Abstract
This review challenges an earlier view that the intervertebral joint could not be classified as a diarthrodial joint and should remain as an amphiarthrosis. However, a careful analysis of the relevant literature and in light of more recent studies, it is clear that while some differences exist between the spinal articulation and the generic synovial joint, there are clear structural, functional and developmental similarities between the joints that in sum outweigh the differences. Further, since the intervertebral motion segment displays movement in three dimensions and the whole spine itself provides integrated rotatory movements, it is proposed that it should be classified not as an amphiarthrose, “a slightly moveable joint” but as a complex polyaxial joint. Hopefully, reclassification will encourage further analysis of the structure and function of the two types of overlapping joints and provide common new insights into diseases that afflict the many joints of the human skeleton.
Keywords: diarthrodial joint, nucleus pulposus, annulus fibrosus, intervertebral disc, arthrotome, synovial cells, lubricin, Ext1, notochord, hyaluronan, aggrecan, joint biomechanics
Introduction
He who rejects change is the architect of decay. The only human institution which rejects progress is the cemetery.
(Harold Wilson)
That the spine is supple is obvious to all that watch the movements of young children at play, the power service of professional tennis players and the sinuous movements of ballet dancers. Undoubtedly, flexibility decreases with age, while spinal movements at all stages of life can be severely limited by disease. Since vertebrae themselves are relatively inelastic, movement in the spine is mediated notably by the tissues of the intervertebral disc. Although the mobility of contiguous vertebrae (motion segments) can be viewed as limited, the integrated movements of the thirty three intervertebral discs permits all of the critical movements of the spine without compromising nerve or muscle function.
The importance of the intervertebral disc was first noted by the great European anatomist Versallius. In his treatise De Humani Corporis Fabrica published in 1555, he remarked that the fluid filled cushions of the disc permitted the movement of the head and presumably the remainder of the spine. The famous English anatomist Henry Gray (1827 – 1861) confirmed this view and classified articulations between vertebrae as “amphiarthroses in which the contiguous bony surfaces are either connected by broad flattened disks of fibrocartilage, of a more or less complex structure” [1]. Analysis of the articulations themselves indicated that vertebrae were interconnecting by three joints; the intervertebral joint, and the right and left zygapophyseal (facet) joints. Linking two contiguous vertebrae, the joint complex has six degrees of freedom since it can deform in three planes: axially, medial/lateral shear and anterior/posterior shear [2]. At the same time, less discursive coupled rotatory or translatory movements are evident in the other joints of the motion segment [3].
Just prior to the death of Gray, the debate was joined by the German anatomist Hubert von Luschka, (1820 – 1875) who had already described the clefting joints in the articulation of cervical vertebrae (uncovertebral joints also called von Luschka joints). He remarked that the spinal motion segment was equivalent to a synovial joint [4]. Now over 125 years later, it is tempting to think that the definition of the spinal joint being diarthrodial still holds. However, possibly because of the large differences in anatomic structure coupled with its unique developmental origin and overall limitation in movement, Grignon and Roland concluded “it would appear difficult to consider the IVD [intervertebral disc] as a diarthrodial joint” [5].
Since publication of the review by Grignon and Roland (2000) a considerable number of new findings related to both diarthrodial as well as spinal joints have emerged prompting a reexamination of the conclusion that the intervertebral disc should not be classified as a diarthodial joint. Certainly, on simple structural and functional criteria, the intervertebral joint deserves to be in a separate category; this view is reinforced by observations from the developmental literature where it has been shown that axial morphogenesis is governed by cues which may be separate from those of the appendicular skeleton [6, 7]. Nevertheless, more recent findings indicate a much closer functional and genetic relationship to a diarthrodial joint than has hitherto been recognized.
Finally, the question is raised, why should we care about joint classification? From a health perspective, if the joints share a number of phenotypic and functional characteristics, this information could be used to provide a deeper understanding of the pathogenesis of disease. For example, if degenerative disc disease is linked to changes in the nutritional status of the nucleus pulposus due to diffusion problems in the endplate cartilage, then could a similar defect modify the normal functioning of the articular cartilage of the synovial joint? Likewise, in osteoarthritis where there is accumulating evidence that this condition is associated with defects in subchondral bone, it begs the question, does a similar change in vertebral structure promote disc degeneration? In addition, the issue could be raised, why is the disc more likely to fail though herniation than the synovial joint; this latter structure maintains its stability even when its function is severely degraded. Hopefully, reclassification will encourage further analysis of the structure and function of the two types of overlapping joints and access disease-related information that has been previously associated with a specific type of joint. In this way, common new insights can be generated into the major diseases that afflict the many joints of the human skeleton.
Structural and Biomechanical Considerations of Diarthrodial and Intervertebral Joints
While at first glance, the structure of the diarthrodial and the intervertebral joints appear to be substantially different, obvious similarities exist. Figure 1 shows an idealized diarthrodial joint which comprises a thin layer of cartilage that covers both the articulating bone surfaces, and an innervated but relatively avascular fibrous joint capsule that is often a site of ligament anchorage. The inner surface of the capsule is lined by a synovial membrane that secretes the synovial fluid. This fluid is of considerable biomechanical importance as it serves as a low-friction lubricant for the cartilage surface, and provides an articulating interface. There is some evidence to indicate that synovial fluid is required for maintenance of the functionality of joint cells [8]. Like diarthrodial joints, the intervertebral disc has two articulating cartilaginous surfaces, the endplate cartilages that are separated by a second proteoglycan rich tissue the nucleus pulposus (Fig. 1). Nucleus pulposus cells secrete and organize a complex extracellular matrix that mainly contains the proteoglycan aggrecan and a small proportion of fibrillar proteins mainly collagen type II. The gel-like nucleus pulposus is surrounded circumferentially by a fibrocartilagenous annulus fibrosus. Sharpey fibers of the annulus are inserted into the end plate cartilages and the vertebral bone [9].
Figure 1.
Schematic showing similarities between an idealized diarthrodial join and an intervertebral disc. The diarthrodial joint comprises a thin layer of cartilage that covers both the articulating bone surfaces, and an innervated but relatively avascular fibrous joint capsule. The inner surface of the capsule is lined by a synovial membrane that secretes the synovial fluid. The intervertebral disc has two articulating cartilaginous surfaces, the endplate cartilages that are separated by a second proteoglycan rich tissue the nucleus pulposus. Nucleus pulposus cells secrete and organize a complex extracellular matrix. The gel-like nucleus pulposus is surrounded circumferentially by a fibrocartilagenous annulus fibrosus. Sharpey fibers of the annulus are inserted into the end plate cartilages and the vertebral bone.
Superficially, there appears to be considerable differences in the gross structure of the two joints. However, if the bones are viewed as being of equal dimensions then the two joints are remarkably similar (Fig 1). At the gross level it could be argued that the biggest difference is in the presence of the intermediary tissue. In the mature intervertebral disc, the nucleus pulposus forms a tongue of tissue across the joint surrounded on all sides by a cell-free zone rich in proteoglycans. A similar type of anatomical division is evident in diarthrodial joints. For example, in the knee joint, the C shaped meniscal cartilage divides the diarthrodial joint cavity into an upper and lower division. In the temperomandibular joint, an articular disc composed of fibrocartilage is positioned between the two bones that form the joint. The disc divides each joint into a lower joint compartment formed by the mandible and the articular disc and an upper compartment formed by the articular disc and the temporal bone. An even more complex articulation exists at the shoulder between the axial skeleton and the pectoral girdle. Thus, the sternoclavicular joint forms the articulation between the ribs and clavicle and the sternum and contains a fibrocartilaginous disc that serves to increase the range of joint movements. Based on the range and complexities of joints in human skeleton to which could be added many more examples from the vertebrate animal kingdom, the presence of an intervening tissue within the articulation is not out of keeping with the anatomy of existing diarthrodial joints.
Not surprisingly, the range of motion of an intervertebral disc in the spine varies considerably from region to region and from species to species. Thus, in the human spine, the lumber and cervical motion segments display a greater range of movements than in the thoracic region. In the animal kingdom, the vertebrae accommodate to the functional requirements of the species. For example, in frogs and reptiles, the procoelous vertebrae are anteriorly concave and posteriorly convex allowing extensive movements in all directions; in some whale species the cervical vertebrae are fused, vestigial disc is removed forming a composite vertebra [10], whereas in giraffes, the anatomy of the cervical vertebrae can be likened to a ball and socket articulation [11]. From this perspective, it is unreasonable to assume that all intervertebral joints exhibit a similar and limited degree of motion. We would argue that if force is plotted against the range of motion, then diaphyseal joints would define one extremity, while synarthrosis the other. The position of an intervertebral joint on this continuum would be variable depending on each of the factors mentioned above. Mirroring the variability in motion, it would not be surprising to find that the cellular and extracellular components of the intervertebral disc would reflect these alterations in functional activity.
It should be noted that compared with cartilage, little is known of the molecular biology of cells of the annulus fibrosus, the endplate cartilage or the nucleus pulposus. In the mature nucleus, the cell content is sparse while the extracellular matrix is rich in aggrecan and other proteoglycans. The annulus fibrosus is morphologically distinct from the fibrous joint capsule and the synovial membrane. However, the outer one third does resemble the capsular tissue in that it is infiltrated with blood vessels and nerves [12]. Moreover, the collagen fibrils are parallel and like the fibrous joint capsule orientated to accommodate the applied mechanical forces. Since the force vectors are different from joint to joint, not-surprisingly, the unique hooping of the fibrils of the annulus fibrosus is not seen in the diarthrodial joint capsule, As far as the innermost aspect of the annulus is concerned, unlike the joint capsule which is lined by a synovial membrane, a distinct lamellar structure is not evident. However, in the inner one quarter of the thickness of the annulus, defined by the lack of annular layers, swollen cells exist in an amorphous matrix that is chemically distinct from that of the overlying outer annulus fibrosus and entirely separate from the cells of the nucleus pulposus. The question is raised are these cells together with those of the nucleus pulposus equivalent in function to the synovial cells of the diarthodial joint?
Similarities Between the Synovium and the Tissues of the Intervertebral Disc
Probably the most overt difference between the conventional diarthrodial joint and that of the intervertebral disc is the presence of a synovium. In the diarthrodial joint, a fine synovial membrane extends across the whole area of the capsule. It is composed of an adherent lining possibly one- to three-cells deep, embedded in a loose connective tissue. While forming a distinct layer of cells with an abundant extracellular matrix, the synovium does not possess a basement membrane. However, there is evidence that a plethora of cellular connections exist between cell layers in the lining as well as robust interactions with components of the synovial cavity [13].
Morphologically two types of synoviocytes have been identified: type A or macrophage-like synoviocytes; type B or fibroblast-like synoviocytes also known as synovial fibroblasts. The A cells are phagocytic and present even in the immature state; the macrophages and in some cases T cells most probably migrate into the tissue from bone-marrow myeloid precursors. These cells have an immunoprotective function and therefore few are noted in the non inflamed healthy synovium. In contrast, the synovial fibroblasts provide innate immunity by secreting products that include cytokines and matrix metalloproteinases. The activities of the both the cell types is significantly increased during inflammation [14].
The discontinuous synovial lining provides a limited barrier to the accumulation of immune complexes, bacterial cell wall products and other phlogistic agents. Development of a diarthrodial synovitis is characterized by changes in the cellular composition of the joint fluid including a leucocytosis driven by the local synthesis of proinflammatory cytokines and chemokines, which cause erosion of the articular cartilage [15]. Barrier-like activities are provided by the type A bone marrow derived mononuclear cells. These migratory cells express class II major histocompatability antigens, and Fc, Rc and CD68 receptors [16]. In addition, the presence of lysosomal vacuoles indicates functional phagocytic activity [17].
The expression of many of the proteins mentioned above has received cursory study in disc cells. However, there has been considerable interest in the expression of CD68, a protein that serves as an integral part of the lysosomal membrane around which intracellular degradation of phagocytosed material takes place. Unlike healthy diarthrodial joints, CD68-positive cells are not seen in healthy discs, either in the nucleus pulposus or the annulus fibrosus. In contrast, CD68-positive cells are seen in discs exhibiting evidence of disc degeneration [18]. Significantly, these cells do not exhibit a morphology suggestive of a migratory cell, leading the authors to suggest that the CD68-positive cells are not invading monocytes or macrophages, but transformed resident cells that are involved in phagocytosis. The phagocytic activity of these cells was further studied by evaluating their function in vitro. Like articular cartilage cells, the disc cells were capable of performing phagocytosis and, possibly as a reflection of their large size, retain the internalized ingested material longer than committed phagocytes [19]. Based on these studies, it is clear that in the healthy state, like the diarthrodial joint, cells of the inner annulus with or without the nucleus pulposus, are capable of mounting a phagocytic response; unlike synovial cells, there appears to be little contribution from exogenous systemic cells. It should be added that in advanced degenerative disc disease, all of the cells found in diarthrodial joint synovitis are present in the nucleus pulposus. We conclude from these studies that both types of joints have similar although not the identical mechanisms to combat inflammation. More specifically, in the diarthrodial joint, while a functional membrane underlies the joint capsule, a similar membrane does not exist in the intervertebral disc. In the disc, possibly, this role is assumed by cells of the inner annulus fibrosus together with cells of the nucleus pulposus.
Lubrication Mechanisms and Chondrocyte Function
If the underlying hypothesis is valid and that the intervertebral disc functions in the same way as the synovial joint, then the overall composition of critical boundary lubricants should be similar and lubrication activities should be assigned to similar macromolecules. As was mentioned earlier, a major component of both diarthrodial joints and the intervertebral disc is aggrecan. The glycosoaminoglycan side chains of this polyelectrolyte are mostly charged and, by binding sodium and other ions, are responsible for the extremely high osmotic swelling pressures of cartilage and the disc. Thus, aggrecan permits the tissues to resist compressive loads and determines its mechanical properties. Aside from aggrecan, hyaluronan and lubricin, are two macromolecules that have critical roles in diarthrodial joint function. These two molecules form a cross linked network with aggrecan which reduced frictional forces and eliminates wear damage due to the shearing of opposing surfaces. Lubricin is a large glycoprotein that acts as a boundary lubricant for synovial fluid and cartilage [20]. In the diarthrodial joint, this protein is synthesized and secreted by superficial zone chondrocytes and cells lining the synovium [21] (Fig. 2). Moreover, since the protein is also synthesized by tendon and ligament cells, it has been suggested that it affects the viscoelastic properties of the tendon fascicles and may play a role in permitting sliding motion of the collagen fibers as they take up applied forces [22, 23].
Figure 2.
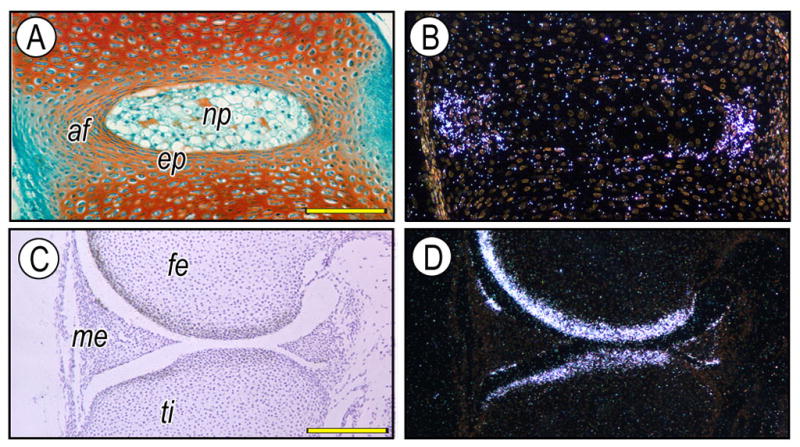
Intervertebral disc (A, B) and knee joint (C, D) from newborn mice were analyzed by in situ hybridization with isotope-labeled RNA probes for lubricin (B, D). It is evident that lubricin is expressed by the annulus fibrosus (af) and superficial zone chondrocytes of the articular cartilage. af, annulus fibrosus; np, nucleus pulposus; ep, endplate cartilage; fe, femur; ti, tibia, me, meniscus. Scale bars: 85 mm for A, B; 150 mm for C, D.
Lubricin is present in the intervertebral disc (Fig. 2). In a study of the goat disc, Shine et al identified the protein in the extracellular matrix of the inner as well as the outer annulus fibrosus suggesting a role in interlamellar tribology1 [24]. Moreover, cells of the annulus and the nucleus were capable of synthesizing lubricin in culture. In the human disc, these workers describe “a discrete layer of lubricin between lamellae of the annulus and nucleus pulposus”. Differences in levels of lubricin expression were thought to be due to variations in mechanical forces, which can alter expression of this molecule [25].
With respect to hyaluronan, aside from its interaction with lubricin, this hydrated polymer is seen in the extracellular matrix of a number of joint tissues, especially synovium and articular cartilage where it stabilizes proteoglycan aggregates in a type II collagen matrix, forming a resilient and compliant hydrated tissue [26, 27]. Not surprisingly, hyaluronan is present in the adult human and canine intervertebral disc and cartilage endplate. It is evident in the interterritorial matrix of the nucleus pulposus and annulus fibrosus and present between the collagenous lamellae in the annulus fibrosus [28]. Noteworthy, conditional deletion of Has2, an enzyme required for the synthesis of hyaluronan resulted in abnormalities of the spine, including vertebral cartilage as well as the intervertebral disc [29]. Moreover, CD44 a hyaluronan receptor has been shown to be present in the disc and articular cartilage where it interacts with ankyrin [30]. In summary, both joints share at least three key biomolecules that are all required for normal function.
Developmental Regulation of Joint and Disc Formation
Work performed in a number of labs has shown that mechanical factors play a major role in regulating the maintenance of cartilage structure and when forces are abnormal they promote tissue breakdown. It has also been shown that mechanical cues influence cartilage growth, phenotype and joint development [31, 32]. Thus, it is not surprising that there are profound differences in the development of “amphiarthroses” of the intervertebral regions of the spine and the morphogenesis of diarthodial joints. The synovial joint develops from predetermined limb patterning events specified by the Hox gene code. Accordingly, combinations of homeotic Hox genes are expressed in each portion of the developing limb (stylopod, zeugopod and autopod) and the patterns of expression dictate the unique characteristics of each limb and each limb region. Embedded in regions of mesenchyme, cell proliferation, synthesis of extracellular matrix components and subsequent tissue expansion specifies the morphology and the positionality of pairs of opposing cartilage anlagen. Between these analage, a region of mesenchyme forms the interzone, the region that ultimately specifies synovial joint formation. Evidence supporting this role for the interzone is based on studies that show that its removal leads to joint ablation and fusion [33]. Pacifici and colleagues reported that the opposing articular cartilages, ligaments and synovial lining cells are derived from interzone cells [34]. Subsequently, expression of CD44 and changes in hyaluronan synthesis causes tissue separation and joint cavity formation. This process of generating the diarthrodial joint cavity is regulated by morphogens like GDF5 (a TGFβ family member), BMP and BMP antagonists like chordin and noggin [35, 36]. Accordingly, deletion of a one of these required genes say noggin, leads to complete fusion of the limb bones with no joint formation [37].
In contrast to the synovial joint, the intervertebral joint is specified by paraxial mesoderm and notochord. The notochord is a rod-like midline structure of mesodermal origin that forms during gastrulation and represents a primitive axial skeleton [38, 39]. Signals from the notochord induce sclerotome cell migration, condensation, and differentiation to generate a peri-notochordal sheath [40]. The sheath exhibits a metameric pattern of more condensed and less condensed zones that give rise to the outer and inner annulus and the vertebrae. Thus, each vertebral body is generated by sclerotomal cells. Concerning the origin of the annulus fibrosus, it is known that each somite contains an arthrotome comprising an outer spherical group of cells that form an epithelial-like layer enclosing mesenchymal cells or a somitocoele [41]. Mittapalli et al (2005) showed that the cells of the somitocoele gives rise to the joint forming cells of the vertebrae: cells in the dorsal region develop into the zygophosphyaeal (synovial) joint whereas the ventral cells form the intervertebral disc [42]. Pacifici and colleagues commented that the somatocoele may play a role in specification of the annulus [43]. Thus, if these somatocoele (arthrotome) cells are ablated, there is abnormal annulus fibrosus formation and joint fusion. From that perspective, these cells would represent a cohort of somitic cells with annulus fibrosus-forming properties. These workers suggested that these arthrotome cells would behave and function similarly to interzone cells that are required for synovial joint formation. Thus, for both the diarthrodial joint and the intervertebral disc, significant developmental similarities exist.
Further evidence of the similarities between synovial joint and the intervertebral joint has been provided recently by a study that focused on the role of Ext1, the gene that encodes a transmembrane glycosyltransferase the enzyme that modifies heparan sulfate [43]. Using Gdf-5 Cre transgenic mice, Mundy et al. showed that the decrease in heparan sulfate that follows deletion of Ext1 results in aberrant synovial joint and spine formation, defective lubricin expression and some apoptosis of the superficial cells [43]. In the spine, fusions occur between vertebrae due to the missing intervertebral discs [43]. It should be noted that the discs of haploinsufficient GDF-5 mutant mice appear to be normal except when challenged with agents that include collagenase and papain treatment and mechanical conditions that induced osteoarthritis [44]. Very recently, a single nucleotide polymorphism in GDF5 was found to be associated with lumbar disc degeneration in a human population [45]. Clearly, from a developmental perspective, based on the limited number of studies of disc and limb formation, considerable similarities exist in the regulation of development of the two joint systems.
Perspective, Summary and Recommendations
This review challenges the conclusion of an earlier paper by Grinon and Roland that categorically stated that the intervertebral joint could not be classified as a diarthrodial joint and presumably it should remain as an amphiarthrosis [5]. This conclusion was reached after some consideration of development, structure and function of the intervertebral joint. However, in light of more recent studies, it is clear that while some differences exist between the spinal articulation and the generic synovial joint, there are clear similarities between the organs that in sum outweigh the differences. These are briefly summarized below.
With respect to structure-function relationships, on first consideration, the intervertebral disc could be seen as being very different from the generic synovial joint. However, on reflection, the separate tissues of the intervertebral disc are very similar to those of the diarthrodial joint: both types of joints are lined by cartilage, they are limited by an external ligament and the joint space contains molecules that promote lubrication (lubricin and hyaluronan) and elevate the osmotic pressure (aggrecan). Indeed, even the presence of the nucleus pulposus is not out of line with what is known of complex diarthrodial joints which contains cartilage and fibrocartilage discs and menisci. Related to the function of the nucleus pulposus and the inner annulus, it is clear that a distinct synovial membrane does not exist in the intervertebral disc. Nevertheless, like the cells of the synovium, the resident disc cells do have the ability to mount a robust response against inflammatory insults.
In terms of movement, the current status of the disc as an amphiarthosis indicates very limited mobility. However, biomechanical studies of the motion segment with or without contributions from the zygapophyses supports the view that the vertebral joint displays a wide range of movements. Moreover, movements of the cervical, thoracic and lumbar vertebrae include flexion-extension, axial rotation and lateral bending. These multi- dimensional excursions are more in line with those of a diarthrodial joint than an amphiarthosis where movements are slow and motion is limited. Noteworthy, while a diarthrodial joint suggest a larger range of motion, some diarthrodial joints such as the sacroiliac joints in the pelvic region and joints in the hands and feet can have motion more limited than that of the intervertebral disc. Thus, rather than being confined by a definition that posits synovial joints in one corner and amphiarthrodial joints in the other, we suggest that in accordance with biological function, there is enormous overlap in joint function that precludes it being confined to one particular category.
Probably the major difference between appendicular diarthrodial joints and the axial intervertebral joints lies in their development. Although the joints originate from different mesenchymal elements, deletion studies indicate that here too there are considerable similarities in the expression of genes that govern organ development and maturation. Recent investigations show that joint formation and even function is dependent on the expression of a number of genes including those of the Hox family, BMP’s, GDF5 and Ext1. Indeed, deletion of Ext1 influences not just the development of limb joints, but also the formation of the intervertebral disc.
Based on the overt structural and functional similarities between the intervertebral disc and the synovial joint and recognizing that while some differences exist between these articulations, it would seem logical to place the disc in the same grouping as the diarthrodial joint. Further, since the intervertebral motion segment displays movement in three dimensions and the whole spine itself provides integrated rotatory movements, we are of the opinion that it should be classified not as an amphiarthrose, “a slightly moveable joint” but as a complex polyaxial joint. We call on our colleagues who are the gate keepers of classifications, taxonomies and assemblages to re-characterize the intervertebral disc.
The time is out of joint; of cursed spite,
that I was ever born to set it right!
(Hamlet, William Shakespeare)
Table 1.
CLASSIFICATION OF JOINTS (after Gray)
| Type of Joints | Examples | |
|---|---|---|
| 1 | Synarthrosis- an almost immoveable articulation. | Sutures - most commonly seen in bones of the skull. |
| Gomphosis - the specialized union between the | ||
| periodontal ligament and alveolar bone. | ||
| Schindylesis - insertion of bony plate into another bone (sphenoid into the ethmoid and vomer) | ||
| Synchondroses - epiphyseal cartilage that unites the epiphysis and diaphysis of a long bone | ||
|
| ||
| 2 | Amphiarthrosis- a slightly moveable joint. | Symphysis - a cartilaginous joint holding together bones at the pubis. The intervertebral disk is currently considered to be an amphiarthrosis |
| Syndesmosis - interosseus ligament that holds the distal ends of the tibia and fibula together | ||
|
| ||
| 3 | Diarthrosis – a moveable joint. Characteristically, it is the articulation between two bone surfaces covered with articular cartilage. They can be subdivided according to the range of movement: |
Uniaxial - movement in one plane. ginglymus or hinge-joint – knee and elbow articulations trochoid or pivot-joint – atlas with axis |
|
Biaxial - movement in two planes condyloid and saddle joints – articulations involving the wrist joint and carpometacarpal joint of the thumb | ||
|
Polyaxial - movement in multiple planes enarthosis joints of the hip and shoulder; possibly, the intervertebral joint | ||
|
Gliding Joints articular processes of the vertebrae and many of the carpal and tarsal joints | ||
Highlights.
We critique the assumption that the intervertebral disc is an amphiarthosis
We compare the development, structure and function of the disc with a synovial joint
The intervertebral disc has many similarities with synovial joints
The intervertebral disc should be re-classified as a polyaxial diarthrodial joint
Acknowledgments
Supported by grants from the National Institutes of Health R01-AR050087 and R01-AR055655. We thank Dr. Maurizio Pacifici for critically reading the manuscript and Dr. Eiki Koyama for providing images shown in Figure 2. We thank Bradley Snyder for illustration shown in Figure 1.
Footnotes
Tribology is the study of friction wear and lubrication
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gray H. Anatomy: descriptive and surgical. Philadelphia: Blanchard and Lea; 1859. [Google Scholar]
- 2.Yong-Hing K, Kirkaldy-Willis WH. The pathophysiology of degenerative disease of the lumbar spine. Orthop Clin North Am. 1983;14:491–504. [PubMed] [Google Scholar]
- 3.Panjabi MM, Oxland TR, Yamamoto I, Crisco JJ. Mechanical behavior of the human lumbar and lumbosacral spine as shown by three-dimensional load-displacement curves. J Bone Joint Surg Am. 1994;76:413–24. doi: 10.2106/00004623-199403000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Luschka Hubert. Die Halbgelenke des menschlichen Körpers: Mit 6 Kupfertafeln. Druck Und Verlag Von Georg Reimer; Berlin: 1858. [Google Scholar]
- 5.Grignon B, Roland J. Can the human intervertebral disc be compared to a diarthrodial joint? Surg Radiol Anat. 2000;22:101–5. doi: 10.1007/s00276-000-0101-8. [DOI] [PubMed] [Google Scholar]
- 6.Eames BF, Helms JA. Conserved molecular program regulating cranial and appendicular skeletogenesis. Dev Dyn. 2004;231:4–13. doi: 10.1002/dvdy.20134. [DOI] [PubMed] [Google Scholar]
- 7.Winslow BB, Takimoto-Kimura R, Burke AC. Global patterning of the vertebrate mesoderm. Dev Dyn. 2007;236:2371–81. doi: 10.1002/dvdy.21254. [DOI] [PubMed] [Google Scholar]
- 8.Lee DA, Salih V, Stockton EF, Stanton JS, Bentley G. Effect of normal synovial fluid on the metabolism of articular chondrocytes in vitro. Clin Orthop Relat Res. 1997;342:228–38. [PubMed] [Google Scholar]
- 9.Humzah MD, Soames RW. Human intervertebral disc: structure and function. Anat Rec. 1988;220:337–56. doi: 10.1002/ar.1092200402. [DOI] [PubMed] [Google Scholar]
- 10.Ogden JA, Lee KE, Conlogue GJ, Barnett JS. Prenatal and postnatal development of the cervical portion of the spine in the short-finned pilot whale Globicephala macrorhyncha. Anat Rec. 1981;200:83–94. doi: 10.1002/ar.1092000108. [DOI] [PubMed] [Google Scholar]
- 11.van Sittert SJ, Skinner JD, Mitchell G. From fetus to adult-an allometric analysis of the giraffe vertebral column. J Exp Zool B Mol Dev Evol. 2010;314:469–79. doi: 10.1002/jez.b.21353. [DOI] [PubMed] [Google Scholar]
- 12.Hassler O. The human intervertebral disc. A micro-angiographical study on its vascular supply at various ages. Acta Orthop Scand. 1969;40:765–72. doi: 10.3109/17453676908989540. [DOI] [PubMed] [Google Scholar]
- 13.Kolomytkin OV, Marino AA, Sadasivan KK, Meek WD, Wolf RE, Hall V, et al. Gap junctions in human synovial cells and tissue. J Cell Physiol. 2000;184:110–7. doi: 10.1002/(SICI)1097-4652(200007)184:1<110::AID-JCP12>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 14.Palmer DG, Selvendran Y, Allen C, Revell PA, Hogg N. Features of synovial membrane identified with monoclonal antibodies. Clin Exp Immunol. 1985;59:529–38. [PMC free article] [PubMed] [Google Scholar]
- 15.Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625–35. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 16.Athanasou NA, Quinn J. Immunocytochemical analysis of human synovial lining cells: phenotypic relation to other marrow derived cells. Ann Rheum Dis. 1991;50:311–5. doi: 10.1136/ard.50.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Etherington DJ, Taylor MA, Henderson B. Elevation of cathepsin L levels in the synovial lining of rabbits with antigen-induced arthritis. Br J Exp Pathol. 1988;69:281–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Nerlich AG, Weiler C, Zipperer J, Narozny M, Boos N. mmunolocalization of phagocytic cells in normal and degenerated intervertebral discs. Spine. 2002;27:2484–90. doi: 10.1097/00007632-200211150-00012. [DOI] [PubMed] [Google Scholar]
- 19.Jones P, Gardner L, Menage J, Williams GT, Roberts S. Intervertebral disc cells as competent phagocytes in vitro: implications for cell death in disc degeneration. Arthritis Res Ther. 2008;10:R86. doi: 10.1186/ar2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan SM, Neu CP, Duraine G, Komvopoulos K, Reddi AH. Atomic force microscope investigation of the boundary-lubricant layer in articular cartilage. Osteoarthritis Cartilage. 2010;18:956–63. doi: 10.1016/j.joca.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Rhee DK, Marcelino J, Baker M, Gong Y, Smits P, Lefebvre V, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115:622–31. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Y, Berger EJ, Zhao C, An KN, Amadio PC, Jay G. Mapping lubricin in canine musculoskeletal tissues. Connect Tissue Res. 2006;47:215–21. doi: 10.1080/03008200600846754. [DOI] [PubMed] [Google Scholar]
- 23.Reuvers J, Thoreson AR, Zhao C, Zhang L, Jay GD, An KN, et al. The mechanical properties of tail tendon fascicles from lubricin knockout, wild type and heterozygous mice. J Struct Biol. 2011;176:41–5. doi: 10.1016/j.jsb.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shine KM, Simson JA, Spector M. Lubricin distribution in the human intervertebral disc. J Bone Joint Surg Am. 2009;91:2205–12. doi: 10.2106/JBJS.H.01344. [DOI] [PubMed] [Google Scholar]
- 25.Grad S, Lee CR, Gorna K, Gogolewski S, Wimmer MA, Alini M. Surface motion upregulates superficial zone protein and hyaluronan production in chondrocyte-seeded three-dimensional scaffolds. Tissue Eng. 2005;11:249–56. doi: 10.1089/ten.2005.11.249. [DOI] [PubMed] [Google Scholar]
- 26.Yasui T, Tsukise A, Sakurai S, Habata I, Meyer W, Hirabayashi Y. Ultrastructural localization of hyaluronic acid in the synovium of the goat knee joint. Ann Anat. 2004;186:379–84. doi: 10.1016/S0940-9602(04)80068-2. [DOI] [PubMed] [Google Scholar]
- 27.Poole AR, Pidoux I, Reiner A, Rosenberg L. An immunoelectron microscope study of the organization of proteoglycan monomer, link protein, and collagen in the matrix of articular cartilage. J Cell Biol. 1982;93:921–37. doi: 10.1083/jcb.93.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inkinen RI, Lammi MJ, Agren U, Tammi R, Puustjärvi K, Tammi MI. Hyaluronan distribution in the human and canine intervertebral disc and cartilage endplate. Histochem J. 1999;31:579–87. doi: 10.1023/a:1003898923823. [DOI] [PubMed] [Google Scholar]
- 29.Roughley PJ, Lamplugh L, Lee ER, Matsumoto K, Yamaguchi Y. The role of hyaluronan produced by Has2 gene expression in development of the spine. Spine. 2011;36:E914–20. doi: 10.1097/BRS.0b013e3181f1e84f. [DOI] [PubMed] [Google Scholar]
- 30.Stevens JW, Kurriger GL, Carter AS, Maynard JA. CD44 expression in the developing and growing rat intervertebral disc. Dev Dyn. 2000;219:381–90. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1060>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 31.Carter DR, Beaupré GS, Wong M, Smith RL, Andriacchi TP, Schurman DJ. The mechanobiology of articular cartilage development and degeneration. Clin Orthop Relat Res. 2004;427(Suppl):S69–77. doi: 10.1097/01.blo.0000144970.05107.7e. [DOI] [PubMed] [Google Scholar]
- 32.Beaupré GS, Stevens SS, Carter DR. Mechanobiology in the development, maintenance, and degeneration of articular cartilage. J Rehabil Res Dev. 2000;37:145–51. [PubMed] [Google Scholar]
- 33.Holder N. An experimental investigation into the early development of the chick elbow joint. J Embryol Exp Morphol. 1977;39:115–27. [PubMed] [Google Scholar]
- 34.Koyama E, Shibukawa Y, Nagayama M, Sugito H, Young B, Yuasa T, et al. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev Biol. 2008;316:62–73. doi: 10.1016/j.ydbio.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Archer CW, Dowthwaite GP, Francis-West P. Development of synovial joints. Birth Defects Res C Embryo Today. 2003;69:144–55. doi: 10.1002/bdrc.10015. [DOI] [PubMed] [Google Scholar]
- 36.Pacifici M, Koyama E, Iwamoto M. Mechanisms of synovial joint and articular cartilage formation: recent advances, but many lingering mysteries. Birth Defects Res C Embryo Today. 2005;75:237–48. doi: 10.1002/bdrc.20050. [DOI] [PubMed] [Google Scholar]
- 37.Brunet LJ, McMahon JA, McMahon AP, Harland RM. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280:1455–57. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- 38.Adams DS, Keller R, Koehl MA. The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development. 1990;110:115–30. doi: 10.1242/dev.110.1.115. [DOI] [PubMed] [Google Scholar]
- 39.Stemple DL. Structure and function of the notochord: an essential organ for chordate development. Development. 2005;132:2503–12. doi: 10.1242/dev.01812. [DOI] [PubMed] [Google Scholar]
- 40.Choi KS, Harfe BD. Hedgehog signaling is required for formation of the notochord sheath and patterning of nuclei pulposi within the intervertebral discs. Proc Natl Acad Sci U S A. 2011;108:9484–9. doi: 10.1073/pnas.1007566108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solursh M, Fisher M, Meier S, Singley CT. The role of extracellular matrix in the formation of the sclerotome. J Embryol Exp Morphol. 1979;54:75–98. [PubMed] [Google Scholar]
- 42.Mittapalli VR, Huang R, Patel K, Christ B, Scaal M. Arthrotome: a specific joint forming compartment in the avian somite. Dev Dyn. 2005;234:48–53. doi: 10.1002/dvdy.20502. [DOI] [PubMed] [Google Scholar]
- 43.Mundy C, Yasuda T, Kinumatsu T, Yamaguchi Y, Iwamoto M, Enomoto-Iwamoto M, et al. Synovial joint formation requires local Ext1 expression and heparan sulfate production in developing mouse embryo limbs and spine. Dev Biol. 2011;351:70–81. doi: 10.1016/j.ydbio.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daans M, Luyten FP, Lories RJ. GDF5 deficiency in mice is associated with instability-driven joint damage, gait and subchondral bone changes. Ann Rheum Dis. 2011;70:208–13. doi: 10.1136/ard.2010.134619. [DOI] [PubMed] [Google Scholar]
- 45.Williams FM, Popham M, Hart DJ, de Schepper E, Bierma-Zeinstra S, Hofman A, et al. GDF5 single nucleotide polymorphism rs143383 is associated with lumbar disc degeneration in Northern European women. Arthritis Rheum. 2011;63:708–12. doi: 10.1002/art.30169. [DOI] [PMC free article] [PubMed] [Google Scholar]



