion groups. Excitotoxic lesions were made by lowering a microinjection syringe (701R 10 µl syringe, Hamilton, Reno, NV, USA) under stereotaxic control (Microinjection Unit, Model 5002, David Kopf Instruments, Tujunga, CA, USA) into target sites and injecting N-methyl-D-aspartic acid (NMDA, 20 mg/ml, Sigma, St. Louis, MO, USA) (Table 1). In sham surgeries, the injector was lowered to 1 mm above the target and no excitotoxin was injected. Subjects were allowed to recover from lesion surgery for at least one week prior to behavioral testing.
Table 1. Stereotaxic coordinates and injection volumes.
Unilateral, excitotoxic lesions were made by injecting N-methyl-D-aspartic acid (NMDA, 20 mg/ml) into sites targeting MA, BNST, or MPOA. Anterior-posterior (A-P) and medial-lateral (M-L) coordinates are relative to bregma, whereas dorsal ventral (D-V) coordinates are relative to dura.
| A-P (mm) | M-L (mm) | D-V (mm) | NMDA (nl) | |
|---|---|---|---|---|
| MA | − 0.85 | ± 2.85 | − 7.60 | 40 |
| BNST | + 1.85 | ± 1.35 | − 5.90 | 30 |
| MPOA | + 2.00 | ± 0.60 | − 7.10 | 40 |
Behavioral Testing
All behavior testing took place during the first six hours of the dark phase and under red illumination. Video recordings of tests were scored using the Observer for Windows, Version 9.0 (Noldus Information Technology, Wageningen, The Netherlands).
Odor Preference
The stimuli and procedures for odor preference tests have been described in detail elsewhere (Been and Petrulis, 2010a; Been and Petrulis, 2010b). Briefly, flank and anogenital samples, soiled litter/bedding, and cage wall rubbings were collected from group-housed, same-sexed odor donor cages that had not been changed for four days prior to odor collection. Odor stimuli therefore reflected the composite sexual identity of the odor, rather than the individual identity of a single animal. Subjects were tested in a series of three tests, each separated by 24 hours: Clean, Non-Contact preference, and Contact preference. During each test, subjects were placed into a testing aquarium containing three acrylic odor presentation boxes with holes drilled along the front surface that allowed volatile odors to pass, but prevented contact with the odor sources. For Clean tests, clean odor stimuli were placed into each of the three odor boxes. For Non-Contact and Contact tests, female and male odor stimuli were placed into each of the two outer odor boxes, and clean odor stimuli were placed into the center odor box. The side on which each sexual odor was placed (left or right) was alternated between consecutive subjects. Non-Contact and Contact tests were identical except that during Contact tests, a single odor slide matching the type of odor stimulus in that container (female, male, clean) was secured to the center of the front surface of each odor presentation box.
Copulatory Behavior
Following the completion of odor preference tests, subjects were tested for their copulatory behavior (i.e. all subjects were sexually-naïve at the time of odor preference and copulatory behavior tests). The procedures for copulatory behavior tests have been described in detail elsewhere (Been and Petrulis, 2010a; Been and Petrulis, 2010b). Briefly, subjects were placed into a clear testing arena with a receptive stimulus female for 20 minutes. An angled mirror was placed below the testing arena to provide a view of the ventral surface of the animals. The total number, durations, and latencies of mating events (anogenital investigation, mounts, intromissions, ejaculations, long intromissions) were scored.
Histology and Lesion Verification
Following the last test, subjects were injected with an overdose of sodium pentobarbital (100 mg/kg; Sleep Away, Ft. Dodge, IA, USA) and transcardially perfused with 200 ml of 0.1M phosphate-buffered saline (PBS, pH 7.4) followed by 200 ml of 10% neutral-buffered formalin (Richard Allan Scientific, Kalamazoo, MI, USA). Brains were post-fixed in 10% formalin overnight and then cryoprotected for at least 24 hours in 30% sucrose in PBS solution. Coronal sections (30-µm) of brain tissue were sectioned using a cryostat (−20°C) and stored in cryoprotectant until immunohistochemical localization of Neuronal Nuclei protein (NeuN, see (Been and Petrulis, 2010a; Been and Petrulis, 2010b) for immunohistochemistry procedures). NeuN-stained sections were examined under a light microscope for the location and extent of lesion damage as compared with published hamster neuroanatomical plates (Morin and Wood, 2001). The minimum and maximum extents of lesion damage were traced onto anatomical plates using Adobe Illustrator CS 11.0 software.
Data Analysis
All data were analyzed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA) for Windows and significance was determined as P < 0.05. To establish investigatory preferences, 3 × 3 (lesion condition × odor) mixed-design ANOVAs were performed separately for MA-MPOA-X and MA-BNST-X groups. Significant interactions were explored using simple effects analysis and pair-wise comparisons with Bonferroni alpha adjustments. Furthermore, separate one-way ANOVAs were used to compare the levels of investigation of each stimulus directly across experimental groups. Group differences in most copulatory measures were detected using one-way ANOVAs, whereas group differences in the proportion of animals displaying copulatory measures were detected using z-tests for independent proportions.
Results
Lesion Reconstruction
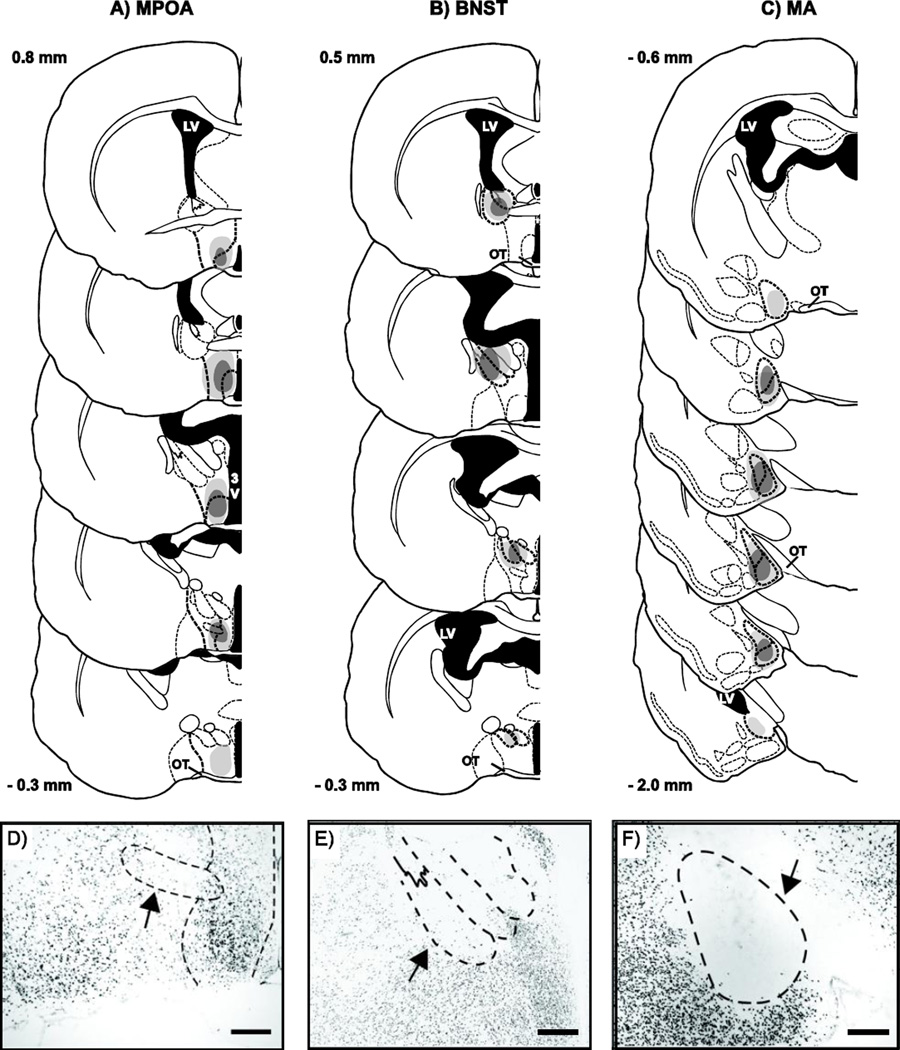
Lesion damage to MA was centered at the juncture between the anterior and posterior subdivisions of MA (Bregma −1.35 mm, Figure 1C). All subjects included in the MA-MPOA-X (CONTRA n = 12; IPSI n = 12) or MA-BNST-X (CONTRA n = 12; IPSI n = 14) experimental groups sustained at least 60% damage in two atlas plates of the anterior MA (Bregma − 0.09 to −1.2) and two atlas plates of the posterior MA (Bregma − 1.5 mm to −1.8 mm). Most subjects sustained additional, albeit lesser (≤ 40%) damage to more anterior and posterior aspects of MA (Bregma − 0.06 mm and −2.0 mm, respectively).
Figure 1.
Lesion reconstructions. Coronal sections through the rostral to caudal extent of A) MPOA, B) BNST, and C) MA showing the largest (light gray) and smallest (dark gray) unilateral lesions included in the IPSI and CONTRA groups. Measurements indicate mm relative to bregma, 3V, 3rd ventricle; LV, lateral ventricle; OT, optic tract. Atlas images modified from (Morin and Wood, 2001). Excitotoxic lesion damage was assessed using immunohistochemical (IHC) localization of NeuN protein. Photomicrographs of NeuN IHC show representative depletion of neurons in D) MPOA, E) BNST, and F) MA. Scale bars = 200 µm.
Lesion damage to MPOA was centered below the most caudal extent of the anterior commissure (Bregma 0.5 mm, Figure 1A). All subjects included in the MA-MPOA-X lesion groups (CONTRA and IPSI) sustained at least 60% damage to MPOA, including the medial preoptic nucleus (MPN) in at least two atlas plates between Bregma 0.8 mm and Bregma −0.1 mm. Many (CONTRA n = 7; IPSI n = 8) subjects had lesion damage that extended more caudally into the anterior hypothalamus (Bregma −0.3 mm).
Lesion damage to BNST was centered at the juncture where the lateral and third ventricles fuse (Bregma 0.2 mm, Figure 1B). All subjects included in the MA-BNST-X lesion groups (CONTRA and IPSI) sustained at least 60% damage to posterointermediate BNST (BNSTpi), posteromedial BNST (BNSTpm), and posterolateral BNST (BNSTpl) in at least two atlas plates between Bregma 0.5 mm and Bregma −0.3 mm. Many subjects (CONTRA n = 5; IPSI n = 8) also sustained lesser (≥ 40%) damage to the anterior BNST.
Subjects were excluded from analysis if their lesions failed to damage a significant portion (≤ 60%) of the target nucleus or if their lesions damaged a significant portion (≥ 40%) of nuclei outside of the target nucleus. To control for non-specific effects of brain surgery, CONTRA and IPSI males were also compared to males that received a sham lesion surgery (SHAM). Only needle tracks were visible in all SHAM subjects (MA-MPOA-X n = 12; MA-BNST-X n = 11).
Odor Preference
Clean Test
In both MA-MPOA-X and MA-BNST-X experimental groups, there was a general bias to investigate the outside boxes more than the center box, but there was no bias towards either of the outside boxes that were used to present male and female odors in subsequent tests (SHAM t(21) = −2.31; IPSI t(24) = −0.37; CONTRA t(22) = 0.28, all P > 0.05). There was also no difference in the total number of midline crosses between lesion groups (MA-MPOA-X F(1, 33) = 0.75; MA-BNST-X, F(1, 34) = 0.81, both P > 0.05), indicating similar levels of activity.
Non-Contact preference test
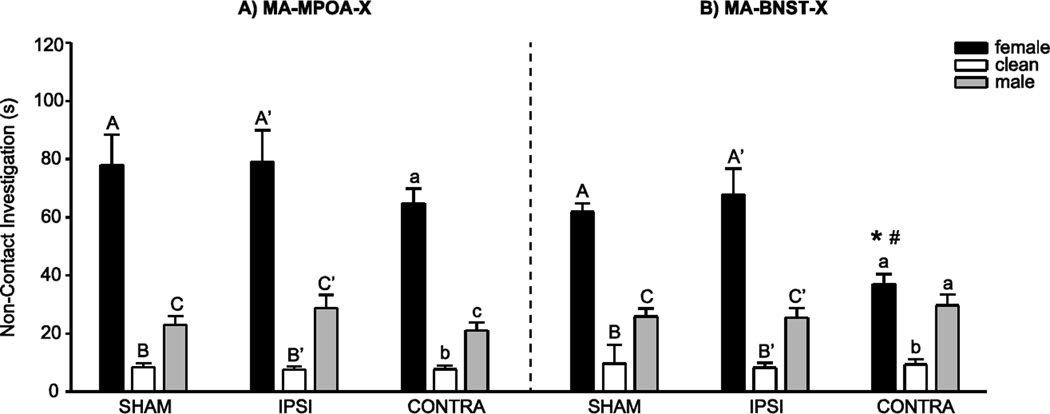
In the MA-MPOA-X group, there was a significant main effect of odor stimulus (F(1, 33) = 112.89 , P < 0.001), but no significant interaction between lesion group and investigation durations across the three odor stimuli (Figure 2A). Indeed, all experimental groups investigated female odors longer than male odors (t(35) = 8.50), female odors longer than clean odors (t(35) = −13.02), and male odors longer than clean odors (t(35) = −7.61) (all P < 0.001).
Figure 2.
Non-Contact Odor Preference. A) In the MA-MPOA-X lesion group, SHAM, IPSI, and CONTRA males all investigated female odors more than male or clean odors. B) In the MA-BNST-X lesion group, SHAM and IPSI males investigated female odors more than male or clean odors, but CONTRA males investigated female and male odors equally. Furthermore, CONTRA males investigated female odors significantly less than SHAM or IPSI males. Data presented as mean ±SEM. Different letters indicate significant differences between odor conditions, * and # indicate significant differences between lesion groups (P < 0.05).
In contrast, in the MA-BNST-X group, there was a significant interaction between lesion group and investigation durations across the three odor stimuli (F(1, 34) = 4.12, P = 0.02; Figure 2B). Whereas SHAM and IPSI males spent significantly more time investigating female odors than male odors (SHAM t(10) = 8.22, P < 0.001; IPSI t(13) = 4.04, P < 0.001), males with CONTRA lesions spent equivalent amounts of time investigating female and male odors (t(11) = 1.59, P = 0.14). This elimination of preference was mediated by a decrease in investigation of female odors, as CONTRA males investigated female odors significantly less than SHAM males (t(10) = 4.45, P < 0.001) or IPSI males (t(11) = 3.15, P = 0.009). All lesion groups investigated female odors (SHAM t(10) = −2.28, P = 0.04; IPSI t(13) = −6.35, P < 0.001; CONTRA t(11) = −8.71, P < 0.001) and male odors (SHAM t(10) = −1.13, P = 0.05; IPSI t(13) = −5.03, P < 0.001; CONTRA t(11) = −4.35, P < 0.001) more than clean odors.
Contact preference test
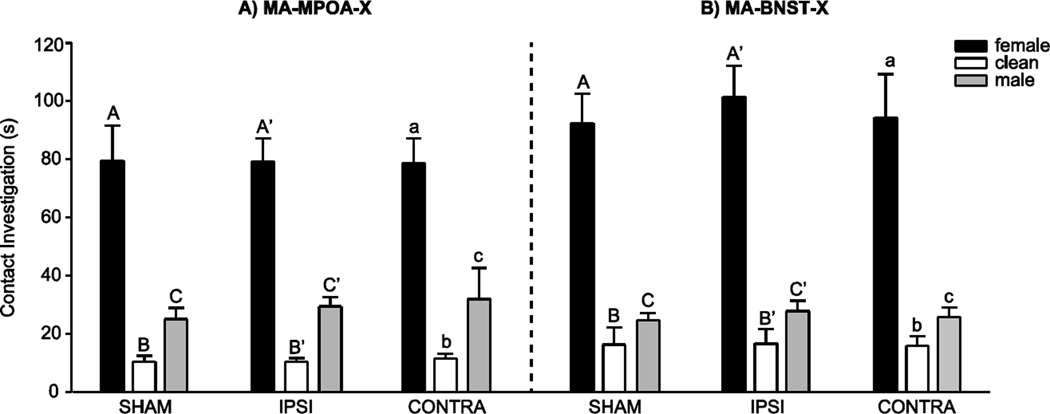
In the MA-MPOA-X and the MA-BNST-X experimental groups, there was there was a significant main effect of odor stimulus (MA-MPOA-X F(1, 33) = 112.56; MA-BNST-X F(1, 34) = 100.74 , both P < 0.001), but no significant interaction between lesion group and investigation durations across the three odor stimuli (Figures 2A and 2B). All experimental groups investigated female odors longer than male odors (MA-MPOA-X t(35) = 10.16; MA-BNST-X t(36) = 7.68), female odors longer than clean odors (MA-MPOA-X t(35) = −11.27; MA-BNST-X t(36) = −12.81), and male odors longer than clean odors (MA-MPOA-X t(35) =−3.98; MA-BNST-X t(36) = −5.51) (all P < 0.001).
Copulatory Behavior
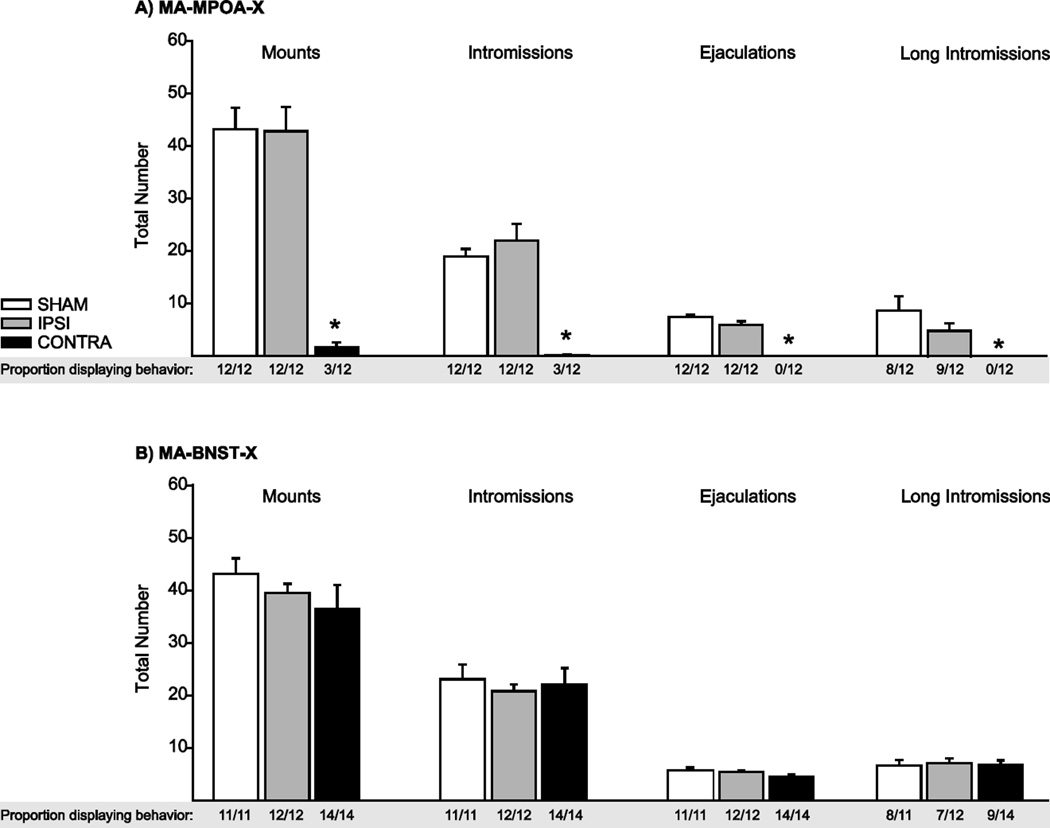
In the MA-MPOA-X experimental groups, males with CONTRA lesions displayed severe deficits in copulatory behavior compared to SHAM and IPSI males (Figure 4A). The proportion of males displaying mounts (z = 3.79), intromissions (z = 3.79), ejaculations (z = 3.79), and long intromissions (z = 3.79) was significantly lower in CONTRA males than in SHAM males (all P < 0.001), whereas the proportion of males displaying mounts, intromissions, ejaculations, and long intromissions did not differ between SHAM and IPSI lesion groups (all P > 0.05). CONTRA males did not differ from SHAM or IPSI males in the total duration of anogenital investigation (AGI) (F(2,35) = 0.145, P = 0.86).
Figure 4.
Copulatory Behavior. A) In the MA-MPOA-X lesion group, the proportion of CONTRA males displaying mounts, intromissions, ejaculations, or long intromissions was significantly lower than SHAM or IPSI males. B) In contrast, there was no difference between SHAM, IPSI, and CONTRA males in the MA-BNST-X lesion group. Data presented as mean ±SEM. * indicates significant differences between lesion groups (P < 0.05).
In contrast, copulatory behavior measures did not differ within the MA-BNST-X experimental groups (Figure 4B). The total number of mounts (F(2,36) = 0.97), intromissions (F(2,36) = 0.22), ejaculations (F(2,35) = 2.41), and long intromissions (F(2,29) = 0.06) did not differ between SHAM, IPSI, and CONTRA males (all P > 0.05). The total duration of AGI (F(2,36) = 2.19, P = 0.13) also did not differ between lesion groups.
Discussion
It is perhaps not surprising that these data support our hypothesis that the functional connections between MA and MPOA are required for copulatory behavior in hamsters, as lesions of either MA (Heeb and Yahr, 2000; Kondo, 1992; Lehman et al., 1980) or MPOA (Been and Petrulis, 2010b; Hull and Dominguez, 2007) alone severely impair copulation in male hamsters and other species, and functional disconnection of MA and MPOA eliminates copulation in rats (Kondo and Arai, 1995) and gerbils (Heeb and Yahr, 2000). Importantly, the current results extend these findings by demonstrating that functional connections between MA and MPOA cell groups are specific to copulation and can be dissociated from the functional connections required for pre-copulatory aspects of male sexual behavior. These data do not, however, support our hypothesis that the functional connections between MA and MPOA are required for odor preference, suggesting that the elimination of odor preference by bilateral MPOA lesions (Been and Petrulis, 2010b) is mediated by a mechanism that is independent of MA. It is possible that, in addition to sexual odor information transmitted from MA to MPOA, direct AOB information transmitted from BNST to MPOA (Davis et al., 1978) is also sufficient to generate attraction to female odors. Indeed, redundancy of function within a network is a common mechanism to accomplish complex behavioral tasks and also ensures that evolutionarily-important behaviors, such as reproduction, are not easily disrupted (Wood, 1997).
The current results also support our hypothesis that the functional connections between MA and BNST are required for volatile odor preference but not copulation. Several lines of evidence suggest that the functional connections between MA and BNST are well positioned to support the attraction to female odors. First, BNST preferentially receives female-specific odor information directly from MA (Been and Petrulis, 2011) and lesions of the anterior MA decrease the c-Fos response to female odors in BNST (Maras and Petrulis, 2010a), suggesting MA provides sexually-relevant odor information to BNST. Second, lesions of BNST alone eliminate volatile odor preference in male hamsters (Been and Petrulis, 2010a) and decrease non-contact erections in response to remote cues from estrous females in male rats (Liu et al., 1997), supporting the idea that main olfactory information relayed from MA to BNST mediates attraction to volatile female odors. Third, BNST maintains reciprocal connections with AOB and MA (Scalia and Winans, 1975; Wood and Swann, 2005); the bidirectional nature of these connections may allow BNST to provide feedback to earlier stages of olfactory processing (Fan and Luo, 2009). Finally, BNST is also reciprocally connected with the ventral striatum and ventral tegmental area in hamsters (Wood and Swann, 2005), providing a potential substrate for the valuation of odor-stimuli and/or generating the motivation to approach and investigate sexual odors.
Although lesions that disconnected MA from BNST did not decrease investigation of directly-contacted (i.e. non-volatile) female odors in the current study, bilateral lesions of BNST decrease investigation of directly-contacted female odors (Been and Petrulis, 2010a) and large lesions including BNST decrease anogenital investigation during copulation in male hamsters (Powers et al., 1987), suggesting that BNST may mediate attraction to non-volatile female odors independently from MA. There are several alternative connections that could provide the substrate for generating preference for non-volatile odors. One likely possibility is that preference for non-volatile female odors may be mediated by direct AOB projections to BNST (Davis et al., 1978), whereas preference for volatile female odors appears to be mediated by serial projections from MOB to MA to BNST. Alternatively, BNST connections with other nuclei important for chemosensory responding, such as the posteromedial cortical nucleus of the amygdala (PMCo) (Wood and Swann, 2005), may mediate attraction to directly contacted female odors. Indeed, lesions of PMCo cause inappropriate investigation of females during copulatory behavior (Maras and Petrulis, 2008). Finally, it is possible that the extremely sparse, contralateral projections between MA and BNST (Kevetter and Winans, 1981; Wood and Swann, 2005) are sufficient to maintain preference for female odors when contact is involved. Although this possibility is less likely, future studies could test this by combining functional disconnection of MA and BNST with lesions of the anterior commissure, thus preventing cross-hemisphere communication.
The presence of a dissociation between individual brain areas mediating the appetitive and consummatory aspects of reproductive behavior has been suggested before (Everitt, 1990). However, previous studies have relied on second-order conditioning tasks, which measure arbitrary responses to learned associations between neutral stimuli and sexual reinforcers and do not necessarily recruit circuitry required for unconditioned sexual attraction (Kippin et al., 2003). For example, lesions of the basolateral amygdala (BLA) (Everitt et al., 1989), but not MPOA (Everitt and Stacey, 1987), decrease male rats’ bar-pressing response for access to a receptive female. BLA is not, however, required for appetitive aspects of copulatory behavior in rats or hamsters (Lehman et al., 1980; McGregor and Herbert, 1992), but is required for instrumental responding to other non-sexual conditioned stimuli (Cador et al., 1989), suggesting BLA may mediate appetitive responding to conditioned reinforcers generally, not to sexual stimuli specifically. Similarly, although attraction to female odors is an unconditioned response that does not require sexual experience in male hamsters (Ballard and Wood, 2007; Been and Petrulis, 2010a; Been and Petrulis, 2010b; Maras and Petrulis, 2006; Maras and Petrulis, 2008; Maras and Petrulis, 2010b), male hamsters will not bar-press for female hamster vaginal secretion (Coppola and O'Connell, 1988), suggesting that natural appetitive behaviors are not always comparable with conditioned appetitive behaviors. The odor preference tests used in the current study model an unconditioned, appetitive response to female odors and therefore better test the neural substrates required for sexual attraction.
Given our previous finding that copulatory experience can mitigate the requirement of BNST (Been and Petrulis, 2010a) and MPOA (Been and Petrulis, 2010b) for odor guided social behaviors, we predict that functionally disconnecting MA from BNST and/or MPOA would produce less severe behavioral deficits in sexually experienced males. It is possible that sexual experience may lead to a more distributed processing of female odor cues such that functional connections between MA and BNST/MPOA are no longer required to execute the appropriate behavioral response. This type of experience-dependent plasticity may result from associations between volatile odors and copulatory cues learned during sexual experience (Kippin et al., 2003; Pfaus et al., 2001). Of the cues available during copulation, non-volatile cues may be the most critical for the formation of associations with the attractive properties of female odor cues. Indeed, sexual experience (Meredith, 1986) or exposure to non-volatile female odors (Westberry and Meredith, 2003) can rescue deficits in copulatory behavior following removal of the vomeronasal organ. Further experiments are needed to determine how sexual experience can alter the neural pathways required for appetitive and consummatory aspects of male reproductive behavior.
Finally, it is important to note that MA, BNST, and MPOA each consist of anatomically, hodologically, and functionally distinct sub-nuclei that may differentially contribute to reproductive behavior (Wood, 1997). Specifically, differential sensitivity to steroid hormones within MA, BNST, and MPOA subnuclei may contribute to the dissociated regulation of appetitive and consummatory behaviors. Indeed, data from our laboratory suggest that the chemosensory anterior MA and steroid-sensitive posterodorsal MA differentially regulate odor preference (Maras and Petrulis, 2006), and that their interaction is required for appropriate investigation of female odors (Maras and Petrulis, 2010b). Although the functional significance of BNST and/or MPOA subnuclei has not been directly tested in hamsters, studies in rats (Arendash and Gorski, 1983; Claro et al., 1995; De Jonge et al., 1989), gerbils (Finn and Yahr, 1994; Finn and Yahr, 2005; Heeb and Yahr, 2000; Sayag et al., 1994), and Japanese quails (Balthazart and Ball, 2007) lend support to the idea that subnuclei within MPOA may regulate distinct aspects of appetitive and consummatory reproductive behavior. A better understanding of the functional microcircuitry of MA, BNST, and MPOA is fundamental to the progress of research on the neural control of reproductive behavior and, ultimately, may demonstrate that the functional dissociation of the pathways required for appetitive and consummatory sexual behaviors is a common mechanism for organizing the neural control of reproductive behavior.
Highlights.
Lesions that functionally disconnect MA from MPOA eliminate copulation but not odor preference.
Lesions that functionally disconnect MA from BNST eliminate odor preference but not copulation.
The pathways required for appetitive and consummatory reproductive behaviors are dissociated.
Figure 3.
Contact Odor Preference. A) In the MA-MPOA-X lesion group, SHAM, IPSI, and CONTRA males all investigated female odors more than male or clean odors. B) Similarly, in the MA-BNST-X lesion group, SHAM, IPSI, and CONTRA males all investigated female odors more than male or clean odors. Data presented as mean ±SEM. Different letters indicate significant differences between odor conditions (P < 0.05).
Acknowledgements
The authors would like to thank Alix Pijeaux for her assistance collecting behavioral data. This work was supported by NIH grant MH072930 to A.P., GSU dissertation grant to L.B., and in part by the Center for Behavioral Neuroscience under the STC program of the NSF, under agreement IBN 9876754.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arendash GW, Gorski RA. Effects of discrete lesions of the sexually dimorphic nucleus of the preoptic area or other medial preoptic regions on the sexual behavior of male rats. Brain Res Bull. 1983;10:147–154. doi: 10.1016/0361-9230(83)90086-2. [DOI] [PubMed] [Google Scholar]
- Ballard CL, Wood RI. Partner preference in male hamsters: steroids, sexual experience and chemosensory cues. Physiol Behav. 2007;91:1–8. doi: 10.1016/j.physbeh.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors. Front Neuroendocrinol. 2007;28:161–178. doi: 10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum MJ, Kelliher KR. Complementary roles of the main and accessory olfactory systems in mammalian mate recognition. Annu Rev Physiol. 2009;71:141–160. doi: 10.1146/annurev.physiol.010908.163137. [DOI] [PubMed] [Google Scholar]
- Been LE, Petrulis A. Lesions of the posterior bed nucleus of the stria terminalis eliminate opposite-sex odor preference and delay copulation in male Syrian hamsters: role of odor volatility and sexual experience. The European journal of neuroscience. 2010a;32:483–493. doi: 10.1111/j.1460-9568.2010.07277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been LE, Petrulis A. The role of the medial preoptic area in appetitive and consummatory reproductive behaviors depends on sexual experience and odor volatility in male Syrian hamsters. Neuroscience. 2010b;170:1120–1132. doi: 10.1016/j.neuroscience.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been LE, Petrulis A. Chemosensory and hormone information are relayed directly between the medial amygdala, posterior bed nucleus of the stria terminalis, and medial preoptic area in male Syrian hamsters. Horm Behav. 2011;59:536–548. doi: 10.1016/j.yhbeh.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cador M, Robbins TW, Everitt BJ. Involvement of the amygdala in stimulus-reward associations: interaction with the ventral striatum. Neuroscience. 1989;30:77–86. doi: 10.1016/0306-4522(89)90354-0. [DOI] [PubMed] [Google Scholar]
- Claro F, Segovia S, Guilamon A, Del Abril A. Lesions in the medial posterior region of the BST impair sexual behavior in sexually experienced and inexperienced male rats. Brain Res Bull. 1995;36:1–10. doi: 10.1016/0361-9230(94)00118-k. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. J Comp Neurol. 1998;399:189–209. doi: 10.1002/(sici)1096-9861(19980921)399:2<189::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Coppola DM, O'Connell RJ. Are pheromones their own reward? Physiol Behav. 1988;44:811–816. doi: 10.1016/0031-9384(88)90067-4. [DOI] [PubMed] [Google Scholar]
- Davis BJ, Macrides F, Youngs WM, Schneider SP, Rosene DL. Efferents and centrifugal afferents of the main and accessory olfactory bulbs in the hamster. Brain Res Bull. 1978;3:59–72. doi: 10.1016/0361-9230(78)90062-x. [DOI] [PubMed] [Google Scholar]
- De Jonge FH, Louwerse AL, Ooms MP, Evers P, Endert E, van de Poll NE. Lesions of the SDN-POA inhibit sexual behavior of male Wistar rats. Brain Res Bull. 1989;23:483–492. doi: 10.1016/0361-9230(89)90194-9. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol. 2004;471:396–433. doi: 10.1002/cne.20002. [DOI] [PubMed] [Google Scholar]
- Everitt BJ. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev. 1990;14:217–232. doi: 10.1016/s0149-7634(05)80222-2. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Cador M, Robbins TW. Interactions between the amygdala and ventral striatum in stimulus-reward associations: studies using a second-order schedule of sexual reinforcement. Neuroscience. 1989;30:63–75. doi: 10.1016/0306-4522(89)90353-9. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Stacey P. Studies of instrumental behavior with sexual reinforcement in male rats (Rattus norvegicus): II. Effects of preoptic area lesions, castration, and testosterone. J Comp Psychol. 1987;101:407–419. [PubMed] [Google Scholar]
- Fan S, Luo M. The organization of feedback projections in a pathway important for processing pheromonal signals. Neuroscience. 2009;161:489–500. doi: 10.1016/j.neuroscience.2009.03.065. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fewell GD, Meredith M. c-fos expression in vomeronasal pathways of mated or pheromone-stimulated male golden hamsters: contributions from vomeronasal sensory input and expression related to mating performance. J Neurosci. 1994;14:3643–3654. doi: 10.1523/JNEUROSCI.14-06-03643.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PD, Yahr P. Projection of the sexually dimorphic area of the gerbil hypothalamus to the retrorubral field is essential for male sexual behavior: role of A8 and other cells. Behav Neurosci. 1994;108:362–378. doi: 10.1037//0735-7044.108.2.362. [DOI] [PubMed] [Google Scholar]
- Finn PD, Yahr P. Projection from the ventral bed nucleus of the stria terminalis to the retrorubral field in rats and the effects of cells in these areas on mating in male rats versus gerbils. Horm Behav. 2005;47:123–138. doi: 10.1016/j.yhbeh.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Gomez DM, Newman SW. Differential projections of the anterior and posterior regions of the medial amygdaloid nucleus in the Syrian hamster. J Comp Neurol. 1992;317:195–218. doi: 10.1002/cne.903170208. [DOI] [PubMed] [Google Scholar]
- Heeb MM, Yahr P. Cell-body lesions of the posterodorsal preoptic nucleus or posterodorsal medial amygdala, but not the parvicellular subparafascicular thalamus, disrupt mating in male gerbils. Physiol Behav. 2000;68:317–331. doi: 10.1016/s0031-9384(99)00182-1. [DOI] [PubMed] [Google Scholar]
- Hull EM, Dominguez JM. Sexual behavior in male rodents. Horm Behav. 2007;52:45–55. doi: 10.1016/j.yhbeh.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Meisel RL, Sachs BD. Male Sexual Behavior. In: Rubin R, editor. Hormones, Brain and Behavior. Burlington, MA: Elsevier Science; 2002. pp. 3–137. [Google Scholar]
- Johnston RE. Chemical communication in golden hamsters: from behavior to molecules and neural mechanisms. In: Dewsbury DA, editor. Contemporary Issues in Comparative Psychology. Sunderland, MA: Sinauer; 1990. pp. 381–412. [Google Scholar]
- Keller M, Baum MJ, Brock O, Brennan PA, Bakker J. The main and the accessory olfactory systems interact in the control of mate recognition and sexual behavior. Behav Brain Res. 2009;200:268–276. doi: 10.1016/j.bbr.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. I. Efferents of the "vomeronasal amygdala". J Comp Neurol. 1981;197:81–98. doi: 10.1002/cne.901970107. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Cain SW, Pfaus JG. Estrous odors and sexually conditioned neutral odors activate separate neural pathways in the male rat. Neuroscience. 2003;117:971–979. doi: 10.1016/s0306-4522(02)00972-7. [DOI] [PubMed] [Google Scholar]
- Kondo Y. Lesions of the medial amygdala produce severe impairment of copulatory behavior in sexually inexperienced male rats. Physiol Behav. 1992;51:939–943. doi: 10.1016/0031-9384(92)90074-c. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Arai Y. Functional association between the medial amygdala and the medial preoptic area in regulation of mating behavior in the male rat. Physiol Behav. 1995;57:69–73. doi: 10.1016/0031-9384(94)00205-j. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Sachs BD, Sakuma Y. Importance of the medial amygdala in rat penile erection evoked by remote stimuli from estrous females. Behav Brain Res. 1997;88:153–160. doi: 10.1016/s0166-4328(97)02287-0. [DOI] [PubMed] [Google Scholar]
- Landauer MR, Banks EM, Carter CS. Sexual preferences of male hamsters (Mesocricetus auratus) for conspecifics in different endocrine conditions. Horm Behav. 1977;9:193–202. doi: 10.1016/0018-506x(77)90055-1. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Winans SS, Powers JB. Medial nucleus of the amygdala mediates chemosensory control of male hamster sexual behavior. Science. 1980;210:557–660. doi: 10.1126/science.7423209. [DOI] [PubMed] [Google Scholar]
- Liu YC, Salamone JD, Sachs BD. Lesions in medial preoptic area and bed nucleus of stria terminalis: differential effects on copulatory behavior and noncontact erection in male rats. J Neurosci. 1997;17:5245–5253. doi: 10.1523/JNEUROSCI.17-13-05245.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrides F, Bartke A, Fernandez F, D'Angelo W. Effects of exposure to vaginal odor and receptive females on plasma testosterone in the male hamster. Neuroendocrinology. 1974;15:355–364. doi: 10.1159/000122326. [DOI] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. Chemosensory and steroid-responsive regions of the medial amygdala regulate distinct aspects of opposite-sex odor preference in male Syrian hamsters. Eur J Neurosci. 2006;24:3541–3552. doi: 10.1111/j.1460-9568.2006.05216.x. [DOI] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. The posteromedial cortical amygdala regulates copulatory behavior, but not sexual odor preference, in the male Syrian hamster (Mesocricetus auratus) Neuroscience. 2008;156:425–435. doi: 10.1016/j.neuroscience.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. The anterior medial amygdala transmits sexual odor information to the posterior medial amygdala and related forebrain nuclei. Eur J Neurosci. 2010a;32:469–482. doi: 10.1111/j.1460-9568.2010.07289.x. [DOI] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. Lesions that functionally disconnect the anterior and posterodorsal sub-regions of the medial amygdala eliminate opposite-sex odor preference in male Syrian hamsters (Mesocricetus auratus) Neuroscience. 2010b;165:1052–1062. doi: 10.1016/j.neuroscience.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor A, Herbert J. Differential effects of excitotoxic basolateral and corticomedial lesions of the amygdala on the behavioural and endocrine responses to either sexual or aggression-promoting stimuli in the male rat. Brain Res. 1992;574:9–20. doi: 10.1016/0006-8993(92)90793-9. [DOI] [PubMed] [Google Scholar]
- Meredith M. Vomeronasal organ removal before sexual experience impairs male hamster mating behavior. Physiol Behav. 1986;36:737–743. doi: 10.1016/0031-9384(86)90362-8. [DOI] [PubMed] [Google Scholar]
- Morin LP, Wood RI. A Stereotaxic Atlas of the Golden Hamster Brain. San Diego: Academic Press; 2001. [Google Scholar]
- Murphy MR, Schneider GE. Olfactory bulb removal eliminates mating behavior in the male golden hamster. Science. 1970;167:302–304. doi: 10.1126/science.167.3916.302. [DOI] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Petrulis A, Johnston RE. Lesions centered on the medial amygdala impair scent-marking and sex-odor recognition but spare discrimination of individual odors in female golden hamsters. Behav Neurosci. 1999;113:345–357. doi: 10.1037//0735-7044.113.2.345. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Kippin TE, Centeno S. Conditioning and sexual behavior: a review. Horm Behav. 2001;40:291–321. doi: 10.1006/hbeh.2001.1686. [DOI] [PubMed] [Google Scholar]
- Pfeiffer CA, Johnston RE. Socially stimulated androgen surges in male hamsters: the roles of vaginal secretions, behavioral interactions, and housing conditions. Horm Behav. 1992;26:283–293. doi: 10.1016/0018-506x(92)90048-z. [DOI] [PubMed] [Google Scholar]
- Powers JB, Fields RB, Winans SS. Olfactory and vomeronasal system participation in male hamsters' attraction to female vaginal secretions. Physiol Behav. 1979;22:77–84. doi: 10.1016/0031-9384(79)90407-4. [DOI] [PubMed] [Google Scholar]
- Powers JB, Newman SW, Bergondy ML. MPOA and BNST lesions in male Syrian hamsters: differential effects on copulatory and chemoinvestigatory behaviors. Behav Brain Res. 1987;23:181–195. doi: 10.1016/0166-4328(87)90019-2. [DOI] [PubMed] [Google Scholar]
- Powers JB, Winans SS. Vomeronasal organ: critical role in mediating sexual behavior of the male hamster. Science. 1975;187:961–963. doi: 10.1126/science.1145182. [DOI] [PubMed] [Google Scholar]
- Rowe FA, Edwards DA. Olfactory bulb removal: influences on the mating behavior of male mice. Physiol Behav. 1972;8:37–41. doi: 10.1016/0031-9384(72)90127-8. [DOI] [PubMed] [Google Scholar]
- Sachs BD, Akasofu K, Citron JH, Daniels SB, Natoli JH. Noncontact stimulation from estrous females evokes penile erection in rats. Physiol Behav. 1994;55:1073–1079. doi: 10.1016/0031-9384(94)90390-5. [DOI] [PubMed] [Google Scholar]
- Sayag N, Hoffman NW, Yahr P. Telencephalic connections of the sexually dimorphic area of the gerbil hypothalamus that influence male sexual behavior. Behav Neurosci. 1994;108:743–757. doi: 10.1037//0735-7044.108.4.743. [DOI] [PubMed] [Google Scholar]
- Scalia F, Winans SS. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol. 1975;161:31–55. doi: 10.1002/cne.901610105. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. The organization of neural inputs to the medial preoptic nucleus of the rat. J Comp Neurol. 1986;246:312–342. doi: 10.1002/cne.902460304. [DOI] [PubMed] [Google Scholar]
- Stark CP, Alpern HP, Fuhrer J, Trowbridge MG, Wimbish H, Smock T. The medial amygdaloid nucleus modifies social behavior in male rats. Physiol Behav. 1998;63:253–259. doi: 10.1016/s0031-9384(97)00438-1. [DOI] [PubMed] [Google Scholar]
- Wang J, Swann JM. The magnocellular medial preoptic nucleus I. Sources of afferent input. Neuroscience. 2006;141:1437–1456. doi: 10.1016/j.neuroscience.2006.04.079. [DOI] [PubMed] [Google Scholar]
- Westberry JM, Meredith M. Pre-exposure to female chemosignals or intracerebral GnRH restores mating behavior in naive male hamsters with vomeronasal organ lesions. Chem Senses. 2003;28:191–196. doi: 10.1093/chemse/28.3.191. [DOI] [PubMed] [Google Scholar]
- Wood RI. Thinking about networks in the control of male hamster sexual behavior. Horm Behav. 1997;32:40–45. doi: 10.1006/hbeh.1997.1403. [DOI] [PubMed] [Google Scholar]
- Wood RI, Brabec RK, Swann JM, Newman SW. Androgen and estrogen concentrating neurons in chemosensory pathways of the male Syrian hamster brain. Brain Res. 1992;596:89–98. doi: 10.1016/0006-8993(92)91536-n. [DOI] [PubMed] [Google Scholar]
- Wood RI, Coolen LM. Integration of chemosensory and hormonal cues is essential for sexual behaviour in the male Syrian hamster: role of the medial amygdaloid nucleus. Neuroscience. 1997;78:1027–1035. doi: 10.1016/s0306-4522(96)00629-x. [DOI] [PubMed] [Google Scholar]
- Wood RI, Newman SW. Androgen and estrogen receptors coexist within individual neurons in the brain of the Syrian hamster. Neuroendocrinology. 1995a;62:487–497. doi: 10.1159/000127039. [DOI] [PubMed] [Google Scholar]
- Wood RI, Newman SW. Integration of chemosensory and hormonal cues is essential for mating in the male Syrian hamster. J Neurosci. 1995b;15:7261–7269. doi: 10.1523/JNEUROSCI.15-11-07261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI, Newman SW. The medial amygdaloid nucleus and medial preoptic area mediate steroidal control of sexual behavior in the male Syrian hamster. Horm Behav. 1995c;29:338–353. doi: 10.1006/hbeh.1995.1024. [DOI] [PubMed] [Google Scholar]
- Wood RI, Swann JM. The bed nucleus of the stria terminalis in the Syrian hamster: subnuclei and connections of the posterior division. Neuroscience. 2005;135:155–179. doi: 10.1016/j.neuroscience.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Nyby J, Whitney G, Beauchamp GK, Katz Y. The vomeronasal organ: primary role in mouse chemosensory gender recognition. Physiol Behav. 1982;29:315–327. doi: 10.1016/0031-9384(82)90021-x. [DOI] [PubMed] [Google Scholar]