Abstract
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disorder in the U.S.; however, few data are available about racial and ethnic variation. We investigated relationships between ethnicity, NAFLD severity, metabolic derangements and socio-demographic characteristics in a well-characterized cohort of adults with biopsy-proven NAFLD. Data were analyzed from 1026 adults (≥18 years) in the Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) from 2004–2008 for whom liver histology data were available within 6-months of enrollment. Associations between ethnicity (Latino versus Non-Latino White) and NAFLD severity (NASH versus Non-NASH histology; and mild versus advanced fibrosis) were explored with multiple logistic regression analysis. We also investigated effect modification of ethnicity on metabolic derangements for NAFLD severity. Within the NASH CRN, 77% (N=785) were Non-Latino White and 12% (N=118) Latino. Sixty-one percent (N=668) had NASH histology and 29% (N=291) had advanced fibrosis. Latinos with NASH were younger, performed less physical activity and had higher carbohydrate intake compared to Non-Latino Whites with NASH. Gender, diabetes, hypertension, hypertriglyceridemia, aspartate aminotransferase (AST), platelets, and the homeostasis model assessment of insulin resistance (HOMA-IR) were significantly associated with NASH. Age, gender, AST, alanine aminotransferase, alkaline phosphatase, platelets, total cholesterol, hypertension and HOMA-IR, but not ethnicity, were significantly associated with advanced fibrosis. The effect of HOMA-IR on risk of NASH was modified by ethnicity: HOMA-IR was not a significant risk factor for NASH among Latinos (Odds Ratio, OR=0.93 [95% Confidence Interval, CI, 0.85–1.02]), but was significant among Non-Latino Whites (OR 1.06, [95%CI 1.01–1.11]).
Conclusion
Metabolic risk factors and socio-demographic characteristics associated with NASH differ by ethnicity. Additional insights into NASH pathogenesis may come from further studies focused on understanding ethnic differences in this disease.
Keywords: Nonalcoholic Steatohepatitis, Latino/Hispanic, Insulin resistance, Nonalcoholic Steatohepatitis Clinical Research Network, Health Disparities
Nonalcoholic fatty liver disease (NAFLD) is a metabolic disorder characterized by excessive triglyceride accumulation in hepatocytes and is intimately associated with insulin resistance. (1, 2) Although the epidemiology of NAFLD is best characterized in Caucasians, racial and ethnic variation in NAFLD has been investigated in several studies in the U.S. population, the results of which point to a non-uniform distribution of NAFLD, with the disorder being most prevalent among Latino individuals and least prevalent among African Americans.(2–8) The explanation for these differences remains unclear; however, it is not fully explained by variations in the prevalence of the stereotypical metabolic risk factors that are associated with NAFLD.(9) Therefore, further investigations of NAFLD in different racial and ethnic groups may help develop our understanding of disease pathogenesis.(10) The aim of this study was to characterize racial and ethnic variations occurring in the clinical, laboratory, socio-demographic and histological features of NAFLD, with a primary focus on comparisons between Latino versus Non-Latino White ethnicities with respect to NASH histology and advanced fibrosis.
Patients and Methods
Participants
This investigation utilized clinical and histological data obtained from the NIH-funded Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN), which is a multicenter collaborative established to assess the natural history, pathogenesis and treatment of NAFLD in the United States.(11–14) Between 2004 and 2008, the NASH CRN enrolled adults and children with the full spectrum of biopsy-proven NAFLD histology into an observational Database study. Additionally, non-diabetic, non-cirrhotic adults with NASH histology were given the opportunity to enroll into an adult treatment trial of Pioglitazone versus Vitamin E versus Placebo for the management of NASH (PIVENS Trial, Clinical Trial Number NCT00063622).(14) All participating centers’ institutional review boards, and a data and safety monitoring board approved all NASH CRN study protocols. Studies were in compliance with Good Clinical Practice guidelines for human research quality standards. Each participant enrolled in the NASH CRN provided written informed consent.
The current investigation included adults enrolled either in the NAFLD Database or the PIVENS trial between 2004 and 2008. The following criteria were used for inclusion in our analyses (n=1026): (1) age ≥18 years; (2) liver histology data available within 6 months of enrollment; (3) no clinically significant history of alcohol consumption (definition of excessive alcohol use in the NASH CRN Database: >20 or >10 grams/day for men and women, respectively; definition of excessive alcohol consumption in PIVENS: >30 or >20 grams/day for men and women, respectively); and (4) no evidence of other etiologies of chronic liver disease.(13)
Outcomes
The primary outcome of interest in this study was NAFLD histological severity. Liver biopsies were categorized as either NASH or Non-NASH NAFLD. We also modeled the outcome of fibrosis, categorized as either mild (Stage 0–2) or advanced (Stage 3–4).
Individuals with NASH had liver biopsies (within 6-months of enrollment into a NASH CRN study) demonstrating typical histological features of NASH, specifically: steatosis, hepatocyte ballooning and lobular inflammation, +/−fibrosis; and that the NASH CRN Pathology Committee read as being ‘definite’ or ‘borderline’ NASH (see below). Also included in the NASH group were individuals with NASH-induced cirrhosis.
Non-NASH histology refers to individuals with histological evidence of NAFLD, but who did not meet histological criteria for NASH, as determined by the NASH CRN Pathology Committee. Non-NASH histology would include, for example, liver biopsies demonstrating steatosis alone, or steatosis with mild, nonspecific inflammation. The Non-NASH histological outcome did not include individuals with cirrhosis. Individuals with NASH-induced cirrhosis were included in the NASH outcome group.
All liver biopsies were stained with hematoxylin-eosin and Masson’s trichrome stains. A diagnosis of NAFLD was defined as the presence of at least 5% steatosis and absence of evidence for other etiologies of chronic liver disease (e.g., viral hepatitis, autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis, iron overload, Wilson disease, etc.).
The NASH CRN Pathology Committee has previously developed and validated a histological scoring system for grading and staging in NAFLD. The Nonalcoholic Fatty Liver Disease Activity Score (NAS) is based upon the assessment of the following microscopic features: steatosis, hepatocyte ballooning, and lobular inflammation, and the fibrosis scoring system is based on localization of pathologic collagen deposition. This scoring system was uniformly applied to all liver biopsies in this investigation.(15) Liver biopsy slides from study participants were read centrally by the Pathology Committee, during which biopsies were rigorously evaluated in a blinded fashion according to the published scoring system. As NASH histology cannot be diagnosed based solely upon the numerical value of the NAS alone, the ultimate diagnostic determinations for each biopsy were assigned by consensus of the Pathology Committee, as follows: (i) definite steatohepatitis, (ii) definitely not steatohepatitis, (iii) borderline steatohepatitis (zone 3 pattern), and (iv) borderline steatohepatitis (zone 1 pattern).
Fibrosis on liver biopsy was staged from 0 to 4, with 0=none; 1a=mild zone 3 (central) perisinusoidal fibrosis; 1b=moderate zone 3 perisinusoidal fibrosis; 1c=periportal and portal fibrosis (zone 1 only); 2= both perisinusoidal and periportal or portal fibrosis; 3= bridging fibrosis; and 4= cirrhosis.
Variables
Demographics
Demographic information collected at the time of enrollment included age, gender, self-reported racial and ethnic affiliation (categorized in this study as, Non-Latino White, Latino, Non-Latino Black, Asian, ‘Other’), education level (dichotomized as ≤ or > high school [HS]), and annual income (dichotomized as ≤ or > $50,000).
Anthropometrics
Height, weight and waist circumference (WC) were measured at the time of enrollment with participants standing wearing light clothing. Body mass index (BMI) was calculated as weight (kg) divided by the height (meters) squared.
Laboratory Data
Laboratory data were collected at the time of enrollment and included measurements of aspartate and alanine aminotransferases (AST and ALT, respectively), gamma glutamyl transferase (GGT), serum albumin, total bilirubin, alkaline phosphatase, platelet count, lipid profile (including: total cholesterol, low density lipoprotein [LDL], high density lipoprotein [HDL] and triglycerides), fasting insulin and fasting glucose levels. The homeostatic model for insulin resistance (HOMA-IR) was calculated from fasting glucose and insulin levels, using the following equation: (Fasting Insulin [microU/L])*(Fasting Glucose [mg/dL])/ 22.5. HOMA-IR was not calculated for individuals taking insulin.
Clinical Data
Information regarding co-morbidities, including: diabetes mellitus, hypertension, and metabolic syndrome were also included. Diabetes mellitus was defined in individuals with fasting blood glucose > 126 mg/dL, or on drug treatment for diabetes. Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg. Metabolic syndrome was defined according to the National Cholesterol Education Program Adult Treatment Panel III criteria, which require at least three of the following: (i) WC > 102 cm and 88 cm, for men and women, respectively; (ii) fasting triglycerides ≥ 150 mg/dL, or on drug treatment for hypertriglyceridemia; (iii) HDL cholesterol < 40 mg/dL or < 50 mg/dL for men and women, respectively, or on drug treatment for dyslipidemia; (iv) blood pressure ≥ 130/85 mmHg, or on drug treatment for hypertension; and (v) fasting blood glucose ≥ 110 mg/dL, or on drug treatment for diabetes mellitus. Medication usage was recorded in detail at the time of enrollment of each participant into a NASH CRN study. Physical exam findings including vital signs, acanthosis nigricans and palmar erythema were recorded. Data were also collected regarding self-reporting of family history of NAFLD.
Dietary and Physical Activity Data
Dietary information was obtained using a validated dietary questionnaire (Block Food Questionnaire, version 1998) based upon self-reported typical eating habits over the past year. Estimates of total calories consumed and proportion of carbohydrate and fat intake were generated using the method previously published by Block et al.(16) At the time of enrollment, NASH CRN study participants also completed a questionnaire, which was derived from the National Health and Nutrition Examination Survey, on self-reported leisure-time physical activity.(17) In the questionnaire, participants reported the amount of time spent per week performing specific leisure-time activities including: brisk walking, jogging, running, hiking/climbing, biking on hills, biking on flat surfaces, swimming, using a treadmill or step machine, dancing, aerobics, calisthenics, weight lifting, golfing, playing singles or doubles tennis, basketball, football and soccer. Participants were also given the opportunity to enter free text responses for their activities.(18) Using a standard reference for metabolic equivalent (MET) intensities for specific activities and the reported duration of each activity, a score for each individual’s total physical activity expressed as metabolic hours per week (MET hrs/week) was generated.(19)
Data Analysis
Continuous data are reported as mean or median values with associated standard deviations (SD) or interquartile ranges [IQR], respectively. We present 95% Confidence Intervals [CI] for mean and median values, as well. Student’s t-test or non-parametric tests were utilized to compare continuous variables, as appropriate. Categorical data are reported as proportions and comparisons were made using either Fisher’s exact test or Chi-square test, as appropriate.
The descriptive clinical data were initially examined with respect to five racial and ethnic categories (Non-Latino White, Non-Latino Black, Latino, Asian, and ‘Other’) and these data are primarily displayed in Tables 1–3. Smaller sample sizes in the Non-Latino Black, Asian and ‘Other’ racial and ethnic categories precluded more detailed analyses.
Table 1.
Characteristics of Adults with NASH Histology by Race and Ethnicity
| Total Population (N=628) | Non Latino Black (N=14) | Non Latino White (N=483) | Latino (N=74) | Asian (N=28) | Other (N=29) | p- value‡ | |
|---|---|---|---|---|---|---|---|
| Age (years)* | 50.0 (49.1–50.9) | 54.6 (48.5–60.7) | 50.9 (49.9–51.9) | 44.2 (41.2–47.1) | 46.9 (40.9–53.0) | 50.8 (46.5–55.1) | <0.0001 |
| Gender (% male)# | 201 (32) | 2 (14) | 153 (32) | 22 (30) | 15 (54) | 9 (31) | 0.79 |
| BMI (kg/m2)* | 34.4 (33.9–34.9) | 36.2 (33.0–39.5) | 34.6 (34.0–35.1) | 33.9 (32.5–35.3) | 30.8 (28.9–32.7) | 35.4 (32.8–37.9) | 0.81 |
| Waist (cm)* | 108.8 (107.7–109.9) | 110.7 (101.5–120.0) | 109.4 (108.2–110.7) | 106.6 (103.4–109.9) | 101.1 (95.8–106.3) | 110.6 (104.8–116.4) | 0.24 |
| ALT (U/L)** | 61 (58–65) | 72 (49–106) | 59 (56–63) | 69 (49–81) | 78 (57–105) | 73 (48–91) | 0.57 |
| AST (U/L)** | 48 (46–51) | 55 (37–82) | 47 (45–51) | 49 (40–53) | 55 (40–78) | 54 (41–70) | 0.58 |
| GGT (U/L)** | 54 (50–57) | 75 (50–93) | 55 (50–58) | 46 (38–53) | 59 (48–84) | 57 (43–86) | 0.16 |
| Albumin (g/L)** | 4.2 (4.2–4.3) | 4.2 (4.0–4.4) | 4.2 (4.2–4.3) | 4.1 (4.0–4.2) | 4.4 (4.2–4.5) | 4.3 (4.1–4.4) | 0.006 |
| Total bilirubin (mg/dL)** | 0.6 (0.6–0.7) | 0.5 (0.4–0.5) | 0.6 (0.6–0.7) | 0.8 (0.7–0.8) | 0.9 (0.6–0.9) | 0.7 (0.6–1.0) | 0.12 |
| Alkaline phosphatase (U/L)** | 84 (82–88) | 87 (70–113) | 85 (82–89) | 86 (76–93) | 67 (57–79) | 78 (74–96) | 0.70 |
| Platelets (× 103) ** | 231 228–237) | 247 (212–275) | 228 (220–234) | 247 (232–263) | 244 (194–274) | 233 (211–267) | 0.02 |
| Total cholesterol (mg/dL)** | 192 (188–196) | 173 (138–196) | 193 (188–198) | 192 (187–206) | 192 (171–208) | 190 (181–215) | 0.62 |
| Triglyceride (mg/dL)** | 152 (142–161) | 134 (83–174) | 157 (143–166) | 136 (123–160) | 155 (115–213) | 145 (115–178) | 0.17 |
| HDL (mg/dL)** | 42 (41–43) | 41 (36–51) | 42 (41–43) | 43 (40–45) | 45 (38–51) | 41 (36–49) | 0.80 |
| LDL (mg/dL)** | 116 (111–119) | 97 (81–120) | 116 (110–120) | 119 (109–124) | 107 (84–119) | 116 (106–140) | 0.29 |
| Systolic BP (mmHg)** | 131 (130–133) | 126 (119–142) | 132 (130–133) | 130 (123–135) | 130 (123–137) | 130 (124–135) | 0.11 |
| Diastolic BP (mmHg)** | 77 (75–78) | 74 (65–81) | 77 (75–78) | 76 (73–81) | 77 (68–80) | 78 (75–85) | 0.66 |
| Hypertension (%)# | 450 (72) | 12 (86) | 365 (76) | 35 (47) | 16 (57) | 22 (76) | <0.0001 |
| Diabetes mellitus (%)# | 235 (37) | 10 (71) | 181 (37) | 20 (27) | 15 (54) | 9 (31) | 0.09 |
| Fasting Glucose (mg/dL)** | 99 (97.0–101.0) | 98 (76.8–137.7) | 98 (96.0–100.0) | 98 (93.1–103.0) | 103 (96.6–121.7) | 107 (94.1–112.6) | 0.66 |
| Fasting Insulin (microU/L)** | 20.0 (18.6–21.1) | 31.8 (18.6–43.8) | 19.5 (18.0–20.8) | 22.6 (18.9–25.7) | 20.1 (11.6–24.0) | 17.1 (14.6–22.0) | 0.17 |
| HOMA-IR | 4.8 (4.4–5.1) | 6.0 (4.4–11.0) | 4.6 (4.2–5.0) | 5.1 (4.5–6.0) | 4.8 (3.3–6.2) | 4.3 (3.3–6.7) | 0.55 |
| Hyperlipidemia (%)# | 523 (83) | 10 (71) | 404 (84) | 60 (81) | 24 (86) | 25 (86) | 0.62 |
| Metabolic syndrome (%)# | 483 (77) | 12 (86) | 381 (79) | 51 (69) | 19 (68) | 20 (69) | 0.07 |
| Family history of NAFLD (%)# | 48 (8) | 0 (0) | 29 (6) | 11 (15) | 4 (14) | 4 (14) | 0.01 |
| Acanthosis nigricans (%)# | 72 (11) | 5 (36) | 27 (6) | 28 (38) | 9 (32) | 3 (10) | <0.001 |
| Palmar erythema (%)# | 67 (11) | 2 (14) | 55 (11) | 3 (4) | 2 (7) | 5 (17) | 0.06 |
| Education (> HS)# | 424 (68) | 8 (57) | 330 (68) | 44 (59) | 24 (86) | 18 (62) | 0.14 |
| Income (>$50,000)# | 342 (54) | 7 (50) | 275 (57) | 30 (41) | 18 (64) | 12 (41) | 0.01 |
| Total caloric intake (kcal)** | 1597 (1549–1690) | 1261 (1063–2982) | 1601 (1549–1710) | 1649 (1394–1878) | 1513 (1281–1691) | 1920 (1312–2493) | 0.61 |
| % Calories from fat** | 38.7 (38.1–39.4) | 41.8 (34.3–47.3) | 38.7 (38.1–39.5) | 36.4 (33.7–39.2) | 42.1 (35.6–44.1) | 38.7 (33.0–42.2) | 0.02 |
| %Calories from carbohydrates** | 47.7 (46.4–48.5) | 46.4 (42.4–56.2) | 47.6 (46.2–48.5) | 49.7 (47.9–52.7) | 44.4 (40.9–50.0) | 47.2 (42.9–51.1) | 0.008 |
| Physical activity (met hrs/week)** | 24.0 (21.0–26.3) | 14.1 (8.5–23.0) | 25.0 (23.0–30.0) | 16.0 (12.0–21.0) | 22.5 (12.0–35.2) | 18.0 (10.5–31.7) | 0.0006 |
Mean (95% confidence interval)
Median (95% confidence interval)
N (%)
Comparing Latino and Non-Latino White individuals
Table 3.
Histological Characteristics of Adults with NAFLD by Race and Ethnicity*
| Total Population (n=1,026) | Non Latino Black (n=27) | Non Latino White (n=785) | Latino (n=118) | Asian (n=54) | Other (n=42) | p-value** | |
|---|---|---|---|---|---|---|---|
| Fibrosis > Stage 2 (%)* | 291 (29) | 8 (30) | 232 (30) | 20 (23) | 15 (28) | 196 (38) | 0.004 |
| Lobular inflammation ≥ Grade 2 (%) | 505 (49) | 13 (48) | 373 (48) | 72 (61) | 27 (50) | 20 (48) | 0.008 |
| Hepatocyte Ballooning (%) | 702 (68) | 17 (63) | 536 (68) | 83 (70) | 33 (61) | 33 (79) | 0.75 |
| NAS ≥ 4 (%) | 756 (74) | 16 (59) | 577 (74) | 89 (75) | 38 (70) | 36 (86) | 0.74 |
Includes individuals with biopsy proven NASH-induced cirrhosis
Comparison between Latino and Non-Latino White individuals
Detailed analyses and statistical assessments of risk factors associated with NASH histology and advanced fibrosis were conducted in participants who self-identified as either Non-Latino White or Latino. As there was only one individual who self-identified as Latino Black, this person was omitted from the analyses due to the likelihood that Latino Black individuals may be culturally and genetically different from the other Latino individuals included in the NASH CRN studies. Associations between clinical characteristics and NAFLD histology (NASH versus Non-NASH histology; and mild versus advanced fibrosis) among Non-Latino Whites and Latino individuals were investigated using univariate and multivariate logistic regression models. Forward and backward stepwise logistic regression analysis was used to identify significant predictors from among the following candidate predictors at enrollment: AST, ALT, total bilirubin, platelet count, triglycerides, total cholesterol, HDL cholesterol, LDL cholesterol, GGT, albumin, alkaline phosphatase, hyperlipidemia, metabolic syndrome, family history of NAFLD, acanthosis nigricans, palmar erythema, education level, income, total caloric intake, percent of calories from fat, percent of calories from carbohydrate, and physical activity. The p-value for addition or elimination from the models was P<0.05 and the models were forced to include terms for age, gender, and ethnicity. We also explored potential effect modification between ethnicity (Latino versus Non-Latino White) and other covariates on the risk of NASH and advanced fibrosis.
For all analyses, SAS 9.1 (SAS Institute ®, Inc., Cary, NC) was utilized. We considered differences statistically significant when p-values were < 0.05. Statistical interactions were considered statistically significant when p-values were <0.001. Nominal two-sided p-values were used.
Results
Characteristics of the Study Population
Overall Population
There were 1026 adults with liver biopsy histology obtained within 6-months of enrollment, including 77 individuals with NASH-induced cirrhosis. Of the 1026 adults included in this study, 37% (N=377) were men, mean (±SD) age was 48.8 (±12.0) years, mean BMI 34.2 (± 6.3) kg/m2, and mean waist circumference 108 (± 14) cm. The median (IQR) AST was 43 (30–63) and ALT 58 (38–90) IU/L. Among the participants, 77% (N=785) were Non-Latino White; 12% (N=118) Latino; 3% (N=27) Non-Latino Black; 5% (N=54) Asian; and 4% (N=42) ‘Other’ race/ethnicity. Compared with the general U.S. adult population, the NASH CRN had an under-representation of Blacks (12% versus 3%) and an adequate representation of Latinos (14% versus 12%).(20) Within the study cohort, 67% (N=732) had hypertension, 32% (N=355) diabetes mellitus, 81% (N=884) hypercholesterolemia, and 73% (N=804) met criteria for the metabolic syndrome.
Of the 1026 participants with biopsy-proven NAFLD, 61% (N=628) had NASH histology, which included the 77 individuals who had NASH-induced cirrhosis. The remaining 398 individuals (39%) had non-NASH histology (i.e., meeting histological criteria for a diagnosis of NAFLD, but not meeting histological criteria for a diagnosis of NASH or NASH-induced cirrhosis). The frequency of NASH among the different racial and ethnic groups was: 62% for Non-Latino Whites, 63% for Latinos, 52% for Non-Latino Blacks, 52% for Asians and 69% for ‘Other’. With respect to the frequency of fibrosis in the cohort, 29% (N=291) had advanced fibrosis (>Stage 2) with the following racial and ethnic distributions: 30% Non-Latino White; 23% Latino; 30% Non-Latino Black; 28% Asian and 38% ‘Other’.
Participants with NASH Histology
The characteristics of Non-Latino Whites, Latinos, Non-Latino Blacks, Asians and ‘Other’ self-identified racial/ethnic groups with NASH histology are shown in Table 1. Among individuals with NASH histology, Latinos were significantly younger compared to Non-Latino Whites (44.2 years [95% CI 41.2–47.1] versus 50.9 years [95% CI 49.9–51.9], p<0.0001). The frequency of hypertension was much lower among Latinos compared with Non-Latino Whites (47% versus 76%, p<0.0001), although in the overall population, there was no statistically significant difference in the frequency of diabetes mellitus, hyperlipidemia, metabolic syndrome or in the values of fasting glucose, fasting insulin and HOMA-IR between the two groups. On physical examination, acanthosis nigricans was at least six-times more common in Latinos compared with Non-Latino Whites (38% versus 6%, respectively, p<0.0001). Although awareness of a family history of NAFLD was relatively uncommon, Latinos were more than twice as likely to report a positive family history compared to Non-Latino Whites (15% versus 6%, respectively, p=0.01). With respect to socio-cultural factors, there was a statistically significant difference between Latinos and Non-Latino Whites with NASH histology in terms of income, dietary composition and physical activity levels. Specifically, Latinos, when compared to Non-Latino Whites, reported lower annual income (57% versus 41% with annual income > $50,000, respectively, p=0.01), consumed a greater percentage of total calories from carbohydrates (49.7% versus 47.6%, p=0.008), and engaged in less physical activity per week (median met hrs/week [95% CI] = 16.0 [12.0–21.0] and 25.0 [23.0–30.0], respectively, p=0.0006). There was no statistical difference in education levels between the two groups.
Participants with Non-NASH Histology
The characteristics of Non-Latino Whites, Latinos, Non-Latino Blacks, Asians and ‘Other’ self-identified racial/ethnic groups with Non-NASH histology are shown in Table 2. Among participants with Non-NASH histology, Latinos were again younger than Non-Latino Whites (38.0 years [95% CI 34.0–42.0] versus 48.5 years [95% CI 47.1–49.6]; p<0.0001) and were less likely to have hypertension (34% versus 59%, p=0.003) or hyperlipidemia (66% versus 80%, p=0.05). There was no significant difference in the frequency of diabetes mellitus or the metabolic syndrome between the two groups, although there was a trend towards diabetes and the metabolic syndrome being less frequent among Latinos (7% versus 15%, p=0.08; and 55% versus 70%, p=0.06, respectively). However, Latinos had a statistically significantly higher HOMA-IR (median [95% CI]=5.0 [3.7–6.9] versus 3.2 [2.9–3.5], p=0.0002) and higher fasting insulin level (median [95% CI]=20.0 [16.6–30.8] versus 14.0 [13.0–15.4], p<0.0001), compared with Non-Latino Whites. Similar to the trend observed in the NASH histology group, in the Non-NASH histology group, we found that Latinos reported lower annual income (45% versus 68% with annual income > $50,000, p=0.004) compared to Non-Latino Whites, but there was no statistically significant difference between the two groups in terms of dietary composition or weekly physical activity levels. The percentage of Latinos completing at least a high school education was significantly lower compared with Non-Latino Whites (52% versus 76%, p=0.002).
Table 2.
Characteristics of Adults with Non-NASH Histology by Race and Ethnicity
| Total Population (N=398) | Non Latino Black (N=13) | Non Latino White (N=302) | Latino (N=44) | Asian (N=26) | Other (N=13) | p- value‡ | |
|---|---|---|---|---|---|---|---|
| Age (years)* | 46.9 (45.8–48.1) | 51.6 (44.3–58.9) | 48.4 (47.1–49.6) | 38.0 (34.0–42.0) | 43.3 (38.2–48.4) | 47.1 (40.9–53.2) | <0.0001 |
| Gender (%male)# | 176 (44) | 1 (8) | 129 (43) | 23 (52) | 17 (65) | 6 (46) | 0.26 |
| BMI (kg/m2)* | 33.8 (33.2–34.4) | 38.1 (34.5–41.7) | 34.1 (33.4–34.8) | 33.7 (31.7–35.6) | 27.4 (25.8–29.0) | 35.4 (32.0–38.7) | 0.68 |
| Waist (cm)* | 107.3 (105.9–108.6) | 111.0 (104.7–117.3) | 108.2 (106.6–109.7) | 108.0 (103.2–112.8) | 92.3 (87.7–96.9) | 110.5 (103.8–117.3) | 0.90 |
| ALT (U/L)** | 57 (53–59) | 33 (22–57) | 56 (49–58) | 60 (51–75) | 69 (56–85) | 67 (39–123) | 0.26 |
| AST (U/L)** | 36 (33–38) | 27 (22–41) | 36 (33–39) | 34 (31–42) | 39 (32–44) | 60 (29–68) | 0.94 |
| GGT (U/L)** | 40 (36–42) | 34 (28–49) | 39 (35–42) | 38 (31–45) | 57 (41–126) | 38 (23–60) | 0.63 |
| Albumin (g/L)** | 4.3 (4.2–4.3) | 4.3 (4.0–4.4) | 4.3 (4.2–4.3) | 4.2 (4.1–4.3) | 4.5 (4.3–4.6) | 4.5 (4.2–4.8) | 0.15 |
| Total bilirubin (mg/dL)** | 0.7 (0.7–0.7) | 0.5 (0.2–0.7) | 0.7 (0.7–0.7) | 0.7 (0.6–0.9) | 0.9 (0.7–1.1) | 0.6 (0.4–0.8) | 0.53 |
| Alkaline phosphatase (U/L)** | 77 (74–79) | 99 (70–117) | 78 (75–80) | 72 (64–82) | 70 (63–87) | 86 (57–99) | 0.27 |
| Platelets (x 103) ** | 252 (246–259) | 283 (249–359) | 250 (245–258) | 252 (227–283) | 243 (204–277) | 258 (223–329) | 0.85 |
| Total cholesterol (mg/dL)** | 195 (191–200) | 202 (171–234) | 196 (192–202) | 181 (167–197) | 202 (180–209) | 190 (157–229) | 0.02 |
| Triglyceride (mg/dL)** | 147 (135–158) | 102 (64–172) | 151 (137–162) | 122 (103–147) | 178 (125–225) | 141 (97–218) | 0.06 |
| HDL (mg/dL)** | 43 (41–44) | 58 (41–65) | 43 (42–45) | 39 (35–42) | 41 (36–49) | 40 (32–47) | 0.03 |
| LDL (mg/dL)** | 120 (117–125) | 138 (98–162) | 121 (118–126) | 113 (105–132) | 112 (102–122) | 128 (92–151) | 0.39 |
| Systolic BP (mmHg)** | 131 (129–133) | 140 (121–152) | 132 (130–134) | 128 (123–133) | 124 (115–130) | 140 (124–146) | 0.28 |
| Diastolic BP (mmHg)** | 77 (75–78) | 77 (75–86) | 77 (76–79) | 76 (71–78) | 71 (68–75) | 83 (77–88) | 0.17 |
| Hypertension (%)# | 226 (57) | 10 (77) | 178 (59) | 15 (34) | 13 (50) | 10 (77) | 0.003 |
| Diabetes mellitus (%)# | 78 (20) | 7 (54) | 55 (18) | 3 (7) | 9 (35) | 4 (31) | 0.08 |
| Fasting Glucose (mg/dL)** | 95 (93.0–97.0) | 108 (86.3–144.7) | 94 (92.5–96.0) | 92 (86.1–95.0) | 101 (92.5–110.0) | 99 (88.4–156.0) | 0.66 |
| Fasting Insulin (microU/L)** | 15.2 (14.0–16.0) | 20.2 (14.2–38.7) | 14.0 (13.0–15.4) | 20.0 (16.6–30.8) | 14.5 (10.6–21.5) | 17.9 (7.7–26.6) | <0.0001 |
| HOMA-IR** | 3.5 (3.2–3.8) | 5.0 (2.7–10.4) | 3.2 (2.9–3.5) | 5.0 (3.7–6.9) | 3.8 (2.6–5.5) | 5.1 (1.6–8.2) | 0.0002 |
| Hyperlipidemia (%)# | 310 (78) | 9 (69) | 241 (80) | 29 (66) | 21 (81) | 10 (77) | 0.05 |
| Metabolic syndrome (%)# | 270 (68) | 9 (69) | 212 (70) | 24 (55) | 15 (58) | 10 (77) | 0.06 |
| Family history of NAFLD (%)# | 30 (8) | 0 (0) | 21 (7) | 7 (16) | 1 (4) | 1 (8) | 0.07 |
| Acanthosis nigricans (%)# | 47 (12) | 6 (46) | 23 (8) | 13 (30) | 2 (8) | 3 (23) | <0.001 |
| Palmar erythema (%)# | 33 (8) | 1 (8) | 30 (10) | 1 (2) | 0 (0) | 1 (8) | 0.15 |
| Education (> HS)# | 294 (74) | 8 (62) | 231 (76) | 23 (52) | 22 (85) | 10 (77) | 0.002 |
| Income (>$50,000)# | 258 (65) | 4 (31) | 206 (68) | 20 (45) | 20 (77) | 8 (62) | 0.004 |
| Total caloric intake (kcal)** | 1710 (1608–1799) | 1435 (822–2755) | 1732 (1635–1822) | 1801 (1434–2222) | 1230 (1002–1722) | 1778 (1238–2394) | 0.85 |
| % Calories from fat** | 38.9 (38.0–39.9) | 37.0 (32.3–44.3) | 39.6 (38.3–40.4) | 36.8 (34.0–39.7) | 39.2 (32.0–43.5) | 38.1 (29.4–39.6) | 0.06 |
| %Calories from carbohydrates* * | 46.9 (45.8–47.7) | 52.9 (45.0–58.3) | 46.2 (45.2–47.3) | 48.6 (44.8–51.5) | 45.3 (42.0–56.8) | 49.1 (47.4–58.2) | 0.13 |
| Physical activity (met hrs/week)** | 30.0 (27.6–32.0) | 20.0 (15.8–30.6) | 29.0 (26.0–32.0) | 37.0 (29.1–55.9) | 26.9 (11.5–42.5) | 34.0 (16.5–65.8) | 0.25 |
Mean (95% confidence interval)
Median (95% confidence interval)
N (%)
Comparing Latino and Non-Latino White individuals
NAFLD Histology and Ethnicity (Latino versus Non-Latino White)
Histo-Pathological Features of NAFLD
A comparison of the histological features of NAFLD among the different racial and ethnic groups is shown in Table 3. There were significant differences between Latino and Non-Latino White participants, with advanced fibrosis (defined as Stage 3–4 fibrosis) being less frequent (23% versus 30%, p-value 0.004) and pronounced lobular inflammation (≥ grade 2) being more frequent (61% versus 48%, p-value 0.008) among Latinos compared to Non-Latino Whites. However, there was no statistically significant difference in hepatocyte ballooning or proportion of individuals with NAS ≥ 4 between the two ethnic groups.
Predictors of NASH
We investigated associations between NASH and clinical, laboratory and socio-demographic factors among Non-Latino Whites and Latinos using univariate and multivariate logistic regression analyses. There were 785 Non-Latino White participants and 118 Latino participants included in the logistic regression analyses (total N=903).
Univariate Logistic Regression
Univariate logistic regression results are shown in Table 4 and demonstrate that the following risk factors were significantly associated with NASH: age, female gender, lower education level, lower annual income, hypertension, hyperlipidemia, diabetes mellitus, metabolic syndrome, AST, ALT, GGT, total bilirubin, alkaline phosphatase, platelet count, triglyceride level and HOMA-IR.
Table 4.
Univariate Analyses: Factors Associated with NASH among Latino and Non-Latino Whites testing for effect modification with Ethnicity
| Patient Characteristic | (N=903) OR (95% CI) | Univariate Regression p-value | p-value for Effect Modification of Ethnicity on Patient Characteristic ‡ |
|---|---|---|---|
| Demographics | |||
| Ethnicity (Latino vs. non-Latino White) | 1.05 (0.71–1.57) | 0.81 | - |
| Age (years) | 1.02 (1.01–1.03) | <0.001 | 0.29 |
| Gender (female vs. male) | 1.71 (1.30–2.26) | <0.001 | 0.26 |
| Education level (>HS vs. ≤HS) | 0.74 (0.55–1.00) | 0.05 | 0.09 |
| Income (≥$50,000 vs. <$50,000) | 0.64 (0.49–0.85) | <0.01 | 0.50 |
| Family history of NAFLD | 0.88 (0.53–1.46) | 0.62 | 0.90 |
| Comorbidities | |||
| Hypertension | 2.02 (1.52–2.68) | <0.0001 | 0.61 |
| Hyperlipidemia | 1.40 (1.00–1.97) | 0.05 | 0.25 |
| Diabetes mellitus | 2.80 (2.01–3.90) | <0.0001 | 0.35 |
| Anthropometrics, diet and physical activity | |||
| BMI (kg/m2) | 1.01 (0.99–1.03) | 0.32 | 0.88 |
| Waist (cm) | 1.01 (1.00–1.02) | 0.34 | 0.35 |
| Total caloric intake (×10−3) | 0.87 (0.75–1.01) | 0.06 | 0.68 |
| % Calories from total fat | 0.99 (0.98–1.01) | 0.51 | 0.87 |
| % Calories from carbohydrates | 1.01 (1.00–1.03) | 0.14 | 0.77 |
| Physical activity (met hours/week) | 1.000 (0.997–1.003) | 0.94 | 0.85 |
| Clinical data | |||
| Systolic BP (mmHg) | 1.01 (1.00–1.02) | 0.12 | 0.78 |
| Diastolic BP (mmHg) | 0.99 (0.98–1.01) | 0.32 | 0.16 |
| Acanthosis nigricans | 0.94 (0.61–1.47) | 0.80 | 0.16 |
| Palmar Erythema | 1.18 (0.75–1.87) | 0.48 | 0.71 |
| Laboratory measurements | |||
| ALT (per 5 U/L) | 1.02 (1.01–1.04) | <0.01 | 0.95 |
| AST (per 5 U/L) | 1.10 (1.07–1.13) | <0.0001 | 0.88 |
| GGT (per 10 U/L) | 1.03 (1.01–1.05) | 0.01 | 0.37 |
| Albumin (g/L) | 0.82 (0.60–1.12) | 0.22 | 0.44 |
| Total bilirubin (mg/dL) | 0.71 (0.52–0.97) | 0.03 | 0.60 |
| Alkaline phosphatase (per 10 U/L) | 1.08 (1.03–1.12) | <0.0001 | 0.18 |
| Platelets (per 1,0000 cells/μL) | 0.96 (0.95–0.98) | <0.0001 | 0.22 |
| Total cholesterol (per 10 mg/dL) | 1.00 (0.97–1.03) | 0.94 | 0.04 |
| Triglycerides (per 10 mg/dL) | 1.02 (1.01–1.03) | <0.01 | 0.42 |
| HDL (per 10 mg/dL) | 0.91 (0.82–1.02) | 0.10 | 0.07 |
| LDL (per 10 mg/dL) | 0.98 (0.94–1.02) | 0.24 | 0.21 |
| HOMA-IR | 1.08 (1.04–1.12) | 0.0001 | <0.001 |
| Metabolic syndrome | 1.61 (1.19–2.18) | <0.01 | 0.72 |
p-value shown is for the interaction term from the regression model with the following terms: Predictor (X), Ethnicity and Ethnicity*Predictor (X). For the interaction terms, we considered p-values < 0.001 to be statistically significant.
Effect Modification of Ethnicity
Effect modification of ethnicity on patient characteristics for NAFLD histological severity was also investigated (Table 4). For the interaction terms in the statistical models, we considered p-values < 0.001 to be statistically significant. We found a significant interaction between HOMA-IR and ethnicity (p<0.001) and, because of this interaction, we examined the effect of HOMA-IR on NASH separately for Latinos and non-Latino Whites, while adjusting for the variables selected from the stepwise logistic regression model (see below). The interaction between HOMA-IR and ethnicity remained statistically significant when diabetic participants were excluded from the analyses (data not shown).
Multivariate Logistic Regression
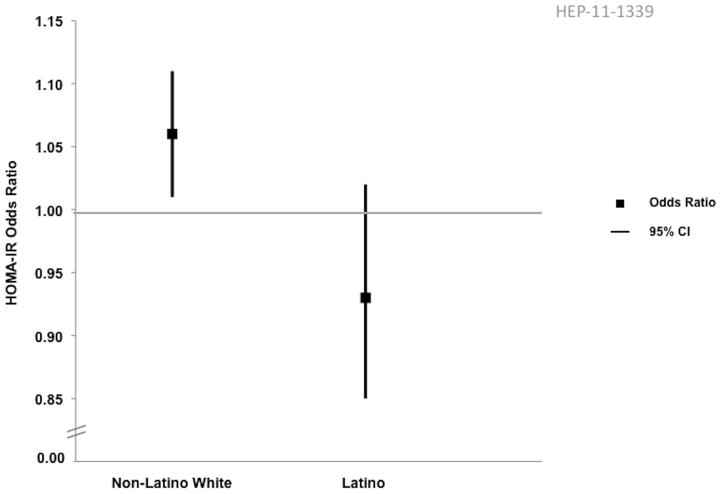
The results from the multivariate logistic regression analysis are show in Table 5. Factors that were positively associated with NASH included: female gender (p=0.001), AST (<0.0001), diabetes mellitus (p=0.01), hypertension (p=0.02) and triglyceride level (p=0.02). Platelet count (p=0.006) was negatively associated with NASH histology. As demonstrated in Figure 1, we also found significant effect modification between ethnicity and HOMA-IR, with HOMA-IR being a significant risk factor for NASH among Non-Latino Whites (OR 1.06 [95% CI 1.01–1.1]), but not among Latinos (OR 0.93 [95% CI 0.85–1.02]).
Table 5.
Factors Associated with NASH from Multivariate Logistic Regression Analysis*
| Patient Characteristic | OR (95% CI) | p |
|---|---|---|
| Ethnicity (Latino vs. non-Latino White) | 1.43 (0.88–2.33) | 0.15 |
| Age (years) | 1.01 (1.00–1.03) | 0.13 |
| Gender (female vs. male) | 1.80 (1.28–2.55) | 0.001 |
| AST (per 5 U/L) | 1.10 (1.07–1.14) | <0.0001 |
| Platelet count (per 10,000 cells/μL) | 0.97 (0.94–0.99) | 0.006 |
| Triglyceride level (per 10 mg/dL) | 1.03 (1.01–1.05) | 0.02 |
| Hypertension | 1.51 (1.06–2.15) | 0.02 |
| Diabetes mellitus | 1.73 (1.14–2.64) | 0.01 |
| HOMA-IR | 1.05 (1.01–1.10) | 0.03 |
Stepwise logistic regression model included the following candidate baseline predictors: AST, ALT, total bilirubin, platelet count, triglycerides, total cholesterol, HDL cholesterol, LDL cholesterol, GGT, albumin, alkaline phosphatase, hyperlipidemia, metabolic syndrome, family history of NAFLD, acanthosis nigricans, palmar erythema, education level, income, total caloric intake, percent of calories from fat, percent of calories from carbohydrate, and physical activity. The model was forced to include terms for age, gender, and ethnicity.
Figure 1.
Differential Effect of HOMA-IR on Risk of NASH by Ethnicity
Predictors of Advanced Fibrosis
We also investigated associations between advanced fibrosis and clinical, laboratory and socio-demographic factors among Non-Latino Whites and Latinos using univariate and multivariate logistic regression analyses. Analyses included 893 participants.
Univariate Logistic Regression
Univariate logistic regression demonstrated that the following risk factors were significantly associated with advanced fibrosis: ethnicity, age, gender, education level, income, hypertension, diabetes, metabolic syndrome, BMI, WC, SBP, DBP, AST, ALT, GGT, alkaline phosphatase, albumin, platelets, LDL, HOMA-IR and palmar erythema. We found no significant evidence for effect modification of ethnicity on patient characteristics with respect to advanced fibrosis.
Multivariate Logistic Regression
The results from the multivariate logistic regression analysis are shown in Table 6. Factors that were positively associated with advanced fibrosis included: age (p=0.01), female gender (p=0.03), AST (p=0.001), alkaline phosphatase (p=0.002), hypertension (p=0.0005) and HOMA-IR (<0.0001). Platelet count (p<0.0001), ALT (p=0.004) and total cholesterol (p=0.004) were significantly inversely associated with risk for advanced fibrosis.
Table 6.
Factors Associated with Advanced Fibrosis from Multivariate Logistic Regression Analysis (N=816)*
| Patient Characteristic | OR (95% CI) | p |
|---|---|---|
| Ethnicity (Latino vs. non-Latino White) | 0.78 (0.42–1.45) | 0.43 |
| Age (years) | 1.02 (1.01–1.04) | 0.01 |
| Gender (female vs. male) | 1.59 (1.04–2.41) | 0.03 |
| AST (per 5 U/L) | 1.07 (1.03–1.12) | 0.001 |
| ALT (per 5 U/L) | 0.95 (0.92–0.99) | 0.004 |
| Platelet count (per 10,000 cells/μL) | 0.91 (0.88–0.94) | <0.0001 |
| Hypertension | 2.20 (1.41–3.42) | 0.0005 |
| HOMA-IR | 1.08 (1.04–1.12) | <0.0001 |
| Alkaline phosphatase (per 10 U/L) | 1.09 (1.03–1.15) | 0.002 |
| Total Cholesterol (per 10 mg/dL) | 0.94 (0.89–0.98) | 0.004 |
Stepwise logistic regression model included the following candidate baseline predictors: AST, ALT, total bilirubin, platelet count, triglycerides, total cholesterol, HDL cholesterol, LDL cholesterol, GGT, albumin, alkaline phosphatase, hyperlipidemia, metabolic syndrome, family history of NAFLD, acanthosis nigricans, palmar erythema, education level, income, total caloric intake, percent of calories from fat, percent of calories from carbohydrate, and physical activity. The model was forced to include terms for age, gender, and ethnicity.
Discussion
The large NASH CRN cohort of patients with well-characterized, biopsy-proven disease allowed for a detailed analysis of the associations of ethnicity and race with clinical and histological features of NAFLD. We found that, among individuals with NASH histology, Latinos were younger, consumed more carbohydrate calories and engaged in less physical activity, compared to Non-Latino Whites. Additionally, Latinos with NASH had lower income and lower prevalence of hypertension compared to Non-Latino Whites, which may be a reflection of similar ethnic trends in the general U.S. adult population with respect to these two characteristics.(21, 22) With respect to specific histological features seen on liver biopsy between Latinos and Non-Latino Whites with NAFLD, Latinos had significantly greater inflammation, but significantly less advanced fibrosis. Although it is conceivable that Latino individuals may have less risk for fibrosis as an ethnic group, it is likely that the lower frequency of advanced fibrosis may be explained, at least in part, by the overall younger age of the Latino population in this study. A notable finding of this study is the differential effect of HOMA-IR on risk of NASH (versus Non-NASH histology), such that HOMA-IR conferred an increased risk of NASH in Non-Latino Whites, but not in Latinos. We did not find differential effect of HOMA-IR on the risk of advanced fibrosis.
Several studies have described racial and ethnic variations in NAFLD, primarily with respect to differences in frequency of the disorder, with consistent reporting of increased frequency in Latino populations and decreased frequency among African Americans.(3, 4, 7, 8, 23) Although the NASH CRN was not designed to be a population-based study, within our group of study participants with NAFLD we found an increased frequency of NASH histology among Latinos (63%) compared to Non-Latino Blacks (52%), which is in keeping with previously published trends.
Although many studies of racial and ethnic variation in NAFLD have focused primarily on the prevalence (or frequency) of the disorder, a few studies have delved further into metabolic associations of NAFLD in different racial and ethnic groups. A recently published study by Lomonaco et al. compared Hispanic and Caucasian individuals with biopsy-proven NASH with respect to several metabolic features, including measures of adipose and hepatic insulin resistance. The investigators found no significant difference between Hispanic and Caucasian individuals with respect to NASH severity, but their data suggested that in Hispanic diabetic patients there may be a trend towards increased risk for fibrosis progression, warranting further investigation.(24) In two separate investigations from the population-based Dallas Heart Study, Browning et al. and Guerrero et al., investigated the well-described dissociation between NAFLD and stereotypical metabolic risk factors, such as insulin resistance and obesity, among different racial and ethnic groups.(3, 9) Using magnetic resonance spectroscopy (MRS) to detect hepatic steatosis in a multi-ethnic population-based sample, Browning et al. reported the highest prevalence of hepatic steatosis among Latinos (45%) and lowest prevalence of steatosis among African Americans (24%), with Whites having an intermediate prevalence of 33%. They also speculated that the increased prevalence of hepatic steatosis among Latinos might be attributable to the high prevalence of obesity and insulin resistance in this ethnic group. However, similar rationale did not apply to the African American population, given that the prevalence of obesity and insulin resistance are actually highest in this segment of the U.S. population.(25) The authors reasonably speculated that the racial and ethnic differences they described in the prevalence of hepatic steatosis were likely a reflection of differences in metabolic responses to obesity and insulin resistance occurring among racial and ethnic groups. Further data supporting the hypothesis that there may be differences in metabolic responses related to NAFLD in different racial and ethnic groups was provided by Guerrero et al. in another investigation from the Dallas Heart Study. They demonstrated that, although intraperitoneal adipose content was linked to the degree of hepatic steatosis regardless of race or ethnicity, there was a difference in the metabolic response in African Americans to the presence of obesity and insulin resistance, such that African Americans appeared to be more resistant to the development of hepatic steatosis and abdominal adiposity, despite having insulin resistance and obesity.(9) Similar to the dissociations between metabolic risk factors and NAFLD that were described in African Americans in the Dallas Heart Study using MRS assessment of hepatic steatosis, we report a differential association between HOMA-IR and the risk of NASH histology among Latinos and Non-Latino Whites, with HOMA-IR being a significant risk factor for NASH among Non-Latino Whites, but not among Latinos. This differential effect of HOMA-IR is intriguing and warrants confirmation in larger studies and further investigation.
Our data suggest that pathogenic differences may exist between Latinos and Non-Latino Whites with respect to the development of NASH. Indeed, this hypothesis is supported by recent data demonstrating a significant association between hepatic steatosis detected with MRS and the PNPLA3 polymorphism (rs738409), an association that was most pronounced among Latinos in the study.(10) The authors found that individuals who were heterozygous for the PNPLA3 polymorphism had higher hepatic triglyceride levels compared to individuals with the wild-type, and that individuals who possessed two copies of the variant allele had a multiplicative effect with respect to hepatic triglycerides. Additional investigations of PNPLA3 suggest that it may play a role, in association with other stressors, in hepatic inflammation and cirrhosis.(26–29)
The limitations of our study should also be acknowledged. As the NASH CRN was not designed to be a population-based study, we cannot draw strong conclusions regarding the frequency of NAFLD histological sub-types (i.e., NASH versus Non-NASH) with respect to race and ethnicity. The participant population consists of consenting individuals with a potential self-selection that is inherent to many large-scale, prospectively enrolled studies. Additionally, it should be noted that the NASH CRN also included a clinical trial (PIVENS), which specifically selected for patients with NASH histology.(14) Despite this, the demographics of our participant population and frequency of metabolic risk factors within the population were entirely consistent with the characteristics of patient populations that have been described in other studies of NAFLD. Additionally, the current study was designed to assess risk factors for NASH histology compared to Non-NASH histology rather than compared to normal liver histology, as the NASH CRN does not currently include a population of individuals without NAFLD. Finally, although the NASH CRN is the largest cohort of NAFLD patients that has been assembled to date with rigorous collection of extensive clinical, laboratory and histological data, we were limited in our ability to reliably assess risk factors for histological severity among other racial groups due to small numbers of individuals self-reporting as African American and Asian.
In summary, the findings of the present study demonstrate differences in metabolic and socio-demographic factors associated with NASH histology between Latino and Non-Latino White adults. HOMA-IR, as a marker of insulin resistance, was not a significant risk factor for NASH among Latinos, but was a significant risk factor among Non-Latino Whites. These findings suggest that there may be pathophysiological variation between the two ethnic groups with respect to the development of NASH and additional investigations are warranted to define this further.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by grants from the National Institutes of Health to the NASH CRN (U01DK61718, U01DK61728, U01DK61730, U01DK61731, U01DK61732, U01DK61734, U01DK61737, U01DK61738, U01DK61713).
List of Abbreviations
- NAFLD
Nonalcoholic Fatty Liver Disease
- NASH
Nonalcoholic Steatohepatitis
- NASH CRN
Nonalcoholic Steatohepatitis Clinical Research Network
- NAS
Nonalcoholic Fatty Liver Disease Activity Score
- HOMA-IR
Homeostasis Model Assessment of Insulin Resistance
- BMI
Body Mass Index
- WC
Waist Circumference
- SBP
Systolic Blood Pressure
- DBP
Diastolic Blood Pressure
- OR
Odds Ratio
- 95% CI
95th Percentile Confidence Interval
- SD
Standard Deviation
- IQR
Interquartile Range
- AST
Aspartate Aminotransferase
- ALT
Alanine Aminotransferase
- GGT
Gamma Glutamyltransferase
- LDL
Low Density Lipoprotein
- HDL
High Density Lipoprotein
- MET
Metabolic Equivalent
- HS
High School
Contributor Information
Kiran Bambha, Email: kiran.bambha@ucdenver.edu.
Patricia Belt, Email: pbelt@jhsph.edu.
Maria Abraham, Email: maabraha@jhsph.edu.
Laura A. Wilson, Email: lwilson@jhsph.edu.
Mark Pabst, Email: mark.pabst@ucsf.edu.
Linda Ferrell, Email: linda.ferrell@ucsf.edu.
Aynur Unalp-Arida, Email: aunalp@jhsph.edu.
Nathan Bass, Email: nathan.bass@ucsf.edu.
References
- 1.Ong JP, Younossi ZM. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis. 2007 Feb;11(1):1–16. vii. doi: 10.1016/j.cld.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003 May;98(5):960–7. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 3.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004 Dec;40(6):1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 4.Weston SR, Leyden W, Murphy R, Bass NM, Bell BP, Manos MM, Terrault NA. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005 Feb;41(2):372–9. doi: 10.1002/hep.20554. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell SH, Harris DM, Patrie JT, Hespenheide EE. Is NASH underdiagnosed among African Americans? Am J Gastroenterol. 2002 Jun;97(6):1496–500. doi: 10.1111/j.1572-0241.2002.05795.x. [DOI] [PubMed] [Google Scholar]
- 6.Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005 May;115(5):e561–5. doi: 10.1542/peds.2004-1832. [DOI] [PubMed] [Google Scholar]
- 7.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011 Jan;140(1):124–31. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 8.Mohanty SR, Troy TN, Huo D, O’Brien BL, Jensen DM, Hart J. Influence of ethnicity on histological differences in non-alcoholic fatty liver disease. J Hepatol. 2009 Apr;50(4):797–804. doi: 10.1016/j.jhep.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology. 2009 Mar;49(3):791–801. doi: 10.1002/hep.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008 Dec;40(12):1461–5. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nonalcoholic steatohepatitis clinical research network. Hepatology. 2003 Feb;37(2):244. doi: 10.1002/hep.510370203. [DOI] [PubMed] [Google Scholar]
- 12.Chalasani NP, Sanyal AJ, Kowdley KV, Robuck PR, Hoofnagle J, Kleiner DE, Unalp A, Tonascia J. Pioglitazone versus vitamin E versus placebo for the treatment of non-diabetic patients with non-alcoholic steatohepatitis: PIVENS trial design. Contemp Clin Trials. 2009 Jan;30(1):88–96. doi: 10.1016/j.cct.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neuschwander-Tetri BA, Clark JM, Bass NM, Van Natta ML, Unalp-Arida A, Tonascia J, Zein CO, Brunt EM, Kleiner DE, McCullough AJ, Sanyal AJ, Diehl AM, Lavine JE, Chalasani N, Kowdley KV. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 2010 Sep;52(3):913–24. doi: 10.1002/hep.23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010 May 6;362(18):1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005 Jun;41(6):1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 16.Block G, Subar AF. Estimates of nutrient intake from a food frequency questionnaire: the 1987 National Health Interview Survey. J Am Diet Assoc. 1992 Aug;92(8):969–77. [PubMed] [Google Scholar]
- 17. [Accessed 6/21/11.]; http://www.cdc.gov/nchs/nhanes.htm.
- 18.Kistler KD, Brunt EM, Clark JM, Diehl AM, Sallis JF, Schwimmer JB. Physical activity recommendations, exercise intensity, and histological severity of nonalcoholic fatty liver disease. Am J Gastroenterol. 2011 Mar;106(3):460–8. doi: 10.1038/ajg.2010.488. quiz 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O’Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, Jacobs DR, Jr, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000 Sep;32(9 Suppl):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 20. [Accessed 6/21/11.]; http://www.census.gov/compendia/statab/cats/population/estimates_and_projections_by_age_sex_raceethnicity.html.
- 21. [Accessed 6/21/11.]; http://www.statehealthfacts.org/index.jsp.
- 22. [Accessed 6/21/11.]; http://www.cdc.gov/mmwr/preview/mmwrhtml/su6001a21.htm?s_cid=su6001a21_w.
- 23.Kallwitz ER, Guzman G, TenCate V, Vitello J, Layden-Almer J, Berkes J, Patel R, Layden TJ, Cotler SJ. The histologic spectrum of liver disease in African-American, non-Hispanic white, and Hispanic obesity surgery patients. Am J Gastroenterol. 2009 Jan;104(1):64–9. doi: 10.1038/ajg.2008.12. [DOI] [PubMed] [Google Scholar]
- 24.Lomonaco R, Ortiz-Lopez C, Orsak B, Finch J, Webb A, Bril F, Louden C, Tio F, Cusi K. Role of ethnicity in overweight and obese patients with nonalcoholic steatohepatitis. Hepatology. 2011 Jun 14; doi: 10.1002/hep.24483. [DOI] [PubMed] [Google Scholar]
- 25. [Accessed 6/11/11.]; www.cdc.gov.
- 26.Sookoian S, Castano GO, Burgueno AL, Gianotti TF, Rosselli MS, Pirola CJ. A nonsynonymous gene variant in the adiponutrin gene is associated with nonalcoholic fatty liver disease severity. J Lipid Res. 2009 Oct;50(10):2111–6. doi: 10.1194/jlr.P900013-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romeo S, Sentinelli F, Dash S, Yeo GS, Savage DB, Leonetti F, Capoccia D, Incani M, Maglio C, Iacovino M, O’Rahilly S, Baroni MG. Morbid obesity exposes the association between PNPLA3 I148M (rs738409) and indices of hepatic injury in individuals of European descent. Int J Obes (Lond) 2010 Jan;34(1):190–4. doi: 10.1038/ijo.2009.216. [DOI] [PubMed] [Google Scholar]
- 28.Tian C, Stokowski RP, Kershenobich D, Ballinger DG, Hinds DA. Variant in PNPLA3 is associated with alcoholic liver disease. Nat Genet. 2010 Jan;42(1):21–3. doi: 10.1038/ng.488. [DOI] [PubMed] [Google Scholar]
- 29.Wagenknecht LE, Palmer ND, Bowden DW, Rotter JI, Norris JM, Ziegler J, Chen YD, Haffner S, Scherzinger A, Langefeld CD. Association of PNPLA3 with non-alcoholic fatty liver disease in a minority cohort: the Insulin Resistance Atherosclerosis Family Study. Liver Int. 2011 Mar;31(3):412–6. doi: 10.1111/j.1478-3231.2010.02444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.