Abstract
Hypoxia is increasingly recognized as an important contributing factor to the development of brain diseases such as Alzheimer’s disease (AD). In the periphery, hypoxia is a powerful regulator of angiogenesis. However, vascular endothelial cells are remarkably heterogeneous and little is known about how brain endothelial cells respond to hypoxic challenge. The objective of this study is to characterize the effect of hypoxic challenge on the angiogenic response of cultured brain-derived microvascular endothelial cells. Brain endothelial cell cultures were initiated from isolated rat brain microvessels and subjected to hypoxia (1% O2) for various time periods. The results showed that hypoxia induced rapid (≤ 0.5 h) expression of hypoxia-inducible factor 1α (HIF-1α) and that cell viability, assessed by MTT assay, was unaffected within the first 8 h. Examination of brain endothelial cell cultures for pro- and anti-angiogenic proteins by western blot, RT-PCR and ELISA revealed that within 0.5 to 2 h of hypoxia levels of vascular endothelial growth factor and endothelin-1 mRNA and protein were elevated. The expression of heme oxygenase-1 also increased but only after 8 h of hypoxia. In contrast, similar hypoxia exposure evoked a decrease in endothelial nitric oxide synthase and thrombospondin-2 levels. Exposure of brain endothelial cell cultures to hypoxia resulted in a significant (p<0.001) decrease (94%) in tube length, an in vitro index of angiogenesis, compared to control cultures. The data indicate, despite a shift toward a pro-angiogenic phenotype, hypoxia inhibited vessel formation in brain endothelial cells. These results suggest that in brain endothelial cells expression of angiogenic factors is not sufficient for the development of new vessels. Further work is needed to determine what factors/conditions prevent hypoxia-induced angiogenic changes from culminating in the formation of new brain blood vessels and what role this may play in the pathologic changes observed in AD and other diseases characterized by cerebral hypoxia.
Keywords: Hypoxia, brain microvascular endothelial cells, endothelin-1, vascular endothelial growth factor, heme oxygenase-1, Alzheimer’s, cerebral hypoperfusion
Introduction
The human brain, although only 2% of total body weight, accounts for 20% of oxygen consumption, reflecting its high rate of metabolic activity (Pimenta de Castro et al., 2010). The high energy demands of the brain render it especially susceptible to the deleterious effects of hypoxia. Hypoxia is increasingly recognized as an important contributing factor to the development of neurodegenerative diseases in the brain (Kaur and Ling, 2008; Peers et al., 2007; 2009; Quaegebeur and Carmeliet, 2010). Emerging evidence suggests that hypoxia is an important risk factor for the development of dementias, since patients suffering from cerebral ischemia or stroke are much more susceptible to dementias, particularly Alzheimer’s disease (AD) (Desmond et al., 2002; Kalaria, 2000; Kokmen et al., 1996; Moroney et al., 1997). Furthermore, cerebral hypoperfusion is one of the major clinical features of AD and pathological changes caused by chronic hypoxia in the CNS are similar to those observed in AD (de la Torre, 2000; Lee et al., 2011; Miklossy, 2003). Despite data which suggest a strong link between cerebral hypoxia and AD, the mechanisms whereby hypoxia contributes to neurodegenerative disease processes are unknown.
In the periphery, hypoxia is a powerful regulator of angiogenesis. The angiogenic process is complex and involves a sequence of discrete steps beginning with endothelial activation and culminating in the formation of new blood vessels. Genes involved in the different stages of angiogenesis have been shown to be responsive to hypoxia in tissue culture (Pugh and Ratcliffe, 2003). Hypoxia regulates angiogenesis by modulating a large number and variety of pro- and anti-angiogenic factors (Enholm et al., 1997; Liu et al., 1995; Oh et al., 1999). Regulation of genes that encode proteins involved in angiogenesis occurs via activation of hypoxia-inducible factor (HIF). HIF, a heterodimeric complex consisting of an oxygen-destructable α subunit and an oxygen-indestructable β subunit, is a sequence-specific DNA-binding protein that affects transcription of a broad range of genes (Brahimi-Horn and Pouysségur, 2009). The oxygen sensitive α subunit ensures a quick response to minute changes in oxygen concentration by regulating proteasomal degradation of HIF-1 (Semenza, 1999). One of the most versatile angiogenic factors stimulated by hypoxia is vascular endothelial growth factor (VEGF) (Forsythe et al., 1996; Liu et al., 1995). VEGF is induced and regulated in a strictly dose-dependent manner by HIF-1 (Ferrara et al., 2003). Other angiogenic factors such as thrombin, endothelin-1 (ET-1), and heme oxygenase-1 (HO-1) have also been reported as factors associated with hypoxia (Landau et al., 2000; Motterlini et al., 2000; Yamashita et al., 2001). In contrast, anti-angiogenic factors such as thrombospondins (TSPs) are inhibited by hypoxia (Laderoute et al., 2000), although this varies depending on cell type (Phelan et al., 1998).
Despite advancements in research on hypoxia-induced angiogenesis in the periphery, little is known about how brain endothelial cells respond to hypoxic challenge. The vascular endothelium is a remarkably heterogeneous organ. Endothelial cells have the ability to differentiate both in structure and function in response to the needs of diverse tissue environments (Craig et al., 1998; Molema, 2010). Endothelial cells from different vascular beds differ in their morphology, cellular behavior and responses to injury (Langenkamp and Molema, 2009). Brain endothelial cells are a highly differentiated, specialized blood-brain barrier endothelial phenotype possessing unique biochemical and structural features not found in other vascular beds (Abbott et al., 2006; Zlokovic, 2008). The cerebrovasculature is increasingly implicated as contributory to the development of neurodegenerative diseases such as AD (Bell and Zlokovic, 2009; Brown and Thore, 2011; Grammas, 2011; Nation et al., 2011). A growing literature shows biochemical and functional changes in the cerebrovasculature in AD including expression and/or release of proteins related to vascular endothelial activation and angiogenesis such as VEGF, ET-1, HIF-1α, and thrombin (Grammas et al., 2006; Thirumangalakudi et al., 2006; Luo and Grammas, 2010; Yin et al., 2010). However, the function of angiogenic proteins in the AD brain is unknown and their significance is controversial (Bell and Zlokovic, 2009; Grammas, 2011; Paris et al., 2010., Wu et al., 2005). Understanding the effects of hypoxia on brain endothelial cells is important to determining mechanisms of hypoxia-induced damage in the brain and its links to AD.
The objective of this study is to characterize the effect of hypoxic challenge on the angiogenic response of cultured brain-derived microvascular endothelial cells
Methods
Treatment of endothelial cell cultures
Rat brain endothelial cell cultures were isolated from rat brain microvessels, as previously described (Diglio et al., 1993). The purity of these cultures was confirmed using antibodies to the endothelial cell surface antigen factor VIII. Endothelial cells used in this study (passages 8 to 15) were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Sigma-Aldrich, St Louis, MO) supplemented with 10% fetal bovine serum (FBS), 1% antibiotic/antimycotic and 2 mM glutamine. Confluent endothelial cell cultures were washed 3 times with Hank’s balanced salt solution (HBSS, Gibco, Grand Island, NY) and then incubated with serum-free DMEM. Cells were exposed to hypoxia (1% O2) for different periods of time in a humidified incubator at 37 °C. Cells grown under normoxic conditions (21% O2) served as a positive control.
Measurement of cell survival by MTT assay
Cell viability was determined using the MTT reagent (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide, Promega, Madison, WI) as follows. Treatment medium was replaced with fresh treatment medium containing 20 μl/ml of the Cell Titer 96 Aqueous One Solution and incubated for 10 min at 37°C after which optical density was measured at 490 nm using a microplate reader. The quantity of soluble formazan product, as measured by the amount of absorbance, was directly proportional to the number of viable cells. The number of viable cells after treatment was determined by measuring optical density and viability expressed as percent of untreated controls.
Measurement of tube formation
Geltrex™ reduced growth factor basement membrane matrix (Invitrogen, Carlsbad, CA, #12760) was added to wells of a 24-well plate (150 μl/well) and incubated at 37 °C for 30 min. After Geltrex™ matrix was polymerized, brain microvascular endothelial cells were seeded onto the matrix at 105 cells/well, and maintained in DMEM supplemented with 10% FBS. Plates were exposed to hypoxia (1% O2) or normoxia (21% O2) at 37°C for 4 h (Kourembanas et al., 1994; Loboda et al., 2006). After staining with fluorescent dye Calcein (Invitrogen, #C3099) for 30 min, the tube-like structures were visualized and captured using Olympus IX71 microscope at 10X magnification. Tube length was analyzed and quantitated using image processing software (ImageJ) available from the National Institutes of Health.
Western blot analysis
Total protein from cell cultures was extracted using lysis buffer (0.1% SDS, 1% Triton X-100, and 0.5% phenylmethyl-sulfonyl fluoride), and protein concentration determined by the Bradford method using Bio-Rad protein reagents (Bio-Rad, Hercules, CA). Samples were resolved in 10% poly-acrylamide gel (25 – 30 μg/lane) using SDS-PAGE and transferred to PVDF membranes. The blots were blocked with 5% nonfat milk in Tris-buffered saline containing 0.1% Tween 20 (TBST) for 1 h. Membranes were then incubated with primary antibodies (HIF-1α: Abcam, Cambridge, MA, #1; VEGF: Abcam, #1316; TATA-binding protein (TBP): Abcam, #818; β-actin: Abcam, #6276; endothelial nitric oxide synthase (eNOS): Abcam, #66127; inducible nitric oxide synthase (iNOS); Santa Cruz Biologicals, Santa Cruz, CA, #651) diluted 1:500 in TBST for 2 h, washed 3 times with TBST, and incubated with peroxidase-conjugated secondary antibodies (HIF-1α, VEGF, TBP and β-actin: anti-mouse antibody, Bio-Rad, #170-6516; eNOS and iNOS: anti-rabbit antibody, Bio-Rad, #172-1019) in TBST (1:2000 dilution) for 1 h. Membranes were washed 3 times with TBST, developed with chemiluminescent reagents and visualized on film. The average intensities over the area of the bands were measured using Quantity One software (Bio-Rad).
RT-PCR analysis of mRNA expression
Total RNA was extracted using the Trizol method (Invitrogen), and 4 μg of RNA were reverse transcribed into cDNA using random primers (Roche, Indianapolis, IN) according to manufacturer’s instructions. cDNA strands (0.2 μg) were amplified by PCR using the gene specific primers listed in Table 1. The reaction was performed by GoTaq Green master mix (Promega, #M7123) for 30 cycles, each at 95°C for 40 s, 55°C for 40 s and 72°C for 1 min. The PCR products were visualized on a 1.2 % agarose gel using UV transillumination.
Table 1.
Primers
| Gene | Orientation | Sequence | Amplicon |
|---|---|---|---|
| HIF-1α | Left primer | TGCATCTCCACCTTCTACCC | 384 bp |
| Right primer | CTGCTCCATTCCATCCTGTT | ||
|
| |||
| VEGF | Left primer | GCCCATGAAGTGGTGAAGTT | 360 bp |
| Right primer | TTTCTTGCCCTTTCGTTTTT | ||
|
| |||
| ET-1 | Left primer | AGAAACAGCTGTCTTGGGAGCAGA | 466 bp |
| Right primer | TGCTGATGGCCTCCAACCTTCTTA | ||
|
| |||
| HO-1 | Left primer | TGCTCGCATGAACACTCTG | 123 bp |
| Right primer | TCCTCTGTCAGCAGTGCCT | ||
|
| |||
| TSP-1 | Left primer | TTCCTGTTGCATGTGTGTGGAAGC | 696 bp |
| Right primer | AAGGGTGAGAAGGACGTTGGTTGA | ||
|
| |||
| TSP-2 | Left primer | ATGAGTGTGCTGTGGTCGCAGATA | 412 bp |
| Right primer | TCCTGCCCAGAATTTGGCAGTTTG | ||
|
| |||
| β-actin | Left primer | TGTCACCAACTGGGACGATA | 165 bp |
| Right primer | GGGGTGTTGAAGGTCTCAAA | ||
Detection of VEGF or ET-1 by ELISA
Endothelial cell culture medium was collected and concentrations of VEGF or ET-1 in the medium were determined using ELISA kits from R&D Systems (Minneapolis, MN). According to manufacturer’s instructions, 100 μl of standard or sample were added to each well. After incubation at room temperature on a shaker for 2 h, wells were washed 4 times and then incubated with 200 μl conjugate/detection antibody for 2 h at room temperature on the shaker. For VEGF detection, the wells were coated with the capture antibody prior to adding the samples. For ET-1, plates pre-coated with the capture antibody were used. After streptavidin-HRP was conjugated to target molecules, it was detected by addition of 200 μl of O-phenylene diamine-H2O2 (Pierce, Rockford, IL) for 20 min. Optical density was measured at 450 nm using a microplate reader.
Assessment of HIF-1α expression by immunofluorecence
Endothelial cells were fixed in cold acetone for 5 min and washed 3 times with phosphate buffered saline. After 30 min incubation with 1% BSA in phosphate buffered saline tween to block nonspecific binding, cells were incubated (4°C) simultaneously with two primary antibodies (HIF-1α: Abcam #1, 1:200; VWF: Santa Cruz #14014, 1:50) overnight. Cells were washed then incubated with two secondary antibodies HIF-1α: Alexa Fluor 488 goat anti-mouse IgG (H+L), Invitrogen #A11001; for VWF: Alexa Fluor 594 goat anti-rabbit IgG (H+L), Invitrogen #A11012) diluted 1:400 with antibody diluent for 1 h. After stringent washing with TBST, cells were counter-stained with nuclear staining DAPI solution for 20 min. After washing and drying at room temperature, samples were observed with an Olympus IX71 microscope.
Statistical analysis
Data from each experiment are expressed as mean ± standard deviation (SD). Comparisons between control and treatment groups were conducted using a two-tailed Student’s t-test. For multiple comparisons among control and treatment groups, the one-way ANOVA followed by Bonferroni’s multiple comparison test for multiple samples were used. Statistical significance was determined at p<0.05.
Results
Time-dependent effects of hypoxia on cell survival and induction of HIF-1α in cultured brain endothelial cells
Confluent brain microvascular endothelial cell cultures were subjected to hypoxia (1% O2) for various periods of time and cell viability measured by MTT assay. The results showed that hypoxic stress did not affect cell viability within the first 8 h. In contrast, exposure of cells to hypoxia for ≥ 8 h evoked significant (p<0.001) cell death (45%). After 8 h of hypoxia, reoxygenation of cell cultures for an additional 8 h significantly increased cell death an additional 40% (p<0.01) compared to 8 h hypoxia-only treated cultures.
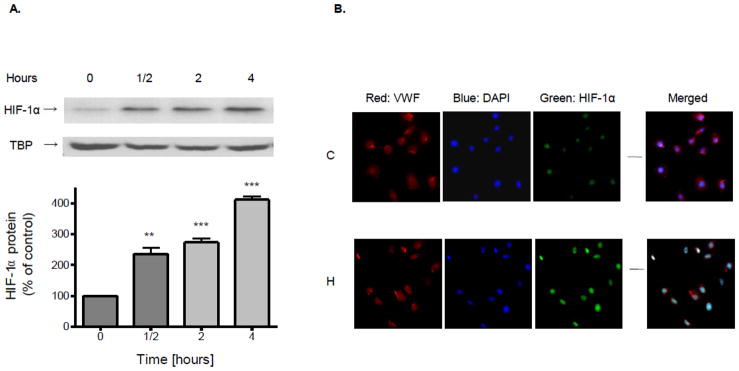
Exposure of cultured brain endothelial cells to hypoxia caused expression (p<0.01) of HIF-1α protein within 0.5 h (Fig. 1A). Expression of HIF-1α was highly significant (p<0.001) at 2 and 4 h of hypoxia. Induction of HIF-1α protein expression was confirmed by immunofluorescent comparison of cultures exposed to normoxic or hypoxic conditions (4 h) (Fig. 1B). Hypoxia increase HIF-1α mRNA expression (177%) but not until 8 h. Reoxygenation for 2 h significantly reduced the increase evoked by hypoxia on protein (p<0.01) (45%) and mRNA (p<0.05) (61%) levels, respectively.
Figure 1.
Confluent brain microvascular endothelial cell cultures were subjected to hypoxia (1% O2) for various periods of time. (A) Nuclear proteins were extracted and resolved using SDS-PAGE. HIF-1α or TBP protein was detected by Western blot probed with corresponding antibodies. Data in the bottom panel are the means ± SD of 3 experiments and expressed as percent of untreated control. **p<0.01, ***p< 0.001 vs. 0 h. (B) After exposure to normoxia (C) or 1% O2 hypoxia (H) for 4 h, brain microvascular endothelial cell cultures were processed for immunofluorescence. Cultures were fixed, incubated with antibodies to VWF and HIF-1α and counter stained with DAPI.
Exposure of endothelial cells to hypoxia induces VEGF expression and secretion
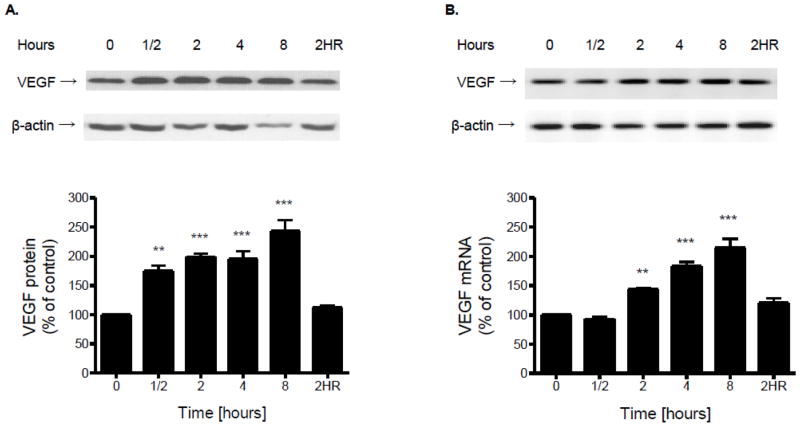
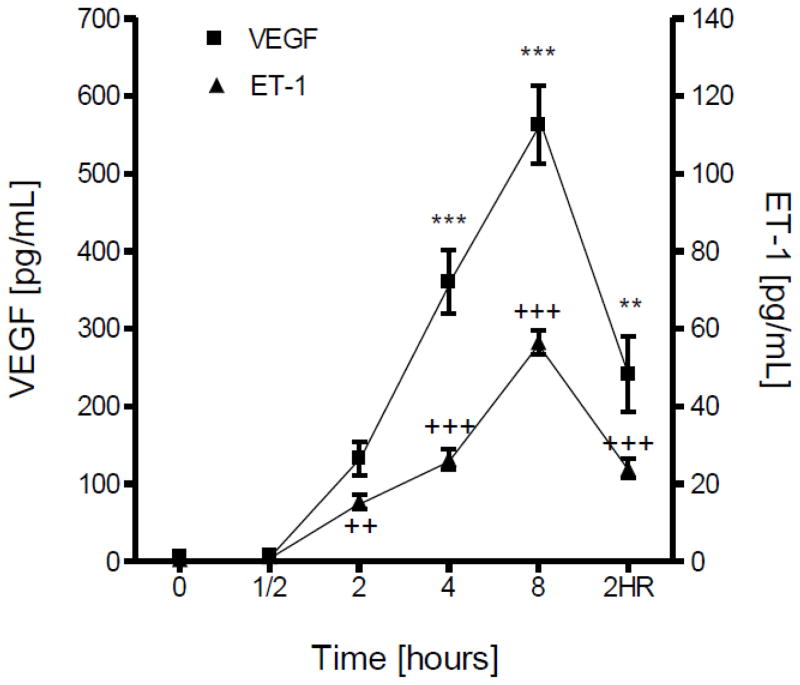
The effect of hypoxia on the expression and release of VEGF from cultured brain microvascular endothelial cells was determined by Western blot, RT-PCR and ELISA. Both cell-associated protein (Fig. 2A) and mRNA (Fig. 2B) levels of VEGF were significantly increased by hypoxia treatment in a time-dependent manner. Two hours of reoxygenation restored VEGF expression to control levels at both protein and mRNA levels (Fig. 2). Similarly, exposure of cultured microvascular brain endothelial cells to hypoxia resulted in elevated release of VEGF into culture medium compared to normoxic controls at the same time points (Fig. 3).
Figure 2.
Confluent brain microvascular endothelial cell cultures were subjected to hypoxia (1% O2) for various periods of time. (A) Total protein was extracted and resolved using SDS-PAGE. VEGF or β-actin protein was detected by Western blot probed with corresponding antibodies. Protein or mRNA levels of VEGF were determined by normalizing the densities of their bands to those of β-actin. (B) Total RNA was extracted, reverse transcribed and amplified with gene specific primers for VEGF or β-actin. Data in the bottom panel are the means ± SD of 3 experiments and expressed as percent of untreated control. 2HR: 2 h hypoxia followed by 2 h reoxygenation. **p<0.01, ***p <0.001 vs. 0 h.
Figure 3.
Confluent brain microvascular endothelial cell cultures were subjected to hypoxia (1% O2) for various periods of time. ELISA was performed to determine VEGF or ET-1 concentration (pg/ml) in the culture medium. The amount of induced VEGF or ET-1 by hypoxia was compared to normoxia control cultures at the same time points. Results are means ± SD of 3 experiments performed in triplicate. 2HR: 2 h hypoxia followed by 2 h reoxygenation. **/++p<0.01, ***/+++p <0.001 vs. 0 h.
Hypoxia induces an increase in ET-1 and a decrease in eNOS
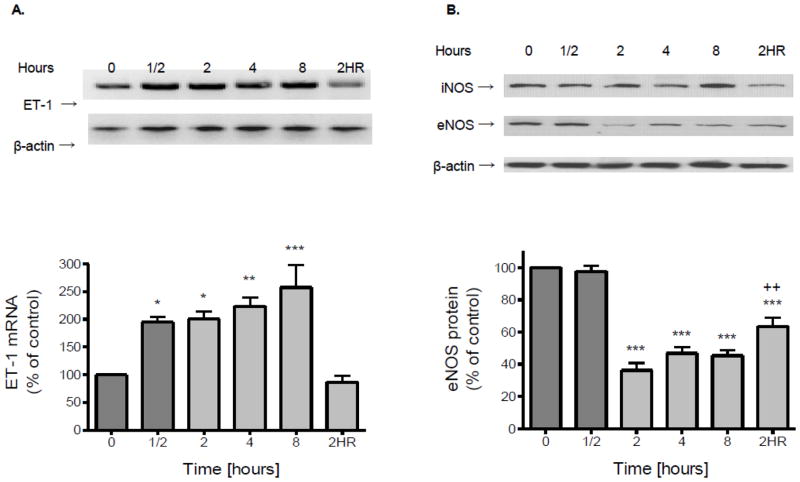
In vascular endothelial cells, the regulation of ET-1 and nitric oxide (NO) is often coordinated (Boulanger and Luscher, 1990). Hypoxic stress evoked a significant increase of ET-1 released into culture medium compared to normoxic controls examined at the same time points (Fig. 3). A two-fold increase in mRNA levels of ET-1 was observed with hypoxia treatment as early as 0.5 h (Fig. 4A). With 2 h of reoxygenation ET-1 mRNA levels recovered to that of normoxic controls (Fig. 4A). In contrast to ET-1, 2 h of hypoxia treatment resulted in a significant (p<0.001) decrease in expression of eNOS (Fig. 4B). Two hours of reoxygenation was able to partially blunt the reduction in eNOS protein levels but did not restore expression to control levels (Fig. 4B). Exposure of endothelial cells to hypoxia did not affect expression of iNOS (Fig. 4B).
Figure 4.
Confluent brain microvascular endothelial cell cultures were subjected to hypoxia (1% O2) for various periods of time. (A) Total RNA was extracted, reverse transcribed and amplified with gene specific primers for ET-1 or β-actin. (B) Total protein was extracted and resolved using SDS-PAGE. eNOS or β-actin protein was detected by Western blot probed with corresponding antibodies. Expression levels of ET-1 or eNOS were determined by normalizing the densities of their bands to those of β-actin. Data in the bottom panel are the means ± SD of 3 experiments and expressed as percent of untreated control. 2HR: 2 h hypoxia followed by 2 h reoxygenation. *p<0.5, **p<0.01, ***p<0.001 vs. 0 h; ++p<0.01 vs. 2 h.
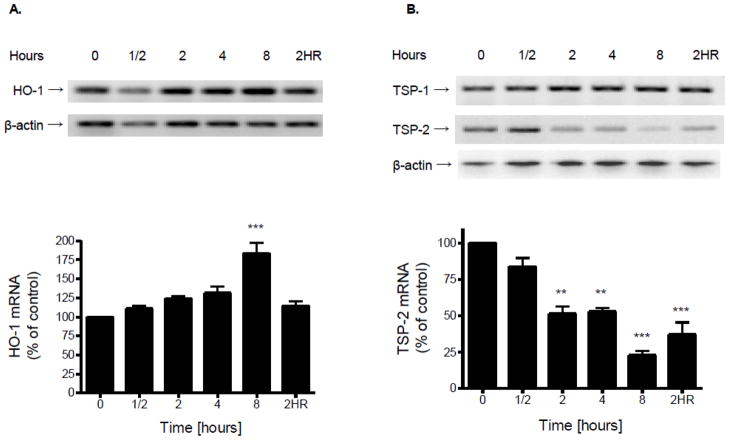
Hypoxia induces expression of HO-1 and reduces levels of TSP-2
Exposure of cultured endothelial cells to hypoxia did not affect expression of mRNA for HO-1 until 8 h (Fig. 5A). At 8 h of hypoxia there was a significant (p<0.001) increase in levels of HO-1. Reoxygenation (2 h) was able to restore expression of HO-1 to control levels (Fig. 5A).
Figure 5.
Confluent brain microvascular endothelial cell cultures were subjected to hypoxia (1% O2) for various periods of time. Total RNA was extracted, reverse transcribed and amplified with gene specific primers for HO-1 (A); TSP-1 or TSP-2 (B). mRNA levels of HO-1, TSP-1, or TSP-2 were determined by normalizing their band densities to those of β-actin. Data in the bottom panel are the means ± SD of 3 experiments and expressed as percent of untreated control. 2HR: 2 h hypoxia followed by 2 h reoxygenation. **p<0.01, ***p<0.001 vs. 0 h.
A significant (p<0.01) decrease in TSP-2 mRNA expression was detectable at 2 h of hypoxia and by 8 h the level of TSP-2 was less than 25% (p<0.001) of that demonstrable in normoxic controls (Fig. 5B). Reoxygenation (2 h) was unable to restore expression of TSP-2 (Fig. 5B). Exposure of endothelial cells to hypoxia did not affect expression of TSP-1 (Fig. 5B).
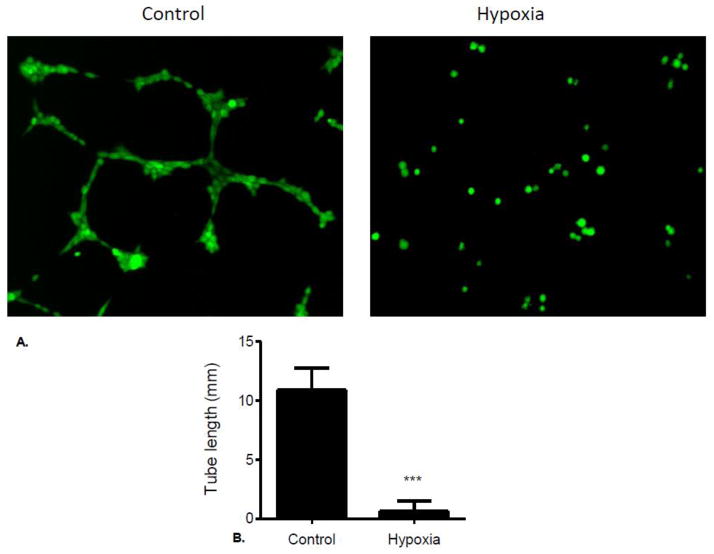
Hypoxia inhibits vessel formation in cultured brain endothelial cells
Experiments performed to determine the effect of hypoxia on brain endothelial cell vessel formation in culture showed that endothelial cells seeded onto extracellular matrix form vessels after 4 (Fig. 6A). In contrast, exposure of cultures to hypoxia significantly inhibited vessel formation (Fig. 6A). Quantification of stained cultures showed a 94% reduction in tube length in hypoxia-exposed endothelial cell cultures compared to control cultures (Fig. 6B).
Figure 6.
(A) Brain microvascular endothelial cells were seeded onto the layer of matrix at 105 cells/well, and maintained in DMEM supplemented with 10% FBS. Plates were incubated at 21% O2 (Control) or 1% O2 (Hypoxia) at 37°C for 4 h and then stained with fluorescent dye Calcein for 30 min. Tube-like structures were visualized and captured using Olympus IX71 microscope at 10x magnification. (B) Tube length was analyzed and quantitated using image processing software (ImageJ) available from the National Institutes of Health.
Discussion
Hypoxic challenge to the brain is a characteristic feature of both acute brain injury and chronic neurodegenerative diseases (Hacket, 1999; Peers et al., 2009). The chain-of-events, initiated by hypoxia, which culminate in neuronal dysfunction and/or death remains unclear. However, a key role for the cerebral endothelium, which is responsible for tightly regulating the CNS milieu and is a primary sensor of blood oxygen levels, is likely (Madri, 2009). HIF-1α, a master regulator of the cellular and physiological response to hypoxia (Semenza, 1999), is elevated in brain blood vessels in AD (Grammas et al., 2006). HIF-1α levels are controlled by multiple transcriptional, post-transcriptional and post-translational mechanisms (Semenza, 1999). In this study we observe a time-dependent increase in accumulation of HIF-1α protein inside the nucleus of brain microvascular endothelial cells challenged with hypoxia; consistent with the stabilization of HIF-1α under hypoxic conditions (Brahimi-Horn and Pouyssegur, 2009). Brain microvascular endothelial cells produce more than a 2-fold increase in HIF-1α protein levels compared to controls within a half hour of hypoxia exposure, suggesting that the initial HIF-1α response to hypoxia is due to post-translational regulation of its expression. In contrast, there is no change in HIF-1α mRNA until 8 h, indicating that transcriptional regulation is delayed in brain microvascular endothelial cells. In the periphery, hypoxia activates HIF-1α which in turn initiates a series of gene expression changes in vascular endothelial cells that are consistent with angiogenesis involving VEGF, ET-1, eNOS, and HO-1 TSPs (Kourembanas et al.,1991; Liu et al., 1995; Strijdom et al., 2009; Sun et al., 2002; Tenan et al., 2000). Therefore, in the current study we examined the effect of hypoxic challenge on expression of these factors by cultured brain endothelial cells as discussed below.
VEGF, a multifunctional cytokine, induces endothelial cell migration and proliferation (Neufeld et al., 1999). Stimulation of VEGF gene expression by hypoxia is thought to be mediated by the specific binding of HIF-1α to hypoxic response elements in the regulatory region of the VEGF gene (Liu et al., 1995). In brain microvascular endothelial cells we demonstrate that HIF-1α stimulates both expression and secretion of VEGF. In the current study we show a significant increase in VEGF protein precedes an increase in mRNA which is consistent with studies in other cell types that document post-transcriptional regulation of VEGF levels (Shenberger et al., 2007). VEGF has been to shown to regulate HO-1 expression and activity in vascular endothelial cells. The cytoprotective protein HO-1 is also pro-angiogenic (Suzuki et al., 2003). In one study, after 6 h of hypoxia HO-1 mRNA expression increases about two-fold while HO-1 mRNA levels are not significantly affected. Here we show an increase in HO-1 mRNA levels, but only after 8 h of hypoxia. Hypoxia-induced HO-1 expression in bovine aortic endothelial cells has also been documented (Motterlini et al., 2000; Sun et al., 2002).
ET-1, a vasoconstrictor produced in vascular endothelial cells, is also recognized as an angiogenic factor (Knowles et al., 2005). In human umbilical vein endothelial cells, hypoxia induces ET-1 gene expression and secretion (Kourembanas et al., 1991) which is consistent with the results obtained herein from brain-derived microvascular endothelial cells. ET-1 stimulation by hypoxia is mediated by HIF-1α (Hu et al., 1998) and antagonized by NO (Blanchard et al., 1992). It is well documented that NO and ET-1 regulate each other in the vascular endothelium and thus modulate vascular tone as well as response to injury (Bourque et al., 2011). In the current study, although there is no change in expression level of iNOS we document a dramatic loss in eNOS protein levels in brain microvascular endothelial cells after exposure to hypoxia for 2 h. Similarly, Strijdom et al. (2006) reported a significant decrease in eNOS levels in rat hypoxic cardiomyocytes exposed to hypoxia. Our data showing an increase in ET-1 and decrease in eNOS are consistent with literature that shows differential regulation of these two mediators in vascular endothelial cells. The mechanism whereby hypoxia reduces eNOS is uncertain; however, it has been reported that hypoxia decreases eNOS at the message level by inducing changes in transcription kinetics and stability of eNOS mRNA (McQuillan et al., 1994). Our data indicate that hypoxia can also reduce eNOS at the protein level. These data are consistent with what has been published in human coronary artery and microvascular endothelial cells (Loboda et al., 2006; Olszewska-Pazdrak et al., 2009;). Finally, we examined expression of TSP-1 and TSP-2, important physiological inhibitors of angiogenesis (Vailhé and Feige, 2003), and find that in brain microvascular endothelial cells hypoxia decreases TSP-2 gene expression but does not affect expression of TSP-1. Taken together, these data demonstrate a pro-angiogenic shift in brain endothelial cells exposed to hypoxia in vitro. The increased expression of pro-angiogenic factors and the decrease in angiogenic inhibitors favor the formation of new blood vessels. However, our data indicate that despite this pro-angiogenic phenotypic shift in brain endothelial cells, vessel formation is inhibited by hypoxia. In our study, culture of brain-derived endothelial cells on a cellular matrix that favors angiogenesis results in tube formation by 4 h. In contrast, in cultures exposed to hypoxia formation of tubes is inhibited. Quantitation of tube formation length shows significantly (p<0.001) less tube formation in hypoxia-exposed cultures compared to normoxic control cultures. These data are in contrast to considerable literature that documents an increase in angiogenesis in response to hypoxia (Yamakawa et al., 2003; Tang et al., 2004). However, an angiogenic response to hypoxia is not a universal finding, as some studies demonstrate that hypoxia inhibits vessel/tube formation (Olszewska-Pazdrak et al., 2009). In this regard, Isner (2002) finds that in patients with myocardial ischemia, angiogenic responses to hypoxia are defective or absent. In a study where human coronary endothelial cells are exposed to 1% O2, similar to the conditions of our study, there is a decrease in both basal and VEGF-mediated tube formation as well as in active eNOS (Olszewska-Pasdrak et al., 2009).
The data obtained in the current study may explain, in part, some paradoxical findings regarding hypoxia in the AD brain. Cerebral hypoperfusion is one of the major clinical features in AD and likely contributes to the clinical and pathological manifestations in this disease (de la Torre, 2000; Kalaria, 2000; Miklossy, 2003). Genome-wide expression profiling in the AD brain has identified a marked upregulation of genes that promote angiogenesis (Pogue and Lukiw, 2004). We have shown AD brain microvessels express or release inflammatory proteins, including thrombin, VEGF, angiopoietin-2, tumor necrosis factor-α, transforming growth factor-β, interleukin (IL) IL-1β, IL-6, IL-8, monocyte chemoattractant protein-1, matrix metalloproteinases, and integrins (Grammas and Ovase, 2001; 2002; Grammas et al, 2006, Thirumangalakudi et al, 2006; Yin et al., 2010), all of which have been implicated in angiogenesis. Despite increases in several pro-angiogenic factors in the AD brain, evidence for increased vascularity in AD is lacking. On the contrary, it has been suggested the angiogenic process is delayed and/or impaired in aged tissues, with several studies showing decreased microvascular density in the AD brain (Buee et al., 1994; 1997; Edelber and Reed, 2003; Paris et al., 2010,). Therefore, lack of vessel formation despite the increase in pro-angiogenic factors evoked by hypoxia suggests these angiogenic factors are not sufficient for the completion of the angiogenic process and the development of new vessels by brain endothelial cells. Further work is needed to determine what factors/conditions prevent hypoxia-induced angiogenic changes from culminating in the formation of new brain blood vessels.
Our results showing that brain-derived endothelial cells respond differently to hypoxia than most endothelial cells derived from peripheral vessels may reflect the functional heterogeneity of endothelial cells (Aird, 2003). Brain endothelial cells are highly differentiated and specialized in their blood-brain barrier function (Zlokovic. 2008; Grammas et al., 2011). Although the effect of astroglial-derived paracrine factors on maintenance of blood-brain barrier properties has been extensively documented (Abbott et al., 2006), other data also indicate there is an intrinsic endothelial identity, depending on vascular bed origin, that is stable and heritable (Aird, 2003; Chi et al., 2003). Indeed, there is evidence certain site-specific properties of endothelial cells are epigenetically programmed such that their maintenance is no longer dependent on signals from the extracellular milieu. For example, DNA microarray studies of multiple passaged endothelial cells cultured from different sites reveal differences in transcriptional profiles (Chi et al., 2003). The brain vasculature appears to be especially sensitive to hypoxia and oxidative stress. This sensitivity may in part be due to higher levels of NAD(P)H-oxidase in brain endothelial cells compared to endothelial cells in peripheral vessels (Closhen et al., 2010). In this regard, the inflammatory protein CRP evokes NAD(P)H-oxidase dependent functional derangements in brain- but not aorta-derived endothelial cells (Closhen et al., 2010). In addition, the higher concentration of mitochondria in cerebrovascular brain endothelial cells relative to other endothelia (Oldendorf et al., 1977) may render them more susceptible to the effects of hypoxia.
The angiogenic factors produced by brain endothelial cells in response to hypoxia have pleiotropic effects in the brain and likely important consequences for brain function. For example, in the brain HO-1 is a mediator with both beneficial and deleterious properties. The mechanisms responsible for excessive iron deposition and mitochondrial insufficiency in the aging and degenerating nervous system remain poorly understood; HO-1 has been implicated in this process (Schipper et al., 2009; Schipper, 2011). In rat astroglia transfected with the human HO-1 gene, mitochondrial iron trapping is abrogated by HO-1 inhibitors. Furthermore, HO-1 immunoreactivity is enhanced greatly in neurons and astrocytes of the hippocampus and cerebral cortex of AD brains as well as in the substantia nigra in Parkinson’s disease (Schipper, 2000). These results suggest HO-1 over-expression contributes to the pathological iron deposition and mitochondrial damage documented in these neurodegenerative disorders.
VEGF has direct neurotrophic effects and can protect neurons from exogenous injury reducing neuronal cell death in vitro evoked by hypoxia, glutamate or serum-deprivation (Tolosa et al., 2008; Tolosa et al., 2009; Wick et al., 2002). In contrast to these neuroprotective effects, VEGF overexpression is associated with many CNS disorders. In the AD brain, VEGF is deposited in the walls of intraparenchymal vessels as well as in clusters of reactive astrocytes (Kalaria et al., 1998; Thirumangalakudi et al., 2006). In addition, intrathecal levels of VEGF in AD are related to clinical severity and to intrathecal levels of Aβ (Tarkowski et al., 2002). Because VEGF has a potentiating effect on CNS inflammation and increases in vascular permeability, continuous upregulation of VEGF at sites of brain injury may drive chronic neuroinflammation. Similarly, ET-1 has been shown to be both neuroprotective as well neurotoxic (Luo and Grammas., 2010). Whether ET-1 is neurotoxic or neuroprotective may depend on the expression of other inflammatory mediators as well as the timing of exposure. In this latter regard, we have shown that the ET-1 significantly increases neuronal survival when cells are challenged with oxidative stress (H2O2) or thrombin, but that this neuroprotective effect requires pretreatment (Luo and Grammas., 2010). The angiogenic factors produced by brain endothelial cells in response to hypoxia have pleiotropic effects in the brain. A thorough understanding of the identity, regulation and function of these proteins could shed light on the basis for cell dysfunction and death in the brain in conditions characterized by cerebral hypoxia.
Conclusions
The results of the current study indicate that despite a shift toward a pro-angiogenic phenotype hypoxia inhibited vessel formation in brain endothelial cells. These results suggest that in brain endothelial cells expression of angiogenic factors is not sufficient for the development of new vessels. Further work is needed to determine what factors/conditions prevent hypoxia-induced angiogenic changes from culminating in the formation of new brain blood vessels and what role this may play in the pathologic changes observed in AD and other diseases characterized by cerebral hypoxia.
Highlights.
Effects of hypoxia on brain microvascular endothelial cells (ECs) were examined
Hypoxia resulted in the increased expression and release of VEGF protein and mRNA
Increased ET-1 was released into the culture medium under hypoxic conditions
Hypoxia resulted in a decrease in expression of eNOS as well
This study suggest hypoxia evokes a pro-angiogenic phenotype in ECs
Acknowledgments
Sources of support: This work was supported in part by grants from the National Institutes of Health (AG15964, AG020569 and AG028367). Dr. Grammas is the recipient of the Shirley and Mildred Garrison Chair in Aging. The authors gratefully acknowledge the secretarial assistance of Terri Stahl.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Aird WC. Endothelial cell heterogeneity. Crit Care Med. 2003;31:5221–5230. doi: 10.1097/01.CCM.0000057847.32590.C1. [DOI] [PubMed] [Google Scholar]
- Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009;118:103–113. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard KL, Acquaviva AM, Galson DL, Bunn HF. Hypoxic induction of the human erythropoietin gene: cooperation between the promoter and enhancer, each of which contains steroid receptor response elements. Mol Cell Biol. 1992;12:5373–5385. doi: 10.1128/mcb.12.12.5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger C, Luscher TF. Release of endothelin from the porcine aorta. Inhibition by endothelium-derived nitric oxide. J Clin Invest. 1990;85:587–590. doi: 10.1172/JCI114477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque SL, Davidge ST, Adams MA. The interaction between endothelin-1 and nitric oxide in the vasculature: new perspectives. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1288–R1295. doi: 10.1152/ajpregu.00397.2010. [DOI] [PubMed] [Google Scholar]
- Brahimi-Horn MC, Pouysségur J. HIF at a glance. J Cell Sci. 2009;122:1055–1057. doi: 10.1242/jcs.035022. [DOI] [PubMed] [Google Scholar]
- Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37:56–74. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buee L, Hof PR, Bouras C, Delacourte A, Perl DP, Morrison JH, Fillit HM. Pathological alterations of the cerebral microvasculature in Alzheimer’s disease and related dementing disorders. Acta Neuropathol. 1994;87:469–480. doi: 10.1007/BF00294173. [DOI] [PubMed] [Google Scholar]
- Buee L, Hof PR, Delacourte A. Brain microvascular changes in Alzheimer’s disease and other dementias. Ann NY Acad Sci. 1997;826:7–24. doi: 10.1111/j.1749-6632.1997.tb48457.x. [DOI] [PubMed] [Google Scholar]
- Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D, Brown PO. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci USA. 2003;100:10623–10628. doi: 10.1073/pnas.1434429100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closhen D, Bender B, Luhmann HJ, Kuhlmann CR. CRP-induced levels of oxidative stress are higher in brain than aortic endothelial cells. Cytokine. 2010;50:117–120. doi: 10.1016/j.cyto.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Craig LE, Spelman JP, Strandberg JD, Zink MC. Endothelial cells from diverse tissues exhibit differences in growth and morphology. Microvasc Res. 1998;55:65–76. doi: 10.1006/mvre.1997.2045. [DOI] [PubMed] [Google Scholar]
- de la Torre JC. Impaired cerebromicrovascular perfusion. Summary of evidence in support of its causality in Alzheimer’s disease. Ann NY Acad Sci. 2000;924:136–152. doi: 10.1111/j.1749-6632.2000.tb05572.x. [DOI] [PubMed] [Google Scholar]
- Desmond DW, Moroney JT, Sano M, Stern Y. Incidence of dementia after ischemic stroke: results of a longitudinal study. Stroke. 2002;33:2254–2260. doi: 10.1161/01.str.0000028235.91778.95. [DOI] [PubMed] [Google Scholar]
- Diglio CA, Liu W, Grammas P, Giacomelli F, Wiener J. Isolation and charaterization of cerebral resistance vessel endothelium in culture. Tissue Cell. 1993;6:833–846. doi: 10.1016/0040-8166(93)90032-g. [DOI] [PubMed] [Google Scholar]
- Edelber JM, Reed MJ. Aging and angiogenesis. Front Biosci. 2003;8:s1199–s1209. doi: 10.2741/1166. [DOI] [PubMed] [Google Scholar]
- Enholm B, Paavonen K, Ristimäki A, Kumar V, Gunji Y, Klefstrom J, Kivinen L, Laiho M, Olofsson B, Joukov V, Eriksson U, Alitalo K. Comparison of VEGF, VEGF-B, VEGF-C and Ang-1 mRNA regulation by serum growth factors, oncoproteins and hypoxia. Oncogene. 1997;14:2475–2483. doi: 10.1038/sj.onc.1201090. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The Biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammas P. Neurovascular dysfunction, inflammation and endothelial activation: implications for the pathogenesis of Alzheimer’s disease. J Neuroinflammation. 2011;8:26. doi: 10.1186/1742-2094-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammas P, Ovase R. Inflammatory factors are elevated in brain microvessels in Alzheimer’s disease. Neurobiol Aging. 2001;6:837–842. doi: 10.1016/s0197-4580(01)00276-7. [DOI] [PubMed] [Google Scholar]
- Grammas P, Ovase R. Cerebrovascular transforming growth factor-beta contributes to inflammation in the Alzheimer’s disease brain. Am J Pathol. 2002;5:1583–1587. doi: 10.1016/s0002-9440(10)61105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammas P, Samany PG, Thirumangalakudi L. Thrombin and inflammatory proteins are elevated in Alzheimer’s disease microvessels: implications for disease pathogenesis. J Alzheimers Dis. 2006;9:51–58. doi: 10.3233/jad-2006-9105. [DOI] [PubMed] [Google Scholar]
- Grammas P, Martinez J, Miller B. Cerebral microvascular endothelium and the pathogenesis of neurodegenerative diseases. Expert Rev Mol Med. 2011;10:e19. doi: 10.1017/S1462399411001918. [DOI] [PubMed] [Google Scholar]
- Hackett PH. High altitude cerebral edema and acute mountain sickness: a pathophysiology update. Adv Exp Med Biol. 1999;474:23–45. doi: 10.1007/978-1-4615-4711-2_2. [DOI] [PubMed] [Google Scholar]
- Hu J, Discher DJ, Bishopric NH, Webster KA. Hypoxia regulates expression of the endothelin-1 gene through a proximal hypoxia-inducible factor-1 binding site on the antisense strand. Biochem Biophys Res Commun. 1998;245:894–899. doi: 10.1006/bbrc.1998.8543. [DOI] [PubMed] [Google Scholar]
- Isner JM. Myocardial gene therapy. Nature. 2002;415:234–239. doi: 10.1038/415234a. [DOI] [PubMed] [Google Scholar]
- Kalaria RN. The role of cerebral ischemia in Alzheimer’s disease. Neurobiol Aging. 2000;21:321–330. doi: 10.1016/s0197-4580(00)00125-1. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Cohen DL, Premkumar DR, Nag S, LaManna JC, Lust WD. Vascular endothelial growth factor in Alzheimer’s disease and experimental ischemia. Brain Res Mol Brain Res. 1998;62:101–105. doi: 10.1016/s0169-328x(98)00190-9. [DOI] [PubMed] [Google Scholar]
- Kaur C, Ling EA. Blood brain barrier in hypoxic-ischemic conditions. Curr Neurovasc Res. 2008;5:71–81. doi: 10.2174/156720208783565645. [DOI] [PubMed] [Google Scholar]
- Knowles J, Loizidou M, Taylor I. Endothelin-1 and angiogenesis in cancer. Curr Vasc Pharmacol. 2005;3:309–314. doi: 10.2174/157016105774329462. [DOI] [PubMed] [Google Scholar]
- Kokmen E, Whisnant JP, O’Fallon WM, Chu CP, Beard CM. Dementia after ischemic stroke: a population-based study in Rochester, Minnesota (1960–1984) Neurology. 1996;46:154–159. doi: 10.1212/wnl.46.1.154. [DOI] [PubMed] [Google Scholar]
- Kourembanas S, Marsden PA, McQuillan LP, Faller DV. Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. J Clin Invest. 1991;88:1054–1057. doi: 10.1172/JCI115367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laderoute KR, Alarcon RM, Brody MD, Calaoagan JM, Chen EY, Knapp AM, Yun Z, Denko NC, Giaccia AJ. Opposing effects of hypoxia on expression of the angiogenic inhibitor thrombospondin 1 and the angiogenic inducer vascular endothelial growth factor. Clin Cancer Res. 2000;6:2941–2950. [PubMed] [Google Scholar]
- Landau E, Tirosh R, Pinson A, Banai S, Even-Ram S, Maoz M, Katzav S, Bar-Shavit R. Protection of thrombin receptor expression under hypoxia. J Biol Chem. 2000;275:2281–2287. doi: 10.1074/jbc.275.4.2281. [DOI] [PubMed] [Google Scholar]
- Langenkamp E, Molema G. Microvascular endothelial cell heterogeneity: general concepts and pharmacological consequences for anti-angiogenic therapy of cancer. Cell Tissue Res. 2009;335:205–222. doi: 10.1007/s00441-008-0642-4. [DOI] [PubMed] [Google Scholar]
- Lee JS, Im DS, An YS, Hong JM, Gwag BJ, Joo IS. Chronic cerebral hypoperfusion in a mouse model of Alzheimer’s disease: an additional contributing factor of cognitive impairment. Neurosci Lett. 2011;489:84–88. doi: 10.1016/j.neulet.2010.11.071. [DOI] [PubMed] [Google Scholar]
- Liu Y, Cox SR, Morita T, Kourembanas S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5′ enhancer. Circ Res. 1995;77:638–643. doi: 10.1161/01.res.77.3.638. [DOI] [PubMed] [Google Scholar]
- Loboda A, Jazwa A, Jozkowicz A, Dorosz J, Balla J, Molema G, Dulak J. Atorvastatin prevents hypoxia-induced inhibition of endothelial nitric oxide synthase expression but does not affect heme oxygenase-1 in human microvascular endothelial cells. Atherosclerosis. 2006;187:26–30. doi: 10.1016/j.atherosclerosis.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Grammas P. Endothelin-1 is elevated in Alzheimer’s disease brain microvessels and is neuroprotective. J Alzheimers Dis. 2010;21:887–896. doi: 10.3233/JAD-2010-091486. [DOI] [PubMed] [Google Scholar]
- Madri JA. Modeling the neurovascular niche: implications for recovery from CNS injury. J Physiol Pharmacol. 2009;60:95–104. [PubMed] [Google Scholar]
- McQuillan LP, Leung GK, Marsden PA, Kostyk SK, Kourembanas S. Hypoxia inhibits expression of eNOS via transcriptional and posttranscriptional mechanisms. Am J Physiol. 1994;267:H1921–1927. doi: 10.1152/ajpheart.1994.267.5.H1921. [DOI] [PubMed] [Google Scholar]
- Miklossy J. Cerebral hypoperfusion induces cortical watershed microinfarcts which may further aggravate cognitive decline in Alzheimer’s disease. Neurol Res. 2003;25:605–610. doi: 10.1179/016164103101202048. [DOI] [PubMed] [Google Scholar]
- Molema G. Heterogeneity in endothelial responsiveness to cytokines, molecular causes, and pharmacological consequences. Semin Thromb Hemost. 2010;36:246–264. doi: 10.1055/s-0030-1253448. [DOI] [PubMed] [Google Scholar]
- Moroney JT, Bagiella E, Desmond DW, Paik MC, Stern Y, Tatemichi TK. Cerebral hypoxia and ischemia in the pathogenesis of dementia after stroke. Ann N Y Acad Sci. 1997;826:433–436. doi: 10.1111/j.1749-6632.1997.tb48498.x. [DOI] [PubMed] [Google Scholar]
- Motterlini R, Foresti R, Bassi R, Calabrese V, Clark JE, Green CJ. Endothelial heme oxygenase-1 induction by hypoxia. Modulation by inducible nitric-oxide synthase and S-nitrosothiols. J Biol Chem. 2000;275:13613–13620. doi: 10.1074/jbc.275.18.13613. [DOI] [PubMed] [Google Scholar]
- Nation DA, Hong S, Jak AJ, Delano-Wood L, Mills PJ, Bondi MW, Dimsdale JE. Stress, exercise, and Alzheimer’s disease: A neurovascular pathway. Med Hypotheses. 2011;76:847–854. doi: 10.1016/j.mehy.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- Oh H, Takagi H, Suzuma K, Otani A, Matsumura M, Honda Y. Hypoxia and vascular endothelial growth factor selectively up-regulate angiopoietin-2 in bovine microvascular endothelial cells. J Biol Chem. 1999;274:15732–15739. doi: 10.1074/jbc.274.22.15732. [DOI] [PubMed] [Google Scholar]
- Oldendorf WH, Cornford ME, Brown WJ. The apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol. 1977;1:409–417. doi: 10.1002/ana.410010502. [DOI] [PubMed] [Google Scholar]
- Olszewska-Pazdrak B, Hein TW, Olszewska P, Carney DH. Chronic hypoxia attenuates VEGF signaling and angiogenic responses by downregulation of KDR in human endothelial cells. Am J Physiol Cell Physiol. 2009;296:C1162–C1170. doi: 10.1152/ajpcell.00533.2008. [DOI] [PubMed] [Google Scholar]
- Paris D, Ganey N, Banasiak M, Laporte V, Patel N, Mullan M, Murphy SF, Yee GT, Bachmeier C, Ganey C, Beaulieu-Abdelahad D, Mathura VS, Brem S, Mullan M. Impaired orthotopic glioma growth and vascularization in transgenic mouse models of Alzheimer’s disease. J Neurosci. 2010;30:11251–11258. doi: 10.1523/JNEUROSCI.2586-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers C, Pearson HA, Boyle JP. Hypoxia and Alzheimer’s disease. Essays Biochem. 2007;43:153–164. doi: 10.1042/BSE0430153. [DOI] [PubMed] [Google Scholar]
- Peers C, Dallas ML, Boycott HE, Scragg JL, Pearson HA, Boyle JP. Hypoxia and neurodegeneration. Ann NY Acad Sci. 2009;1177:169–177. doi: 10.1111/j.1749-6632.2009.05026.x. [DOI] [PubMed] [Google Scholar]
- Phelan MW, Forman LW, Perrine SP, Faller DV. Hypoxia increases thrombospondin-1 transcript and protein in cultured endothelial cells. J Lab Clin Med. 1998;132:519–529. doi: 10.1016/s0022-2143(98)90131-7. [DOI] [PubMed] [Google Scholar]
- Pimenta de Castro I, Martins LM, Tufi R. Mitochondrial quality control and neurological disease: an emerging connection. ERMM. 2010;12:e12. doi: 10.1017/S1462399410001456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue AI, Lukiw WJ. Angiogenic signaling in Alzheimer’s disease. Neuroreport. 2004;15:1507–1510. doi: 10.1097/01.wnr.0000130539.39937.1d. [DOI] [PubMed] [Google Scholar]
- Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- Quaegebeur A, Carmeliet P. Oxygen sensing: a common crossroad in cancer and neurodegeneration. Curr Top Microbiol Immunol. 2010;345:71–103. doi: 10.1007/82_2010_83. [DOI] [PubMed] [Google Scholar]
- Schipper HM. Heme oxygenase-1: role in brain aging and neurodegeneration. Exp Gerontol. 2000;35:821–830. doi: 10.1016/s0531-5565(00)00148-0. [DOI] [PubMed] [Google Scholar]
- Schipper HM. Heme oxygenase-1 in Alzheimer’s disease: a tribute to Moussa Youdim. J Neural Transm. 2011;118:381–387. doi: 10.1007/s00702-010-0436-1. [DOI] [PubMed] [Google Scholar]
- Schipper HM, Song W, Zukor H, Hascalovici JR, Zeligman D. Heme oxygenase-1 and neurodegeneration: expanding frontiers of engagement. J Neurochem. 2009;110:469–485. doi: 10.1111/j.1471-4159.2009.06160.x. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Regulation of mammalian O2 homeostatsis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- Shenberger JS, Zhang L, Powell RJ, Barchowsky A. Hyperoxia enhances VEGF release from A549 cells via post-transcriptional processes. Free Rad Biol Med. 2007;43:844–852. doi: 10.1016/j.freeradbiomed.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strijdom H, Jacobs S, Hattingh S, Page C, Lochner A. Nitric oxide production is higher in rat cardiac microvessel endothelial cells than ventricular cardiomyocytes in baseline and hypoxic conditions: a comparative study. FASEB J. 2006;20:314–316. doi: 10.1096/fj.05-4225fje. [DOI] [PubMed] [Google Scholar]
- Strijdom H, Chamane N, Lochner A. Nitric oxide in the cardiovascular system: a simple molecule with complex actions. Cardiovasc J Afr. 2009;20:303–310. [PMC free article] [PubMed] [Google Scholar]
- Sun J, Hoshino H, Takaku K, Nakajima O, Muto A, Suzuki H, Tashiro S, Takahashi S, Shibahara S, Alam J, Taketo MM, Yamamoto M, Igarashi K. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002;21:5216–5224. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Iso-o N, Takeshita S, Tsukamoto K, Mori I, Sato T, Ohno M, Nagai R, Ishizaka N. Facilitated angiogenesis induced by heme oxygenase-1 gene transfer in a rat model of hindlimb ischemia. Biochem Biophys Res Commun. 2003;302:138–143. doi: 10.1016/s0006-291x(03)00114-1. [DOI] [PubMed] [Google Scholar]
- Tang N, Wang L, Esko J, Giordano FJ, Huang Y, Gerber HP, Ferrara N, Johnson RS. Loss of HIF-1 in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell. 2004;6:485–495. doi: 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Tarkowski E, Issa R, Sjogren M, Wallin A, Blennow K, Tarkowski A, Kumar P. Increased intrathecal levels of the angiogenic factors VEGF and TGF-beta in Alzheimer’s disease and vascular dementia. Neurobiol Aging. 2002;23:237–243. doi: 10.1016/s0197-4580(01)00285-8. [DOI] [PubMed] [Google Scholar]
- Tenan M, Fulci G, Albertoni M, Diserens AC, Hamou MF, El Atifi-Borel M, Feige JJ, Pepper MS, Van Meir EG. Thrombospondin-1 is downregulated by anoxia and suppresses tumorigenicity of human glioblastoma cells. J Exp Med. 2000;191:1789–1798. doi: 10.1084/jem.191.10.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirumangalakudi L, Samany PG, Owoso A, Wiskar B, Grammas P. Angiogenic proteins are expressed by brain blood vessels in Alzheimer’s disease. J Alzheimers Dis. 2006;10:111–118. doi: 10.3233/jad-2006-10114. [DOI] [PubMed] [Google Scholar]
- Tolosa L, Mir M, Asensio VJ, Olmos G, Lladó J. Vascular endothelial growth factor protects spinal cord motoneurons against glutamate-induced excitotoxicity via phosphatidylinositol 3-kinase. J Neurochem. 2008;105:1080–1090. doi: 10.1111/j.1471-4159.2007.05206.x. [DOI] [PubMed] [Google Scholar]
- Tolosa L, Mir M, Olmos G, Lladó J. Vascular endothelial growth factor protects motoneurons from serum deprivation-induced cell death through phosphatidylinositol 3-kinase-mediated p38 mitogen-activated protein kinase inhibition. Neuroscience. 2009;158:1348–1355. doi: 10.1016/j.neuroscience.2008.10.060. [DOI] [PubMed] [Google Scholar]
- Vailhé B, Feige JJ. Thrombospondins as anti-angiogenic therapeutic agents. Curr Pharm Des. 2003;9:583–588. doi: 10.2174/1381612033391342. [DOI] [PubMed] [Google Scholar]
- Wick A, Wick W, Waltenberger J, Weller M, Dichgans J, Schulz JB. Neuroprotection by hypoxic preconditioning requires sequential activation of vascular endothelial growth factor receptor and Akt. J Neurosci. 2002;22:6401–6407. doi: 10.1523/JNEUROSCI.22-15-06401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Guo H, Chow N, Sallstrom J, Bell RD, Deane R, Brooks AI, Kanagala S, Rubiom A, Sagare A, Liu D, Li F, Armstrong D, Gasiewicz T, Zidovetzki R, Song X, Hofman F, Zlokovic BV. Role of MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat Med. 2005;11:959–965. doi: 10.1038/nm1287. [DOI] [PubMed] [Google Scholar]
- Yamakawa M, Liu LX, Date T, Belanger AJ, Vincent KA, Akita GY, Kuriyama T, Cheng SH, Gregory RJ, Jiang C. Hypoxia-inducible factor-1 mediates activation of cultured vascular endothelial cells by inducing multiple angiogenic factors. Circ Res. 2003;93:664–673. doi: 10.1161/01.RES.0000093984.48643.D7. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Discher DJ, Hu J, Bishopric NH, Webster KA. Molecular regulation of the endothelin-1 gene by hypoxia. Contributions of hypoxia-inducible factor-1, activator protein-1, GATA-2, and p300/CBP. J Biol Chem. 2001;276:12645–12653. doi: 10.1074/jbc.M011344200. [DOI] [PubMed] [Google Scholar]
- Yin X, Wright J, Wall T, Grammas P. Brain endothelial cells synthesize neurotoxic thrombin in Alzheimer’s disease. Am J Pathol. 2010;176:1600–1606. doi: 10.2353/ajpath.2010.090406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]