Abstract
The fragile X premutation is a tandem CGG trinucleotide repeat expansion in the Fragile X Mental Retardation 1 (FMR1) gene between 55 and 200 repeats in length. A CGG knock-in (CGG KI) mouse has been developed that models the neuropathology and cognitive deficits reported in fragile X premutation carriers. Previous studies have demonstrated that CGG KI mice have spatiotemporal information processing deficits and impaired visuomotor function that worsen with increasing CGG repeat length. Since skilled forelimb reaching requires integration of information from the visual and motor systems, skilled reaching performance could identify potential visuomotor dysfunction in CGG KI mice. To characterize motor deficits associated with the fragile X premutation, 6 month old female CGG KI mice heterozygous for trinucleotide repeats ranging from 70–200 CGG in length were tested for their ability to learn a skilled forelimb reaching task. The results demonstrate that female CGG KI mice show deficits for learning a skilled forelimb reaching task compared to wildtype littermates, and that these deficits worsen with increasing CGG repeat lengths.
Keywords: Fragile X Premutation, Transgenic Mice, Motor Function, Skilled Reaching, Fragile X-associated Disorders
1. Introduction
The Fragile X Mental Retardation 1 (FMR1) gene is polymorphic for the length of a CGG trinucleotide repeat in the 5’ untranslated region (UTR). In the general population there are fewer than 45 CGG repeats in the FMR1 gene, while in the full mutation underlying fragile X syndrome (FXS) there are greater than 200 CGG repeats and the FMR1 gene is transcriptionally silenced. In the fragile X premutation there are between 55–200 CGG repeats and increased transcription of FMR1 mRNA (Garcia-Arocena & Hagerman, 2010).
To investigate the pathological and behavioral consequences of the fragile X premutation, a transgenic CGG knock-in (KI) mouse was developed in which the 5’ UTR containing 8 CGG repeats in the endogenous murine Fmr1 gene was replaced, via homologous recombination, with a human Nhel-Xhol fragment containing 98 CGG repeats (Willemsen et al., 2003). Behavioral analyses of these mice have demonstrated spatiotemporal processing deficits (Hunsaker et al., 2009, 2010) and an early motor phenotype evaluated by a skilled ladder walking task that was interpreted as impaired visuomotor processing (Hunsaker et al., 2011).
To further characterize the nature of the motor performance deficits in CGG KI mice, female CGG KI mice heterozygous for the fragile X premutation were trained on a skilled reaching task based on work by Whishaw and colleagues (Farr & Whishaw, 2002), among others (Buitrago et al., 2004; Hermer-Vazquez et al., 2007; Kolb & Gibb, 2010; Tennant & Jones, 2009). Female mice heterozygous for the fragile X premutation were chosen for this study over male mice because the frequency of fragile X premutation is higher in females than males (1:113 females vs 1:250 in males; cf., Hagerman, 2008). Additionally, there have also been emerging reports of neurocognitive abnormalities in human females carrying the fragile X premutation and heterozygous female CGG KI mice (Goodrich-Hunsaker et al., 2011a,b,c; Hunsaker et al., 2010, 2011; Lachiewicz et al., 2006). The importance of studying female premutation carriers and CGG KI mice is that the identification of subtle phenotypes in these less affected populations may inform research into the underlying mechanisms subserving the more profound phenotypes observed in males. A skilled forelimb reaching task was chosen as it has been shown that reaching in peripersonal space (i.e., space within reach of a limb) depends upon integration of visuospatial and motor information across widespread neural circuitry involving the basal ganglia, motor and posterior parietal cortices (Kolb & Gibb, 2010; Redish & Touretzky, 1994; Beloozerova & Sirota, 2003; Beurze et al., 2010; Simon, 2008), superior colliculus, and cerebellum (MacKinnon et al., 1976). Each of these structures are affected to some degree in the carriers of the fragile X premutation and the CGG KI mouse (Adams et al., 2007; Hunsaker, et al., 2009, 2010, 2011; Keri & Benedek, 2009, 2010; Lachiewicz et al., 2006; van Dam et al., 2005; Wenzel et al., 2010; Willemsen et al., 2003). Furthermore, previous studies with rats using single pellet reaching tasks demonstrate a relationship between impaired motor control and onset/severity of neurological disease, particularly focusing on the deleterious effects of dopamine depletion on skilled reaching performance (cf., Vergara-Aragon et al., 2003).
In the present study, CGG KI mice were trained to extend their forelimb through an opening to grasp and retrieve a sucrose pellet. We demonstrated that heterozygous female CGG KI mice took between 1 and 2 days longer to reach asymptotic performance on the skilled forelimb reaching task and that the CGG KI mice failed to reach the same asymptotic level of performance as wildtype littermates (i.e., never reached with the same level of success). Furthermore, a CGG repeat length dosage effect was evident within the CGG KI mice: such that mice with longer CGG repeat lengths (136–200) had a lower percentage of successful reaches than CGG KI mice with more intermediate length CGG repeats (70–116), and took longer to acquire the task. Furthermore, Within the CGG KI mice there was a negative association between increasing CGG repeats and performance on the skilled reaching task.
2. Methods and Materials
2.1. Animals
Twelve female CGG KI mice heterozygous for the fragile X premutation at 6 months of age and 6 female wildtype mice of the same age were used as subjects for this task. All wildtype mice were littermates with CGG KI mice included in the study. All CGG KI mice were bred onto a congenic C57BL/6J background over greater than 12 generations from founder mice on a mixed FVB/N×C57BL/6J background (Willemsen et al., 2003). Mice were housed in same sex, mixed genotype groups with three or four mice per cage in a temperature and humidity controlled vivarium on A 12 h light-dark cycle. Mice had ad libitum access to water and were maintained at 90–95% their free feeding weight throughout experimentation. Mouse weights did not differ among genotypes during experimentation. All experiments were conducted during the light phase of the diurnal cycle and conformed to University of California, Davis IACUC approved protocols.
2.2. Genotyping
As somatic instability of CGG repeats among tissues in the CGG KI mouse has been shown to be negligible (under 10 CGG repeats across tissues; Berman & Willemsen, 2009; Willemsen et al., 2003), genotyping was carried out upon tail snips. DNA was extracted from mouse tails by incubating with 10 mg/mL Proteinase K (Roche Diagnostics; Mannheim, Germany) in 300 µL lysis buffer containing 50 mM Tris-HCl, pH 7.5, 10 mM EDTA, 150 mM NaCl, 1% SDS overnight at 55°C. One hundred µL saturated NaCl was then added and the suspension was centrifuged. One volume of 100% ethanol was added, gently mixed, and the DNA was pelleted by centrifugation and the supernatant discarded. The DNA was washed and centrifuged in 500 µL 70% ethanol. The DNA was then dissolved in 100 µL milliQ-H2O. CGG repeat lengths were determined by PCR using the Expanded High Fidelity Plus PCR System (Roche Diagnostics). Briefly, approximately 500–700 ng of DNA was added to 50 µL of PCR mixture containing 2.0 µM/L of each primer, 250 µM/L of each dNTP (Invitrogen; Tigart, OR), 2% dimethyl sulfoxide (Sigma-Aldrich; St. Louis, MO), 2.5 M Betaine (Sigma- Aldrich), 5 U Expand HF buffer with mg (7.5 µM/L). The forward primer was 5’- GCTCAGCTCCGTTTCGGTTTCACTTCCGGT-3’ and the reverse primer was 5’- AGCCCCGCACTTCCACCACCAGCTCCTCCA-3’. PCR steps were 10 min denaturation at 95°C, followed by 34 cycles of 1 min denaturation at 95°C, annealing for 1 min at 65°C, and elongation for 5 min at 75°C to end each cycle. PCR ends with a final elongation step of 10 min at 75 °C. DNA CGG repeat band sizes were determined by running DNA samples on a 2.5% agarose gel and staining DNA with ethidium bromide (Brouwer et al., 2008a; Hunsaker et al., 2011). For female CGG KI mice heterozygous for the fragile X premutation there were two bands present, one corresponding to the wildtype allele (CGG repeat length 8–12), and another corresponding to the premutation allele (CGG repeat length 70–200). For wildtype mice, only the wildtype allele was present. Genotyping was performed twice on each animal, once using tail snips taken at weaning and again on tail snips collected at sacrifice. In all cases the genotypes matched.
2.3. Skilled Forelimb Reaching Apparatus
The apparatus for the skilled forelimb reaching task was a transparent Plexiglas box 19.5 cm long, 8 cm wide, and 20 cm tall. A 1-cm wide vertical window ran up the front of the box centered along the front wall. A .2-cm thick plastic shelf (8.3 cm long and 3.8 cm wide) was mounted 1.1 cm from the floor on the front of the box. Twenty mg banana-flavored sucrose pellets (Bioserve Inc.; Frenchtown, NJ) could be placed in indentations spaced 1 cm away from the window and centered on its edges such that the mouse could only reach each indentation with one paw and could not reach the pellets with their tongue (cf., Farr & Whishaw, 2002).
3. Experimental Methods
3.1. Skilled Forelimb Reaching Task
3.1.1. Pretraining
Mice were food deprived to 90–95% free feeding weight and given access to 20 mg banana flavored sucrose pellets in their home cage to habituate to the food reward for 2 days. Throughout experimentation mice were provided sufficient food to maintain 95% free feeding weight 30 min after experimentation each day.
3.1.2. Training
On days 3–5, mice were placed in the apparatus with sucrose pellets on the floor and in the open window within reach of the mouse’s tongue for 30 min and allowed to consume sucrose pellets. On days 6–10, mice were placed in the apparatus with sucrose pellets available straight ahead immediately outside the open window for 15 min, allowing the mouse to use their tongue to obtain the reward pellet. When mice freely ate rewards, they moved on to task acquisition.
3.1.3. Acquisition
Prior to the first day of acquisition, mice were placed in the apparatus with the indentations on both sides outside the window containing sucrose pellets. The mice were allowed to reach and obtain as many rewards as possible for 15 min. The paw preference of each mouse was determined as the paw used during the majority of individual reaches. Starting the next day, all mice were trained against their paw preference.
Mice were placed in the apparatus for 15 min with one sucrose pellet placed on the side of the open window such that the mouse could only obtain it with the non-preferred paw. Each time the mouse reached, an experimenter blinded to mouse genotype recorded whether the reach was successful or whether or not errors occurred and immediately replaced the reward pellet when displaced. A successful reach was defined as the mouse obtaining and consuming the food pellet. If the mouse knocked the pellet away or dropped it prior to eating it an error was recorded. This acquisition was continued for 15 days.
3.2. Dependent Measures and Statistical Analysis
Because the fragile X premutation is present developmentally and has been shown to alter neurodevelopmental trajectories (Cunningham et al., 2011), data were collected during acquisition of the skilled reaching task rather than during postacquisition performance tests as per the more common approach in brain lesion studies (cf., Farr & Whishaw, 2002).
The number of times that the mouse successfully reached and obtained a sucrose pellet reward was collected as the dependent variable. If the mouse reached and missed/displaced the pellet during a reach or dropped the pellet before consuming it, an error was recorded and the pellet was immediately replaced. Qualitative observations concerning the behavior/strategy of each mouse was also recorded by the observer. For analysis, the percentage of reaches that were successful was calculated for each day: (% successful reaches = [number of successful reaches / total number of reaches] * 100).
To determine whether parametric analyses of variance (ANOVA) were appropriate for the data, tests of normality, homoscedasticity, and sphericity were performed. Once it was determined that parametric statistics were appropriate for the data, the data were plotted and placed into CGG repeat length groups as follows: the mice in the wildtype group all had between 8–12 CGG repeats (mean 10 +/− .2 SEM; n=6), mice included in the Low CGG repeat group ranged between 70–116 CGG repeats (mean 86 +/− 7; n=6), and the mice included in the High CGG repeat group ranged between 136–200 CGG repeats (mean 168 +/− 12; n=6). Similar groupings were also used in previous studies of male and female CGG KI mice (Hunsaker et al., 2010, 2011). A 3 (CGG repeat group) × 15 (Day) repeated measures analysis of covariance (ANCOVA) was used to determine differences among the groups for acquisition of the skilled reaching task with total number of times each mouse reached during each session as a covariate. Similar ANOVA were used to confirm that the total number of reaches did not differ among genotypes.
To specifically determine differences in the day the mice learned the task to asymptotic performance levels, the data for each animal was evaluated for the point at which the learning curved changed from being linear to curvilinear and confirmed using the change point algorithm reported by Gallistel et al. (2004; translated into R from the original MATLAB code). The first change point in the returned change point array corresponded to the first point at which the learning curve statistically significantly changed (discrimination threshold was set at logit=3: odds against = 1,000:1 or p<0.001) was chosen as the index of learning for each mouse. This change point for each animal was then used to compare the learning index across groups using a one way ANOVA.
Subsequent analyses were performed to further characterize all main effects, and Tukey-HSD post hoc pairwise comparisons tests were used to characterize all significant main effects and interactions among factors. To characterize any possible relationship of performance on the skilled forelimb reaching task as a function of CGG repeat length in CGG KI animals with expanded (70–200) CGG trinucleotide repeats, a Pearson’s correlation coefficient was calculated comparing asymptotic performance (performance averaged across days 12–15) and CGG repeat length. To control for the false discovery rate (FDR) given the number of analyses performed on the data, p values were FDR adjusted as outlined by Benjamini et al. (2001). All analyses were considered significant at p(adj)<.05. Statistical analyses were performed in R 2.13.1 language and environment (R Development Core Team, 2011).
4. Results
For all mice, data were grouped by CGG repeat length (wildtype, Low CGG, High CGG) and analyzed across days of training using repeated measures ANCOVA with percent successful reaches as the dependent variable, CGG repeat group as the grouping factor, and day of training as a repeated within-subjects factor with total number of attempted reaches during each session as a covariate. All p values have been FDR adjusted per Benjamini et al. (2001). There was a main effect of CGG repeat length group (F(2,211)= 54.75, p(adj)<.001), an effect for training day (F(14,211)=26.38, p(adj)<.001), and there was a significant interaction between group and day (F(28,211)=1.69, p(adj)=.02), suggesting that the longitudinal performance trajectory differed among CGG repeat groups. Total number of reaches per session did not significantly contribute to skilled forelimb reaching task acquisition (F(14, 211)=1.08, p(adj)=.30), and did not differ among CGG repeat groups (F(28,211)=.94, p (adj)=.56; wildtype mean 46 +/− 12 (SEM) reaches per session; Low CGG repeat group 53 +/− 19 reaches per session; High CGG repeat group 51 +/− 9 reaches per session).
To further characterize the significant interaction, a Tukey-HSD post hoc pairwise comparisons test demonstrated that no groups differed during days 1–6 of training (all p(adj)>.15), on day 7–15 the wildtype group showed a greater percentage of successful reaches than the CGG KI mouse groups (all p(adj)<.001). On day 8–15 the Low CGG group with 70–116 CGG repeats showed a greater percentage of successful reaches than the High CGG repeat group with 136–200 CGG repeats (all p(adj)<.01).
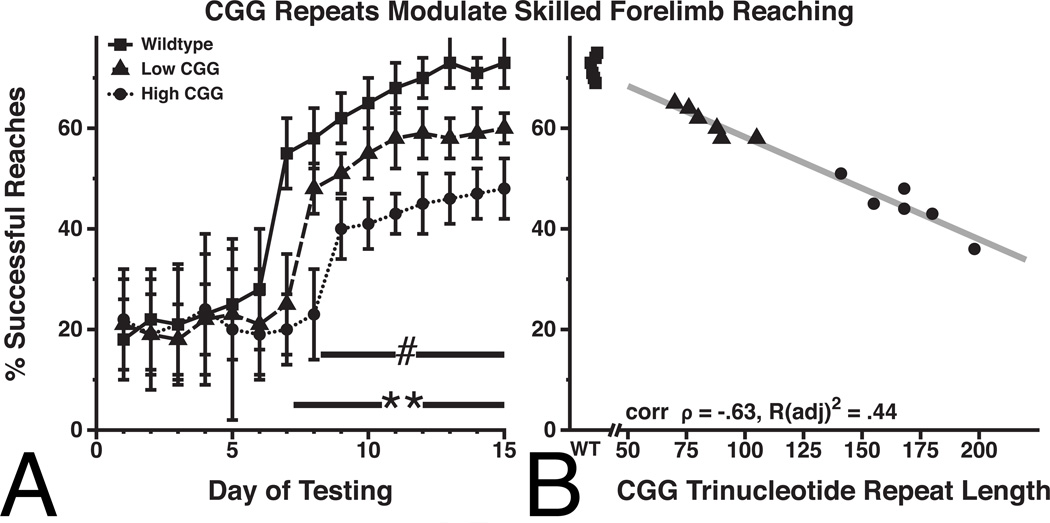
Based on the results of the paired comparisons, it appears that the three groups show differential time-courses for reaching asymptotic performance on the skilled forelimb reaching task (Figure 1A). To evaluate differential learning rates, the day of training at which the learning curve changed from linear to curvilinear was determined for each group, and used to define the day of acquisition during which significant improvement in performance had occurred. All groups showed a clear linear trend for days 1–6 of training (all p(adj)<.01). Beginning on day 7 the learning curve for the wildtype group became curvilinear (i.e., significant quadratic trend emerged; F(1,5)=12.04, p(adj)=.01), reflecting the marked increase in successful reaches beginning day 7. The Low CGG group did not show a curvilinear trend until day 8 (F(1,5)=8.25, p(adj)=.03), and the High CGG group did not show a curvilinear trend until day 9 (F(1,5)=7.13, p(adj)=.04). A one way ANOVA comparing the days when the trend became curvilinear across CGG groups revealed a main effect of CGG repeat grouping (F(2,16)=39.39, p(adj)<.001). A Tukey-HSD post hoc pairwise comparisons test revealed the wildtype group showed a curvilinear trend earlier than the high CGG repeat group (p(adj)<.001) and low CGG repeat group (p(adj)<.01). The low CGG repeat group showed a curvilinear trend earlier than the high CGG repeat group (p(adj)<.01).
Figure 1. CGG repeat length modulates skilled reaching task performance.
A. During the first 6 days of learning, all mice performed similarly. After day 7, CGG KI mice show deficits for skilled forelimb reaching compared to wildtype littermate controls (**). Beginning on day 8, the CGG KI mice with 136–200 CGG repeats (High CGG) are impaired relative to CGG KI mice with 70–116 CGG repeats (Low CGG) (#). ** p<.001, # p<.01.
B. A Pearson’s correlation coefficient was calculated within CGG KI mice performance during days 12–15 demonstrating an inverse linear association between increasing CGG repeat length and asymptotic performance levels of the skilled reaching task (wildtype mice were excluded from the analysis to focus on mice with expanded CGG repeat lengths; corr = −.63, p(adj)=.03; R(adj)2 =.44).
A confirmatory analysis of differences in the learning curve among CGG groups was performed using a change point algorithm described by Gallistel and colleagues (2004). The first change point in the data (corresponding to the first alteration to the learning curve at a p<0.001 threshold) returned by the algorithm was selected for each mouse and compared across groups: the wildtype mice showed a significant change in the slope of the learning curve on day 7 (group mean 6.9 +/− .25 SEM), the Low CGG repeat group showed a change in slope on day 8 (group mean 8.1 +/− .33), and the High CGG repeat group showed a change in slope on day 9 (group mean 9.25 +/− .35), confirming the analysis using the curvilinear trend as the measure of learning rate.
To characterize any possible relationship between CGG repeat length and performance on the skilled forelimb reaching task in CGG KI animals with expanded CGG trinucleotide repeats, a Pearson's correlation coefficient was calculated between CGG repeat length and averaged performance for days 12–15 for CGG KI mice (Figure 1B). A negative association was observed between the CGG trinucleotide repeat length and the asymptotic level of skilled reaching performance in the CGG KI mice (wildtype mice were excluded from the analysis to focus on expanded CGG repeat lengths unique to CGG KI mice; corr = −.63, p(adj)=.03; R(adj)2 =.44).
Qualitative observations collected during the reaching task suggest the CGG KI mice’s reaching patterns differed from the wildtype mice. The wildtype mice reached with a linear trajectory toward the reward pellet, grasped the pellet, and returned the pellet to the mouth for consumption. The CGG KI mice, however, generally reached with a less precise, nonlinear trajectory, specifically using a more sweeping or arcing motion to reach for the reward pellets. Additionally, on the attempts when the CGG KI mice reached with a linear trajectory, they appeared to close the hand either too soon or too late, generally displacing the sucrose pellet. Both of these differences resulted in skilled reaching errors. Once the reward was grasped by a CGG KI mouse; however, the CGG KI mice did not show any tendency to drop the reward prior to consumption.
5. Discussion
The current experimental results provide evidence for impaired reaching abilities in CGG KI mice that are modulated by CGG repeat length in female CGG KI mice modeling the fragile X premutation. These data model subclinical motor features present in female carriers of the fragile X premutation as young as 36 years of age that show no features of FXTAS (Narcisa et al., 2011). These female premutation carriers demonstrated impaired finger tapping used as a measure of manual coordination in both the dominant and non dominant hands, as well as slower reaction time with the non-dominant hand. Importantly, in the same study no gross motor disturbances were identified. As such, the skilled reaching deficits observed in CGG KI mice may serve as a valid behavioral biomarker for studies into progression of motor symptoms in CGG KI mice across age or to evaluate treatment options.
The present deficits for skilled reaching in CGG KI mice suggest CGG KI mice have an impairment along neural circuits involving the basal ganglia, motor and parietal neocortices, cerebellum, and superior colliculus that are critical for integrating visuospatial information with motor efferent copy to guide successful performance of a skillful reaching task (i.e., a basal ganglia-cortical-collicular-cerebellar circuit; cf., Redish & Touretzky, 1994). This hypothesis is supported by observations pertaining to the CGG KI mice’s reaching patterns. Wildtype mice reached with a consistent linear trajectory toward the reward pellet, grasped the pellet, and returned the pellet to the mouth for consumption. The CGG KI mice, however, reached with a less precise, nonlinear trajectory, specifically using a more sweeping or arcing motion to reach for the reward pellets. Additionally, at times the CGG KI mice reached with a linear trajectory, but appeared to close the hand either too soon or too late, resulting in displacing the sucrose pellet. Both of these differences resulted in skilled reaching errors. Once the reward was grasped, however, the CGG KI mice, similar to the wildtype mice, did not show any tendency to drop the reward prior to consumption, suggesting intact motor function sufficient to manipulate foodstuffs. These data are congruent with impaired visuomotor integration leading to an inability to generate, modify, or alter initial reaching trajectories to effectively obtain the reward pellets more reliably than ataxic or cerebellar symptoms--implicating not only functional impairments within the basal ganglia and cerebellum, but also impairments within the parietal lobe and superior colliculus.
Previous research has implicated the superior colliculus as a critical structure underlying much of this visuomotor integration via reciprocal connections with the intraparietal lobule in the parietal cortex, which has been implicated in visuospatial processing necessary to guide skilled reaching and walking behaviors (Beloozerova & Sirota, 2003; Hikosaka et al., 2002; Mutha et al., 2010), as well as reciprocal connectivity with the cerebellum. Furthermore, communication between the cortex and cerebellum (via cortico-cerebellar projections) is required for skilled motor behavior (Bays et al., 2010). Anatomically, the superior colliculus is located in an optimal location to bridge communication between incoming sensory input, visuospatial information, and motor output (Clower et al., 2001; Meredith & Stein, 1986). Specifically, the superior colliculus receives projections from the parietal cortex carrying visuospatial and somatosensory information as well as motor efferent copy via projections from the cerebellum (Goodale et al., 1978; Sprague & Maikle, 1965). It has further been proposed reciprocal connections within the intraparietal lobule that may be sufficient to subserve the reciprocal transfer of visuospatial and motor information necessary to guide skilled reaching. These inputs are integrated in the superior colliculus and feedback projections are sent back to the parietal lobes, frontal lobes (primarily the primary motor and premotor cortices), and cerebellum to guide fine on-line corrections to ongoing skilled motor movements (Bernier & Grafton, 2010).
It is also likely that an interaction between the basal ganglia and the rostral/parietal cortices contribute to the observed deficits. It has been demonstrated that disruptions to the dopaminergic system in the basal ganglia is sufficient to result in impaired skilled reaching (Barneoud et al., 2000; Faraji & Metz, 2007; Galvan et al., 2001; Jeyasingham et al., 2001; Melvin et al., 2005; Whishaw et al., 2007). This is important since both full mutation and premutation length CGG repeat expansions in the FMR1 gene disrupt dopaminergic signaling (Ceravolo et al., 2005; Hall et al., 2006; Scaglione et al., 2008), particularly post-synaptic signaling (Wang et al., 2008). These data suggest that the basal ganglia-cortical interactions provide motor and visuospatial information necessary for effective visually guided reaching (Hikosaka et al., 2002; Redish & Touretzky, 1997). In fact, basal ganglia and cerebellum projections terminate in adjacent portions of the parietal cortex to inputs from the superior colliculus projections (i.e., intraparietal lobule; Clower et al., 2001, 2005).
There are ample evidence for neuropathological features (e.g., intranuclear inclusion bodies in neuronal and astroglial nuclei) in CGG KI mice along the basal ganglia-cortical-collicular-cerebellar circuit described above (Hunsaker et al., 2009; Wenzel et al., 2010, Willemsen et al., 2003). Intranuclear inclusions are present in the cerebellum, specifically in the granule cell layers and Bergmann Glia, inclusions are present in neurons in the superior colliculus at ages as young as 6 months in male and female CGG KI mice, and there are inclusions present in neurons and astrocytes of the rostral and parietal neocortices beginning around 6 months of age in male and female CGG KI mice. Only rarely were inclusions found in the caudate, putamen, or globus pallidus in CGG KI mice. (Hunsaker et al., 2009; Wenzel et al., 2010; Willemsen et al., 2003; MR Hunsaker unpublished observations). Although intranuclear inclusion bodies are less prevalent in the brains of CGG KI mice at 6 months of age compared to 12–17 months of age, it is not correct to assume that the cell populations are unaffected by the premutation prior to the emergence of pathological features, as behavioral deficits have been identified in the CGG KI mousemodel as early as 2 months of age (Hunsaker et al., 2011). Despite these pathologic anatomical features being present, in depth functional analyses of the role for these areas in cognitive function have not been undertaken in CGG KI mice. The present experiment provides experimental rationale toward undertaking such studies using more functional assays more fully characterize the neurocognitive dysfunction reported in carriers of the fragile X premutation (cf., Goodrich-Hunsaker et al., 2011a,b,c; Lachiewicz et al., 2006).
Due to the nature of the present skilled reaching task as an acquisition, rather than performance, task the present experiment did not quantify the specific outcome measures used by Whishaw and colleagues (Farr et al., 2002) such as the sequences of arm and hand movements that may be able to better dissect out the relative contributions of purely motor and cognitive contributions to task performance in CGG KI mice. More specifically, such more sophisticated analyses would reveal whether the mice were demonstrating symptoms congruent with motor dysmetria or else symptoms more reliably associated with general clumsiness or ataxia. Such data would allow for more sophisticated analyses of function in the CGG KI mouse brain, and provide a greater analogy to the measures used to quantify motor symptoms in the human fragile X premutation, such as the CATSYS system (Narcisa, et al., 2011).
In summary, these data provide further evidence that the female heterozygous CGG KI mouse model of the fragile X premutation shows visuomotor processing deficits that appear to derive from neurocognitive impairments, rather than purely motor performance impairments. These data further suggest behavioral tasks emphasizing visuomotor integration may be required to observe mild, prodromal motor phenotypes in carriers of the fragile X premutation prior to the onset of any neurodegenerative processes. As such, the skilled forelimb reaching task can be used as a translational endpoint or biomarker for future therapeutic intervention studies.
Highlights.
CGG KI mice modeling the fragile X premutation show deficits for skilled forelimb reaching
CGG repeat length shows a negative association with skilled reaching performance.
CGG KI mice show visuospatial and visuomotor function impairments
Acknowledgements
The authors wish to thank Lara E. Cardy, Gian G. Greenberg, Lindsey C. Curley, and Ramona E. von Leden for assistance with preliminary versions of this experiment, and Binh T. Ta for assistance with mouse genotyping.
Funding: This work was supported by National Institute of Health (NIH) grants, NINDS RL1 NS062411 and TL1 DA024854. This work was also made possible by a Roadmap Initiative grant (UL1 DE019583) from the National Institute of Dental and Craniofacial Research (NIDCR) in support of the NeuroTherapeutics Research Institute (NTRI) consortium; and by a grant (UL1 RR024146) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JS, Adams PE, Nguyen D, Brunberg JA, Tassone F, Zhang W, Hagerman RJ. Volumetric brain changes in females with fragile X-associated tremor/ataxia syndrome (FXTAS) Neurology. 2007;69:851–859. doi: 10.1212/01.wnl.0000269781.10417.7b. [DOI] [PubMed] [Google Scholar]
- Barneoud P, Descombris E, Aubin N, Abrous DN. Evaluation of simple and complex sensorimotor behaviours in rats with a partial lesion of the dopaminergic nigrostriatal system. European Journal of Neuroscience. 2000;12:322–336. doi: 10.1046/j.1460-9568.2000.00896.x. [DOI] [PubMed] [Google Scholar]
- Bays PM, Singh-Curry V, Gorgoraptis N, Driver J, Husain M. Integration of goal- and stimulus-reated visual signals revealed by damage to human parietal cortex. Journal of Neuroscience. 2010;30:5968–5978. doi: 10.1523/JNEUROSCI.0997-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloozerova IN, Sirota MG. Integration of motor and visual information in the parietal area 5 during locomotion. Journal of Neurophysiology. 2003;90:961–971. doi: 10.1152/jn.01147.2002. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behavioural Brain Research. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- Berman RF, Willemsen R. Mouse Models of Fragile X-Associated Tremor Ataxia. Journal of Investigative Medicine. 2009;57:837–841. doi: 10.231/JIM.0b013e3181af59d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier P-M, Grafton ST. Human posterior parietal cortex flexibly determines reference frames for reaching based on sensory context. Neuron. 2010;68:776–788. doi: 10.1016/j.neuron.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Beurze SM, Toni I, Pisella L, Medendorp WP. Reference Frames for Reach Planning in Human Parietofrontal Cortex. Journal of Neurophysiology. 2010;104:1736–1745. doi: 10.1152/jn.01044.2009. [DOI] [PubMed] [Google Scholar]
- Brouwer JR, Huizer K, Severijnen LA, Hukema RK, Berman RF, Oostra BA, Willemsen R. CGG-repeat length and neuropathological and molecular correlates in a mouse model for fragile X-associated tremor/ataxia syndrome. Journal of Neurochemistry. 2008a;107:176–183. doi: 10.1111/j.1471-4159.2008.05747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer JR, Severijnen E, de Jong FH, Hessl D, Hagerman RJ, Oostra BA, Willemsen R. Altered hypothalamus-pituitary-adrenal gland axis regulation in the expanded CGG-repeat mouse model for fragile X-associated tremor/ataxia syndrome. Psychoneuroendocrinology. 2008b;33:863–873. doi: 10.1016/j.psyneuen.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer JR, Willemsen R, Oostra BA. The FMR1 gene and fragile X-associated tremor/ataxia syndrome. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 2009;150:782–798. doi: 10.1002/ajmg.b.30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitrago MM, Ringer T, Schulz JB, Dichgans J, Luft AR. Characterization of motor skill and instrumental learning time scales in a skilled reaching task in rat. Behavioural Brain Research. 2004;155:249–256. doi: 10.1016/j.bbr.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Ceravolo R, Antonini A, Volterrani D, Rossi C, Goldwurm S, Di Maria E, Murri L. Dopamine transporter imaging study in parkinsonism occurring in fragile X premutation carriers. Neurology. 2005;65:1971–1973. doi: 10.1212/01.wnl.0000188821.51055.52. [DOI] [PubMed] [Google Scholar]
- Clower DM, Dum RP, Strick PL. Basal Ganglia and cerebellar inputs to ‘AIP’. Cerebral Cortex. 2005;15:913–920. doi: 10.1093/cercor/bhh190. [DOI] [PubMed] [Google Scholar]
- Clower DM, West RA, Lynch JC, 7 Strick PL. The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. Journal of Neuroscience. 2001;21:6283–6291. doi: 10.1523/JNEUROSCI.21-16-06283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Martinez-Cerdeno V, Navarro-Porras E, Prakash AN, Angelastro JM, Willemsen R, Noctor SC. Premutation CGG-repeat expansion of the Fmr1 gene impairs mouse neocortical development. Human Molecular Genetics. 2011;20:64–79. doi: 10.1093/hmg/ddq432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Hulst C, Heulens I, Brouwer JR, Willemsen R, De Geest N, Reeve SP, Kooy RF. Expression of the GABAergic system in animal models for fragile X syndrome and fragile X associated tremor/ataxia syndrome (FXTAS) Brain Research. 2009;1253:176–83. doi: 10.1016/j.brainres.2008.11.075. [DOI] [PubMed] [Google Scholar]
- Faraji J, Metz GA. Sequential bilateral lesions have additive effects on single skilled limb use in rats. Behavioural Brain Research. 2007;177:195–204. doi: 10.1016/j.bbr.2006.11.034. [DOI] [PubMed] [Google Scholar]
- Farr TD, Whishaw IQ. Quantitative and qualitative impairments in skilled reaching in the mouse (Mus musculus) after a focal motor cortex stroke. Stroke. 2002;33:1869–1875. doi: 10.1161/01.str.0000020714.48349.4e. [DOI] [PubMed] [Google Scholar]
- Gallistel CR, Fairhurst S, Balsam P. The learning curve: implications of a quantitative analysis. Proceedings of the National Academy of Sciences, USA. 2004;101:13124–13131. doi: 10.1073/pnas.0404965101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Floran B, Erlij D, Aceves J. Intrapallidal dopamine restores motor deficits induced by 6-hydroxydopamine in the rat. Journal of Neural Transmission. 2001;108:153–166. doi: 10.1007/s007020170085. [DOI] [PubMed] [Google Scholar]
- Garcia-Arocena D, Hagerman P. Advances in understanding the molecular basis of FXTAS. Human Molecular Genetics. 2010;19:83–89. doi: 10.1093/hmg/ddq166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodale MA, Foreman NP, Milner AD. Visual orientation in the rat: a double dissociation of deficits following cortical and collicular lesions. Experimental Brain Research. 1978;31:445–457. doi: 10.1007/BF00237301. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Wong LM, McLennan Y, Srivastava S, Tassone F, Harvey D, Simon TJ. Young adult female fragile X permutation carriers show age- and genetically-modulated cognitive impairments. Brain and Cognition. 2011;75:255–260. doi: 10.1016/j.bandc.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Wong LM, McLennan Y, Srivastava S, Tassone F, Harvey D, Simon TJ. Enhanced Manual and Oral Motor Reaction Time in Young Adult Female Fragile X Premutation Carriers. Journal of the International Neuropsychiatric Society. 2011b;21:1–5. doi: 10.1017/S1355617711000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Wong LM, McLennan Y, Tassone F, Harvey D, Simon TJ. Adult female fragile X premutation carriers exhibit age- and CGG repeat length-related impairments on an attentionally based enumeration task. Frontiers in Human Neuroscience. 2011c;5:63. doi: 10.3389/fnhum.2011.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DA, Berry-Kravis E, Hagerman RJ, Hagerman PJ, Rice CD, Leehey MA. Symptomatic treatment in the fragile X-associated tremor/ataxia syndrome. Movement Disorders. 2006;21:1741–1744. doi: 10.1002/mds.21001. [DOI] [PubMed] [Google Scholar]
- Hagerman PJ. The fragile X prevalence paradox. Journal of Medical Genetics. 2008;45:498–499. doi: 10.1136/jmg.2008.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermer-Vazquez L, Hermer-Vazquez R, Chapin JK. The reach-to-grasp-food task for rats: a rare case of modularity in animal behavior? Behavioural Brain Research. 2007;177:322–328. doi: 10.1016/j.bbr.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, sakai K, Nakahara H. Central mechanisms of motor skill learning. Current Opinion in Neurobiology. 2002;12:217–222. doi: 10.1016/s0959-4388(02)00307-0. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Goodrich-Hunsaker NJ, Willemsen R, Berman RF. Temporal Ordering Deficits in Female CGG KI Mice Heterozygous for the Fragile X Premutation. Behavioural Brain Research. 2010;213:263–268. doi: 10.1016/j.bbr.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, von Leden RE, Ta BT, Goodrich-Hunsaker NJ, Arque G, Kim KM, Berman RF. Motor Deficits on a Ladder Rung Task in Male and Female Adolescent CGG Knock-in Mice. Behavioural Brain Research. 2011;222:117–121. doi: 10.1016/j.bbr.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Wenzel HJ, Willemsen R, Berman RF. Progressive spatial processing deficits in a mouse model of the fragile X premutation. Behavioral Neuroscience. 2009;123:1315–1324. doi: 10.1037/a0017616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keri S, Benedek G. Visual pathway deficit in female fragile X premutation carriers: a potential endophenotype. Brain and Cognition. 2009;69:291–295. doi: 10.1016/j.bandc.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Keri S, Benedek G. The perception of biological and mechanical motion in female fragile X premutation carriers. Brain and Cognition. 2010;72:197–201. doi: 10.1016/j.bandc.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gibb R. Tactile stimulation after frontal or parietal cortical injury in infant rats facilitates functional recovery and produces synaptic changes in adjacent cortex. Behavioural Brain Research. 2010;214:115–120. doi: 10.1016/j.bbr.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Jeyasingham RA, Baird AL, Meldrum A, Dunnett SB. Differential effects of unilateral striatal and nigrostriatal lesions on grip strength, skilled paw reaching and drug-induced rotation in the rat. Brain Research Bulletin. 2001;55:541–548. doi: 10.1016/s0361-9230(01)00557-3. [DOI] [PubMed] [Google Scholar]
- Lachiewicz AM, Dawson DV, Spiridigliozzi GA, McConkie-Rosell A. Arithmetic difficulties in females with the fragile X premutation. American Journal of Medical Genetics A. 2006;140:665–672. doi: 10.1002/ajmg.a.31082. [DOI] [PubMed] [Google Scholar]
- MacKinnon DA, Gross CG, Bender DB. A visual deficit after superior colliculus lesions in monkeys. Acta Neurobiologiae Experimentalis. 1976;36:169–180. [PubMed] [Google Scholar]
- Melvin KG, Doan J, Pellis SM, Brown L, Whishaw IQ, Suchowersky O. Pallidal deep brain stimulation and L-dopa do not improve qualitative aspects of skilled reaching in Parkinson's disease. Behavioural Brain Research. 2005;160:188–194. doi: 10.1016/j.bbr.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Visual, auditory, and somatosensory convergence on cells in superior colliculus results in multisensory integration. Journal of Neurophysiology. 1986;56:640–662. doi: 10.1152/jn.1986.56.3.640. [DOI] [PubMed] [Google Scholar]
- Mutha PK, Sainburg RL, Haaland KY. Coordination deficits in ideomotor apraxia during visually targeted reaching reflect impaired visuomotor transformations. Neuropsychologia. 2010;48:3855–3867. doi: 10.1016/j.neuropsychologia.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narcisa V, Aguilar D, Nguyen DV, Campos L, Brodovsky J, White S, Hagerman RJ. A Quantitative Assessment of Tremor and Ataxia in Female FMR1 Premutation Carriers Using CATSYS. Current Gerontology and Genomics Research. 2011 doi: 10.1155/2011/484713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. URL http://www.R-project.org/ [Google Scholar]
- Redish AD, Touretzky DS. The reaching task: evidence for vector arithmetic in the motor system? Biological Cybernetics. 1994;71:307–317. doi: 10.1007/BF00239618. [DOI] [PubMed] [Google Scholar]
- Scaglione C, Ginestroni A, Vella A, Dotti MT, Nave RD, Rizzo G, Mascalchi M. MRI and SPECT of midbrain and striatal degeneration in fragile X-associated tremor/ataxia syndrome. Journal of Neurology. 2008;255:144–146. doi: 10.1007/s00415-007-0711-8. [DOI] [PubMed] [Google Scholar]
- Simon TJ. A new account of the neurocognitive foundations of impairments in space, time and number processing in children with chromosome 22q11.2 deletion syndrome. Developmental Disabilities Research Reviews. 2008;14:52–58. doi: 10.1002/ddrr.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague JM, Meikle TH., Jr The role of the superior colliculus in visually guided behavior. Experimental Neurology. 1965;11:115–146. doi: 10.1016/0014-4886(65)90026-9. [DOI] [PubMed] [Google Scholar]
- Tennant KA, Jones TA. Sensorimotor behavioral effects of endothelin-1 induced small cortical infarcts in C57BL/6 mice. Journal of Neuroscience Methods. 2009;181:18–26. doi: 10.1016/j.jneumeth.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam D, Errijgers V, Kooy RF, Willemsen R, Mientjes E, Oostra BA, De Deyn PP. Cognitive decline, neuromotor and behavioural disturbances in a mouse model for fragile-X-associated tremor/ataxia syndrome (FXTAS) Behavioural Brain Research. 2005;162:233–239. doi: 10.1016/j.bbr.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Vergara-Aragon P, Gonzalez CL, Whishaw IQ. A novel skilled-reaching impairment in paw supination on the "good" side of the hemi-Parkinson rat improved with rehabilitation. Journal of Neuroscience. 2003;23:579–586. doi: 10.1523/JNEUROSCI.23-02-00579.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wu LJ, Kim SS, Lee FJ, Gong B, Toyoda H, Zhuo M. FMRP acts as a key messenger for dopamine modulation in the forebrain. Neuron. 2008;59:634–647. doi: 10.1016/j.neuron.2008.06.027. [DOI] [PubMed] [Google Scholar]
- Wenzel HJ, Hunsaker MR, Greco CM, Willemsen R, Berman RF. Ubiquitin-positive intranuclear inclusions in neuronal and glial cells in a mouse model of the fragile X premutation. Brain Research. 2010;1318:155–166. doi: 10.1016/j.brainres.2009.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, Zeeb F, Erickson C, 7 mcDonald RJ. Neurotoxic lesions of the caudate-putamen on a reaching for food task in the rat: acute sensorimotor neglect and chronic qualitative motor impairment follow lateral lesions and improved success follows medial lesions. Neuroscience. 2007;146:86–97. doi: 10.1016/j.neuroscience.2007.01.034. [DOI] [PubMed] [Google Scholar]
- Willemsen R, Hoogeveen-Westerveld M, Reis S, Holstege J, Severijnen LA, Nieuwenhuizen IM, Oostra BA. The FMR1 CGG repeat mouse displays ubiquitin-positive intranuclear neuronal inclusions; implications for the cerebellar tremor/ataxia syndrome. Human Molecular Genetics. 2003;12:949–959. doi: 10.1093/hmg/ddg114. [DOI] [PubMed] [Google Scholar]