Abstract
As a skin-resident member of the dendritic cell (DC) family, Langerhans cells (LCs) are generally regarded to function as professional antigen presenting cells. Here we report a simple method to visualize the endocytotic activity of LCs in living animals. BALB/c mice received subcutaneous injection of FITC-conjugated dextran (DX) probes into the ear skin and were then examined under confocal microscopy. Large numbers of FITC+ epidermal cells became detectable 12–24 h after injection as background fluorescence signals began to disappear. Most (>90%) of the FITC+ epidermal cells expressed Langerin, and >95% of Langerin+ epidermal cells exhibited significant FITC signals. To assess intracellular localization, Alexa Fluor 546-conjugated DX probes were locally injected into IAβ-EGFP knock-in mice and Langerin-EGFP-DTR mice – three dimensional rotation images showed close association of most of the internalized DX probes with MHC class II molecules, but not with Langerin molecules. These observations support the current view that LCs constantly sample surrounding materials, including harmful and innocuous antigens, at the environmental interface. Our data also validate the potential utility of the newly developed imaging approach to monitor LC function in wild-type animals.
INTRODUCTION
Langerhans cells (LCs) are positioned at the outermost surface of the body, where they extend long dendritic processes through intercellular spaces between adjacent keratinocytes. Moreover, LCs isolated from the skin and/or LC-like dendritic cell (DC) cultures exhibit potent T cell-stimulatory activities. Based on these features (i.e., location, morphology, and in vitro function), LCs are generally believed to serve as the primary antigen presenting cells in the skin (Steinman and Banchereau, 2007; Merad et al., 2008; Nestle et al., 2009). On the other hand, LC depletion studies with genetically engineered mouse lines expressing diphtheria toxin (DT) or high affinity DT receptor (DTR) in a LC-targeted fashion (Bennett et al., 2005; Kissenpfennig et al., 2005; Kaplan et al., 2005) did not necessarily support the above concept because they showed opposing effects on allergic contact hypersensitivity responses, depending upon the mouse lines, timing of LC removal, and skin sensitization protocols (Kaplan et al., 2008). LC-depleted mice even showed markedly reduced incidence of skin cancer development in two-stage chemical carcinogenesis experiments (Strid et al., 2008). Thus, in vivo functions of LCs still remain ambiguous.
Recent advances in mouse genetic engineering and intravital confocal imaging have enabled direct visualization of LC behaviors in living animals. Working with the I-Aβ-EGFP knock-in mice, in which the endogenous MHC class II I-Aβ chain is replaced by an EGFP-tagged version (Boes et al., 2002), we recorded four-dimensional (4D) behaviors of EGFP+ LCs in ear skin (Nishibu et al., 2006). Under the steady state, EGFP+ LCs exhibited a unique motile activity, termed the dendrite surveillance extension and retraction cycling habitude (dSEARCH), characterized by rhythmic extension and retraction of their dendrites. Not only did local skin inflammation augment the dSEARCH movement of dendrites, it also triggered amoeba-like lateral displacement of cell bodies within the epidermal compartment. With regard to underlying mechanisms, two pro-inflammatory cytokines, i.e., IL-1 and TNFα, were found to be required and sufficient for exacerbating both forms of motile activities of LCs (Nishibu et al., 2007). Although our observations have uncovered unique LC responses to inflammatory stimuli, this imaging protocol (which requires genetic engineering) is not applicable to human. Nor do the recorded images represent any functional parameter of LCs. Our primary purpose was, therefore, to overcome these limitations of the currently available intravital LC imaging protocol.
LCs freshly isolated from mouse skin have been shown to internalize a variety of particles, including latex beads, bacteria, yeast, and parasites (Reis e Sousa et al., 1993; Blank et al., 1993). Strikingly, in vivo endocytotic activities of human LCs were reported more than four decades ago. Electron microscopic analyses of human skin samples revealed Birbeck granule-containing LCs that had engulfed melanosomes (Mishima, 1966). The abilities of LCs to ingest and degrade exogenous molecules were demonstrated by injecting horseradish peroxidase locally into healthy volunteers (Wolff and Schreiner, 1970). Therefore, we hypothesized that one might be able to visualize the endocytotic activity of LCs in situ by injecting FITC-conjugated dextran (DX) into the skin of wild-type mice.
RESULTS AND DISCUSSION
Fate of FITC-DX probes after local injection into mouse skin
We injected FITC-DX probes with the molecular size of 70 kDa subcutaneously (s.c.) into the ear skin of anesthetized wild-type BALB/c mice. Immediately after DX administration, we placed the mice under confocal microscopy to record a series of Z-axis scanning images of the injection site. Compiled Z-axis images are shown in Figure 1a, in which the outermost surface of Stratum corneum is labeled as 0 μm depth. We considered the vertical space up to 20 μm from the surface to represent the epidermal compartment, based on the z-axis location of collagen bundles detectable by second harmonic signals (Ward et al., 2007). An actual 1 μm series of scanning images can be seen in Supplementary Movie S1. When examined immediately after administration, the entire injection site (except hair shafts and hair follicles) was filled with strong FITC fluorescence signals.
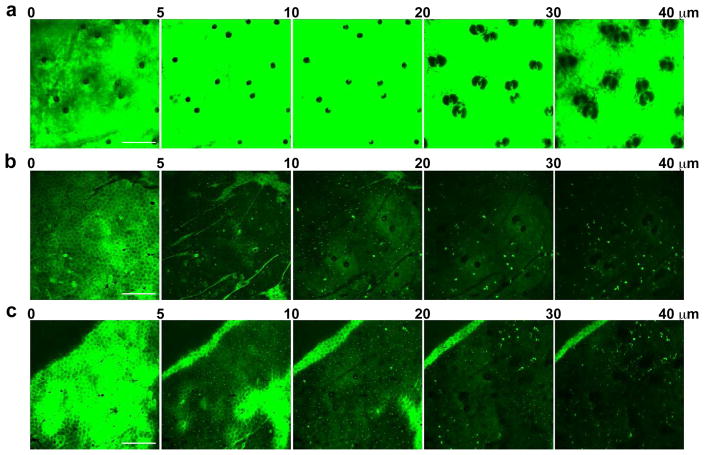
Figure 1. Direct visualization of macromolecule endocytosis by epidermal LCs.
Anesthetized wild-type BALB/c mice received s.c. injection of 70 kDa FITC-DX probes into ear skin. At 0 (panel a), 24 (b), and 48 hours (c) after the injection, the mice were placed under a confocal microscope to record FITC fluorescence signals in the ear skin. Data shown are compiled x-y plane fluorescence images in the indicated z-axis depth ranges from the skin surface, i.e., Stratum corneum. Scale bar: 200 μm.
The robust FITC fluorescence signals disappeared from the injection site at 12–24 hours, reflecting lymphatic drainage of the unbound probes (Bollinger et al., 1981). At the same time, large numbers of FITC+ cells that had incorporated the DX probes became detectable within the epidermal compartment underneath the honeycomb-shaped corneocyte layer (Figure 1b and Movies S2 and S3). The dermal compartment (from 20 to 40 μm in depth) also contained FITC+ cells, which were much larger in size compared to the FITC+ cells found in the epidermis. Numbers and fluorescence intensities of these FITC+ cells remained relatively unchanged until 48 hours after injection (Figure 1c and Movie S4). Although fluorescence signals began to decline thereafter, FITC+ cells remained visible up to 144 hours after injection (data not shown). These observations indicated that locally injected DX probes were captured preferentially by certain populations in the epidermal and dermal compartments, while non-captured probes were drained rapidly from the skin.
Identity of the epidermal cells that have incorporated FITC-DX probes
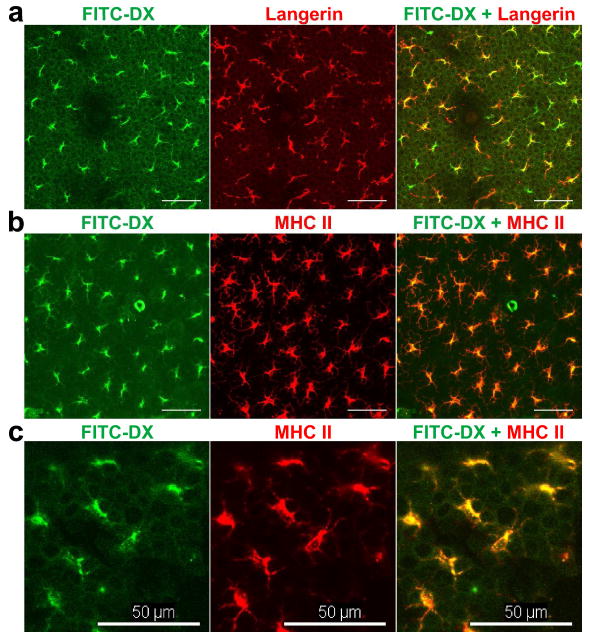
The key question concerned the identity of those cells that had captured the FITC-DX probes. Because our primary purpose was to study the in vivo endocytotic potential of LCs, we focused our efforts on the FITC+ cells in the epidermal compartment. Epidermal sheets prepared from ear skin 24 hours after FITC-DX injection were stained with Alexa Fluor 546 (AF546)-conjugated monoclonal antibody (mAb) against Langerin, which is a LC-specific C-type lectin associated with Birbeck granule formation (Valladeau et al., 2000). Most (>90%) of the FITC+ epidermal cells expressed Langerin (Figure 2a). Conversely, FITC fluorescence signals were detected in more than 95% of Langerin+ epidermal cells. Moreover, most of the FITC+ epidermal cells also expressed MHC class II molecules (Figure 2b). Thus, we have concluded that DX probes are captured primarily by LCs in the epidermis.
Figure 2. Identity of the epidermal cells that have incorporated FITC-DX probes.
BALB/c mice received s.c. injection of 70 kDa FITC-DX probes into ear skin. At 36 hours after the injection, the ear samples were harvested for immunofluorescence staining of the EDTA- separated epidermal sheets with AF546-conjugated mAb against Langerin (panel a) or against MHC class II molecules (panels b and c). Scale bar: 50 μm.
Topical application of FITC has been shown to induce rapid migration of LCs from the epidermis to draining lymph nodes (LNs) with a peak at 18-24 hours (Macatonia et al., 1987; Thomas et al., 1980). However, local injection of FITC-DX did not induce significant LC migration as assessed by counting the epidermal density of LCs (Figure S1) or the number of FITC+ cells expressing a LC marker EpCAM (Strid et al., 2008) in the draining LNs (Figure S2). Concerning the explanation for this discordance, it is conceivable that chemical reactivity of FITC towards nucleophiles was diminished or lost after DX conjugation.
All the images shown thus far were recorded after injecting the 70 kDa FITC-DX formulation. In an attempt to improve the resolution, we next tested FITC probes conjugated to DX polymers of different sizes (Figure S3). The FITC probe without DX was quickly drained without leaving detectable cell-associated signals. FITC-DX probes of 4 kDa and 20 kDa unveiled only faint and few images of epidermal cells. Clear images of epidermal cells were obtained after injection of a FITC-DX probe of 150 kDa (and a 70 kDa DX probe labeled with a red fluorescent tag, AF546). Interestingly, FITC-ovalbumin (OVA) probes showed clear accumulation in dermal cells, but not in epidermal cells (Figure S4a and Movie S5). By contrast, epidermal cells and dermal cells both accumulated FITC-mannan probes, although the resolution of the resulting images was rather limited (Figure S4b and Movie S6). It should be stated that our observations do not necessarily imply that LCs selectively take up polysaccharide polymers, because protein probes may be degraded more rapidly than polysaccharide probes in LCs. Based on these observations, we chose the 70kDa DX probes for the subsequent imaging experiments.
Dual-color, intravital 4D imaging of DX endocytosis by LCs
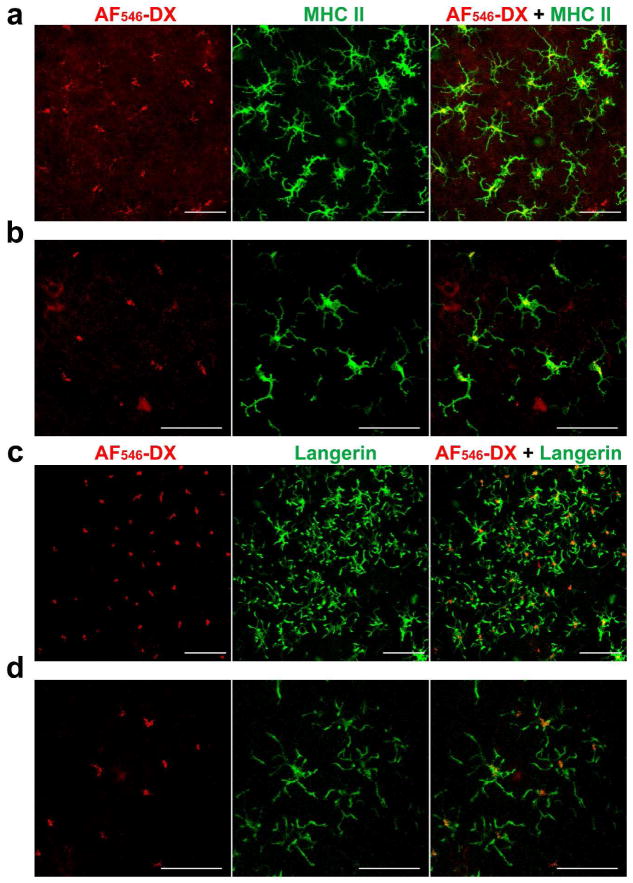
In immunofluorescence staining of the fixed skin samples, FITC-DX signals appeared to be associated with immunoreactivity with anti-MHC II mAb (Figure 2c). If so, it would imply further that the DX probes are delivered to the MHC class II compartments (MIICs), i.e., late-endosomal and lysosomal organelles where antigen processing and peptide loading are known to take place (Tulp et al., 1994). To test this directly, we injected the AF546-labeled DX probe to the skin of I-Aβ-EGFP knock-in mice. Dual-color intravital imaging experiments showed close association of most DX probes with MHC II molecules (Figures 3a and 3b). Co-localization of the two molecules was further confirmed by rotating the 3D images (Movie S7). By contrast, AF546-DX probes injected into Langerin-EGFP-DTR mice (Bennett et al., 2005) showed no significant association with Langerin molecules (Figure 3c and 3d and Movie S8). Thus, it appears reasonable to propose that the internalized DX probes are preferentially delivered into MIICs in LCs.
Figure 3. Association of internalized DX probes with MHC II molecules in LCs.
I-Aβ-EGFP knock-in mice (a and b) and Langerin-EGFP-DTR knock-in mice (c and d) received s.c. injection of 70 kDa AF546-DX probes into the ear skin. At 32 hours, the mice were examined under a confocal microscope. Data shown are compiled x-y plane images within the epidermal component (i.e., 0-19 μm z-axis depth range from the skin surface). Scale bar: 50 μm.
Concluding Remarks
Working with in vitro generated DC cultures, Sallusto et al. (1995) demonstrated the intrinsic abilities of DCs to capture FITC-DX probes in a macrophage mannose receptor (MMR)-dependent manner and to concentrate them in the MIICs. Moreover, LCs freshly isolated from human skin showed significant binding of FITC-DX (Condaminet et al., 1998). We now expand those observations by showing the in vivo ability of LCs to capture FITC-DX in living animals. Of many C-type lectins expressed by DCs (Figdor et al., 2002), MMR exhibits extremely high avidities to both mannose and fucose motifs (Lee et al., 2011), thereby mediating efficient uptake of microbial pathogens and allergens (Royer et al., 2010). Glycosylated antigens incorporated via MMR can be delivered not only to MIICs, but also to early endosomes for cross-presentation to CD8 T cells (Burgdorf et al., 2007). By contrast, Langerin recognizes many carbohydrate moieties including mannose (Stambach and Taylor, 2003; Tada et al., 2006) and it has been shown to mediate LC uptake of non-peptide microbial antigens for CD1a-restricted presentation to CD4/CD8 double-negative T cells (Hunger et al., 2004). Our observations support that MMR, Langerin, and perhaps other C-type lectins facilitate efficient antigen sampling by LCs in situ.
The intravital imaging approach developed in this study has allowed real-time monitoring of endocytotic function of LCs. This simple method, which does not require genetic engineering of test subjects, may be applicable to human. In fact, the lymphatic drainage function was successfully monitored in lymphoedema patients after s.c. injection of the same FITC-DX probe (Bollinger et al., 1981). Thus, it is tempting to speculate that a similar imaging approach can be used to study the behavior and function of epidermal LCs in human.
MATERIALS AND METHODS
Animals
Langerin-EGFP-DTR-knock-in mice (Bennett et al., 2005), I-Aβ-EGFP knock-in mice (Boes et al., 2002), and BALB/c mice were used in this study. All animal experiments were approved by the Institutional Animal Care and Use Committee at University of Toledo College of Medicine and were conducted according to guidelines of the National Institutes of Health (NIH).
Intravital confocal imaging
To reduce auto-fluorescence signals associated with hair follicles, the ear skin was treated with Nair Hair Remover (Church & Dwight) at least 3 days prior to DX injection. Anesthetized mice received s.c. injection of 20 μL of 10 mg/mL FITC-DX or AF546-DX (Sigma-Aldrich) into the ear using a 31G needle. We recorded images only in the center of the ear to avoid the needle insertion site, i.e., tip of the ear. The FITC-DX probes with 4, 20, 70, and 150 kDa had FITC modification at various levels from 0.002 to 0.02 mol FITC per mol glucose. FITC-conjugated mannan and OVA probes were also injected in some experiments. Intravital confocal images were recorded and processed as described before (Nishibu et al., 2006; Nishibu et al., 2007); the protocols are described briefly in Supplementary Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Björn E. Clausen (Erasmus MC, University Medical Center, Rotterdam) and Dr. Marianne Boes (University Medical Center, Wilhelmina Children’s Hospital, Utrecht) for providing Langerin-EGFP-DTR and I-Aβ-EGFP knock-in mice, respectively. This work was supported by grants from the NIH and the Bill and Melinda Gates Foundation.
Abbreviations
- DCs
dendritic cells
- LCs
Langerhans cells
- DX
dextran
- DTR
diphtheria toxin receptor
- D
dimensional
- dSEARCH
dendrite surveillance extension and retraction cycling habitude
- s.c
subcutaneous
- mAb
monoclonal antibody
- LNs
lymph nodes
- AF546
Alexa Fluor 546
- OVA
ovalbumin
- MIICs
MHC class II compartments
- MMR
macrophage mannose receptor
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Bennett CL, van Rijn E, Jung S, et al. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169:569–576. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank C, Fuchs H, Rappersberger K, et al. Parasitism of epidermal Langerhans cells in experimental cutaneous Leishmaniasis with Leishmania major. J Infect Dis. 1993;167:418–425. doi: 10.1093/infdis/167.2.418. [DOI] [PubMed] [Google Scholar]
- Boes M, Cerny J, Massol R, et al. T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature. 2002;418:983–988. doi: 10.1038/nature01004. [DOI] [PubMed] [Google Scholar]
- Bollinger A, Jager K, Sgier F, et al. Fluorescence microlymphography. Circulation. 1981;64:1195–1200. doi: 10.1161/01.cir.64.6.1195. [DOI] [PubMed] [Google Scholar]
- Burgdorf S, Kautz A, Bohnert V, et al. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316:612–616. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- Condaminet B, Peguet-Navarro J, Stahl PD, et al. Human epidermal Langerhans cells express the mannose-fucose binding receptor. Eur J Immunol. 1998;28:3541–3551. doi: 10.1002/(SICI)1521-4141(199811)28:11<3541::AID-IMMU3541>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Figdor CG, van Kooyk Y, Adema GJ. C-type lectin receptors on dendritic cells and Langerhans cells. Nat Rev Immunol. 2002;2:77–84. doi: 10.1038/nri723. [DOI] [PubMed] [Google Scholar]
- Hunger RE, Sieling PA, Ochoa MT, et al. Langerhans cells utilize CD1a and langerin to efficiently present non-peptide antigens to T cells. J Clin Invest. 2004;113:701–708. doi: 10.1172/JCI19655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DH, Jenison MC, Saeland S, et al. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Kaplan DH, Kissenpfennig A, Clausen BE. Insights into Langerhans cell function from Langerhans cell ablation models. Eur J Immunol. 2008;38:2369–2376. doi: 10.1002/eji.200838397. [DOI] [PubMed] [Google Scholar]
- Kissenpfennig A, Henri S, Dubois B, et al. Dynamics and function of Langerhans cells in vivo dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Lee RT, Hsu TL, Huang SK, et al. Survey of immune-related, mannose/fucose-binding C-type lectin receptors reveals widely divergent sugar-binding specificities. Glycobiology. 2011;21:512–520. doi: 10.1093/glycob/cwq193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatonia SE, Knight SC, Edwards AJ, et al. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. J Exp Med. 1987;166:1654–1667. doi: 10.1084/jem.166.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- Mishima Y. Melanosomes in phagocytic vacuoles in Langerhans cells. Electron microscopy of keratin-stripped human epidermis. J Cell Biol. 1966;30:417–423. doi: 10.1083/jcb.30.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle FO, Di MP, Qin JZ, et al. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9:679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibu A, Ward BR, Boes M, et al. Roles for IL-1 and TNFalpha in dynamic behavioral responses of Langerhans cells to topical hapten application. J Dermatol Sci. 2007;45:23–30. doi: 10.1016/j.jdermsci.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibu A, Ward BR, Jester JV, et al. Behavioral responses of epidermal Langerhans cells in situ to local pathological stimuli. J Invest Dermatol. 2006;126:787–796. doi: 10.1038/sj.jid.5700107. [DOI] [PubMed] [Google Scholar]
- Sousa Reise, Stahl PD, Austyn JM. Phagocytosis of antigens by Langerhans cells in vitro. J Exp Med. 1993;178:509–519. doi: 10.1084/jem.178.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer PJ, Emara M, Yang C, et al. The mannose receptor mediates the uptake of diverse native allergens by dendritic cells and determines allergen-induced T cell polarization through modulation of IDO activity. J Immunol. 2010;185:1522–1531. doi: 10.4049/jimmunol.1000774. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Cella M, Danieli C, et al. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: Downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambach NS, Taylor ME. Characterization of carbohydrate recognition by langerin, a C-type lectin of Langerhans cells. Glycobiology. 2003;13:401–410. doi: 10.1093/glycob/cwg045. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- Strid J, Roberts SJ, Filler RB, et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol. 2008;9:146–154. doi: 10.1038/ni1556. [DOI] [PubMed] [Google Scholar]
- Tada Y, Riedl E, Lowenthal MS, et al. Identification and characterization of endogenous Langerin ligands in murine extracellular matrix. J Invest Dermatol. 2006;126:1549–1558. doi: 10.1038/sj.jid.5700283. [DOI] [PubMed] [Google Scholar]
- Thomas WR, Edwards AJ, Watkins MC, et al. Distribution of immunogenic cells after painting with the contact sensitizers fluorescein isothiocyanate and oxazolone. Different sensitizers form immunogenic complexes with different cell populations. Immunology. 1980;39:21–27. [PMC free article] [PubMed] [Google Scholar]
- Tulp A, Verwoerd D, Dobberstein B, et al. Isolation and characterization of the intracellular MHC class II compartment. Nature. 1994;369:120–126. doi: 10.1038/369120a0. [DOI] [PubMed] [Google Scholar]
- Valladeau J, Ravel O, Dezutter-Dambuyant C, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- Ward BR, Jester JV, Nishibu A, et al. Local thermal injury elicits immediate dynamic behavioural responses by corneal Langerhans cells. Immunology. 2007;120:556–572. doi: 10.1111/j.1365-2567.2006.02533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff K, Schreiner E. Uptake, intracellular transport and degradation of exogenous protein by Langerhans cells. An electron microscopic-cytochemical study using peroxidase as tracer substance. J Invest Dermatol. 1970;54:37–47. doi: 10.1111/1523-1747.ep12551501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.