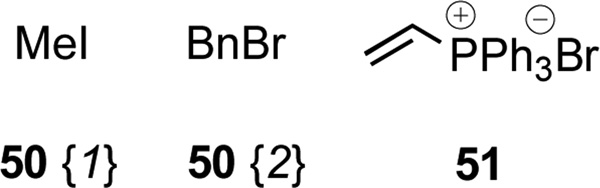Abstract
A strategy featuring a multicomponent assembly process followed by an intramolecular azide–alkyne dipolar (Huisgen) cycloaddition was implemented for the facile synthesis of three different 1,2,3-triazolo-1,4-benzodiazepine scaffolds. A diverse library of 170 compounds derived from these scaffolds was then created through N-functionalizations, palladium-catalyzed cross-coupling reactions, and several applications of α-aminonitrile chemistry.
Keywords: Triazole, dipolar cycloaddition, azide–alkyne, benzodiazepine, multicomponent reaction, α-aminonitrile, diversity oriented synthesis
The identification of biologically active small molecules through high-throughput screening (HTS) is a common strategy in campaigns to discover both novel drug leads and molecular probes that may be used to study aberrant biological pathways leading to disease.1 Because of their high hit rates in HTS and ‘drug-like’ pharmacological profiles, derivatives of privileged structures are uniquely well-suited as molecular scaffolds in drug discovery.2 Straightforward variation of the substituents on privileged scaffolds often leads to potent and selective binders for multiple biological targets from a single library.
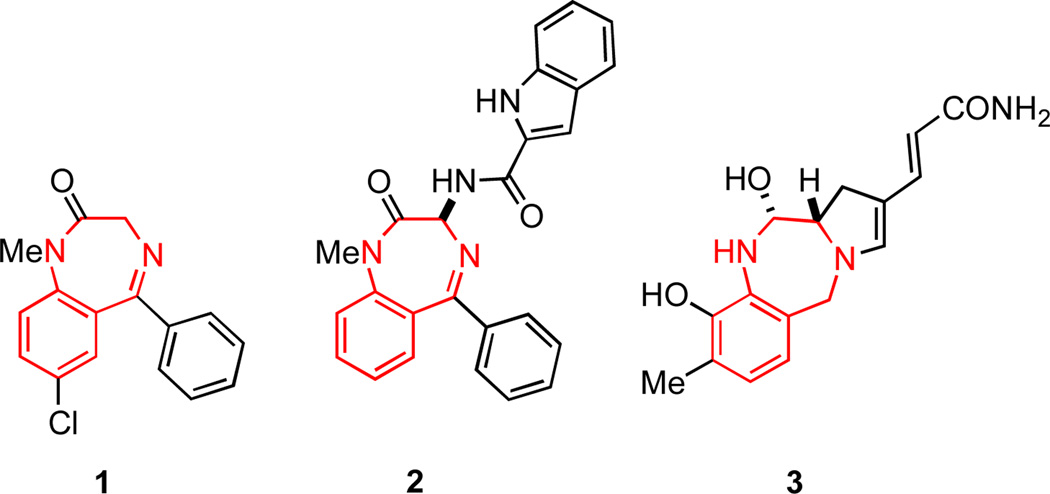
More than 20 years ago Evans identified the benzodiazepine ring system as the archetypal privileged structure, thereby introducing the term into medicinal chemistry.3,4 Compounds derived from the 1,4-benzodiazepine ring system bind to a multitude of targets, including G protein-coupled receptors (GPCRs), ligand-gated ion channels, and enzymes.2a The propensity of compounds derived from the benzodiazepine nucleus to bind to GPCRs has been suggested to arise from the ability of the scaffold to act as a structural mimic of peptide β-turns5 and α-helices.6 Such secondary structural elements orient substituents in a manner that enhances protein binding. Indeed, virtually all GPCR-binding ligands adopt α-, β- or γ-turns.7 It is therefore unsurprising that the 1,4-benzodiazepine substructure is a common structural subunit in numerous pharmaceutical agents, biological probes, and bioactive natural products such as diazepam (1),8 devazepide (2)9 and anthramycin (3)10 respectively (Figure 1).
Figure 1.
Derivatives of the 1,4-benzodiazepine ring system.
A key consideration inherent in designing and preparing novel compound libraries for HTS is the efficient synthesis of core scaffolds that bear functionality to enable facile diversification. In this context, we have recently developed a Mannich-type multicomponent assembly process (MCAP) to construct aryl aminomethyl adducts that are suitably functionalized for use in subsequent cyclizations to afford a variety of nitrogen-containing heterocyclic structures.11 This sequential MCAP/cyclization strategy has been successfully utilized to rapidly access natural products11,12,13a and unnatural heterocyclic frameworks.13,14 We also applied this approach to the syntheses of diverse, medicinally-relevant scaffolds based upon the 1,2,3-triazolo-1,4-benzodiazepine core 6 (Scheme 1),15,16 2-aryl piperidines,16,17 tetrahydroisoquinolines,16,18 as well as isoindolinones, norbenzomorphans, benzazocines, and benzoxacines.16,19 We now report the application of this general approach for diversity oriented synthesis (DOS) to the preparation of libraries of 1,2,3-triazolo-1,4-benzodiazepines, a subclass of the benzodiazepine privileged structure whose biological properties have not been widely explored.20
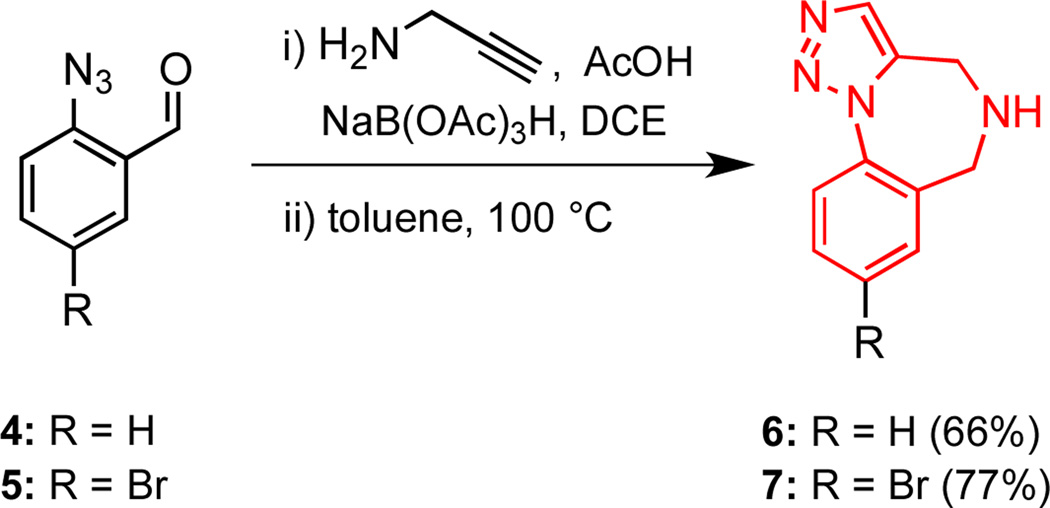
Scheme 1.
Synthesis of Scaffolds 6 and 7.
As described previously, the parent 1,2,3-triazolo-1,4-benzodiazepine 6 and brominated analog 7 were readily prepared on multi-gram scale by a reductive amination and azide–alkyne dipolar (Huisgen) cycloaddition sequence (Scheme 1).15 This approach afforded scaffolds 6 and 7 in high purity after recrystallization and represents a considerably more expedient access to compound 6 than previously reported.21
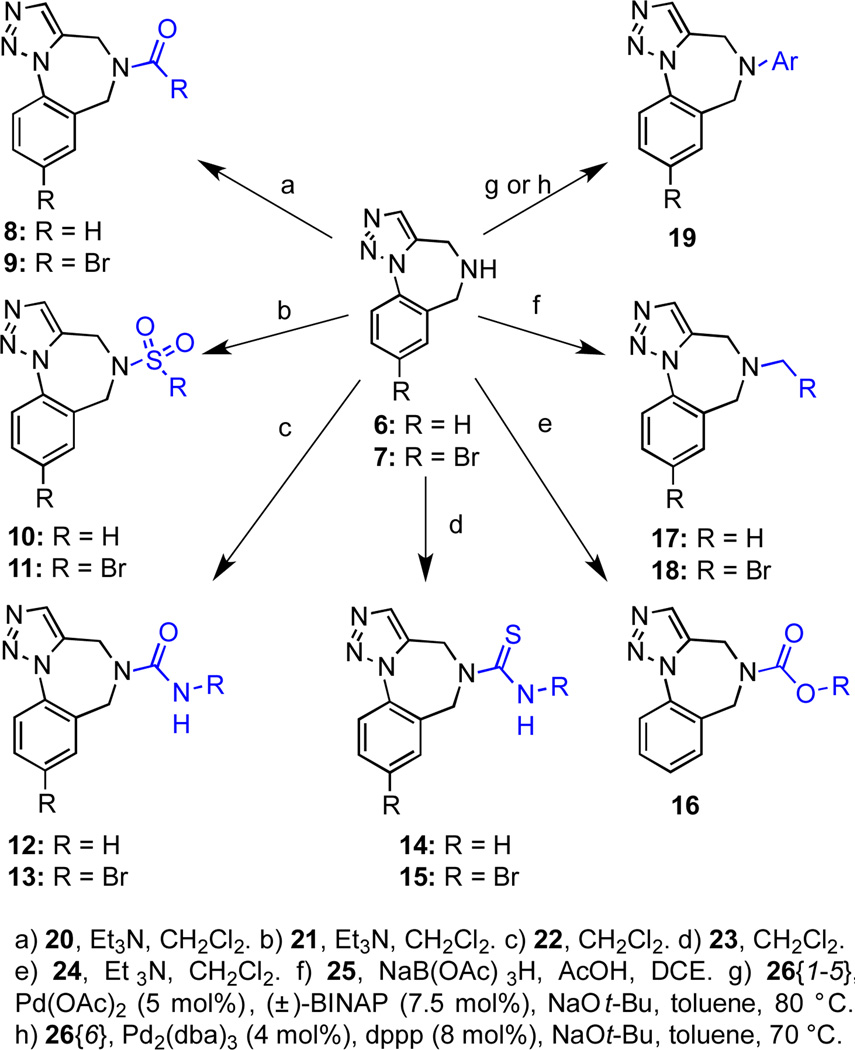
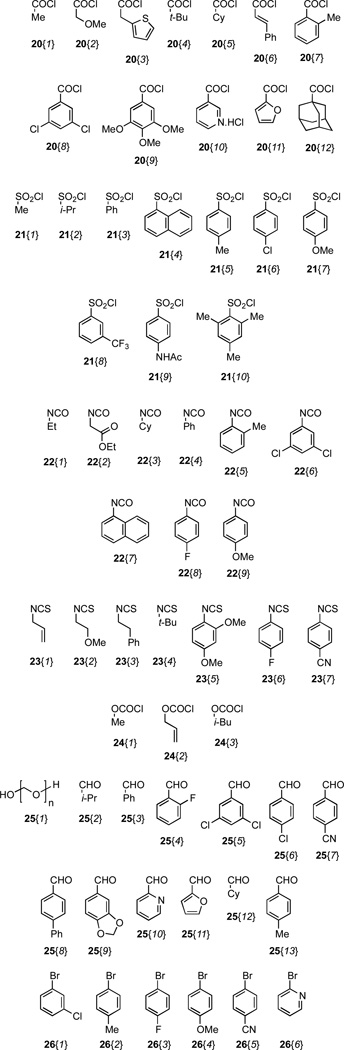
The two heterocycles 6 and 7 were then subjected to various tactics for N-functionalization to diversify the scaffolds into libraries of amides 8 and 9, sulfonamides 10 and 11, ureas 12 and 13, thioureas 14 and 15, carbamate 16, and amine derivatives 17–19 (Scheme 2, Figure 2, Table 1). Reagents were typically used in excess to mitigate for variations in the purity of reagents, which were used without purification, and reaction efficiency. Functionalizing agents were selected to contain a mixture of aliphatic and aromatic groups. Different aliphatic substituents were chosen to vary the nonpolar surface area, whereas aromatic groups were varied by selecting substituents that would modulate the electrostatic properties of the aromatic ring. In this manner, it would be possible to acquire preliminary structure activity relationship (SAR) data from a small set of compounds. N-Arylation procedures to access chemset 19 were explored for the formation of p-toluidine derivative 19{2}. We discovered that conditions reported by Buchwald utilizing Pd(OAc)2, (±)-BINAP and NaOt-Bu were superior to alternative protocols in which Pd2(dba)3 was employed as the Pd source.22 Use of this method allowed the introduction of aryl halides onto the parent scaffold (B{1} and 19{3}) without the intervention of observable side reactions involving secondary cross-couplings. The 2-amino pyridine derivative 19{6} was also prepared using a procedure described by Buchwald, but a catalyst loading of 4 mol % Pd2dba3 was necessary for complete conversion.23
Scheme 2.
Nitrogen functionalization of scaffolds 6 and 7.
Figure 2.
Nitrogen-functionalizing agents used to derivatize scaffolds 6 and 7.
Table 1.
Nitrogen functionalization of scaffolds 6 and 7.
| Entry | Scaffold | Functionalizing agent |
Product | Yield (%) |
|---|---|---|---|---|
| 1 | 6 | 20{1} | 8{1} | 95 |
| 2 | 6 | 20{2} | 8{2} | 96 |
| 3 | 6 | 20{3} | 8{3} | Quant. |
| 4 | 6 | 20{4} | 8{4} | 93 |
| 5 | 6 | 20{5} | 8{5} | 98 |
| 6 | 6 | 20{6} | 8{6} | 96 |
| 7 | 6 | 20{7} | 8{7} | 98 |
| 8 | 6 | 20{8} | 8{8} | 98 |
| 9 | 6 | 20{9} | 8{9} | 98 |
| 10 | 6 | 20{10} | 8{10} | 82a,b |
| 11 | 6 | 20{11} | 8{11} | 95 |
| 12 | 7 | 20{9} | 9{9} | 90 |
| 13 | 7 | 20{12} | 9{12} | 67 |
| 14 | 6 | 21{1} | 10{1} | 97 |
| 15 | 6 | 21{2} | 10{2} | 64 |
| 16 | 6 | 21{3} | 10{3} | 91 |
| 17 | 6 | 21{4} | 10{4} | 98 |
| 18 | 6 | 21{5} | 10{5} | 98 |
| 19 | 6 | 21{6} | 10{6} | 89 |
| 20 | 6 | 21{7} | 10{7} | 94 |
| 21 | 6 | 21{8} | 10{8} | 88 |
| 22 | 6 | 21{9} | 10{9} | 81 |
| 23 | 6 | 21{10} | 10{10} | 84 |
| 24 | 7 | 21{1} | 11{1} | 85 |
| 25 | 7 | 21{4} | 11{4} | 53 |
| 26 | 7 | 21{6} | 11{6} | 55 |
| 27 | 6 | 22{1} | 12{1} | 99 |
| 28 | 6 | 22{2} | 12{2} | 79 |
| 29 | 6 | 22{3} | 12{3} | 96 |
| 30 | 6 | 22{4} | 12{4} | 99 |
| 31 | 6 | 22{5} | 12{5} | 99 |
| 32 | 6 | 22{6} | 12{6} | 86 |
| 33 | 6 | 22{7} | 12{7} | 91 |
| 34 | 6 | 22{8} | 12{8} | Quant. |
| 35 | 6 | 22{9} | 12{9} | Quant. |
| 36 | 7 | 22{2} | 13{2} | 58 |
| 37 | 7 | 22{3} | 13{3} | 55 |
| 38 | 7 | 22{4} | 13{4} | Quant. |
| 39 | 6 | 23{1} | 14{1} | 99 |
| 40 | 6 | 23{2} | 14{2} | 98 |
| 41 | 6 | 23{3} | 14{3} | 98 |
| 42 | 6 | 23{4} | 14{4} | 83 |
| 43 | 6 | 23{5} | 14{5} | 94 |
| 44 | 6 | 23{6} | 14{6} | 95 |
| 45 | 6 | 23{7} | 14{7} | 90 |
| 46 | 7 | 23{1} | 15{1} | 79 |
| 47 | 7 | 23{3} | 15{3} | 83 |
| 48 | 7 | 23{7} | 15{7} | Quant. |
| 49 | 6 | 24{1} | 16{1} | 88 |
| 50 | 6 | 24{2} | 16{2} | 77 |
| 51 | 6 | 24{3} | 16{3} | 98 |
| 52 | 6 | 25{1} | 17{1} | 71 |
| 53 | 6 | 25{2} | 17{2} | Quant. |
| 54 | 6 | 25{3} | 17{3} | 98 |
| 55 | 6 | 25{4} | 17{4} | 83 |
| 56 | 6 | 25{5} | 17{5} | Quant. |
| 57 | 6 | 25{6} | 17{6} | 82 |
| 58 | 6 | 25{7} | 17{7} | 65 |
| 59 | 6 | 25{8} | 17{8} | 60 |
| 60 | 6 | 25{9} | 17{9} | 96 |
| 61 | 6 | 25{10} | 17{10} | 96 |
| 62 | 6 | 25{11} | 17{11} | 56c |
| 63 | 7 | 25{5} | 18{5} | 98 |
| 64 | 7 | 25{12} | 18{12} | 55 |
| 65 | 7 | 25{13} | 18{13} | 70 |
| 66 | 6 | 26{1} | 19{1} | 96 |
| 67 | 6 | 26{2} | 19{2} | 83 |
| 68 | 6 | 26{3} | 19{3} | 73 |
| 69 | 6 | 26{4} | 19{4} | 48 |
| 70 | 6 | 26{5} | 19{5} | 0 |
| 71 | 6 | 26{6} | 19{6} | 85c |
An additional 3.0 equivalents of Et3N were employed.
Isolated as the free-base.
No AcOH added.
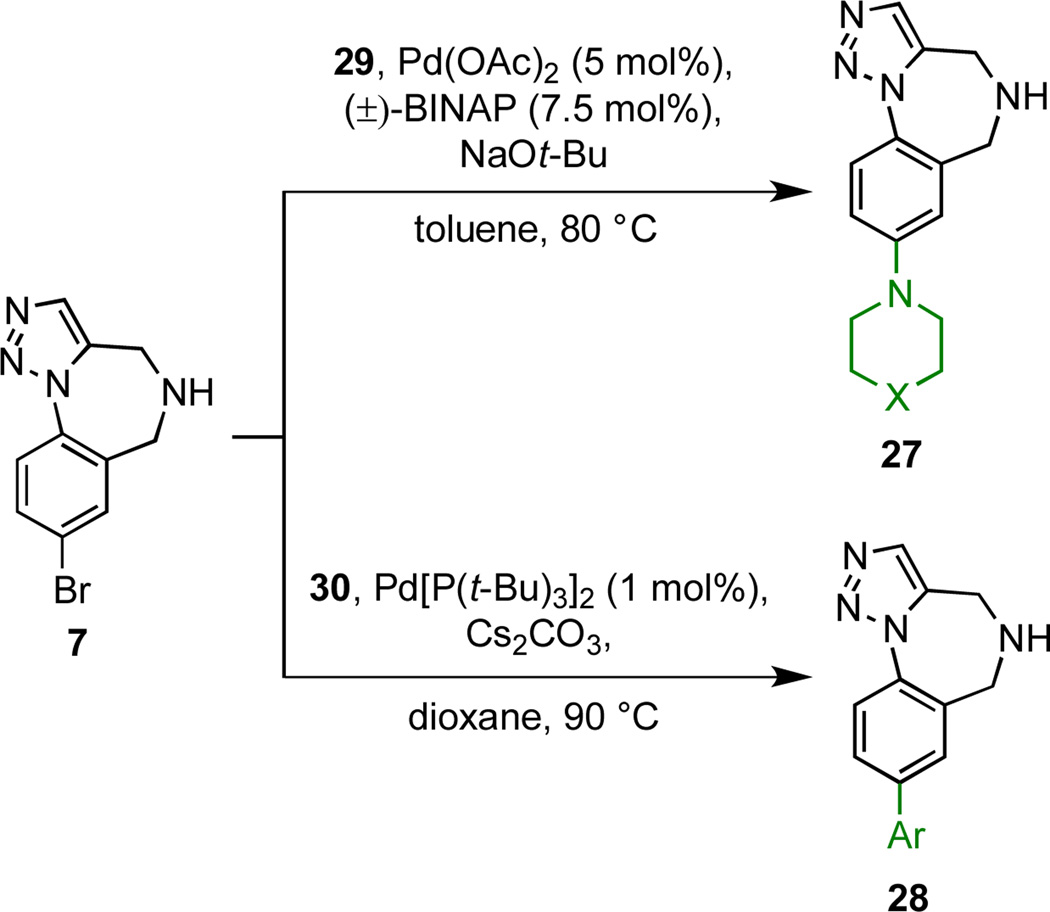
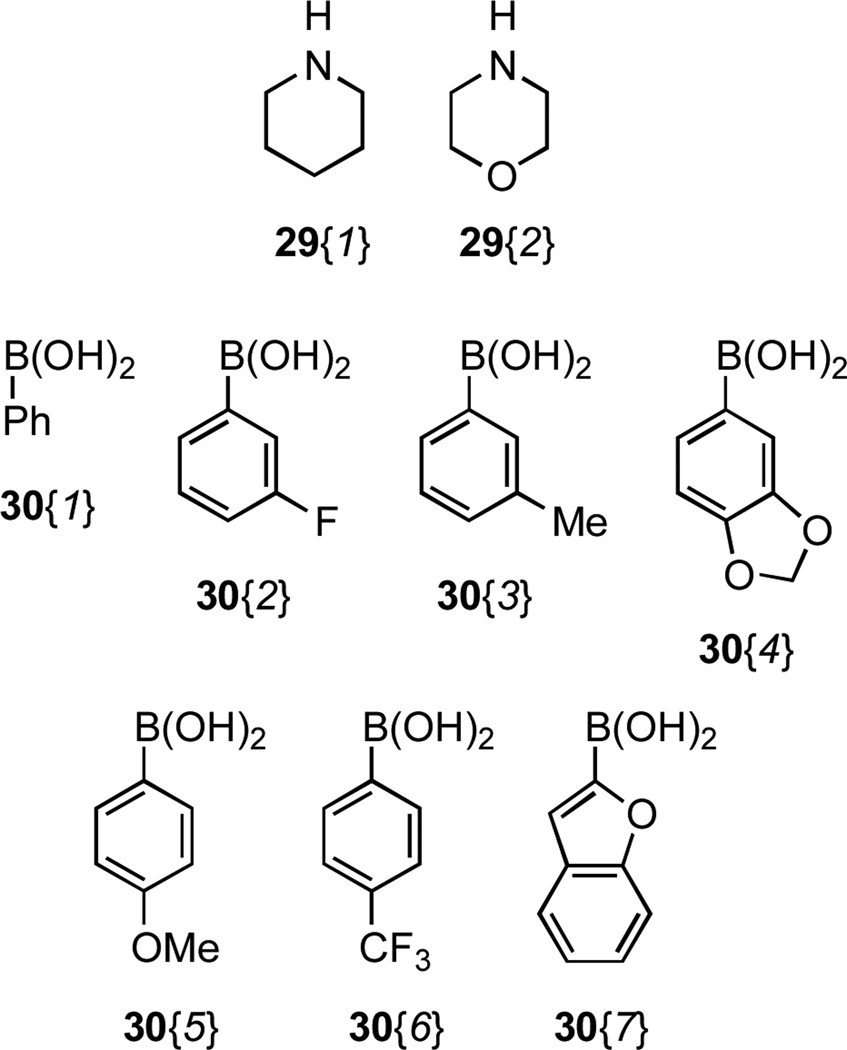
The aryl bromide functionality present within scaffold 7 was exploited as a handle for diversification through several palladium catalysed cross-couplings (Scheme 3, Figure 3, Table 2). In order to avoid homocoupling of 7 during Buchwald-Hartwig aminations, it was necessary to employ a 10-fold excess of amines 29 to afford the aniline derivatives 27 (Figure 3).22 Suzuki cross-couplings of 7 with boronic acids 30 afforded biaryl products 28 bearing substituents having a broad range of electronic properties. The use of heteroaromatic boronic acid derivatives is exemplified by the synthesis of 28{7}.
Scheme 3.
Cross-coupling reactions with scaffold 7.
Figure 3.
Cross-coupling partners employed with scaffold 7.
Table 2.
Cross-coupling reactions with scaffold 7.
| Entry | Coupling Partner | Product | Yield (%) |
|---|---|---|---|
| 1 | 29{1} | 27{1} | 75 |
| 2 | 29{2} | 27{2} | 69 |
| 3 | 30{1} | 28{1} | 84 |
| 4 | 30{2} | 28{2} | 73 |
| 5 | 30{3} | 28{3} | 74 |
| 6 | 30{4} | 28{4} | 78 |
| 7 | 30{5} | 28{5} | 78 |
| 8 | 30{6} | 28{6} | 76 |
| 9 | 30{7} | 28{7} | 92 |
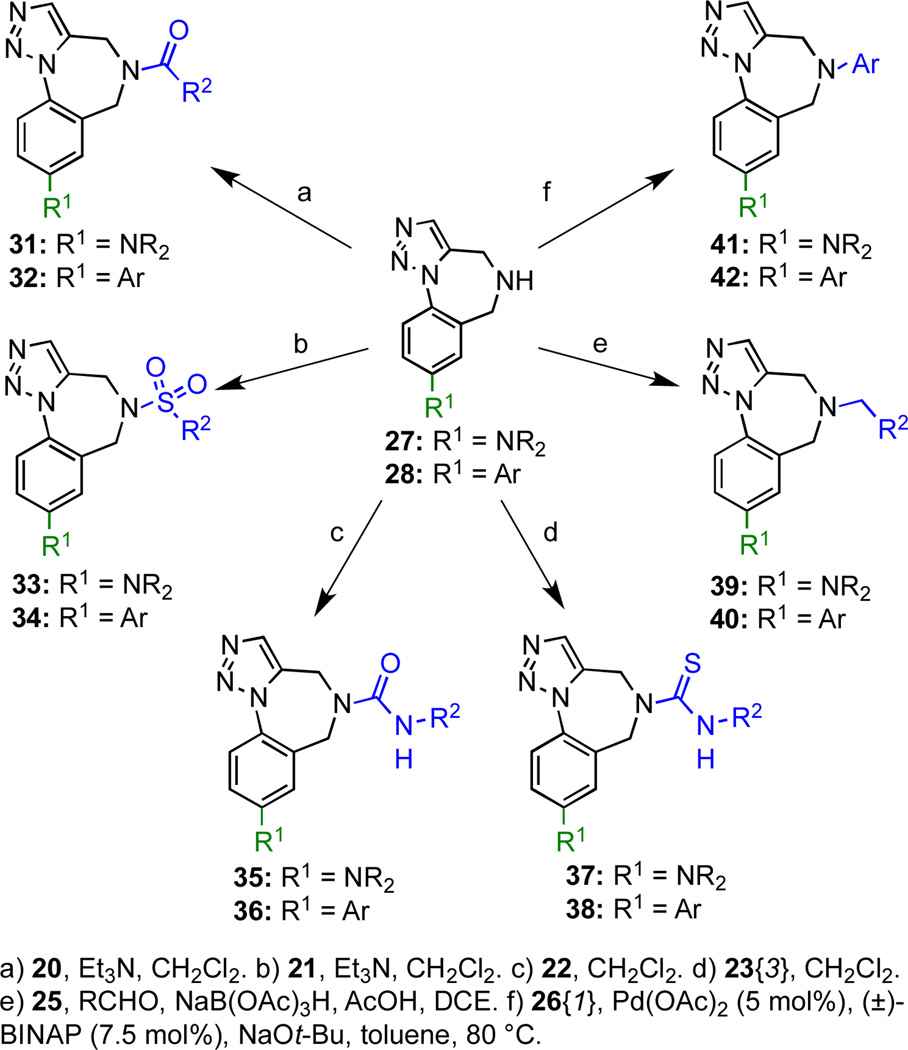
Five cross-coupled products (27{1–2}, 28{1–2} and 28{5}) were subjected to ten independent N-functionalizations to afford a ‘two-dimensionally’ functionalized collection of 50 compounds (chemsets 31–42) in generally high yields (Scheme 4, Figure 4, Table 3).
Scheme 4.
Two-dimensional library derived from scaffold 7.
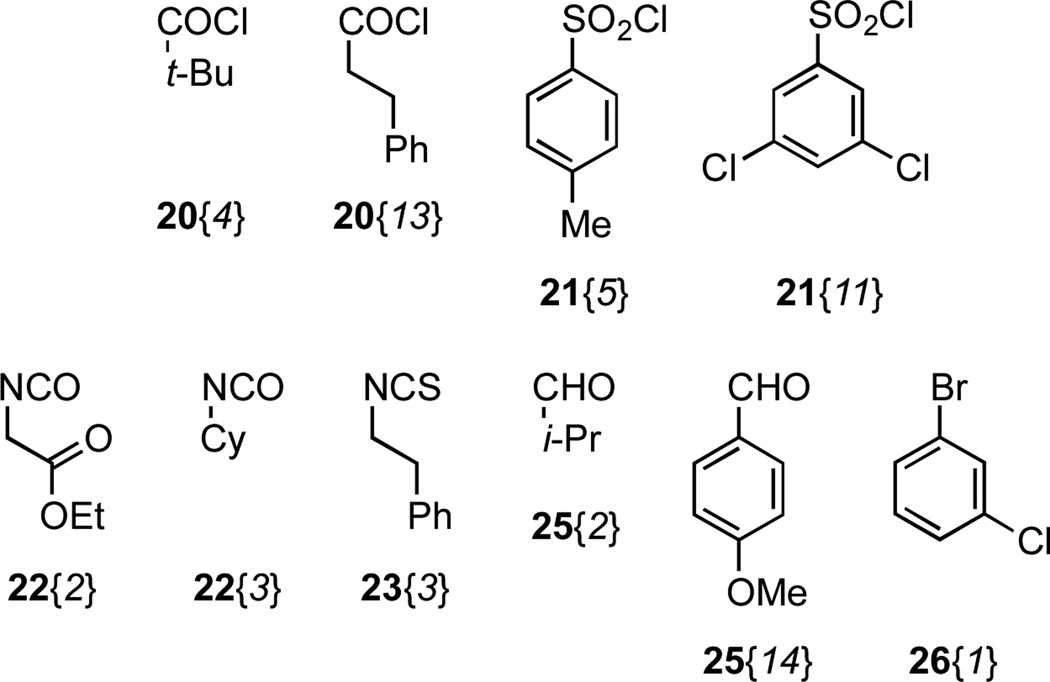
Figure 4.
Nitrogen-functionalizing agents used in the two-dimensional library derived from scaffold 7.
Table 3.
Two-dimensional library derived from scaffold 7.
| Entry | Amine input |
Functionalizing agent |
Product | Yield (%) |
|---|---|---|---|---|
| 1 | 27{1} | 20{4} | 31{1,4} | Quant. |
| 2 | 27{1} | 20{13} | 31{1,13} | 86 |
| 3 | 27{1} | 21{5} | 33{1,5} | 86 |
| 4 | 27{1} | 21{11} | 33{1,11} | 87 |
| 5 | 27{1} | 22{2} | 35{1,2} | 92 |
| 6 | 27{1} | 22{3} | 35{1,3} | 7 |
| 7 | 27{1} | 23{3} | 37{1,3} | 91 |
| 8 | 27{1} | 25{2} | 39{1,2} | 78 |
| 9 | 27{1} | 25{14} | 39{1,14} | 88 |
| 10 | 27{1} | 26{1} | 41{1,1} | 83 |
| 11 | 27{2} | 20{4} | 31{2,4} | 94 |
| 12 | 27{2} | 20{13} | 31{2,13} | 73 |
| 13 | 27{2} | 21{5} | 33{2,5} | 87 |
| 14 | 27{2} | 21{11} | 33{2,11} | 88 |
| 15 | 27{2} | 22{2} | 35{2,2} | 76 |
| 16 | 27{2} | 22{3} | 35{2,3} | 90 |
| 17 | 27{2} | 23{3} | 37{2,3} | 94 |
| 18 | 27{2} | 25{2} | 39{2,2} | 88 |
| 19 | 27{2} | 25{14} | 39{2,14} | 79 |
| 20 | 27{2} | 26{1} | 41{2,1} | 90 |
| 21 | 28{1} | 20{4} | 32{1,4} | Quant. |
| 22 | 28{1} | 20{13} | 32{1,13} | 88 |
| 23 | 28{1} | 21{5} | 34{1,5} | 91 |
| 24 | 28{1} | 21{11} | 34{1,11} | Quant. |
| 25 | 28{1} | 22{2} | 36{1,2} | 86 |
| 26 | 28{1} | 22{3} | 36{1,3} | 97 |
| 27 | 28{1} | 23{3} | 38{1,3} | 98 |
| 28 | 28{1} | 25{2} | 40{1,2} | 85 |
| 29 | 28{1} | 25{14} | 40{1,14} | 39 |
| 30 | 28{1} | 26{1} | 42{1,1} | 94 |
| 31 | 28{2} | 20{4} | 32{2,4} | Quant. |
| 32 | 28{2} | 20{13} | 32{2,13} | 73 |
| 33 | 28{2} | 21{5} | 34{2,5} | Quant. |
| 34 | 28{2} | 21{11} | 34{2,11} | Quant. |
| 35 | 28{2} | 22{2} | 36{2,2} | 90 |
| 36 | 28{2} | 22{3} | 36{2,3} | Quant. |
| 37 | 28{2} | 23{3} | 38{2,3} | Quant. |
| 38 | 28{2} | 25{2} | 40{2,2} | 94 |
| 39 | 28{2} | 25{14} | 40{2,14} | 35 |
| 40 | 28{2} | 26{1} | 42{2,1} | 96 |
| 41 | 28{5} | 20{4} | 32{5,4} | Quant. |
| 42 | 28{5} | 20{13} | 32{5,13} | 85 |
| 43 | 28{5} | 21{5} | 34{5,5} | 97 |
| 44 | 28{5} | 21{11} | 34{5,11} | Quant. |
| 45 | 28{5} | 22{2} | 36{5,2} | 94 |
| 46 | 28{5} | 22{3} | 36{5,3} | 98 |
| 47 | 28{5} | 23{3} | 38{5,3} | 99 |
| 48 | 28{5} | 25{2} | 40{5,2} | 94 |
| 49 | 28{5} | 25{14} | 40{5,14} | 40 |
| 50 | 28{5} | 26{1} | 42{5,1} | 99 |
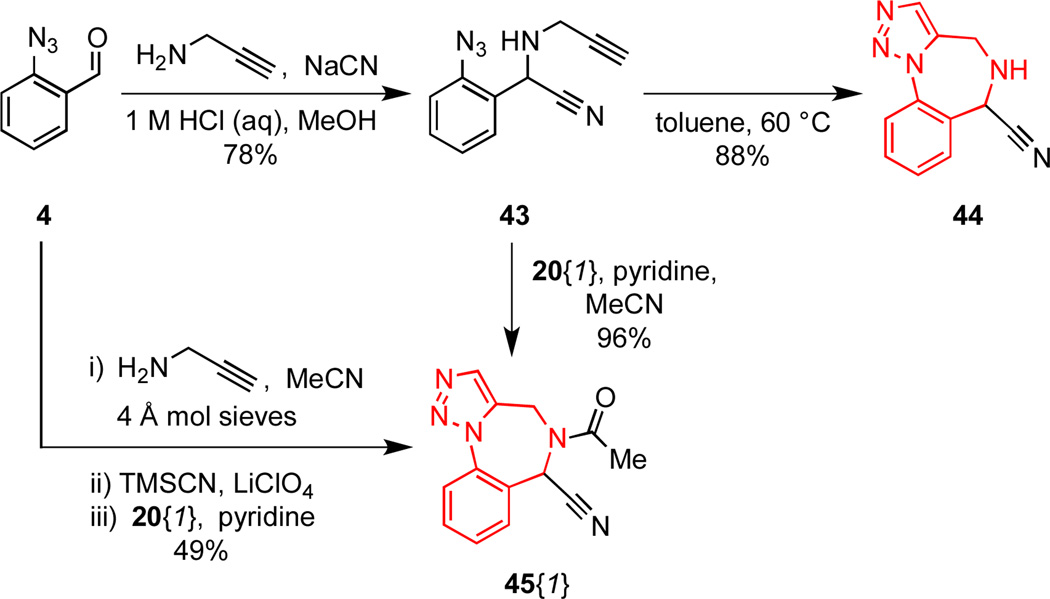
Because compounds bearing the α-aminonitrile functionality are synthetically versatile intermediates, we envisioned that a scaffold containing this subunit might facilitate diversification through a number of strategies.24 The Strecker MCAP employing benzaldehyde 4 and propargylamine furnished the α-aminonitrile 43 in 78% yield (Scheme 5). Upon mild heating, 43 underwent facile Huisgen cycloaddition to deliver the cyano-substituted scaffold 44 in 88% yield. It was necessary to perform this reaction under dilute conditions and at a temperature not exceeding 60 °C in order to avoid elimination of HCN from the product 44. Interestingly, when 43 was N-acylated, the intermediate amide underwent facile cycloaddition at ambient temperature to yield the amide derivative of the scaffold 45{1}. This observation led to the development of a convenient, 4-component process to afford 45{1} directly from benzaldehyde 4 in 49% yield (Scheme 5). This MCAP approach is an expedient method to access amide derivatives of 44. However, N-functionalization of this scaffold is a better strategy for the library synthesis outlined in Scheme 6, because this approach allows the use of a number of parallel diversification reactions to give products that may be easily purified.
Scheme 5.
Synthesis of nitrile substituted scaffold 44.
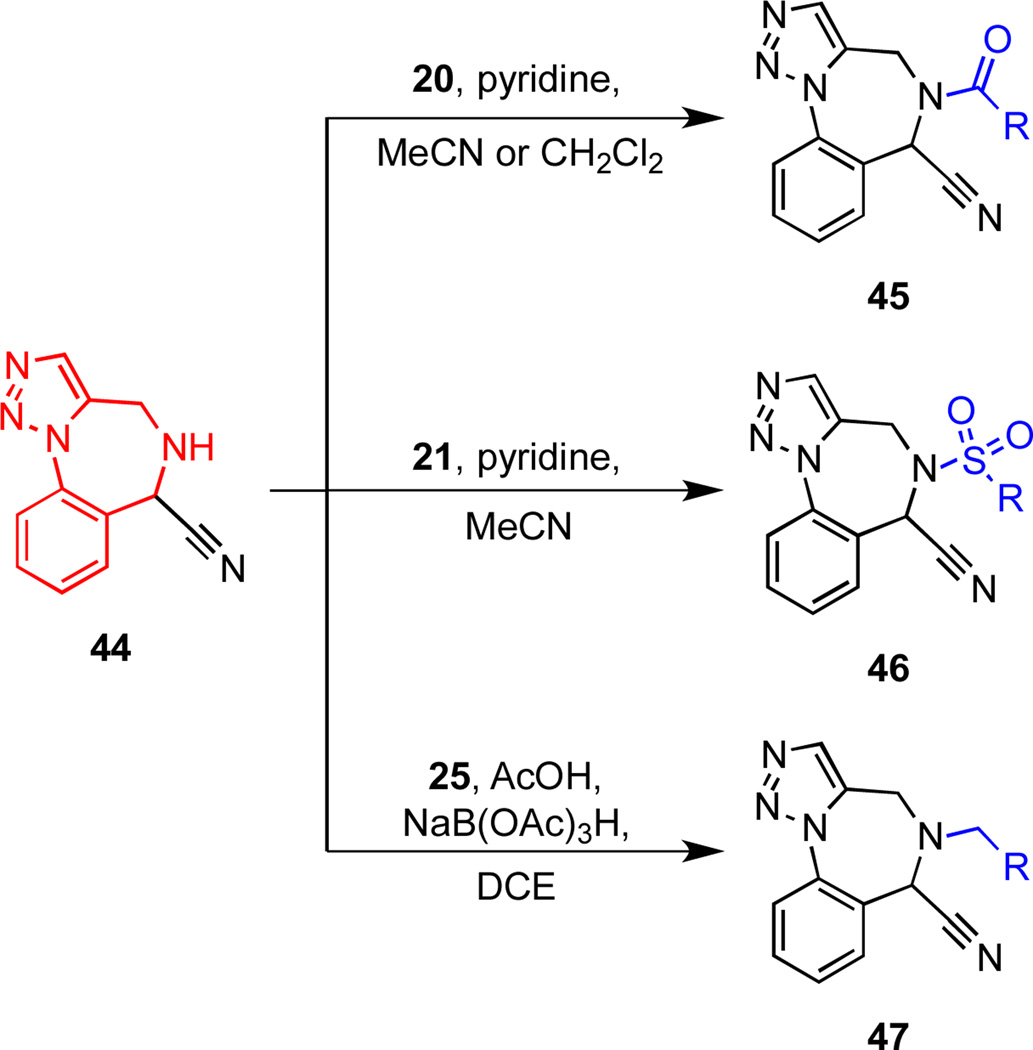
Scheme 6.
Nitrogen functionalization of scaffold 44.
N-Acylations and N-sulfonylations of scaffold 44 to produce chemsets 45 and 46 were conducted using pyridine as the base; when triethylamine was employed, elimination of HCN was a significant side-reaction (Scheme 6, Figure 5, Table 4). Reductive amination of 44, which was achieved under the same conditions as those described for the preparation of 17 and 18 (Scheme 2), generally proceeded in good yields. However, the reaction of 44 with the electron poor aldehyde 25{5} required the presence of trifluoroacetic acid and additional quantities of NaBH(OAc)3 to consume the starting material 44 (Table 4, entry 19).
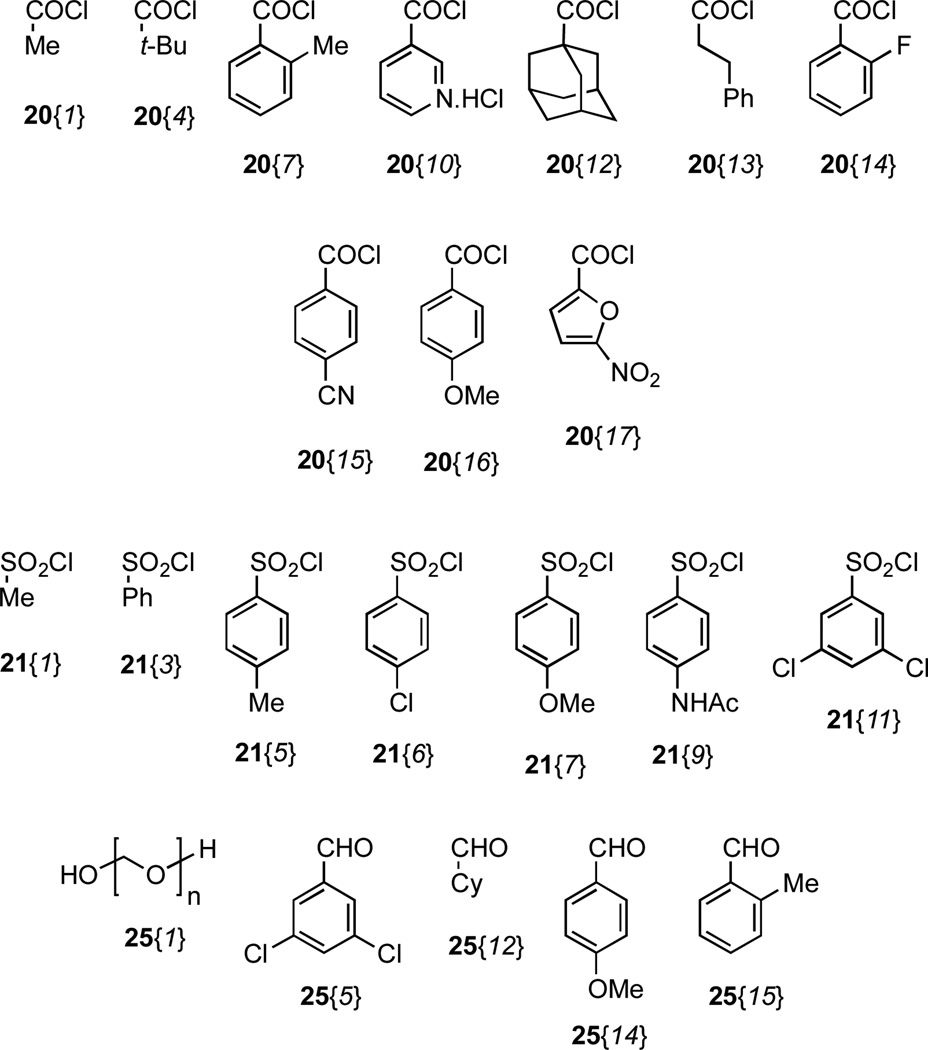
Figure 5.
Nitrogen functionalizing agents used with scaffold 44.
Table 4.
Nitrogen functionalization of scaffold 44.
| Entry | Functionalizing Agent | Product | Yield (%) |
|---|---|---|---|
| 1 | 20{1} | 45{1} | 92 |
| 2 | 20{4} | 45{4} | 92 |
| 3 | 20{7} | 45{7} | 93 |
| 4 | 20{10} | 45{10} | 92a |
| 5 | 20{12} | 45{12} | 49b |
| 6 | 20{13} | 45{13} | 95 |
| 7 | 20{14} | 45{14} | 98 |
| 8 | 20{15} | 45{15} | 66c |
| 9 | 20{16} | 45{16} | Quant. |
| 10 | 20{17} | 45{17} | Quant. |
| 11 | 21{1} | 46{1} | 97 |
| 12 | 21{3} | 46{3} | 91 |
| 13 | 21{5} | 46{5} | 86 |
| 14 | 21{6} | 46{6} | 90 |
| 15 | 21{7} | 46{7} | 91 |
| 16 | 21{9} | 46{9} | 85 |
| 17 | 21{11} | 46{11} | 80 |
| 18 | 25{1} | 47{1} | 63 |
| 19 | 25{5} | 47{5} | 41d |
| 20 | 25{12} | 47{12} | 84 |
| 21 | 25{14} | 47{14} | 74 |
| 22 | 25{15} | 47{15} | 87 |
Isolated as the free-base
Yield after recrystallization from toluene (following chromatography).
Yield after the product was washed with hexanes (3 × 5 mL) following chromatography.
The addition of TFA (1.0 eq.) and extra NaBH(OAc)3 (4.0 eq.) was required to fully consume 44.
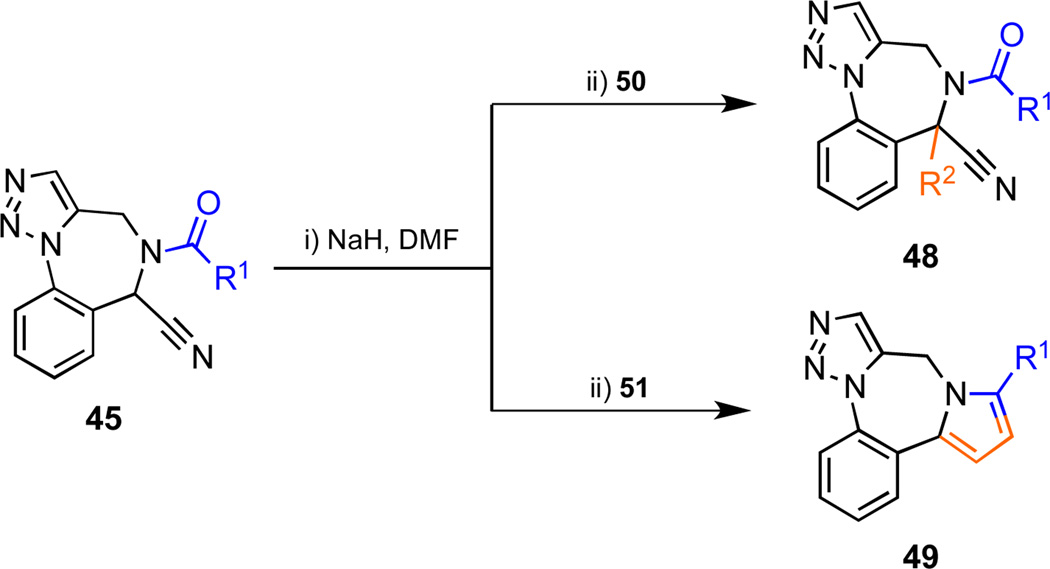
The acidic nature of the proton α to the cyano group in amide derivatives 45 was exploited to facilitate further elaboration of the scaffold (Scheme 7, Figure 6, Table 5). In the event, deprotonation of 45 with NaH, followed by trapping the intermediate carbanion with alkylating agents 50 delivered a small library of compounds bearing fully substituted carbon centers (chemset 48). Following literature precedent, vinyltriphenylphosphonium bromide (Schweizer’s reagent) (51) was utilized as an electrophile in order to prepare a novel collection of pyrrole-fused structures (chemset 49) through an addition/intramolecular Wittig reaction/elimination sequence.25 Notably, the Wittig reaction and aromatization steps were complete within 90 min at ambient temperature. With the sole exception of the pivalamide 45{4}, N-alkyl substituted amides were good substrates for this sequence of reactions. Aryl-substituted amides such as 45{15}, 45{16} and 45{7} also participated in this transformation, as did the nicotinamide 45{10}, which represents the first example of the use of a heteroaryl-substituted amide in this reaction. On the other hand, treating the anion of 5-nitro-furanyl amide 45{17} with the vinyl phosphonium salt 51 formed a complex mixture of products; no pyrrole was observed. It should be noted that the bromo-substituted benzaldehyde 5 might be used as a starting material to prepare the corresponding bromo derivatives of 44, 45, 48, and 49. These compounds could then be further derivatized by palladium catalyzed cross-coupling reactions to introduce aryl and amine substituents onto the benzodiazepine rings of 44, 45, 48, and 49.
Scheme 7.
Anion derived diversification of amides 45.
Figure 6.
Electrophiles employed to trap anions derived from amides 45.
Table 5.
Libraries derived from amides 45.
| Entry | Amide | Electrophile | Product | Yield(%) |
|---|---|---|---|---|
| 1 | 45{1} | 50{1} | 48{1,1} | 80 |
| 2 | 45{1} | 50{2} | 48{1,2} | 86 |
| 3 | 45{15} | 50{1} | 48{15,1} | 80 |
| 4 | 45{15} | 50{2} | 48{15,2} | 76 |
| 5 | 45{1} | 51 | 49{1} | 72 |
| 6 | 45{13} | 51 | 49{13} | 66 |
| 7 | 45{4} | 51 | 49{4} | Tracea |
| 8 | 45{15} | 51 | 49{15} | 95 |
| 9 | 45{16} | 51 | 49{16} | 85 |
| 10 | 45{7} | 51 | 49{7} | 44 |
| 11 | 45{10} | 51 | 49{10} | 91 |
| 12 | 45{17} | 51 | 49{17} | 0 |
Trace formation of the pyrrole product 49{4} was observed in the 1H NMR spectrum of the crude reaction mixture.
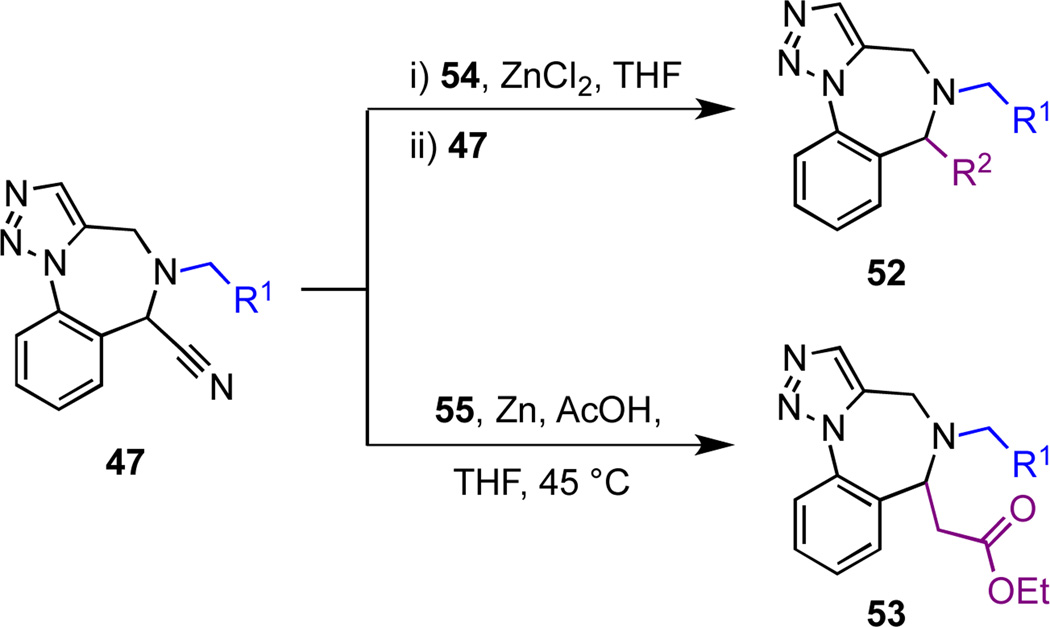
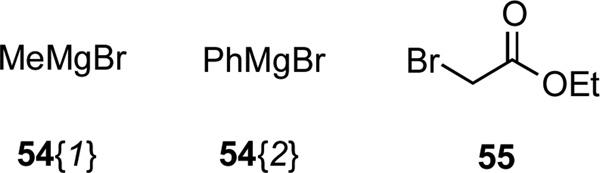
We were also interested in exploiting the Bruylants reaction to generate substituted benzylamine derivatives 52 by displacing cyanide ion from α-aminonitriles 47 with organometallic reagents (Scheme 8, Figure 7, Table 6).26 After conducting a brief series of exploratory studies, we discovered that transmetallation of Grignard reagents to their corresponding organo-zinc species was necessary to avoid competitive deprotonation of the acidic proton α to the cyano group.27 Displacement of cyanide ion under ‘Reformatsky-type’ conditions with the enolate of ethyl acetate afforded chemset 53; these compounds bear an ethyl ester group that might serve as a further point of diversification.28
Scheme 8.
Diversification of aminonitriles 47 using the Bruylants reaction.
Figure 7.
Precursors to organo-zinc reagents used in the Bruylants reaction.
Table 6.
Amines derived from the Bruylants reaction.
| Entry | Aminonitrile | Organo-zinc precursor |
Product | Yield (%) |
|---|---|---|---|---|
| 1 | 47{1} | 54{1} | 52{1,1} | 73 |
| 2 | 47{1} | 54{2} | 52{1,2} | 86 |
| 3 | 47{12} | 54{1} | 52{12,1} | 75 |
| 4 | 47{12} | 54{2} | 52{12,2} | 73 |
| 5 | 47{14} | 54{1} | 52{14,1} | 80 |
| 6 | 47{14} | 54{2} | 52{14,2} | 81 |
| 7 | 47{1} | 55 | 53{1} | 74 |
| 8 | 47{12} | 55 | 53{12} | 77 |
| 9 | 47{14} | 55 | 53{14} | 75 |
In summary, a diverse collection of 170 1,2,3-triazolo-1,4-benzodiazepines was prepared. A sequential MCAP/Huisgen cycloaddition strategy was instrumental for the rapid construction of the parent scaffolds. N-Functionalization and cross-coupling reactions were then applied to these scaffolds to enable the rapid production of derivatives through a combinatorial style approach, whereas exploitation of α-aminonitrile chemistry facilitated the introduction of significant structural diversity via a DOS approach. A complete Lipinski analysis of a representative selection of 80 compounds containing at least two examples of each structural subtype and having a maximal diversity of substituents was performed, and no compound was found that violated the Lipinski rule of five.29 Hence, the library members have physiochemical properties that are likely to be associated with good solubility, membrane permeability, and bioavailability. All compounds have been submitted to the National Institutes of Health (NIH) Molecular Libraries Small Molecule Repository (MLSMR) and are being distributed to the Molecular Libraries Probe Production Centers Network (MLPCN) for HTS in a wide range of biological assays. Selected compounds have also been sent to the National Institute of Mental Health, Psychoactive Drug Screening Program (NIMH PDSP). The further development and application of our sequential MCAP/cyclization strategy to the synthesis of medicinally relevant small molecules is ongoing and will be reported in due course.
Supplementary Material
ACKNOWLEDGMENT
We thank the National Institutes of Health (GM 86192) and the Robert A. Welch Foundation (F-0652) for their generous support of this work.
Footnotes
ASSOCIATED CONTENT:
Experimental procedures, full characterization data for representative compounds, LCMS data for representative compounds and tabulated Lipinski’s rule parameters for representative compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Jenkins JL, Kao RYT, Shapiro R. Virtual Screening to Enrich Hit Lists From High-Throughput Screening: A Case Study on Small-Molecule Inhibitors of Angiogenin. Prot. Struct. Funct. Genet. 2003;50:81–93. doi: 10.1002/prot.10270. [DOI] [PubMed] [Google Scholar]
- 2.For reviews, see: Horton DA, Bourne GT, Smythe ML. The Combinatorial Synthesis of Bicyclic Privileged Structures or Privileged Substructures. Chem. Rev. 2003;103:893–930. doi: 10.1021/cr020033s. DeSimone RW, Currie KS, Mitchell SA, Darrow JW, Pippin DA. Privileged Structures: Applications in Drug Discovery. Comb. Chem. High T. Scr. 2004;7:473–493. doi: 10.2174/1386207043328544. Costantino L, Barlocco D. Privileged Structure as Leads in Medicinal Chemistry. Curr. Med. Chem. 2006;13:65–85. Duarte CD, Barreiro EJ, Fraga CAM. Privileged Structures: A Useful Concept for the Rational Design of New Lead Drug Candidates. Mini-Rev. Med. Chem. 2007;7:1108–1119. doi: 10.2174/138955707782331722. Welsch ME, Snyder SA, Stockwell BR. Privileged Scaffolds for Library Design and Drug Discovery. Curr. Opin. Chem. Biol. 2010;14:347–361. doi: 10.1016/j.cbpa.2010.02.018.
- 3.Evans BE, Rittle KE, Bock MG, Dipardo RM, Freidinger RM, Whitter WL, Lundell GF, Veber DF, Anderson PS, Chang RSL, Lotti VJ, Cerino DJ, Chen TB, Kling PJ, Kunkel KA, Springer JP, Hirshfield J. Methods for Drug Discovery: Development of Potent, Selective, Orally Effective Cholecystokinin Antagonists. J. Med. Chem. 1988;31:2235–2246. doi: 10.1021/jm00120a002. [DOI] [PubMed] [Google Scholar]
- 4.For examples of libraries based upon benzodiazepine core structures, see references 2 and 3.
- 5.Ripka WC, De Lucca GV, Bach AC, II, Pottorf RS, Blaney JM. Protein β-Turn Mimetics I. Design, Synthesis, and Evaluation in Model Cyclic Peptides. Tetrahedron. 1993;49:3593–3608. [Google Scholar]
- 6.Grasberger BL, Lu T, Schubert C, Parks DJ, Carver TE, Koblish HK, Cummings MD, LaFrance LV, Milkiewicz KL, Calvo RR, Maguire D, Lattanze J, Franks CF, Zhao S, Ramachandren K, Bylebyl GR, Zhang M, Mathney CL, Petrella EC, Pantoliano MW, Deckman IC, Spurlino JC, Maroney AC, Tomczuk BE, Molloy CJ, Bone RF. Discovery and Cocrystal Structure of Benzodiazepinedione HDM2 Antagonists That Activate p53 in Cells. J. Med. Chem. 2005;48:909–912. doi: 10.1021/jm049137g. [DOI] [PubMed] [Google Scholar]
- 7.Tyndall JDA, Pfeiffer B, Abbenante G, Fairlie DP. Over One Hundred Peptide-Activated G Protein-Coupled Receptors Recognize Ligands with Turn Structure. Chem. Rev. 2005;105:793–826. doi: 10.1021/cr040689g. [DOI] [PubMed] [Google Scholar]
- 8.Sternbach LH. The Benzodiazepine Story. J. Med. Chem. 1979;22:1–7. doi: 10.1021/jm00187a001. [DOI] [PubMed] [Google Scholar]
- 9.Chang RSL, Lotti VJ. Biochemical and Pharmacological Characterization of an Extremely Potent and Selective Nonpeptide Cholecystokinin Antagonist. Proc. Natl. Acad Sci. USA. 1986;83:4923–4926. doi: 10.1073/pnas.83.13.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Leimgruber W, Stefanovic V, Schenker F, Karr A, Berger J. Isolation and Characterization of Anthramycin, a New Antitumor Antibiotic. J. Am. Chem. Soc. 1965;87:5791–5793. doi: 10.1021/ja00952a050. [DOI] [PubMed] [Google Scholar]; (b) Leimgruber W, Batcho AD, Schenker F. The Structure of Anthramycin. J. Am. Chem. Soc. 1965;87:5793–5795. doi: 10.1021/ja00952a051. [DOI] [PubMed] [Google Scholar]
- 11.For the first example of this process, see: Martin SF, Benage B, Geraci LS, Hunter JE, Mortimore M. Unified Strategy for Synthesis of Indole and 2-Oxindole Alkaloids. J. Am. Chem. Soc. 1991;113:6161–6171.
- 12.Cheng B, Sunderhaus JD, Martin SF. Concise Total Synthesis of Pseudotabersonine via Double Ring-Closing Metathesis Strategy. Org. Lett. 2010;12:3622–3625. doi: 10.1021/ol101356u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Sunderhaus JD, Dockendorff C, Martin SF. Applications of Multicomponent Reactions for the Synthesis of Diverse Heterocyclic Scaffolds. Org. Lett. 2007;9:4223–4226. doi: 10.1021/ol7018357. [DOI] [PubMed] [Google Scholar]; (b) Sunderhaus JD, Dockendorff C, Martin SF. Synthesis of Diverse Heterocyclic Scaffolds via Tandem Additions to Imine Derivatives and Ring-Forming Reactions. Tetrahedron. 2009;65:6454–6469. doi: 10.1016/j.tet.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.For a review of related strategies, see: Sunderhaus JD, Martin SF. Applications of Multicomponent Reactions to the Synthesis of Diverse Heterocyclic Scaffolds. Chem.–Eur. J. 2009;15:1300–1308. doi: 10.1002/chem.200802140.
- 15.Donald JR, Martin SF. Synthesis and Diversification of 1,2,3-Triazole-Fused 1,4- Benzodiazepine Scaffolds. Org. Lett. 2011;13:852–855. doi: 10.1021/ol1028404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donald JR, Granger BA, Hardy S, Sahn JJ, Martin SF. Applications of Multicomponent Assembly Processes to the Facile Syntheses of Diversely Functionalized Nitrogen Heterocycles. Heterocycles. 2012;84 doi: 10.3987/COM-11-S(P)92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardy S, Martin SF. Multicomponent Assembly and Diversification of Novel Heterocyclic Scaffolds Derived from 2-Arylpiperidines. Org. Lett. 2011;13:3102–3105. doi: 10.1021/ol201010s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granger BA, Kaneda K, Martin SF. Multicomponent Assembly Strategies for the Synthesis of Diverse Tetrahydroisoquinoline Scaffolds. Org. Lett. 2011;13:4542–4545. doi: 10.1021/ol201739u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.(a) Sahn JJ, Su JY, Martin SF. Facile and Unified Approach to Skeletally Diverse, Privileged Scaffolds. Org. Lett. 2011;13:2590–2593. doi: 10.1021/ol200709h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sahn JJ, Martin SF. Facile Syntheses of Substituted, Conformationally-Constrained Benzoxazocines and Benzazocines via Sequential Multicomponent Assembly and Cyclization. Tetrahedron Lett. 2011;52:6855–6858. doi: 10.1016/j.tetlet.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.For reports of the biological activity of 1,2,3-triazole fused benzodiazepines, see: Bertelli L, Biagi G, Giorgi I, Livi O, Manera C, Scartoni V, Martini C, Giannaccini G, Trincavelli L, Barili PL. 1,2,3-Triazolo[1,5-a][1,4]- and 1,2,3-Triazolo[1,5-a]-[1,5]benzodiazepine Derivatives: Synthesis and Benzodiazepine Receptor Binding. Farmaco. 1998;53:305–311. doi: 10.1016/s0014-827x(98)00025-1. Mohapatra DK, Maity PK, Shabab M, Khan MI. Click Chemistry Based Rapid One-Pot Synthesis and Evaluation for Protease Inhibition of new Tetracyclic Triazole Fused Benzodiazepine Derivatives. Bioorg. Med. Chem. Lett. 2009;19:5241–5245. doi: 10.1016/j.bmcl.2009.06.107.
- 21.(a) Alajarín M, Cabrera J, Pastor A, Villalgordo JM. A New Modular and Flexible Approach to [1,2,3]Triazolo[1,5-a][1,4]benzodiazepines. Tetrahedron Lett. 2007;48:3495–3499. [Google Scholar]; (b) Jain R, Trehan S, Singh N, Nanda GK, Magadi SK, Sharma SK, Das J. Novel Heterocyclic Compounds. Panacea Biotech Limited. 093269 (A1) World Patent WO. 2009
- 22.Wolfe JP, Buchwald SL. Scope and Limitations of the Pd/BINAP-Catalyzed Amination of Aryl Bromides. J. Org. Chem. 2000;65:1144–1157. doi: 10.1021/jo9916986. [DOI] [PubMed] [Google Scholar]
- 23.Wagaw S, Buchwald SL. The Synthesis of Aminopyridines: A Method Employing Palladium- Catalyzed Carbon-Nitrogen Bond Formation. J. Org. Chem. 1996;61:7240–7241. doi: 10.1021/jo9612739. [DOI] [PubMed] [Google Scholar]
- 24.For recent reviews on the use of α-amino nitriles in the generation of molecular diversity, see: Opatz T. The Chemistry of Deprotonated α-Aminonitriles. Synthesis. 2009;12:1941–1959. González-Vera JA, García-López MT, Herranz R. Potential of Amino Acid-Derived α-Amino Nitriles for Generating Molecular Diversity. Mini-Rev. Org. Chem. 2008;5:209–221. Enders D, Shilvock JP. Some Recent Applications of α-Amino Nitrile Chemistry. Chem. Soc. Rev. . 2000;29:359–373.
- 25.(a) Cooney JV, McEwen WE. Synthesis of Substituted Pyrroles by Intramolecular Condensation of a Wittig Reagent with the Carbonyl Group of a Tertiary Amide. J. Org. Chem. 1981;46:2570–2573. [Google Scholar]; (b) Cooney JV, Beaver BD, McEwen WE. Synthesis of Nitrogen Heterocycles by Condensation of the Conjugate Base of Open-Chain Reissert Compound Analogs with Vinyltriphenylphosphonium Bromide (Schweizer’s salt) J. Het. Chem. 1985;22:635–642. [Google Scholar]
- 26.Bruylants P. The Reaction of Organic-Magnesium Compounds on α-Amino Nitriles. Bull. Soc. Chim. Belg. 1924;33:467–478. [Google Scholar]
- 27.Treatment of α-aminonitrile 47{14} with 54{2} followed by quenching with d4-AcOD gave the desired Bruylants reaction product in 56% yield along with starting material in 20% yield which exhibited >95% D incorporation, as determined by 1H NMR spectroscopy.
- 28.Bernardi L, Bonini BF, Capitò E, Dessole G, Fochi M, Comes-Franchini M, Ricci A. A New and Practical Procedure for the Bruylants Reaction. Zinc-Mediated Synthesis of Tertiary Homoallylamines and β-Aminoesters. Synlett. 2003:1778–1782. [Google Scholar]
- 29.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug. Deliv. Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.