Abstract
Objective
To examine the negative statistical relationship between educational level and risk of anxiety disorders, and to estimate to what extent this relationship may be explained by genes or environmental factors influencing both phenotypes.
Method
Registry data on educational level for 3339 young adult Norwegian twin pairs and diagnostic data on anxiety disorders for 1385 of these pairs were analyzed, specifying structural equations models using MX software.
Results
In the best-fitting model genes accounted for 59% of the variance in education. 18% of the variance was due to environmental factors shared by co-twins, and the remaining 23% to nonshared environment. 46% of the variance in liability to anxiety disorders was genetic, the remaining variance was due to nonshared environment. A phenotypic polychoric correlation of −0.30 between educational level and ‘any anxiety disorder’ was estimated to be primarily (83% in the best-fitting model) caused by genes common to the two traits.
Conclusion
The relationship between low education and risk of anxiety disorders appears to be primarily determined by genetic effect common to educational level and anxiety disorders.
Keywords: anxiety disorders, education, twins, population genetics
Introduction
As recognized for many decades, mental health varies with socioeconomic status (1). The literature shows strong statistical relationships between socioeconomic indicators and schizophrenia specifically (2,3) and mental disorders more generally (4). The social gradient for risk of mood disorders has also been a subject of considerable interest and investigation (5,6). Anxiety disorders have received somewhat less attention. Yet large-scale epidemiological studies indicate that most anxiety disorders are more strongly related to education and income than are mood disorders (7–9). The social gradient for mental health is found in all studied western countries (5). Paradoxically, the social gradient seems to be small or absent in developing countries, in which social and economic differences are usually quite large (10,11). Judging from these results a quite strong social gradient should be expected – and has been observed (12,13) - in a relatively egalitarian and prosperous society like that in Norway.
The discussion about the causal direction underlying this relationship has also persisted for generations (14,15). To what extent does poor mental health hamper education and career, and thus, cause downward social mobility (the social selection hypothesis)? In contrast, to what extent does stress associated with low social status and poverty increase the risk of poor mental health (social causality hypothesis)? An alternative approach to understanding the association between socio-economic status (SES) and mental health is to consider possible effects from risk factors common to mental health and SES. The same genes or environmental factors could influence both social status and mental health, in a direct manner or indirectly through personal characteristics, boosting educational attainment and career as well as protecting against poor mental health. Data from twin studies are informative on the relative contribution to the relationship between two or more phenotypes of genetic, family (shared) environmental, and individual (non-shared) environmental factors. While decomposing the correlation between mental health and SES into genetic and environmental components does not resolve the question of causal direction, such results can still contribute to the understanding of the SES-mental health relationship.
Aims of the study
We expected to observe a negative relationship between a strong indicator of socio-economic status, educational attainment, and anxiety disorders. Using data from a population-based twin sample, the primary aim was to estimate the relative contribution of genes and environmental factors to this relationship.
Material and methods
Sample
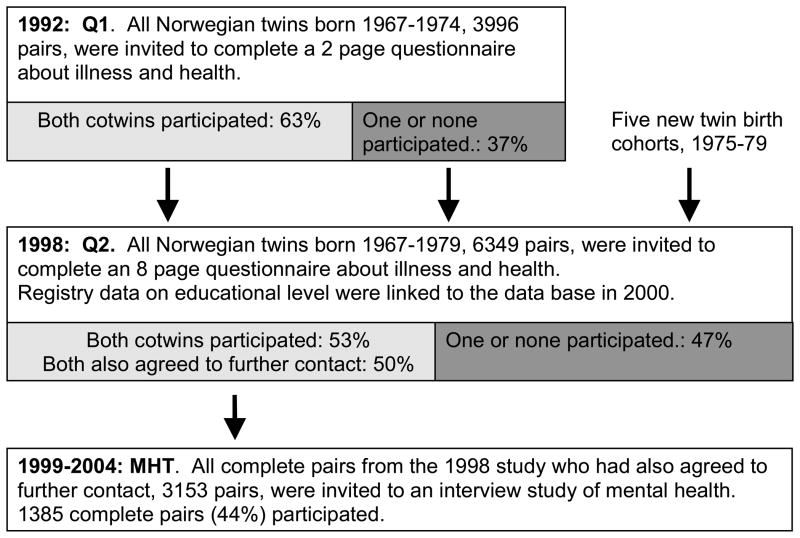
Twins were ascertained for this study from the Norwegian Institute of Public Health Twin Panel (NIPHTP), established in 1992. They were identified through the Medical Birth Registry, established January 1, 1967, which receives mandatory notification of all births in Norway. The first NIPHTP questionnaire study was conducted in 1992. A follow up study of all Norwegian twins born 1967–1979 was conducted in 1998. Altogether, 12,698 twins (6,349 pairs) received the second questionnaire (Q2), and 8,045 (63%) responded after one reminder. The Q2 sample used in the present study includes all the 3,334 complete pairs (53%). The NIPHTP is described in detail elsewhere (16, 17).
An interview study of DSM-IV axis I and axis II mental disorders – “Mental Health in Twins”, (MHT) - was started in 1999 as an extension of the Q2 study. All complete pairs in which both twins had agreed to further contact, 3,153 pairs, were invited. Altogether there were valid anxiety data for 1385 complete pairs and 21 single twins or 44% of the invited twins. Figure 1 shows the various phases of the NIPHTP/MHT study. Age when interviewed was 19–36 years, mean=28.1.
Figure 1.
The Norwegian Institute of Public Health Twin Panel, including the Mental Health in Twins Study
There were valid education data for all except three of the MHT participants. Education data were also available for all but a few of the remaining complete pairs in the Q2 sample, and for a few incomplete Q2-pairs who due to technical problems were accidentally invited to the MHT-study. Altogether there were data on education in 3339 pairs. There were also education data available for almost all Norwegian twins at the same age that did not participate in the study. There were no large differences in mean educational level between genders (difference in favor of women 0.05 SD, p=0.006) or zygosity (difference in favor of MZ twins 0.11 SD, p<0.001). The mean education was 0.17 SD higher for participants than for non-participants.
Table 1 shows the numbers of male and female MZ and DZ pairs with valid data on education and anxiety disorders, respectively. Zygosity was determined by molecular methods based on the genotyping of 24 microsatellite markers in all but 385 of the interviewed pairs, for whom zygosity was determined from questionnaire information. Estimated misclassification rate in the interview sample, based on comparison between questionnaire information and genetic analyses, was 0.7%. Estimated misclassification rate in the entire Q2 sample, in which zygosity classification was based on questionnaire data in a much larger proportion of the pairs, was 2.0%.
Table 1.
Number of pairs by zygosity, sex and valid education and anxiety data.
| N of pairs
|
||
|---|---|---|
| Education | Anxiety | |
| MZM | 529 | 219 |
| DZM | 395 | 117 |
| MZF | 796 | 446 |
| DZF | 638 | 264 |
| DZO | 981 | 339 |
Note:
MZM=monozygotic males
DZM=dizygotic males
MZF=monozygotic females
DZF=dizygotic females
DZO=dizygotic, opposite sex
Informed consent was obtained from all MHT participants after complete description of the study. An invitation letter informed about the purpose of the Q2 study, and return of the questionnaire was regarded as informed consent.
Measures
The education data were exported from Statistics Norway in 2003. Practically all the twins, born 1967–1979 had then finished their education. Educational level was scored as one of nine values: 0=no education, 1=lowest public school, seven years (lowest obligatory level before around 1966), 2=obligatory public school after around 1966, nine years, 3=public school (nine years) and one or two years vocational school, 4=high school (12 years), 5=public school and four or more years with general/vocational education, 6= one to four years at university or college, 7=five to seven years at university or college, 8= doctoral degree (18). All twins in our sample, born after 1966, had at least nine years public school. Category eight includes only 0.2% of the sample and was collapsed with category seven. Thus, the range of educational level was 2 – 7 in our sample. DSM-IV Axis I disorders were assessed using a computerized version of the Composite International Diagnostic Interview (CIDI) (19). The interviews took place between June 1999 and May 2004 and were conducted face-to-face except for 231 telephone interviews (8.3%). The majority of the 28 interviewers were psychology students in their final training or experienced psychiatric nurses. All received a standardized training program by teachers certified by the WHO and passed a user license test for the CIDI. They were supervised during the data collection period. Each twin in a pair was interviewed by a different interviewer blind to the results of the co-twin.
The anxiety disorders here examined were panic attack/disorder, generalized anxiety disorder, social phobia, agoraphobia, specific phobias (details by subtype below), obsessive-compulsive disorder, and post-traumatic stress disorder. DSM-IV diagnoses were assigned without hierarchical rules.
Among the content of the questionnaire from 1998 were five items from the SCL-25 (20), tapping symptoms of anxiety and depression and commonly used as an index of global mental health. The five item short-version has been shown to correlate at 0.92 with the original instrument (21). There was a time lag from one to five years between the completion of SCL-5 and the CIDI interview, and whereas the latter gives life-time diagnoses, SCL-5 refers to the last 14 days. Despite this difference, SCL-5 scores were used as a crude indicator of the severity of the various CIDI-based anxiety diagnoses.
Results from previous analyses (22) demonstrated that without allowing highly relaxed subthreshold scores, the individual anxiety disorders are not sufficiently prevalent to permit testing in multivariate genetic models. Therefore, we chose to use a phenotypically global “any anxiety disorder” variable based on full diagnostic data in bivariate genetic analyses with education. To justify this, we tested for phenotypic unidimensionality via confirmatory factor analysis (CFA). A single factor solution showed a good fit, CFI=0.96. All but two variables showed loadings from 0.47 to 0.89 (mean=0.67). The remaining two, animal phobia and “other specific” phobia, showed loadings under 0.4. A CFA performed excluding these variables showed an even better fit, CFI=1.00. Furthermore, mean SCL-5 case/non-case differences for theses types of phobias were lowest or close to lowest of all the anxiety diagnoses, namely 0.7 SD and 0.8 SD, as compared to 1.8 SD for PTSD and 1.7 fro GAD. The relatively low SCL-5 scores for people diagnosed with these phobias indicate that most of them do not suffer from a severe anxiety disorder. Results from another twin study (23) have also indicated that animal phobia is influenced by other genes than most of the remaining phobia sub-types and other anxiety disorders. On the basis of these results we chose to exclude the phobias “specific, animal type”, “specific, other type”, and a few cases categorized as “phobia not otherwise specified” from the variable “any anxiety disorder” used in the genetic analyses. The dichotomous “any anxiety” variable was scored ‘1’ if any of the remaining anxiety diagnoses were present, otherwise ‘0’.
Statistical analysis
We first assessed the phenotypic relationships between educational level and each separate anxiety disorder and “any anxiety disorder” via polychoric correlations.
Our twin modelling assumed that, although our education variable is ordinal rather than scored at an interval level, the underlying distribution of educational attainment is normally distributed in the population. Likewise, liability to anxiety is assumed to be continuous and normally distributed, with individuals who exceed a theoretical threshold expressing a disorder. Resemblance in twin pairs is assessed using a tetrachoric/polychoric correlation (24.). Using the multiple threshold model in the PRELIS program (25), we tested for deviations from an underlying bivariate normal distribution of the two variables.
As is standard in twin modelling (26), individual differences in education and liability to anxiety are assumed to arise from three sources: additive genetic ‘A’, from genes whose allelic effects combine additively, shared (or “common”) environment ‘C’, which includes all sources increasing similarity between the twins, and specific environment ‘E’, which includes environmental experiences not shared by co-twins and measurement error. Co-twin similarity in MZ pairs is due to identical genes, A, and, by definition, shared environment, C. DZ pairs share (on average) half of their A, and all of their C, similar to other full sibling pairs.
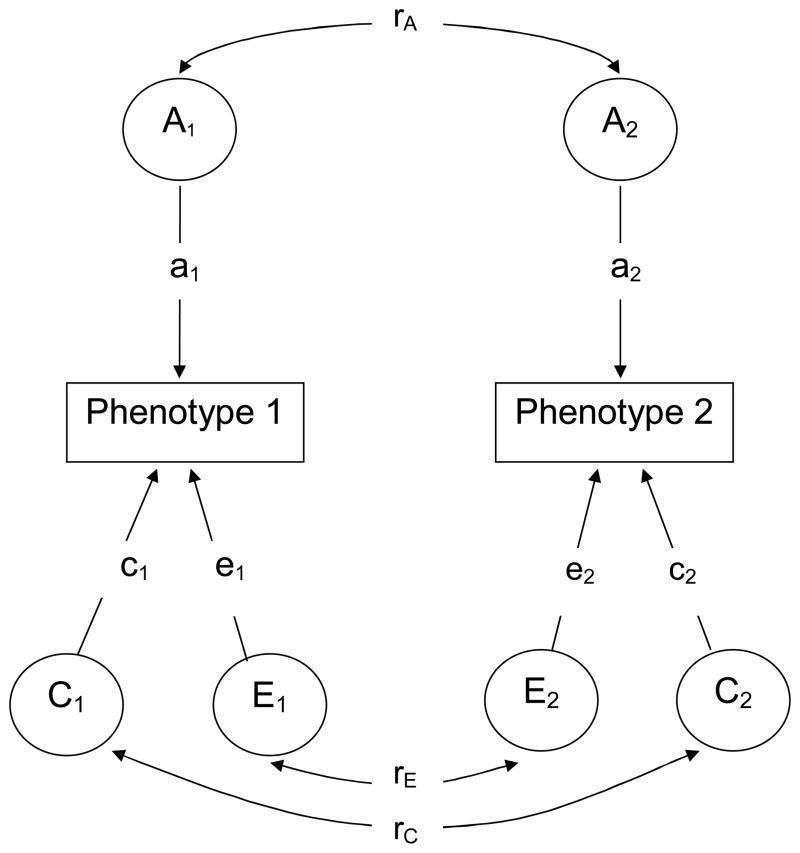
Univariate twin studies use data on co-twin similarity for a single trait or illness. Bivariate studies utilize observed similarity both between the same trait in co-twins, between one trait in one twin and the other trait in the co-twin, and between the two traits observed within persons. Besides yielding estimates of the effects of A, C and E (path coefficients a, c, and e, respectively) for each of the two specific phenotypes, bivariate analyses estimate the correlations between the latent factors for each of the phenotypes. Figure 2 shows a bivariate model specifying the correlations rA, rE, and rC between each of the traits’ causal factors, A, C, and E. (To avoid confusion, we will use the term “shared” for environment shared by – or common to- co-twins (C) and reserve the term “common” for common causality for the two phenotypes.) Full and nested ‘correlated factor’ models were tested. In the nested models some of the parameters a, c and e are fixed at zero or the correlations are fixed to unity. The best fitting of the models was chosen using Akaike’s Information Criterion (AIC) (27).
Figure 2.
Path diagram of bivariate genetic twin model
A=additive genetic effect, C=environmental effect shared by co-twins, E=environmental effect not shared by co-twins
Under qualitative sex specific effects, termed general sex-limitation, different genetic or environmental sources may be operating among males and females, and the genetic or environmental factors, A, C, or E, are permitted to correlate freelybetween males and females. Under quantitative sex specific effects (common sex-limitation), the genes and environmental factors are the same in males and females, but the strengths of the effects may vary (e.g. 26). In principle, one could test for qualitative and quantitative sex-specific effects as part of the bivariate modeling. However, in order to keep the bivariate model as simple as possible, we conducted preliminary univariate analyses to test for sex-specific effects. The analyses did not show evidence of generalized sex specific effects, implying that the specification of such effects would also not be required in our bivariate modeling.
The Mx computer program (28) was used for these analyses. Raw data analysis was performed and the thresholds (prevalences) were permitted to vary between men and women. The full Q2 dataset, for which there were data on education in the full sample and anxiety data only for the MHT participants, was entered in the analyses.
Results
Mean values and standard deviations for education were 5.01 (SD=1.37) for the participants and 4.51 (SD=1.39) for the total Norwegian twin population born 1967–1979. The distribution of educational level was: 9 years public school 3.5%, public school + 1–2 years vocational school 15.1%, high school 35.8%, public school +≥4 years general/vocational education 3.6%, 1–4 years university or college, 33.6%, ≥5 years university or college 8.4%. There were no mean sex differences in educational level (t=0.02, p=0.99), but slightly higher variance among women than among men (SD=1.38 and SD=1.36, respectively, p<0.001). Prevalences for the anxiety disorders are shown in Table 2. 20.4% of the sample had “any anxiety disorder”. The prevalence estimate adjusted for overrepresentation of women in the sample was 18.6%. Differences between cases and non-cases in mean global mental problem z-scores were 1.8 for PTSD, 1.7 for GAD, 1.4 for social phobia, 1.3 for agoraphobia and OCD, 1.2 for panic and 1.0 or lower for the remaining phobias. Mean age of onset ranged from 11.2 years for blood phobia to 19.8 years for GAD. Mean age of onset for any disorder was 12. 6 years (SD=6.6).
Table 2.
Polychoric correlations with 95% confidence intervals between educational level and various anxiety disorders.
| Prevalence
|
Polychoric correlation anxiety-education
|
OR (95%CI)B | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N cases | %A | Males (N=1018)
|
Females (n=1772)
|
Total (n=2790)
|
|||||
| r | SE | r | SE | r | SE | ||||
| Panic disorder/attackC | 154 | 5.0 | −0.31 | 0.08 | −0.23 | 0.05 | −0.25 | 0.04 | 1.44 (1.27, 1.64) |
| GAD | 55 | 1.7 | −0.23 | 0.12 | −0.15 | 0.06 | −0.17 | 0.05 | 1.28 (1.06, 1.56) |
| Agoraphobia | 139 | 4.2 | −0.43 | 0.07 | −0.39 | 0.05 | −0.39 | 0.04 | 1.85 (1.61, 2.13) |
| Social phobia | 112 | 3.6 | −0.20 | 0.09 | −0.30 | 0.05 | −0.28 | 0.05 | 1.55 (1,34, 1.79) |
| Animal phobia | 269 | 8.4 | 0.08 | 0.08 | −0.10 | 0.04 | −0.08 | 0.03 | 1.10 (1.00, 1.20) |
| Natural environm. phobia | 153 | 5.0 | −0.22 | 0.07 | −0.20 | 0.04 | −0.21 | 0.04 | 1.35 (1.20, 1.52) |
| Blood phobia | 107 | 3.5 | −0.27 | 0.08 | −0.26 | 0.05 | −0.26 | 0.04 | 1.51 (1.30, 1.75) |
| Situational phobia | 113 | 3.6 | −0.06 | 0.09 | −0.17 | 0.05 | −0.15 | 0.04 | 1.23 (1.08, 1.42) |
| Other specific phobiasD | 101 | 3.3 | −0.05 | 0.08 | −0.10 | 0.05 | −0.10 | 0.04 | 1.17 (1.01, 1.36) |
| OCD | 21 | 0.7 | −0.11 | 0.15 | −0.12 | 0.10 | −0.13 | 0.08 | 1.22 (0.89, 1.67) |
| PTSD | 63 | 2.1 | −0.21 | 0.10 | −0.38 | 0.06 | −0.33 | 0.05 | 1.76 (1.44, 2.14) |
| Any ADE | 569 | 18.6 | −0.27 | 0.05 | −0.32 | 0.03 | −0.30 | 0.03 | 1.45 (1.35, 1.55) |
Adjusted for over-representation of women
Odds ratio per educational unit score (range 2–7), adjusted by sex
87 panic disorders, 67 panic attacks
Includes a few cases of “other phobias, not specified”
Not including animal phobia, other specific phobias or “other phobias, not specified”
Table 2 also shows correlations between anxiety disorders and educational level. The prevalence increased monotonically with decreasing education for five of the disorders and for “any disorder”. For the other six tabulated disorders altogether nine of 36 cells in total (six anxiety variables x six education categories) departed from perfect monotonic relationships. The association with education was strongest for agoraphobia and PTSD and weakest for the types not included in the genetic analysis, animal phobia and other specific phobias. The correlation between education and any anxiety disorder (not including animal phobias or “other” specific phobias) was −0.30 (95% CI ±0.05) for the total sample, 0.27 (±0.10) for males and 0.32 (±0.06) for females. Age correlated lower than 0.02 with education and “any anxiety”, suggesting that this variable did not produce a spurious relationship between the other two.
Odds ratios displayed in the table reflect increased odds for each increment on the six-step ordinal education variable. Thus, assuming equal increase in anxiety for each increment in education, the estimated OR from the highest to the lowest educational level is calculated as the tabulated OR raised to the fifth power. Accordingly, agoraphobia is estimated to be 1.855=21.7 times more common in the least educated than in the most educated group, while the observed ratio, comparing the two extreme groups, was 31.3. The corresponding value for “any anxiety” was 1.455=6.4, while the observed ratio between the two extreme groups was 5.4.
The strength of the relationship between education and each of the anxiety diagnoses tends to parallel the severity of the disorders in terms of self-reported mental distress. The correspondence between mean mental distress for each disorder and the strength of correlation with education, as measured by the Spearman rank-order correlation, was 0.59 (p=0.057).
We tested the 30 observed bivariate statistical associations (within individuals, between twins, and across twins-across traits in five data groups) for deviations from underlying bivariate normality. The results showed 2 of 30 possible significant deviations (6.6%), close to the 5% chance expectation.
The twin correlations and “cross twin – cross trait” correlations (cross-correlations) for anxiety and education are shown in Table 3. The twin correlation pattern is quite regular for education, with highly similar values for the two MZ groups and for the three DZ groups, respectively. The pattern for anxiety is different, with same-sex DZ twin correlations almost as high as the MZ correlations and an opposite-sex DZ correlation close to zero. This pattern suggests modest genetic effects and strong effects of shared environment that differs by sex. In contrast, the cross correlations for the MZ pairs are on average approximately 1.5 to 1.6 those of the same-sex DZ pairs, and the cross-correlations for opposite sex twins do not depart very much from the other DZ cross-correlations, quite similar to the pattern seen for education.
Table 3.
Polychoric twin-cotwin, within-trait and cross-trait correlations (with 95% CIs) between “any anxiety disorder” and educational level
| Within-Trait
|
Cross-Trait
|
|||
|---|---|---|---|---|
| Education | Anxiety | Education T1- anxiety T2 | Education T2- anxiety T1 | |
| MZM | 0.78 (0.07) | 0.44 (0.29) | 0.18 (0.23) | 0.24 (0.22) |
| DZM | 0.47 (0.20) | 0.36 (0.49) | 0.21 (0.35) | 0.07 (0.59) |
| MZF | 0.75 (0.07) | 0.46 (0.14) | 0.26 (0.14) | 0.29 (0.13) |
| DZF | 0.50 (0.10) | 0.44 (0.18) | 0.19 (0.19) | 0.15 (0.17) |
| DZO | 0.49 (0.10) | 0.06 (0.23) | 0.14 (0.16) | 0.10 (0.17) |
Note:
MZM=monozygotic males, DZM=dizygotic males, MZF=monozygotic females, DZF=dizygotic females, DZO=dizygotic, opposite sex T1=(randomly assigned) twin 1, T2=twin2
Univariate analyses of anxiety
Whereas the twin correlations for education data do not suggest any sex specific effects, the correlations for anxiety are consistent with general sex-limitation effects, that is, whether there are genetic effects unique to men and/or women. We tested for such possible sex specific effects by conducting preliminary univariate analyses of the anxiety data. First we specified a full ACE model with general sex-limitation effects and a free correlation, rAMF, between genes affecting anxiety in males and females. A corresponding general sex-limitation model with sex specific effects of shared environment and free rCMF was also tested. The fit for the two models was almost identical, with chi-square value 0.13 higher for the former than for the latter. In accordance with conventions, and because previous results doesn’t show evidence of strong effects of shared environment on anxiety disorders (23), we chose the ACE model with rAMF as the more realistic basic model, under which nested models were tested. These included ACE models with common sex limitation effects (the same genes affects the phenotype in men and women, but to a different extent) and without sex limitation. For AE models we also specified general sex limitation, in which different genes affect the same phenotype in men and women.
The fit statistics for the univariate analyses of anxiety data are shown in Table 4. Although dropping c from an ACE model with common sex limitation (Model 5 vs. Model 2) resulted in a just-significant (p=0.04) change in model fit, there was no significant loss of fit when dropping general or common sex limitation effects for either ACE models (Models 1–3) or AE models (Models 4–6). Model 6, an AE model without sex limitation, showed the best fit in terms of AIC value. Model 2, a common sex limitation ACE model, showed the second best AIC value.
Table 4.
Model fit statistics for testing of univariate models for anxiety.
| Model | Compared with model # | Δχ2 | Δ df | p | Δ AIC |
|---|---|---|---|---|---|
| 1. General sex-limitation ACE | - | - | - | - | |
| 2. Common sex-limitation ACE | 1 | .01 | 1 | .92 | -1.99 |
| 3. No sex-limitation ACE | 2 | 6.56 | 3 | .09 | -1.44 |
| 4. General sex-limitation AE | 1 | 3.91 | 2 | .14 | -.09 |
| 5. Common sex-limitation AE | 4 | 2.47 | 1 | .12 | .38 |
| 2 | 6.37 | 2 | .04 | ||
| 6. No sex-limitation AE | 5 | .40 | 2 | .82 | -3.22 |
| 3 | .22 | 1 | .64 |
Note:
Δχ2, Δdf, and p show tests against the models under which they are directly nested.
ΔAIC, change in chi square value minus 2 df compared to model 1, is an overall measure of model fit. Low values indicate good fit. Models: A=additive genetic effect, C=environmental effect shared by co-twins, E=environmental effect not shared by co-twins
Bivariate analyses
In spite of a correlation structure suggestive of sex specific genetic or common environmental effects on anxiety, the univariate results do not show clear evidence of such effects. Rather than choosing a highly complex bivariate model with both general sex-limitation and free correlations between the causal factors for each of the phenotypes, we chose a basic bivariate ACE model with common sex-limitation only. The fit statistics from the testing of bivariate models are shown in Table 5. Effects of shared environment, c, for anxiety could be fixed at zero, whereas dropping c for education significantly worsened the fit.
Table 5.
Model fit statistics for testing of bivariate models for education and anxiety.
| Model | Δχ2 | Δ df | p | Δ AIC |
|---|---|---|---|---|
| 1. CSL ACE | - | - | - | - |
| 2. ACE | 6.53 | 4 | 0.16 | -1.47 |
| 3. ACEDUE | 0.87 | 2 | 0.65 | -4.60 |
| 4. AE | 20.12 | 1 | <0.001 | 13.52 |
Note:
Models: CSL= common sex-limitation, A=additive genetic effect, C=environmental effect shared by co-twins, E=environmental effect not shared by co-twins, CEDU= environmental effect for education only shared by co-twins.
Δχ2, Δdf, and p for each model show the model fit compared to the model above.
ΔAIC shows value compared with Model 1, low value indicates good fit.
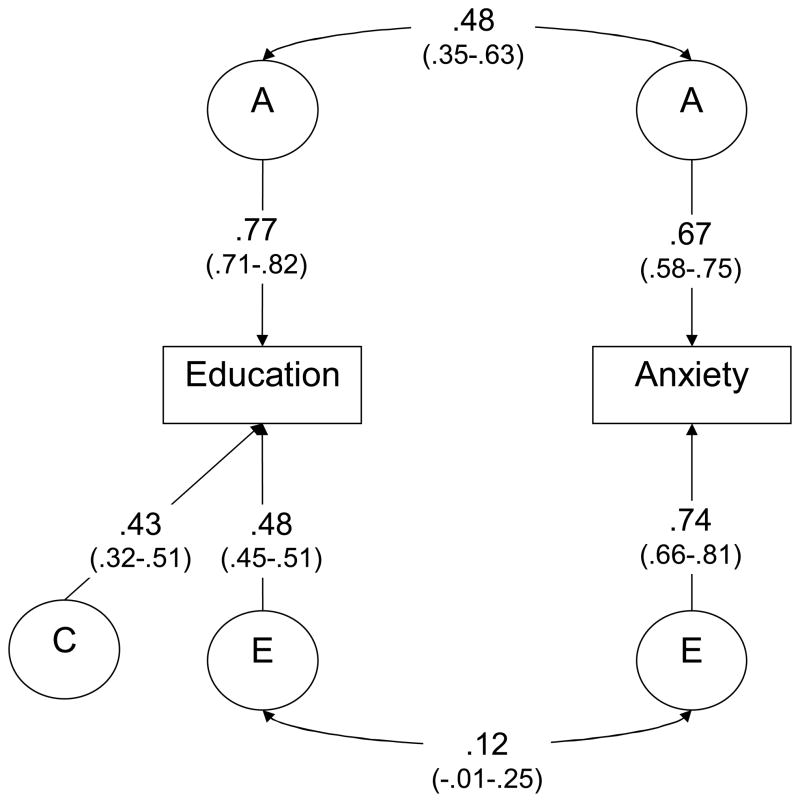
The parameter estimates are shown in Table 6. Most of the estimates from the bivariate models do not vary much between the two models. One exception is the correlation between shared environmental factors, rC, which is reduced from close to unity in the basic model to being fixed at zero in the best-fitting model. However, since the estimates of c are moderate for education and low for anxiety, rC does not contribute much to the phenotypic correlation even when taking on a high value. The estimated correlation between genetic factors for education and anxiety, rA, is 0.48 for the best-fitting model, whereas the correlation between non-shared environmental factors affecting each of the two phenotypes, rE, is 0.12.
Table 6.
Parameter estimates for the various bivariate models for education and anxiety
| Model | Education
|
Anxiety
|
Correlations between factors
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| a2 | c2 | e2 | a2 | c2 | e2 | rA | rC | rE | |
| 1. ACE, CSL (M) | .64 | .14 | .22 | .38 | .03 | .59 | .36 | .53 | .16 |
| (F) | .53 | .24 | .24 | .16 | .31 | .53 | .36 | .53 | .16 |
| 2. ACE | .58 | .19 | .23 | .38 | .07 | .55 | .38 | .59 | .14 |
| 3. ACeduE | .59 | .18 | .23 | .46 | - | .54 | .48 | - | .12 |
Note:
Models: CSL= common sex-limitation, A=additive genetic effect, C=environmental effect shared by co-twins, E=environmental effect not shared by co-twins, CEDU= environmental effect for education only, shared by co-twins.
a2=proportion additive genetic variance, c2=proportion environmental variance shared by co-twins, e2=proportion environmental variance not shared by co-twins.
The results for the best-fitting model with 95% CIs are also shown in Figure 3. The genetic contribution to the total phenotypic correlation between education and “any anxiety” (r =−0.3 from Table 2) can be calculated as rA multiplied by the genetic loadings, a, for education and anxiety, or 0.48*0.77*0.67=0.25. The environmental contribution is similarly calculated: 0.12*0.48*0.74=0.04. These values differ only slightly from those calculated from the basic ACE model: 0.18 for genetic, 0.07 for shared environmental, and 0.05 from non-shared environmental correlations.
Figure 3.
Best-fitting bivariate model with parameter estimates and 95% confidence intervals
A=additive genetic effect, C=environmental effect shared by co-twins, E=environmental effect not shared by co-twins
Discussion
The phenotypic polychoric correlation of −0.3 between educational level and anxiety implies that almost one tenth of individual variance in liability to anxiety disorders can be predicted by educational level. Although not directly comparable, this result from a Norwegian sample suggests a somewhat stronger association between education and mental health than in most other countries (7,9,29, 30). Norway is a relatively egalitarian society, where, for instance, public funding makes higher education available to anyone with sufficient aptitude. It may seem counter-intuitive to find a strong social gradient for mental health in this society, but this finding conforms to results showing little or no social gradient for mental health in developing countries (10, 11), in which social differences are usually large.
The results suggest that the correlation between education and anxiety is to a large extent caused by common genes and not much by common environment shared by co-twins. The estimate of the correlation between nonshared environmental factors is low and only marginally significant.
The relationship between socio-economic status and health has traditionally been explained by two different mechanisms, “social causation” and “social selection”. Under social causation, lower educational level represents a risk for, and higher education represents a protection against, poor mental health. Age of onset of anxiety disorders is typically in childhood or adolescence (31), which is confirmed by our results based on retrospective self-reports. Such early onset might seem incompatible with a direct protective effect of high education on anxiety, but the variation in age of onset is high, and some persons will have their anxiety disorder after finished education. Also, anxiety disorders are not necessarily lifelong, and it is not unthinkable that a high level of education could increase the chances of getting cured. Finally, risk of anxiety disorders could well be associated with scholastic success or failure during the whole school period, rather than with final educational attainment per se. A direct implication of social causation is that better education (or school performance), either in an absolute sense or relative to societal norms, reduces the risk of mental health problems. In contrast, our results, suggesting that most of the phenotypic correlation between anxiety and education is caused by common genetic effects, imply that extra education will not necessarily protect much against risk of anxiety. Of course that does not mean that anxiety disorders cannot be efficiently prevented or treated in other ways.
The implications of our findings are also not fully consistent with those of the selection hypothesis. The latter states that morbidity hampers educational attainment and, accordingly, that curing anxiety helps improving education. If the phenotypic association primarily reflects common genes, however, preventing and treating anxiety among young people may be of limited significance for increasing educational success.
Unlike common genetic effects, a contribution to the phenotypic correlation from shared environmental factors could be consistent with a social causality model, in which environmental factors associated with the family could influence both education and liability to anxiety. Examples of possible shared environmental factors are neighborhood and school qualities, including social as well as intellectual and educational aspects.
Limitations
To our knowledge this is the first attempt to disentangle the statistical relationship between indicators of socioeconomic level and mental health into genetic and environmental components. The most obvious limitation of this study is the moderate statistical power. The results are consistent with a model in which all phenotypic correlation is explained by familial causal factors. However, they cannot be taken as clear evidence against a contribution to this correlation from a direct phenotypic causal relationship. In principal, phenotypic causality can be tested with twin data such as ours, but simulation studies have shown that we have far from sufficient statistical power for such testing (32).
Furthermore, even under the assumption that the commonality is primarily due to common effects from causal factors, rather than from phenotypic causality, limited statistical power still forbids drawing firm conclusions about some of our results until they are replicated. If our analyses have failed to identify true effects of sex specific genes, we may have wrongly specified the expected value of the opposite sex twin correlation to be equal to that of the like-sex DZ twin correlations. Attributing the much lower opposite sex twin correlation than the MZ correlations to genetic effects could have inflated the strength of the genetic effects at the expense of effects of shared environment. This could also have inflated the estimate of the genetic correlation between education and anxiety and deflated the correlation due to shared environment. Our conclusion that the correlation between education and anxiety primarily reflects effects of common genes, can only be inferred leaning on previous evidence which shows little evidence of nonshared environmental effects on anxiety (23).
Sampling bias may have influenced our findings. The mean difference in education between participants and non-participants was only 0.17 SD, however. A recent attrition study of our twin sample indicates that a moderate selection of mentally healthy twins has taken place, but this selection did not appear to substantively affect results from genetic twin analyses (33). The prevalence rates are as high as, or a little higher than expected. A Norwegian prevalence study (34) did not report rates for “any anxiety disorders”, but the rates for specific disorders are mostly similar or a little lower than ours. Our estimate of “any anxiety disorder”, 18.6%, is slightly higher than an estimate from the National Comorbidity Survey Replication, 18.1% (35), in spite of the fact that we have excluded some types of phobias. Like the results from the attrition study, our relatively high prevalence estimates suggest no strong selection towards healthy twins. But even if our sample should not be fully representative, a serious attrition bias of our results is unlikely.
Our sample consists of young adult Norwegian twins. These results may not fully generalize to other populations, both due to possible age and cohort specific effects. Furthermore, the generalizability of our results is limited to populations with approximately the same distribution of genes and environmental factors as in ours. Even if almost all the correlation between education and anxiety is caused by common genes, new sources of environmental variation in the society could introduce an environmental contribution to the correlation.
Significant outcomes.
The heritability of educational level was estimated to be 0.59, and the proportion of variance due to environmental factors shared by co-twins was 0.18. The heritability of ‘any anxiety disorder’ was 0.46.
The polychoric correlation between educational level and ‘any anxiety disorder’ was −0.30.
The phenotypic relationship between educational level and anxiety disorders seems to be caused mainly by genes common to the two traits.
Limitations.
Although the sample is large compared to most other twin studies of diagnosed mental disorders, the statistical power for the present purpose is limited. Some of the contribution to the phenotypic correlation estimated to be genetic may in fact be due to environmental factors shared by co-twins.
The results do not exclude a causal effect of education on anxiety disorder or vice versa, or a bidirectional causation.
Acknowledgments
Supported by NIH grants MH-068643 (principal investigator Kendler) and MH-65322 (principal investigator Neale). The twin program of research at the Norwegian Institute of Public Health is supported by grants from the Norwegian Research Council, the Norwegian Foundation for Health and Rehabilitation, The Foundation of Borderline Research, and the European Commission under the program Quality of Life and Management of the Living Resources of the 5th Framework Program (number QLG2-CT-2002-01254). Genotyping on the twins was performed at the Starr Genotyping Resource Centre at Rockefeller University. The authors thank Statistics Norway for access to registry data on educational level and the twins for their participation.
Footnotes
Declaration of interest
None of the authors have conflicts of interest to report.
References
- 1.DOHRENWEND BP, DOHRENWEND BS. Social status and psychological disorder: a causal inquiry. Wiley, NY: 1969. [Google Scholar]
- 2.ALLARDYCE J, BOYDELL J. The wider social environment and schizophrenia. Schizophrenia Bulletin. 2006;32:592–598. doi: 10.1093/schbul/sbl008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CANTOR-GRAAE E, SELTEN JP. Schizophrenia and migration: a meta-analysis and review. Am J Psychiatry. 2005;162:12–24. doi: 10.1176/appi.ajp.162.1.12. [DOI] [PubMed] [Google Scholar]
- 4.MUNTANER C, EATON WW, MIECH R, O’CAMPO P. Socioeconomic position and major mental disorders. Epidemiologic Reviews. 2004;26:53–62. doi: 10.1093/epirev/mxh001. [DOI] [PubMed] [Google Scholar]
- 5.LORANT V, DELIÈGE D, EATON W, ROBERT A, PHILIPPOT P, ANSSEAU M. Socioeconomic inequalities in depression: A meta-analysis. Am J Epidemiol. 2003;157:98–112. doi: 10.1093/aje/kwf182. [DOI] [PubMed] [Google Scholar]
- 6.LEHTINEN V, JOUKAMAA M. Epidemiology of depression: prevalence, risk factors and treatment situation. Acta Psychiatr Scand. 1994;377(Suppl):7–10. doi: 10.1111/j.1600-0447.1994.tb05794.x. [DOI] [PubMed] [Google Scholar]
- 7.KESSLER RC, CHIU WT, DEMLER O, WALTERS EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.BIJL RV, RAVELLI A, VAN ZESSEN G. Prevalence of psychiatric disorder in the general population: results of the Netherlands Mental Health Survey and Incidence Study (NEMESIS) Soc Psychiatry Psychiatr Epidemiol. 1998;33:587–595. doi: 10.1007/s001270050098. [DOI] [PubMed] [Google Scholar]
- 9.REGIER DA, NARROW WE, RAE DS. The epidemiology of anxiety disorders: the epidemiologic catchment area (ECA) experience. J of Psychiat Res. 1990;24(Suppl):23–14. doi: 10.1016/0022-3956(90)90031-k. [DOI] [PubMed] [Google Scholar]
- 10.DAS HJ, DO QT, FRIEDMAN J, MCKENZIE D, SCOTT K. Mental health and poverty in developing countries: Revisiting the relationship. Social Science and Medicine. 2007;5:467–480. doi: 10.1016/j.socscimed.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 11.CHO MJ, KIM J, JEON HJ, et al. Lifetime and 12-Month Prevalence of DSM-IV Psychiatric Disorders Among Korean Adults. J Nerv Ment Dis. 2007;195:203–210. doi: 10.1097/01.nmd.0000243826.40732.45. [DOI] [PubMed] [Google Scholar]
- 12.KRINGLEN E, TORGERSEN S, CRAMER V. A Norwegian psychiatric epidemiologic study. American Journal of Psychiatry. 2001;158:1091–1098. doi: 10.1176/appi.ajp.158.7.1091. [DOI] [PubMed] [Google Scholar]
- 13.DALGARD OS, MYKLETUN A, ROGNERUD M, JOHANSEN R, ZAHL PH. Education, sense of mastery and mental health: results from a nation wide health monitoring study in Norway. BMC Psychiatry. 2007;7:20. doi: 10.1186/1471-244X-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.FARRIS REL, DUNHAM HW. Mental Disease in Urban Areas. University of Chicago Press; IL: 1939. [Google Scholar]
- 15.LEIGHTON AH, MURPHY JM. Psychiatric Disorder. The Problem of Cultural Distortion. Milbank Mem Fund Q. 1965;43:189–198. [PubMed] [Google Scholar]
- 16.HARRIS JR, MAGNUS P, TAMBS K. The Norwegian Institute of Public Health Twin Panel: a description of the sample and program of research. Twin Research. 2002;5:415–423. doi: 10.1375/136905202320906192. [DOI] [PubMed] [Google Scholar]
- 17.HARRIS JR, MAGNUS P, TAMBS K. The Norwegian Institute of Public Health Twin Program of Research: An update. Twin Research and Human Genetics. 2006;9:858–64. doi: 10.1375/183242706779462886. [DOI] [PubMed] [Google Scholar]
- 18.STATISTICS NORWAY. Norwegian Standard Classification of Education. Statistics Norway; Norway: 2003. Revised 2000. [Google Scholar]
- 19.WITTCHEN HU, PFISTER H. DIA-X Interviews (M-CIDI): Manual fur Screening-Verfahren und Interview: Interviewheft Langsschnittuntersuchung (DIA-X-Lifetime); Erganzungsheft (DIA-X-Lifetime); Interviewheft Querschnittuntersuchung (DIA-X 12 Monate); Erganzungsheft (DIA-X 12 Monate); PC-Programm zur Durchfuhrung des Interviews (Langs- und Querschnittuntersuchung); Auswertungsprogramm. Swets & Zeitlinger; Austria: 1997. [Google Scholar]
- 20.HESBACHER PT, RICKELS R, MORRIS RJ, NEWMAN H, ROSENFELD MD. Psychiatric illness in family practice. Journal of Clinical Psychiatry. 1980;41:6–10. [PubMed] [Google Scholar]
- 21.TAMBS K, MOUM T. How Well Can a Few Questionnaire Items Indicate Anxiety and Depression. Acta Psychiatrica Scandinavica. 1993;87:364–367. doi: 10.1111/j.1600-0447.1993.tb03388.x. [DOI] [PubMed] [Google Scholar]
- 22.TAMBS K, CZAJKOWSKY N, RØYSAMB E, et al. The Structure of Genetic and Environmental Risk Factors for Dimensional Representations of DSM-IV Anxiety Disorders. British Journal of Psychiatry. 2009;195:301–307. doi: 10.1192/bjp.bp.108.059485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.HETTEMA JM, NEALE MC, KENDLER KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. American Journal of Psychiatry. 2001;158:1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- 24.OLSSON U. Maximum-likelihood estimation of polychoric correlation coefficient. Psychometrica. 1979;44:443–460. [Google Scholar]
- 25.JÖRESKOG KG, SÖRBOM D. Prelis 2. User’s Reference Guide. Scientific Software International; IL: 2002. [Google Scholar]
- 26.NEALE MC, CARDON LR. Methodology for Genetic Studies of Twins and Families. Kluwer; The Netherlands: 1992. [Google Scholar]
- 27.AKAIKE H. Statistical predictor identification. Ann Inst Stat Math. 1970;21:243–247. [Google Scholar]
- 28.NEALE MC, BOKER SM, XIE G, MAES H. Mx: Statistical Modeling. 5. Department of Psychiatry, Virginia Commonwealth University; VA: 1999. [Google Scholar]
- 29.KESSLER RC, MCGONAGLE KA, ZHAO S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 30.DE GRAAF R, BIJL RV, SMIT F, VOLLEBERGH WAM, SPIJKER J. Risk factors for 12-month comorbidity of mood, anxiety, and substance abuse disorders: Findings from the Netherlands Mental Health Survey and Incidence Study. Am J Psychiatry. 2002;159:620–629. doi: 10.1176/appi.ajp.159.4.620. [DOI] [PubMed] [Google Scholar]
- 31.KESSLER RC, RUSCIO AM, SHEAR K, WITTCHEN HU. Epidemiology of anxiety disorders. Curr Top Behav Neurosci. 2010;2:21–35. [PubMed] [Google Scholar]
- 32.HEATH AC, KESSLER RC, NEALE MC, HEWITT JK, EAVES LJ, KENDLER KS. Testing hypotheses about direction of causation using cross-sectional family data. Behavior Genetic. 1993;23:29–50. doi: 10.1007/BF01067552. [DOI] [PubMed] [Google Scholar]
- 33.TAMBS K, RØNNING T, PRESCOTT CA, et al. The Norwegian Institute of Public Health Twin Study of Mental Health: Examining Recruitment and Attrition Bias. Twin Research and Human Genetics. 2009;9:185–193. doi: 10.1375/twin.12.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.KRINGLEN E, TORGERSEN S, CRAMER V. A Norwegian Psychiatric Epidemiological Study. American Journal of Psychiatry. 2001;158:191–198. doi: 10.1176/appi.ajp.158.7.1091. [DOI] [PubMed] [Google Scholar]
- 35.KESSLER RC, CHIU WT, DEMLE O, WALTERS EE. Prevalence, Severity, and Comorbidity of 12-Month DSM-IV Disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]