Abstract
The down regulation of mitochondrial electron transport is an emerging mechanism of cytoprotective intervention that is effective in pathologic settings such as myocardial ischemia and reperfusion when the continuation of mitochondrial respiration produces reactive oxygen species, mitochondrial calcium overload, and the release of cytochrome c to activate cell death programs. The initial target of deranged electron transport is the mitochondria themselves. In the first part of this review, we describe this concept and summarize different approaches used to regulate mitochondrial respiration by targeting complex I as a proximal site in the electron transport chain (ETC) in order to favor the cytoprotection. The second part of the review highlights the emerging role of signal transducer and activator of transcription 3 (STAT3) in the direct, non-transcriptional regulation of ETC, as an example of a genetic approach to modulate respiration. Recent studies indicate that a pool of STAT3 resides in the mitochondria where it is necessary for the maximal activity of complexes I and II of the electron transport chain (ETC). The over expression of mitochondrial-targeted STAT3 results in a partial blockade of electron transport at complexes I and II that does not impair mitochondrial membrane potential nor enhance the production of reactive oxygen species (ROS). The targeting of transcriptionally-inactive STAT3 to mitochondria attenuates damage to mitochondria during cell stress, resulting in decreased production of ROS and retention of cytochrome c by mitochondria. The overexpression of STAT3 targeted to mitochondria unveils a novel protective approach mediated by modulation of mitochondrial respiration that is independent of STAT3 transcriptional activity. The limitation of mitochondrial respiration under pathologic circumstances can be approached by activation and over expression of endogenous signaling mechanisms in addition to pharmacologic means. The regulation of mitochondrial respiration comprises a cardioprotective paradigm to decrease cellular injury during ischemia and reperfusion.
1. Introduction
Mitochondria are crucial for the production of cellular energy through oxidative phosphorylation (Henze and Martin, 2003). They also participate in a variety of other homeostatic processes, including calcium homeostasis, fatty acid oxidation, heme synthesis, steroid synthesis, and cell signaling (McBride et al., 2006). Mitochondrial dysfunction impairs not only energy generation but also cell homeostasis. Not surprisingly, defects in mitochondrial function are found in aging and multiple diseases, including congenital metabolic disorders, and cardiac dysfunction (Edmond, 2009; Hoppel et al., 2009; Lesnefsky et al., 2001c). In normal conditions, mitochondrial ATP production is coupled with oxygen consumption. However, in pathological states, an imbalance in oxygen utilization occurs, which leads to the generation of reactive oxygen species (ROS) and oxidative damage to mitochondrial constituents, setting the stage for cellular injury. Enhanced cell death as a result of mitochondrial dysfunction impedes organ function, which occurs in numerous cardiac pathologies, including cardiomyopathy, congestive heart failure and ischemia/reperfusion injury.
Although modest mitochondrial ROS production serves as a signaling mechanism that preserves oxygen homeostasis (Chandel, 2010; Chandel et al., 1998), more extensive, “cytotoxic” ROS production causes damage first to the mitochondria themselves followed by cellular injury. This review focuses on emerging genetic approaches to modulate the activity of the electron transport chain during cell stress conditions in order to attenuate cell injury. Modulation of electron transport is protective during myocardial ischemia, when mitochondria are sources of cell injury. Cytoprotection achieved by the blockade of electron transport during pathologic processes is in stark contrast to the blockade of electron transport during normal aerobic metabolism. Inhibition of respiration at complex I under aerobic conditions leads to cellular injury (Li et al., 2003) and activates programmed cell death (Kushnareva et al., 2002). Thus, in pathologic settings such as ischemia or early reperfusion, modulation of mitochondrial metabolism can be beneficial.
2. Mitochondria as Sources of Cardiac Injury
2.1. Mitochondrial Damage
Mitochondrial electron transport sustains progressive damage during myocardial ischemia (reviewed in (Chen and Lesnefsky, 2009b; Lesnefsky et al., 2001d)). Initial damage to the electron transport chain involves complex I (Flameng et al., 1991; Rouslin, 1983). As ischemia progresses, damage occurs to complex III (Lesnefsky et al., 2001a) and complex IV (cytochrome oxidase) (Lesnefsky et al., 2001d; Lesnefsky et al., 1997; Paradies et al., 1998; Piper et al., 1985; Ueta et al., 1990).
Complex I activity decreases during ischemia. In isolated perfused rat heart, ischemia decreases complex I activity without alternation of the NADH dehydrogenase component (Ohnishi et al., 2005). The site of ischemic damage within complex I was further localized as discussed below. Ischemia damages complex III by inactivation of the Rieske iron-sulfur protein component, a key catalytic center (Lesnefsky et al., 2001a). A decrease in respiration through cytochrome oxidase occurs due to a selective decrease in cardiolipin content (Lesnefsky et al., 2001e), rather than functional inactivation or damage to a catalytic or regulatory subunit (Lesnefsky et al., 1997). Cardiolipin is a critical factor for the optimal complex IV activity (Robinson et al., 1980; Vik and Capaldi, 1977).
Ischemic damage to complex I limits respiration with NADH-linked substrates and produces ROS (Genova et al., 2001; Ohnishi et al., 2005). The FMN in NADH dehydrogenase (Kudin et al., 2004; Kushnareva et al., 2002), iron sulfur cluster N2 and the two tightly bound ubiquinones located distal in the complex (Genova et al., 2001; Ohnishi et al., 2005) are key catalytic sites that are potential targets of ischemic injury. Preserved NADH dehydrogenase activity following ischemia localizes the site of damage distal to FMN (Chen et al., 2008). Ischemia increased ROS generation from complex I in the presence of unaltered NADH dehydrogenase activity, consistent with a distal site of damage. Rotenone blocks electron transport at the ubiquinone acceptor site (Chance et al., 1963) and increases ROS generation from ischemic hearts, bracketing the site for ROS generation proximal to the ubiquinone binding site. This functional localization suggests the N2 iron-sulfur site or the tightly bound quinones as the locus for production of ROS from ischemia-damaged complex I (Chen et al., 2008). In contrast, when reversed electron flow within complex I was studied, ROS generation was paradoxically decreased by ischemia, again supporting a defect in the distal portion of complex I. Thus, ischemia inactivates complex I by altering the segment that contains the two tightly bound quinones Qnf and Qns. Functional assessment using forward and reverse electron flow best localizes the site of net H2O2 production following ischemia to the iron-sulfur clusters proximal to the proposed site of ischemic damage at the tightly bound quinones (Chen et al., 2008). Others, using different models of ischemia, such incubation of blocks of canine myocardium in plastic bags at 37 °C, observed decreases in both complex I activity and the NADH dehydrogenase component (Rouslin, 1983). These differences are likely due to differences in species and models of ischemia.
2.2. Mitochondrial-Driven Cellular Damage from Electron Transport
Mitochondria generate ROS at the onset of reperfusion (Ambrosio et al., 1993; Bolli and McCay, 1990; Kevin et al., 2003; Maupoil et al., 1990; Merrill, 2002; Pietri et al., 1989). It was originally thought that during cardiac ischemia, cytochrome oxidase would rapidly consume all available oxygen content and lead to complete anoxia, arresting ROS production. Instead, during the initial progression of ischemia, oxygen remains available (Ilangovan et al., 2004). Simulated ischemia in cardiomyocytes, under conditions of severe hypoxia but not anoxia, increases ROS production (Becker et al., 1999; Vanden Hoek et al., 1997), as observed in the intact heart under conditions of low flow ischemia (Merrill, 2002). In situ ROS production, consistent with persistence of oxygen, increases during stop flow ischemia (Kevin et al., 2003; Lee et al., 2000; Riess et al., 2004; Stowe et al., 2006).
Mitochondria are a primary source for ROS production during ischemia (Becker et al., 1999; Davies, 1989; Vanden Hoek et al., 1997). Ischemia increased the capacity for ROS production from both complex I and complex III (Chen et al., 2008). Since mitochondrial damage mainly occurs during ischemia (Chen et al., 2006b; Lesnefsky et al., 2004c), ischemia-damaged electron transport chains are responsible for the enhanced ROS generation during the reoxygenation of early reperfusion (Becker, 2004; Chen et al., 2007b; O’Rourke et al., 2005).
In addition to oxidant-mediated cell injury, ischemic damage to mitochondria sets the stage for the activation of cell death processes during reperfusion. Ischemia leads to cytochrome c release from mitochondria (Lesnefsky et al., 2001e; Lesnefsky et al., 1997). The decrease in cytochrome c content occurs concomitant with the loss of cardiolipin content in the inner membrane (Borutaite and Brown, 2003; Borutaite et al., 2001; Borutaite et al., 2003; Lesnefsky et al., 2001e; Lesnefsky et al., 1997). Cardiolipin contributes a key role in the retention of cytochrome c at the inner membrane via non-ionic and electrostatic mechanisms (Ott et al., 2002). Following displacement of cytochrome c from the inner membrane (Ott et al., 2002; Shidoji et al., 1999), an increase in permeability of the outer mitochondrial membrane, usually via the insertion of proapoptotic peptides into the outer membrane (Korsmeyer et al., 2000), allows the release of delocalized cytochrome c (Ott et al., 2002) and the activation of apoptotic programs. Cytochrome c release is initiated during ischemia.
During the activation of cell death programs, outer membrane permeation occurs through the activation of the pro-apoptotic effector proteins bax and bak (Adams and Cory, 1998) via interaction with activator peptides including truncated-bid (t-bid) or bim. Activator peptides are initially sequestered and inhibited by the antiapoptotic proteins bcl-2 and bcl-xl (Gottlieb, 2003; Gustafsson and Gottlieb, 2007; Kroemer et al., 2007; Uren et al., 2007; Willis and Adams, 2005). Bcl-2 and bcl-xL also interact with and sequester sensitizer BH3-only domain peptides. Thirty minutes of stop flow ischemia decreased the content of bcl-2 in mitochondria. At the time of bcl-2 depletion, the mitochondria remain functionally and morphologically intact (Lesnefsky et al., 2001e; Lesnefsky et al., 1997). Respiration remains coupled, with unchanged state 4 rates of respiration (Lesnefsky et al., 2001e; Lesnefsky et al., 1997). Outer membrane intactness is unaltered as assessed by oxidation of exogenous reduced cytochrome c (Chen et al., 2006b) and the retention of intermembrane space proteins including apoptosis inducing factor (AIF). Thus, based on morphologic, functional and respiration data, mitochondria remain intact when bcl-2 is depleted, consistent with a regulated permeation of the outer membrane to facilitate cytochrome c release. Release of cytochrome c during ischemia activates downstream caspase 3 leading to an increase cardiomyocyte apoptosis (Borutaite et al., 2003) that becomes increasingly evident during reperfusion (Borutaite et al., 2003; Chen et al., 2001; Gottlieb et al., 1994; Hausenloy and Yellon, 2003).
2.3. Attenuation of Mitochondrial Injury
Mitochondria generate cytotoxic reactive oxygen species during ischemia (Becker et al., 1999; Kevin et al., 2003; Vanden Hoek et al., 1997). Our group proposed that persistent, unregulated activity of the electron transport chain during ischemia would produce oxidative damage, likely from complex III, leading to damage to the electron transport chain and the loss of cytochrome c (Chen et al., 2007b). In order to test this hypothesis, blockade of electron flow was employed during ischemia to interrupt this pathogenic sequence.
As an initial proof of concept, electron transport into complex III was inhibited with rotenone, an irreversible inhibitor of complex I. The blockade of electron transport during ischemia preserved cardiolipin content, cytochrome c content, and respiration through cytochrome oxidase (Lesnefsky et al., 2004b). Thus, ischemic damage to mitochondria is mediated by the mitochondria themselves (Lesnefsky et al., 2004b). A rapidly reversible inhibitor of electron transport, amobarbital (Amytal®) (Spiegel and Wainio, 1969), a short-acting barbiturate that inhibits complex I at the rotenone-site (Degli Esposti, 1998; Horgan et al., 1968), was utilized to evaluate if preservation of electron transport during ischemia decreased myocardial injury following reperfusion. Amobarbital treatment of the isolated heart immediately before ischemia led to a reversible inhibition of respiration during ischemia with prevention of damage to electron transport chain complexes (Chen et al., 2006a).
Reversible blockade of respiration at complex I during ischemia protected against damage to complex I (Chen et al., 2006a). Protection of complexes III and IV, as well as retention of cytochrome c, was also observed (Chen et al., 2006a). Studies of intact hearts provided additional support for the specificity of amobarbital treatment (Aldakkak et al., 2008). In isolated, perfused guinea pig hearts, amobarbital treatment immediately before ischemia decreased production of reactive oxygen species and decreased Ca2+ accumulation in mitochondria evident in situ in the intact heart (Aldakkak et al., 2008). NADH contents observed in the amobarbital-treated hearts supported the in situ inhibition of complex I in the intact heart (Aldakkak et al., 2008). The blockade of electron transport during ischemia preserves respiratory function in isolated mitochondria (Chen et al., 2006a) and intact hearts (Aldakkak et al., 2008). Thus, in pathologic settings such as ischemia or early reperfusion, modulation of mitochondrial metabolism can be beneficial, when the result of mitochondrial respiration is cellular injury.
2.4. Attenuation of Cellular Injury
The protection of electron transport during ischemia allows a critical test of the contribution of ischemic mitochondrial damage to reperfusion injury. Reversible blockade of electron transport during ischemia with amobarbital demonstrated that protection of electron transport during ischemia was carried forward into reperfusion, indicated by the preservation of oxidative phosphorylation following 30 minutes of reperfusion (Chen et al., 2006b). Indeed, the reversible blockade of electron transport during ischemia protected complex I, as well as the distal electron transport chain (Chen et al., 2006b). Blockade of respiration during ischemia preserved the integrity of the inner and outer mitochondrial membranes following reperfusion, with continued retention of cytochrome c by mitochondria (Chen et al., 2006b). H2O2 production from both complex I and complex III during reperfusion was markedly decreased (Chen et al., 2006b). In situ in the intact heart, reperfusion of myocardium without ischemic damage to electron transport led to decreased generation of reactive oxygen species and less mitochondrial Ca2+ loading (Aldakkak et al., 2008) with improved contractile recovery and decreased infarct size (Aldakkak et al., 2008; Chen et al., 2006b). Thus, protection of mitochondria during ischemia is sustained during reperfusion, leading to decreased mitochondrial-driven oxidative and calcium-mediated injury with improved functional recovery and less cell death.
Ischemic damage to electron transport decreased bcl-2 content whereas protection of electron transport by amobarbital during ischemia preserves the bcl-2 content, providing strong support that the electron transport chain contributes to bcl-2 depletion during ischemia (Chen and Lesnefsky, 2011). At the onset of reperfusion, reoxygenation leads to production of reactive oxygen species from the ischemia-damaged electron transport chain (Aldakkak et al., 2008; Chen and Lesnefsky, 2008; Chen et al., 2006b). Oxidative stress occurs in the setting of decreased mitochondrial bcl-2 content and favors opening of the mitochondrial permeability transition pore (Chen and Lesnefsky, 2011). Thus, preservation of bcl-2 content via reversible blockade of electron transport should enhance cell survival.
The susceptibility to permeability transition pore opening during early reperfusion was studied in mitochondria from hearts that underwent ischemia in the presence or absence of amobarbital blockade of electron transport. The calcium retention capacity (CRC) was used as an index of susceptibility to permeability transition following 30 minutes of reperfusion. Compared to untreated ischemic-reperfused hearts, amobarbital treatment only during ischemia improved CRC measured after reperfusion (Chen and Lesnefsky, 2011). The cardiac protection observed when the heart is reperfused in the setting of preserved mitochondrial function provides strong support that ischemic damage to mitochondria is a key mechanism of myocardial injury during reperfusion.
Damage to the electron transport chain occurs mainly during ischemia (Chen et al., 2007b; Lesnefsky et al., 2001a; Lesnefsky et al., 1997) and persists during reperfusion (Lesnefsky et al., 2004c; Paillard et al., 2009). Interventions that protect myocardium during reperfusion, including ischemic postconditioning (Gomez et al., 2008; Hausenloy and Yellon, 2004; Paillard et al., 2009) or brief inhibition of electron transport (Ambrosio et al., 1993; Stewart et al., 2009), reduce myocardial injury independent of the recovery of oxidative phosphorylation (Lesnefsky et al., 2004c; Paillard et al., 2009). These unexpected observations point to the key role of electron transport chain-dependent mechanisms, including the production of reactive oxygen species (Chen and Lesnefsky, 2006), shift to a pro-apoptotic balance via depletion of bcl-2 (Chen and Lesnefsky, 2011), and increased tendency to permeability transition (Chen and Lesnefsky, 2011), and mechanistically link electron transport chain damage from ischemia to cardiomyocyte death during reperfusion.
3. Mechanisms of Modulation
3.1. Site Matters
Blockade of complex I protects mitochondria against ischemic damage (Ambrosio et al., 1993; Chen et al., 2006b; Park et al., 1997). In contrast, blockade of electron transport during ischemia at cytochrome oxidase did not protect the electron transport chain (Chen et al., 2010). Blockade of distal electron transport will lead to the accumulation of electrons at upstream complexes and increase ROS generation (Chen and Lesnefsky, 2006; Chen et al., 2003). Thus, the central segment of the electron transport chain, encompassing complex III and cytochrome c, leads to mitochondrial damage during ischemia.
Complex III is a major site for the production and extra-mitochondrial release of ROS during ischemia (Chen et al., 2003; Han et al., 2003). Cytochrome c and cardiolipin form a complex (Belikova et al., 2006; Kagan et al., 2005). In the presence of H2O2, perhaps generated from complex III, the cardiolipin-cytochrome c peroxidase complex consumes H2O2 and simultaneously generates peroxidized cardiolipin (Belikova et al., 2006; Kagan et al., 2005; Vlasova et al., 2006), directing damage to the inner membrane. This process, in turn, favors cytochrome c release from mitochondria. Blockade of proximal electron transport decreases the H2O2 production (Chen and Lesnefsky, 2007; Chen et al., 2006b) and prevents cardiolipin depletion (Lesnefsky et al., 2004b), consistent with this scheme. Thus, limitation of electron flow into the complex III-cytochrome c region of the electron transport chain is critical to temper ischemic damage to mitochondria, and perhaps to attenuate mitochondrial-driven injury during reperfusion (Ambrosio et al., 1993; Stewart et al., 2009).
The presence of pre-existing cytochrome oxidase defects before ischemia appears to enhance cardiac injury and blunt cytoprotective responses. The elderly heart contains defect in cytochrome oxidase in interfibrillar mitochondria (Fannin et al., 1999; Lesnefsky et al., 2001b) and sustains increased injury during ischemia and reperfusion (Lesnefsky et al., 1994; Lucas and Szweda, 1998). Aging hearts are also resistant to endogenous cytoprotection provided by ischemic preconditioning and postconditioning (Boengler et al., 2007; Schulman et al., 2001; Vessey et al., 2009). A similar phenotype is observed in mice deficient in sphingosine kinase 2. In the baseline state, mitochondria isolated from sphk2−/− hearts contain a decrease in respiration due to a defect in cytochrome oxidase (Strub et al., 2010). In these mice, ischemic preconditioning failed to improve the resistance of myocardium to subsequent infarction in vitro (Vessey et al., 2011) and in vivo (Gomez et al., 2010). A likely mechanism involves an increased susceptibility to permeability transition pore opening (Gomez et al., 2010). Thus, blockage of electron transport at complex IV provides mitochondria that cannot respond to cytoprotective modulation.
3.2. Pharmacologic Inhibition: Nitrite, Nitrate and Hydrogen Sulfide Modulate Electron Transport
Blockade of electron transport at complex I during ischemia using other inhibitors of complex I including HMR-1098 (Pasdois et al., 2007) and cell permeable nitric oxide (NO) donors (Nadtochiy et al., 2007) leads to cardioprotection. NO reacts with sulfhydryl groups of complex I and decreases complex I activity (Tompkins et al., 2006). The protective effect of NO via blockade of complex I occurs in intact hearts (Shiva et al., 2007). Similarly, the generation of NO via nitrite (Shiva et al., 2007) or nitrate (Zhu et al., 2011) protects the heart via inhibition of complex I. Nitric oxide inhibits both complex I and cytochrome oxidase (Antunes and Cadenas, 2007; Cooper and Brown, 2008; Murphy and Steenbergen, 2007). Inhibition of complex I by nitric oxide decreases myocardial injury, whereas inhibition of cytochrome oxide by nitric oxide augments tissue injury (Cooper et al., 2003), but clearly the protective effect of proximal blockade of electron transport dominates.
Hydrogen sulfide (H2S) is cardioprotective. Either exogenous administration or induction of endogenous H2S at the time of reperfusion limits the extent of myocardial infarction (Elrod et al., 2007; Salloum et al., 2009). It is possible that this molecule modulates also the activities of complexes I and II. These studies provide additional support for the concept that blockade of the proximal electron transport chain during ischemia decreases myocardial injury following reperfusion.
3.3. Modulation of Electron Transport by Post-Translational Modifications
Inhibition of electron transport chain complexes can also be achieved by site-specific posttranslational modifications. Complex I is reversibly acetylated in response to nutrient deprivation and oxidative stress (Verdin et al., 2010). There are three class-III, NAD+-dependent deacetylases found in the mitochondria: sirtuins-3, 4 and 5 (SIRT3, 4, 5) (Verdin et al., 2010). They were initially characterized as enzymes deacetylating nuclear histones and transcription factors involved in the regulation of survival pathways, stress and metabolism (Sauve et al., 2006). Deletion of SIRT3 leads to the increased acetylation and specific inhibition of complex I and II (Ahn et al., 2008; Cimen et al., 2009). Fourteen subunits of complex I contain acetylation domains, including NDUFV1, NDUFS1, NDUFA9 and NDUFA13/GRIM-19 (Koopman et al., 2009). Recently it has been shown that a major catalytic subunit of complex II, SDHa, is also reversibly acetylated by SIRT3 (Cimen et al., 2009).
The activities of complexes I and II are modified by protein S-glutathionylation (Pr-GSSG), which is responsive to changes in redox state (GSH/GSSG ratio) in the mitochondrial matrix. NDUFS1 and NDUFV1 subunits of complex I are glutathionylated upon exposure to GSSG (Taylor et al., 2003) or a low GSH/GSSG ratio (Beer et al., 2004). This modification is reversible upon addition of glutharedoxin-2 (Grx2) or glutathione (GSH) (Beer et al., 2004). The correlation between glutathionylation and activity of complex I is unclear. Taylor et. al showed that the glutathionylation decreased complex I activity and increased ROS production in vitro (Taylor et al., 2003). However, others reported that complex I activity is augmented and superoxide generation reduced (Chen et al., 2007a). Whether complex I is regulated by Pr-GSSG in the intact heart has not yet been explored. It is reported that mitochondrial complex II is constitutively glutathionylated in the SDHa subunit, which is altered by ischemia and reperfusion in isolated rat hearts (Chen et al., 2007c). Moreover, studies of isolated complex II indicate that Pr-GSSG increases enzymatic activity and decreases the superoxide generation (Chen et al., 2007c). These results suggest that ischemia/reperfusion-mediated deglutathionylation leads to a decrease in complex II activity.
Another posttranslational modification of electron transport that modulates electron transport and protects during cardiac ischemia and reperfusion is S-nitrosation of complex I (Burwell et al., 2006; Nadtochiy et al., 2007). Administration of S-nitrosating agent S-nitroso-2-mercaptopropionyl glycine (SNO-MPG) either before cardiac ischemia or at reperfusion rescued complex I activity and decreased infarct size (Nadtochiy et al., 2009). Elevated mitochondrial S-nitrosation and associated inhibition of complex I activity during ischemia and reperfusion were reversed after two hours of reperfusion (Nadtochiy et al., 2007; Nadtochiy et al., 2009).
3.4. Genetic Approaches to Modulate Electron Transport
Since pharmacologic inhibition of a proximal site in ETC is protective against ischemia/reperfusion injury, one may hypothesize that a genetic approach to knock down complex I should result in a similar cardioprotection. A transgenic mouse with truncation of the terminal 10–15 amino acids of NDUFS4 subunit of complex I has been generated (Ingraham et al., 2009). While homozygous mutation (NDUFSTR/TR) is embryonic lethal, heterozygous NDUFSWT/TR mice exhibited a 30% decrease in complex I activity and increased levels of lactic acid. Interestingly, the previously discussed beneficial effects of SNO-MPG were abolished in hearts of NDUFS4WT/TR mice (Ingraham et al., 2009), meaning that fully functional complex I is necessary for SNO-MPG-mediated cardioprotection (Nadtochiy et al., 2009). Another group reported generation of a mouse with a conditional knock out of the NDUFS4 gene using a Cre/loxP approach (Kruse et al., 2008). Mice with a deletion of both alleles, NDUFS4−/−, manifested encephalomyopathy, including retarded growth rate, loss of motor skill, lethargy, blindness, and died at 7 weeks of age. No complex I activity was detected in mitochondria with this phenotype. However, mice with one allele still present were indistinguishable from the wild type. This was in contrast with heterozygous NDUFS4WT/TR mice showing 30% decrease in complex I activity (Ingraham et al., 2009). The explanation of these differences might be that the truncated NDUFS4 protein acts as a dominant negative and partially represses function of the protein product of the wild type allele. Thus, a severe permanent inhibition of complex I activity seems to be detrimental to mitochondrial function, consistent with studies that the pharmacologic inhibition of complex I under aerobic conditions leads to apoptosis (Kushnareva et al., 2002).
3.4.1. STAT3 knockout
STAT3 is a transcription factor originally identified as an IL-6-induced activator of acute phase genes (Levy and Lee, 2002). Other members of the IL-6 family, which utilize the gp130 receptor also activate STAT3 (Levy and Lee, 2002). Analysis of tissue-specific conditional STAT3 knock-out mice has provided strong evidence that STAT3 contributes a central role in the control of cell growth and host responses to inflammation and cellular stress (Levy and Lee, 2002). STAT3 positively regulates expression of antiapoptotic (Levy and Lee, 2002) and antioxidant genes (Negoro et al., 2001; Oshima et al., 2005). The cardioprotective role of STAT3 that activates expression of antiapoptotic and antioxidative genes has been previously reviewed (Boengler et al., 2008b; Hilfiker-Kleiner et al., 2005).
Selective deletion of STAT3 in mouse cardiomyocytes leads to enhanced cardiac inflammation with fibrosis, dilated cardiomyopathy and premature death due to congestive heart failure (Hilfiker-Kleiner et al., 2005). Female mice, where STAT3 is not expressed in cardiomyocytes, develop post-partum cardiomyopathy (Hilfiker-Kleiner et al., 2007). Ventricles from STAT3-null hearts show elevated ROS levels (Hilfiker-Kleiner et al., 2007). Ischemic and pharmacologic preconditioning protected the viability of wild type but not STAT3−/− cardiomyocytes (Hilfiker-Kleiner et al., 2005). Moreover, STAT3−/− cardiomyocytes demonstrate increased sensitivity to endotoxin/LPS-mediated toxic shock (Jacoby et al., 2003). Alternatively, if STAT3 is over expressed in cardiomyocytes, mice are less sensitive to treatment with doxorubicin, which exerts cardiotoxicity in part via the generation of ROS (Kunisada et al., 2000). Doxorubicin treatment decreases complex I activity in heart mitochondria (Xiong et al., 2006; Zhu et al., 2011), which in turn leads to increased ROS production from complex I. Clearly, the expression of STAT3 in the heart protects from a variety of stresses that induce the formation of ROS.
Recent reports have shown that STAT3 also localizes to the mitochondria (Boengler et al., 2010; Phillips et al., 2010; Wegrzyn et al., 2009). STAT3 deletion in cardiomyocytes attenuates integrated respiration due to a 50% decrease in the enzymatic activities of complexes I and II (Wegrzyn et al., 2009). Similar to NDUFS4−/− mice, the profound complex I defect observed in STAT3 knockout cells is detrimental to mitochondrial function. Interestingly, STAT3 has also been shown to co-immunoprecipitate with matrix-localized cyclophilin D, a target of mitochondrial permeability transition pore (MPTP) inhibitor cyclosporine A (Boengler et al., 2010). Opening of the MPTP leads to the mitochondrial swelling and permeation of both inner and outer mitochondrial membranes with cytochrome c release, resulting in cardiomyocyte death. Mitochondria isolated from STAT3−/− hearts tolerate lower levels of calcium before undergoing MPTP opening (Boengler et al., 2010). Therefore, one would predict that STAT3−/− mitochondria would be more susceptible to damage sustained during reperfusion, when elevated calcium levels trigger MPTP opening. Not surprisingly, it has been shown that the reduction of infarct size by ischemic preconditioning and postconditioning, where blocking of MPTP opening is one of the major mechanisms, is abolished in STAT3−/− hearts (Boengler et al., 2008a; Lacerda et al., 2009; Smith et al., 2004).
3.4.2. The Role of Mitochondrial-Targeted STAT3 in the Control of Cellular Respiration
Reconstitution of STAT3-null cells with mitochondria-localized STAT3 containing a mutated DNA-binding domain or tyrosine 705 restored deficits in the respiration indicating that mitochondrial-localized STAT3 modulates the electron transport chain through a non-transcriptional mechanism (Wegrzyn et al., 2009). Since STAT3 regulates mitochondrial respiration, we examined the contribution of mitochondrial STAT3 to oxidative physiology, including during stress. In order to address this issue, a transgenic mouse was generated that selectively over expressed in cardiomyocytes a DNA binding mutant of STAT3 containing a mitochondrial targeting sequence (MLS-STAT3E) (Szczepanek et al., 2011). The effect of transcriptionally inactive mitochondrial STAT3 on both mitochondrial respiration and oxidant production was examined in the basal condition and under ischemia. Transgenic mice were phenotypically normal and fertile. The mutations in the DNA binding domain blocked STAT3 function as a transcription factor. The presence of the transgene did not alter the expression of STAT3-dependent genes in the baseline state, under ischemic conditions and following exposure to leukemia inhibitory factor (LIF), a member of IL-6 family of STAT3 activators (Szczepanek et al., 2011).
In mitochondria from MLS-STAT3E mice, respiration was tightly coupled with normal rates of state 4 respiration and respiratory control ratios (RCRs) (Szczepanek et al., 2011). The maximal rate of respiration was modestly decreased by approximately 20% in transgenic hearts when glutamate+malate or succinate were used as substrates. Uncoupled respiration was also reduced, excluding complex V and the phosphorylation apparatus as a source of the defect. Oxidative phosphorylation through complex IV using TMPD/ascorbate as substrate was preserved, excluding a defect in cytochrome oxidase. MLS-STAT3E decreased the enzyme activities of complexes I and II, whereas the activities of complexes III and IV were unchanged. Thus, transgenic mice exhibit a modest, chronic partial blockade of the proximal electron transport chain. Interestingly, this decrease in respiration is less severe than the impact in hearts of STAT3−/− mice discussed above (Figure 1A).
Figure 1. STAT3 expression in heart mitochondria affects mitochondrial function.
(A) Cardiac-specific deletion of STAT3 results in a 50% decrease in complex I activity (Wegrzyn et al., 2009), whereas mitochondria-targeted over expression of STAT3 (MLS-STAT3E) leads to a modest 20% reduction in complex I activity (Szczepanek et al., 2011). Results are means ± SEM, n=4 for both groups. (B) Over expression of mitochondria-targeted, transcriptionally inactive STAT3 (MLS-STAT3E) decreases ROS production and blocks cytochrome c release during ischemia (Szczepanek et al., 2011). Results are means ± SEM, n=4 for both groups. Y axis depicts the ischemia-induced increase in net release of H2O2 from mitochondria respiring with glutamate+malate as complex I substrate, expressed as percent increase compared to time control (mitochondria from non-ischemic hearts). X axis depicts cytochrome c release and is shown as a percent decrease in signal compared to cytochrome c in time control samples.
Functional analysis of complex I activity (Chen et al., 2008), localized the defect to the distal portion of complex I that includes the chain of iron-sulfur clusters or the quinone-binding site (Figure 2). Of even greater interest, transgenic mice contain a site of partial block potentially proximal to or at the segment of complex I that sustains damage during ischemia. Therefore, this blockade may attenuate the production of reactive oxygen species from complex I during the stress of ischemia (Figure 1B). Since complex I and II activities were decreased, the mRNA and protein expression of representative subunits of the complexes were tested and found to be unaltered. The exact mechanism of STAT3 interaction with complexes I and II remains to be elucidated. Recently, it has been shown that the ratio of complexes I and II to mitochondrial STAT3 is in an order of 105 (Phillips et al., 2010). This finding makes it unlikely that STAT3 is a structural part of these complexes, yet does not exclude possibility that STAT3 modulates respiration through regulation of other proteins responsible for posttranslational modifications of ETC complexes. Boengler et. al suggested protein kinases as a possible target of STAT3 (Boengler et al., 2010). Interestingly, a recent study suggests that the mitochondrial ETC complexes contain a mechanism that allows them to autophosphorylate their own subunits, and may play a role in the regulation of respiration (Phillips et al., 2011).
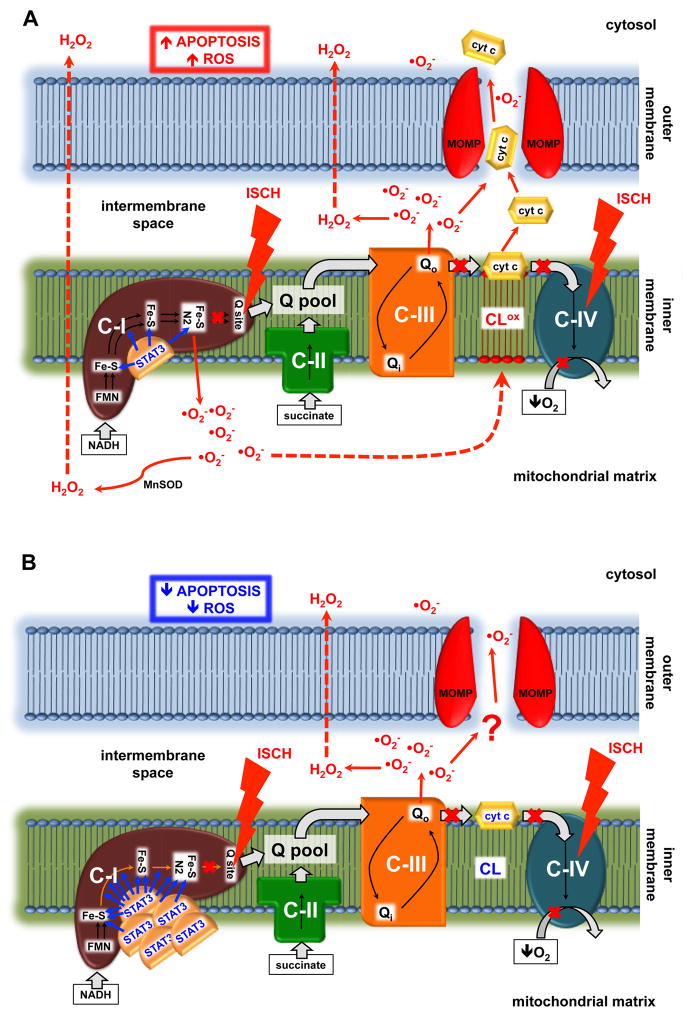
Figure 2. Postulated mechanism of the protective role of STAT3 in heart mitochondria during ischemia.
(A) In wild type mitochondria ischemia increases superoxide production from complex I that is directed toward the matrix. This results in cardiolipin oxidation and cytochrome c delocalization from the inner membrane. Further damage to the mitochondria leads to outer membrane permeabilization, which allows the release of cytochrome c from mitochondria and the subsequent induction of apoptosis. (B) Over expression of MLS-STAT3E in the mitochondria partially blocks electron flow through iron-sulfur clusters within complex I, resulting in blockade of superoxide generation from complex I during ischemia. This in turn decreases cardiolipin oxidation and preserves cytochrome c retention in the inner membrane. Less ROS and the lack of cytochrome c translocation into the cytosol attenuates apoptosis and increases cell viability during oxidative stress. STAT3, signal transducer and activator of transcription 3; C-I, II, III and IV, respiratory complex I, II, III and IV; cyt c, cytochrome c; Q, ubiquinone; FMN, flavin mononucleotide; Fe-S, iron-sulfur cluster; NADH, nicotinamide adenine dinucleotide; ISCH, ischemia; ROS, reactive oxygen species; MnSOD, mitochondrial manganese superoxide dismutase; CL, cardiolipin; CLox, oxidized cardiolipin; MOMP, mitochondrial outer membrane permeabilization.
Mitochondrial membrane potential (ΔΨ) is a key indicator of inner mitochondrial membrane intactness (Chen, 1988). The maximum membrane potential that mitochondria can generate was similar in mitochondria from MLS-STAT3E mice and wild type littermates (Szczepanek et al., 2011). Thus, despite the defect in complex I, mitochondrial targeted STAT3 over expression preserves a normal resting membrane potential. This finding is in contrast to impaired generation of membrane potential observed in mitochondria from STAT3−/− astrocytes (Sarafian et al., 2010). In addition to the decreased ΔΨ, these cells exhibit also attenuated ATP production, and demonstrate increased generation of ROS, which is even greater following rotenone administration (Sarafian et al., 2010). However, Gough et. al. reported no change in mitochondrial membrane potential in untransformed fibroblasts isolated from mouse STAT3−/− embyos (Gough et al., 2009). H-RasV12-transformed cells exhibited increased ΔΨ (Gough et al., 2009), which is a characteristic of cancer cells (Fantin et al., 2006). Interestingly though, the deletion of STAT3 enhanced already increased membrane potential of H-RasV12-transformed cells, which was further suppressed by mitochondrial-targeted STAT3 expression. The discrepancy between results obtained by both groups is unclear but might be a result of different cell types used and different methods employed for ΔΨ measurement. Unfortunately, there are no reports available that show the level of a membrane potential in the mitochondria isolated from hearts with deleted STAT3.
Since a partial blockade of electron transport was discovered in mitochondrial-targeted STAT3 mice, the response of these mitochondria to stress conditions was of substantial interest. Could targeting of STAT3 to mitochondria modulate the electron transport chain in a manner similar to pharmacologic approaches and post-translational modification? To answer this question, isolated hearts from the transgenic and wild type littermates underwent ischemia (Chen and Lesnefsky, 2009a; Szczepanek et al., 2011) in order to assess mitochondrial function following ischemic stress. As expected, ischemia decreased state 3 respiration in wild type controls with a complex I substrate (Szczepanek et al., 2011). In contrast, mitochondria containing the transgene exhibited a minimal additional decrease in complex I-driven state 3 respiration, indicating a protective role of mitochondrial targeted STAT3 during ischemia. Ischemia decreased the activity of complex I in wild type but not in transgenic hearts. Ischemia did not alter the activity of NADH dehydrogenase in wild type or transgenic hearts, indicating that the transgene attenuated ischemic damage to the distal part of complex I (Figure 2), protecting complex I-dependent respiration against ischemic damage.
Ischemia decreased mitochondrial cytochrome c content by greater than 30% in wild type mitochondria, whereas mitochondrial targeted STAT3 expression abrogated cytochrome c release during ischemia (Figure 1B). The retention of cytochrome c is expected to result in less cardiac injury following ischemia and reperfusion, as discussed earlier in this review.
The net release of H2O2 did not differ between transgenic and wild type mitochondria in the baseline state. Ischemia increased the production and release of H2O2 from wild type mitochondria respiring on glutamate+malate. In contrast, production was not increased in transgenic mitochondria (Figure 1B). In contrast to complex I, net production of ROS from complex III after ischemia was similar.
The lack of ischemia-mediated increase in H2O2 release from complex I observed in transgenic mitochondria suggests that the site of STAT3 interaction with complex I may be located proximal to the N2 cluster, providing a site of relative blockade within complex I immediately prior to the likely site of ROS production caused by ischemia. In sum, mitochondrial-targeted STAT3 contributes an important role in abolishing complex I-mediated production of reactive oxygen species during ischemia (Figure 2).
The oxidative physiology of mitochondrial-targeted over expression of STAT3 initially appeared to contradict the observation of mitochondrial function of STAT3 heart knockout mice (Wegrzyn et al., 2009). As discussed above, the complete absence of STAT3 in mitochondria results in a profound inhibition of complexes I and II (Wegrzyn et al., 2009) accompanied by a substantially decreased membrane potential, decreased ATP production, and enhanced ROS generation (Sarafian et al., 2010). In contrast, over expression of mitochondria-localized STAT3 leads to a partial blockade of complexes I and II without increased basal ROS production. Potentially more important, the ability to generate and maintain a membrane potential is preserved. In view of these contrasts, it is not surprising that hearts with mitochondrial-targeted over expression of STAT3 do not exhibit any signs of cardiomyopathy at one year of age (Szczepanek et al., 2011), in contrast to STAT3−/− mice (Jacoby et al., 2003). These distinctive models are reminiscent of the contrasts of a deleterious model of mitochondrial disease leading to cardiomyopathy (complete STAT3 knockout) compared to signaling-pathway mediated modulation of mitochondrial respiration (mitochondrial-targeted over expression of STAT3). The response of mitochondrial-targeted over expression of STAT3 during ischemia is reminiscent of approaches using pharmacologic blockade of complex I, which results in protection of mitochondria and the heart against ischemic injury (Chen et al., 2006b; Nadtochiy et al., 2009).
Although this incomplete blockade of complex I in mitochondrial-targeted STAT3 mitochondria protects against ischemic damage to complex I and inhibits the release of cytochrome c, it does not rescue cytochrome oxidase dysfunction (Szczepanek et al., 2011). This extent of protection is unlike the protection of respiration through cytochrome oxidase that is observed following essentially complete inhibition of complex I in hearts perfused with rotenone or amobarbital prior to ischemia, which prevents the damage to cytochrome oxidase (Chen et al., 2006b; Lesnefsky et al., 2004a). We speculate that STAT3 in the mitochondria attenuates generation of superoxide from complex I that is directed toward the matrix during ischemia (Figure 2), in turn decreasing the probability of cardiolipin oxidation and the subsequent delocalization of cytochrome c from the inner membrane. Further study is obviously needed to address these intriguing questions.
4. Future perspectives
Ischemia activates cytosolic STAT3 resulting in its subsequent translocation to the nucleus where it drives the expression of antiapoptotic and antioxidative genes (Levy and Lee, 2002; Negoro et al., 2001; Oshima et al., 2005). Interestingly, we have shown for the first time that ischemia also induced the translocation of STAT3 from cytosol to mitochondria (Szczepanek et al., 2011). We speculate that the activation of cytosolic STAT3 that results in nuclear import and execution of its cytoprotective transcriptional program, also enhances the mitochondrial localization of STAT3 to exert an acute cytoprotective modulation of respiration. We propose that the mitochondrial-targeted transgenic mouse is a model of the potential of STAT3 to protect mitochondria during stress. Future work is needed to understand the mechanisms that activate mitochondrial translocation of STAT3 during ischemia and reperfusion. Conceptually, while the metabolic “wiring” of the heart is being changed over the course of hours to days by the transcriptional responses of STAT3, the immediate effects of stress on the metabolic “machinery” are adjusted by the acute actions of mitochondrial STAT3. We propose a novel protective and transcriptionally-independent mechanism mediated by mitochondrial STAT3. The STAT3-dependent partial blockade of electron flow through complex I results in lower ROS production in the mitochondria and less cytochrome c release during ischemia. We postulate that during ischemia STAT3 is an antiapoptotic factor in the heart that works both as a signaling molecule involved in regulation of gene expression (Levy and Lee, 2002; Negoro et al., 2001; Oshima et al., 2005), and as a direct modulator of the electron transport chain (Szczepanek et al., 2011; Wegrzyn et al., 2009).
The potential beneficial effects of mitochondrial STAT3 in protecting the myocardium against ischemia/reperfusion-mediated damage need further investigation. It has been already reported that cardiotropin-1 administration prior to ischemia, which primarily activates STAT3, resulted in protection of both neonatal and adult cardiomyocytes against ischemia and reperfusion-induced apoptosis (Liao et al., 2002; Stephanou et al., 1998). A second approach that may be feasible is a gene therapy approach (Hajjar et al., 2000; Hajjar and Samulski, 2006) where an MLS-STAT3E-containing viral vector could be expressed in the heart in an inducible fashion. In addition to the endogenous STAT3 in cardiac tissue, the over expression of mitochondria-localized STAT3 that does not exert transcriptional activity but exhibits cardioprotective features is an intriguing candidate, especially in the elderly population, in which ischemic injury is increased (Lesnefsky et al., 1994; Lesnefsky et al., 2006; Lesnefsky et al., 1996; Lucas and Szweda, 1998).
Highlights.
This review addresses the importance of the down regulation of mitochondrial electron transport as a cytoprotective mechanism in response to pathologic settings where continued mitochondrial respiration produces cellular injury.
There are two parts of this review: the first summarizes different approaches used to regulate mitochondrial respiration by targeting complex I of ETC in order to promote cytoprotection.
The second part highlights the emerging role of signal transducer and activator of transcription 3 (STAT3) in the direct, non-transcriptional regulation of ETC.
The over expression of mitochondrial-targeted STAT3 results in a partial blockade of electron transport at complexes I and II that attenuates damage to mitochondria during cell stress.
The limitation of mitochondrial respiration under pathologic circumstances can be approached by activation and over expression of endogenous signaling mechanisms in addition to pharmacologic means.
Acknowledgments
This work was supported by NIH grants CA098924 (to A.C.L.) and 2PO1AG15885 (to E.J.L.), and the Office of Research and Development, Medical Research Service, Department of Veterans Affairs (to E.J.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldakkak M, Stowe DF, Chen Q, Lesnefsky EJ, Camara AK. Inhibited mitochondrial respiration by amobarbital during cardiac ischaemia improves redox state and reduces matrix Ca2+ overload and ROS release. Cardiovasc Res. 2008;77:406–415. doi: 10.1016/j.cardiores.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Ambrosio G, Zweier JL, Duilio C, Kuppusamy P, Santoro G, Elia PP, Tritto I, Cirillo P, Condorelli M, Chiariello M. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J Biol Chem. 1993;268:18532–18541. [PubMed] [Google Scholar]
- Antunes F, Cadenas E. The mechanism of cytochrome C oxidase inhibition by nitric oxide. Front Biosci. 2007;12:975–985. doi: 10.2741/2118. [DOI] [PubMed] [Google Scholar]
- Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004;61:461–470. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Becker LB, vanden Hoek TL, Shao ZH, Li CQ, Schumacker PT. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am J Physiol. 1999;277:H2240–2246. doi: 10.1152/ajpheart.1999.277.6.H2240. [DOI] [PubMed] [Google Scholar]
- Beer SM, Taylor ER, Brown SE, Dahm CC, Costa NJ, Runswick MJ, Murphy MP. Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: implications for mitochondrial redox regulation and antioxidant DEFENSE. J Biol Chem. 2004;279:47939–47951. doi: 10.1074/jbc.M408011200. [DOI] [PubMed] [Google Scholar]
- Belikova NA, Vladimirov YA, Osipov AN, Kapralov AA, Tyurin VA, Potapovich MV, Basova LV, Peterson J, Kurnikov IV, Kagan VE. Peroxidase activity and structural transitions of cytochrome c bound to cardiolipin-containing membranes. Biochemistry. 2006;45:4998–5009. doi: 10.1021/bi0525573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boengler K, Buechert A, Heinen Y, Roeskes C, Hilfiker-Kleiner D, Heusch G, Schulz R. Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circ Res. 2008a;102:131–135. doi: 10.1161/CIRCRESAHA.107.164699. [DOI] [PubMed] [Google Scholar]
- Boengler K, Hilfiker-Kleiner D, Drexler H, Heusch G, Schulz R. The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Ther. 2008b;120:172–185. doi: 10.1016/j.pharmthera.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Boengler K, Hilfiker-Kleiner D, Heusch G, Schulz R. Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol. 2010;105:771–785. doi: 10.1007/s00395-010-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boengler K, Konietzka I, Buechert A, Heinen Y, Garcia-Dorado D, Heusch G, Schulz R. Loss of ischemic preconditioning’s cardioprotection in aged mouse hearts is associated with reduced gap junctional and mitochondrial levels of connexin 43. Am J Physiol Heart Circ Physiol. 2007;292:H1764–1769. doi: 10.1152/ajpheart.01071.2006. [DOI] [PubMed] [Google Scholar]
- Bolli R, McCay PB. Use of spin traps in intact animals undergoing myocardial ischemia/reperfusion: a new approach to assessing the role of oxygen radicals in myocardial "stunning". Free Radic Res Commun. 1990;9:169–180. doi: 10.3109/10715769009145674. [DOI] [PubMed] [Google Scholar]
- Borutaite V, Brown GC. Mitochondria in apoptosis of ischemic heart. FEBS Lett. 2003;541:1–5. doi: 10.1016/s0014-5793(03)00278-3. [DOI] [PubMed] [Google Scholar]
- Borutaite V, Budriunaite A, Morkuniene R, Brown GC. Release of mitochondrial cytochrome c and activation of cytosolic caspases induced by myocardial ischaemia. Biochim Biophys Acta. 2001;1537:101–109. doi: 10.1016/s0925-4439(01)00062-x. [DOI] [PubMed] [Google Scholar]
- Borutaite V, Jekabsone A, Morkuniene R, Brown GC. Inhibition of mitochondrial permeability transition prevents mitochondrial dysfunction, cytochrome c release and apoptosis induced by heart ischemia. J Mol Cell Cardiol. 2003;35:357–366. doi: 10.1016/s0022-2828(03)00005-1. [DOI] [PubMed] [Google Scholar]
- Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J. 2006;394:627–634. doi: 10.1042/BJ20051435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, Williams GR, Hollunger G. Inhibition of electron and energy transfer in mitochondria. I. Effects of Amytal, thiopental, rotenone, progesterone, and methylene glycol. J Biol Chem. 1963;238:418–431. [PubMed] [Google Scholar]
- Chandel NS. Mitochondrial regulation of oxygen sensing. Adv Exp Med Biol. 2010;661:339–354. doi: 10.1007/978-1-60761-500-2_22. [DOI] [PubMed] [Google Scholar]
- Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CL, Zhang L, Yeh A, Chen CA, Green-Church KB, Zweier JL, Chen YR. Site-specific S-glutathiolation of mitochondrial NADH ubiquinone reductase. Biochemistry. 2007a;46:5754–5765. doi: 10.1021/bi602580c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LB. Mitochondrial membrane potential in living cells. Annu Rev Cell Biol. 1988;4:155–181. doi: 10.1146/annurev.cb.04.110188.001103. [DOI] [PubMed] [Google Scholar]
- Chen M, He H, Zhan S, Krajewski S, Reed JC, Gottlieb RA. Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. J Biol Chem. 2001;276:30724–30728. doi: 10.1074/jbc.M103701200. [DOI] [PubMed] [Google Scholar]
- Chen Q, Camara AK, Stowe DF, Hoppel CL, Lesnefsky EJ. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol. 2007b;292:C137–147. doi: 10.1152/ajpcell.00270.2006. [DOI] [PubMed] [Google Scholar]
- Chen Q, Hoppel CL, Lesnefsky EJ. Blockade of electron transport before cardiac ischemia with the reversible inhibitor amobarbital protects rat heart mitochondria. J Pharmacol Exp Ther. 2006a;316:200–207. doi: 10.1124/jpet.105.091702. [DOI] [PubMed] [Google Scholar]
- Chen Q, Lesnefsky E. Incubation model for the study of mouse heart mitochondria during ischemia (Abstract) J Investig Med. 2009a;57:558. [Google Scholar]
- Chen Q, Lesnefsky E. BOOK CHAPTER. The Research Signpost/Transworld Research Network; 2009b. Mitochondria and cardiac injury: A journey from reperfusion to ischemia and back again. [Google Scholar]
- Chen Q, Lesnefsky EJ. Depletion of cardiolipin and cytochrome c during ischemia increases hydrogen peroxide production from the electron transport chain. Free Radic Biol Med. 2006;40:976–982. doi: 10.1016/j.freeradbiomed.2005.10.043. [DOI] [PubMed] [Google Scholar]
- Chen Q, Lesnefsky EJ. Blockade of electron transport preserves the contents of bcl-2 and cytochrome c in subsarcolemmal mitochondria during ischemia. Circulation. 2007;115:abstratct 16690. [Google Scholar]
- Chen Q, Lesnefsky EJ. Ischemic damage to the mitochondrial electron transport chain favors opening of the permeability transition pore. FASEB J. 2008;22:E345, abstract 750.346. [Google Scholar]
- Chen Q, Lesnefsky EJ. Blockade of electron transport during ischemia preserves bcl-2 and inhibits opening of the mitochondrial permeability transition pore. FEBS Lett. 2011;585:921–926. doi: 10.1016/j.febslet.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J Pharmacol Exp Ther. 2006b;319:1405–1412. doi: 10.1124/jpet.106.110262. [DOI] [PubMed] [Google Scholar]
- Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol. 2008;294:C460–466. doi: 10.1152/ajpcell.00211.2007. [DOI] [PubMed] [Google Scholar]
- Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: Central role of complex III. J Biol Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- Chen Q, Yin G, Stewart S, Hu Y, Lesnefsky EJ. Isolating the segment of the mitochondrial electron transport chain responsible for mitochondrial damage during cardiac ischemia. Biochem Biophys Res Commun. 2010;397:656–660. doi: 10.1016/j.bbrc.2010.05.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YR, Chen CL, Pfeiffer DR, Zweier JL. Mitochondrial complex II in the post-ischemic heart: oxidative injury and the role of protein S-glutathionylation. J Biol Chem. 2007c;282:32640–32654. doi: 10.1074/jbc.M702294200. [DOI] [PubMed] [Google Scholar]
- Cimen H, Han MJ, Yang Y, Tong Q, Koc H, Koc EC. Regulation of Succinate Dehydrogenase Activity by SIRT3 in Mammalian Mitochondria. Biochemistry. 2009 doi: 10.1021/bi901627u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J Bioenerg Biomembr. 2008;40:533–539. doi: 10.1007/s10863-008-9166-6. [DOI] [PubMed] [Google Scholar]
- Cooper CE, Davies NA, Psychoulis M, Canevari L, Bates TE, Dobbie MS, Casley CS, Sharpe MA. Nitric oxide and peroxynitrite cause irreversible increases in the K(m) for oxygen of mitochondrial cytochrome oxidase: in vitro and in vivo studies. Biochim Biophys Acta. 2003;1607:27–34. doi: 10.1016/j.bbabio.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Davies MJ. Direct detection of radical production in the ischaemic and reperfused myocardium: current status. Free Radic Res Commun. 1989;7:275–284. doi: 10.3109/10715768909087952. [DOI] [PubMed] [Google Scholar]
- Degli Esposti M. Inhibitors of NADH-ubiquinone reductase: an overview. Biochim Biophys Acta. 1998;1364:222–235. doi: 10.1016/s0005-2728(98)00029-2. [DOI] [PubMed] [Google Scholar]
- Edmond JC. Mitochondrial disorders. Int Ophthalmol Clin. 2009;49:27–33. doi: 10.1097/IIO.0b013e3181a8de58. [DOI] [PubMed] [Google Scholar]
- Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fannin SW, Lesnefsky EJ, Slabe TJ, Hassan MO, Hoppel CL. Aging selectively decreases oxidative capacity in rat heart interfibrillar mitochondria. Arch Biochem Biophys. 1999;372:399–407. doi: 10.1006/abbi.1999.1508. [DOI] [PubMed] [Google Scholar]
- Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Flameng W, Andres J, Ferdinande P, Mattheussen M, Van Belle H. Mitochondrial function in myocardial stunning. J Mol Cell Cardiol. 1991;23:1–11. doi: 10.1016/0022-2828(91)90034-j. [DOI] [PubMed] [Google Scholar]
- Genova ML, Ventura B, Giuliano G, Bovina C, Formiggini G, Parenti Castelli G, Lenaz G. The site of production of superoxide radical in mitochondrial Complex I is not a bound ubisemiquinone but presumably iron-sulfur cluster N2. FEBS Lett. 2001;505:364–368. doi: 10.1016/s0014-5793(01)02850-2. [DOI] [PubMed] [Google Scholar]
- Gomez L, Paillard M, Allegood JC, Hait NC, Price MM, Chen Q, Milstien S, Spiegel S, Lesnefsky EJ. Failure to Precondition SK2-KO Mice: Not Without RISK! (BCVS abstract P173. [Accessed: December 23, 2010];2010 Published online: http://www.americanheart.org/presenter.jhtml?identifier=3074181.
- Gomez L, Paillard M, Thibault H, Derumeaux G, Ovize M. Inhibition of GSK3beta by postconditioning is required to prevent opening of the mitochondrial permeability transition pore during reperfusion. Circulation. 2008;117:2761–2768. doi: 10.1161/CIRCULATIONAHA.107.755066. [DOI] [PubMed] [Google Scholar]
- Gottlieb RA. Mitochondrial signaling in apoptosis: mitochondrial daggers to the breaking heart. Basic Res Cardiol. 2003;98:242–249. doi: 10.1007/s00395-003-0404-0. [DOI] [PubMed] [Google Scholar]
- Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest. 1994;94:1621–1628. doi: 10.1172/JCI117504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough DJ, Corlett A, Schlessinger K, Wegrzyn J, Larner AC, Levy DE. Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science. 2009;324:1713–1716. doi: 10.1126/science.1171721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson AB, Gottlieb RA. Bcl-2 family members and apoptosis, taken to heart. Am J Physiol Cell Physiol. 2007;292:C45–51. doi: 10.1152/ajpcell.00229.2006. [DOI] [PubMed] [Google Scholar]
- Hajjar RJ, del Monte F, Matsui T, Rosenzweig A. Prospects for gene therapy for heart failure. Circ Res. 2000;86:616–621. doi: 10.1161/01.res.86.6.616. [DOI] [PubMed] [Google Scholar]
- Hajjar RJ, Samulski RJ. Heart failure: a silver bullet to treat heart failure. Gene Ther. 2006;13:997. doi: 10.1038/sj.gt.3302747. [DOI] [PubMed] [Google Scholar]
- Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278:5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. The mitochondrial permeability transition pore: its fundamental role in mediating cell death during ischaemia and reperfusion. J Mol Cell Cardiol. 2003;35:339–341. doi: 10.1016/s0022-2828(03)00043-9. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Henze K, Martin W. Evolutionary biology: essence of mitochondria. Nature. 2003;426:127–128. doi: 10.1038/426127a. [DOI] [PubMed] [Google Scholar]
- Hilfiker-Kleiner D, Hilfiker A, Drexler H. Many good reasons to have STAT3 in the heart. Pharmacol Ther. 2005;107:131–137. doi: 10.1016/j.pharmthera.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, Quint A, Landmesser U, Doerries C, Luchtefeld M, Poli V, Schneider MD, Balligand JL, Desjardins F, Ansari A, Struman I, Nguyen NQ, Zschemisch NH, Klein G, Heusch G, Schulz R, Hilfiker A, Drexler H. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128:589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- Hoppel CL, Tandler B, Fujioka H, Riva A. Dynamic organization of mitochondria in human heart and in myocardial disease. Int J Biochem Cell Biol. 2009;41:1949–1956. doi: 10.1016/j.biocel.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horgan DJ, Singer TP, Casida JE. Studies on the respiratory chain-linked reduced nicotinamide adenine dinucleotide dehydrogenase. 13. Binding sites of rotenone, piericidin A, and amytal in the respiratory chain. J Biol Chem. 1968;243:834–843. [PubMed] [Google Scholar]
- Ilangovan G, Liebgott T, Kutala VK, Petryakov S, Zweier JL, Kuppusamy P. EPR oximetry in the beating heart: myocardial oxygen consumption rate as an index of postischemic recovery. Magn Reson Med. 2004;51:835–842. doi: 10.1002/mrm.20000. [DOI] [PubMed] [Google Scholar]
- Ingraham CA, Burwell LS, Skalska J, Brookes PS, Howell RL, Sheu SS, Pinkert CA. NDUFS4: creation of a mouse model mimicking a Complex I disorder. Mitochondrion. 2009;9:204–210. doi: 10.1016/j.mito.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby JJ, Kalinowski A, Liu MG, Zhang SS, Gao Q, Chai GX, Ji L, Iwamoto Y, Li E, Schneider M, Russell KS, Fu XY. Cardiomyocyte-restricted knockout of STAT3 results in higher sensitivity to inflammation, cardiac fibrosis, and heart failure with advanced age. Proc Natl Acad Sci U S A. 2003;100:12929–12934. doi: 10.1073/pnas.2134694100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- Kevin LG, Camara AK, Riess ML, Novalija E, Stowe DF. Ischemic preconditioning alters real-time measure of O2 radicals in intact hearts with ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2003;284:H566–574. doi: 10.1152/ajpheart.00711.2002. [DOI] [PubMed] [Google Scholar]
- Koopman WJ, Nijtmans LG, Dieteren CE, Roestenberg P, Valsecchi F, Smeitink JA, Willems PH. Mammalian mitochondrial complex I: Biogenesis, Regulation and Reactive Oxygen Species generation. Antioxid Redox Signal. 2009 doi: 10.1089/ars.2009.2743. [DOI] [PubMed] [Google Scholar]
- Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7:1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- Kruse SE, Watt WC, Marcinek DJ, Kapur RP, Schenkman KA, Palmiter RD. Mice with mitochondrial complex I deficiency develop a fatal encephalomyopathy. Cell Metab. 2008;7:312–320. doi: 10.1016/j.cmet.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudin AP, Bimpong-Buta NY, Vielhaber S, Elger CE, Kunz WS. Characterization of superoxide-producing sites in isolated brain mitochondria. J Biol Chem. 2004;279:4127–4135. doi: 10.1074/jbc.M310341200. [DOI] [PubMed] [Google Scholar]
- Kunisada K, Negoro S, Tone E, Funamoto M, Osugi T, Yamada S, Okabe M, Kishimoto T, Yamauchi-Takihara K. Signal transducer and activator of transcription 3 in the heart transduces not only a hypertrophic signal but a protective signal against doxorubicin-induced cardiomyopathy. Proc Natl Acad Sci U S A. 2000;97:315–319. doi: 10.1073/pnas.97.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnareva Y, Murphy AN, Andreyev A. Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem J. 2002;368:545–553. doi: 10.1042/BJ20021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacerda L, Somers S, Opie LH, Lecour S. Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res. 2009;84:201–208. doi: 10.1093/cvr/cvp274. [DOI] [PubMed] [Google Scholar]
- Lee JW, Bobst EV, Wang YG, Ashraf MM, Bobst AM. Increased endogenous ascorbyl free radical formation with singlet oxygen scavengers in reperfusion injury: an EPR and functional recovery study in rat hearts. Cell Mol Biol (Noisy-le-grand) 2000;46:1383–1395. [PubMed] [Google Scholar]
- Lesnefsky EJ, Chen Q, Moghaddas S, Hassan MO, Tandler B, Hoppel CL. Blockade of electron transport during ischemia protects cardiac mitochondria. J Biol Chem. 2004a;279:47961–47967. doi: 10.1074/jbc.M409720200. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, Chen Q, Moghaddas S, Hassan MO, Tandler B, Hoppel CL. Blockade of electron transport during Ischemia protects cardiac mitochondria. J Biol Chem. 2004b;279:47961–47967. doi: 10.1074/jbc.M409720200. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, Chen Q, Slabe TJ, Stoll MS, Minkler PE, Hassan MO, Tandler B, Hoppel CL. Ischemia, rather than reperfusion, inhibits respiration through cytochrome oxidase in the isolated, perfused rabbit heart: role of cardiolipin. Am J Physiol Heart Circ Physiol. 2004c;287:H258–267. doi: 10.1152/ajpheart.00348.2003. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, Gallo DS, Ye J, Whittingham TS, Lust WD. Aging increases ischemia-reperfusion injury in the isolated, buffer-perfused heart. J Lab Clin Med. 1994;124:843–851. [PubMed] [Google Scholar]
- Lesnefsky EJ, Gudz TI, Migita CT, Ikeda-Saito M, Hassan MO, Turkaly PJ, Hoppel CL. Ischemic injury to mitochondrial electron transport in the aging heart: damage to the iron-sulfur protein subunit of electron transport complex III. Arch Biochem Biophys. 2001a;385:117–128. doi: 10.1006/abbi.2000.2066. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, Gudz TI, Moghaddas S, Migita CT, Ikeda_Saito M, Turkaly PJ, Hoppel CL. Aging decreases electron transport complex III activity in heart interfibrillar mitochondria by alteration of the cytochrome c binding site. J Mol Cell Cardiol. 2001b;33:37–47. doi: 10.1006/jmcc.2000.1273. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, He D, Moghaddas S, Hoppel CL. Reversal of mitochondrial defects before ischemia protects the aged heart. Faseb J. 2006;20:1543–1545. doi: 10.1096/fj.05-4535fje. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, Lundergan CF, Hodgson JM, Nair R, Reiner JS, Greenhouse SW, Califf RM, Ross AM. Increased left ventricular dysfunction in elderly patients despite successful thrombolysis: the GUSTO-I angiographic experience. J Am Coll Cardiol. 1996;28:331–337. doi: 10.1016/0735-1097(96)00148-9. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia--reperfusion, aging, and heart failure. J Mol Cell Cardiol. 2001c;33:1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia-reperfusion, aging, and heart failure. J Mol Cell Cardiol. 2001d;33:1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, Slabe TJ, Stoll MS, Minkler PE, Hoppel CL. Myocardial ischemia selectively depletes cardiolipin in rabbit heart subsarcolemmal mitochondria. Am J Physiol. 2001e;280:H2770–2778. doi: 10.1152/ajpheart.2001.280.6.H2770. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, Tandler B, Ye J, Slabe TJ, Turkaly J, Hoppel CL. Myocardial ischemia decreases oxidative phosphorylation through cytochrome oxidase in subsarcolemmal mitochondria. Am J Physiol. 1997;273:H1544–1554. doi: 10.1152/ajpheart.1997.273.3.H1544. [DOI] [PubMed] [Google Scholar]
- Levy DE, Lee CK. What does Stat3 do? J Clin Invest. 2002;109:1143–1148. doi: 10.1172/JCI15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, Robinson JP. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem. 2003;278:8516–8525. doi: 10.1074/jbc.M210432200. [DOI] [PubMed] [Google Scholar]
- Liao Z, Brar BK, Cai Q, Stephanou A, O’Leary RM, Pennica D, Yellon DM, Latchman DS. Cardiotrophin-1 (CT-1) can protect the adult heart from injury when added both prior to ischaemia and at reperfusion. Cardiovasc Res. 2002;53:902–910. doi: 10.1016/s0008-6363(01)00531-4. [DOI] [PubMed] [Google Scholar]
- Lucas DT, Szweda LI. Cardiac reperfusion injury: aging, lipid peroxidation, and mitochondrial dysfunction. Proc Natl Acad Sci U S A. 1998;95:510–514. doi: 10.1073/pnas.95.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maupoil V, Rochette L, Tabard A, Clauser P, Harpey C. Evolution of free radical formation during low-flow ischemia and reperfusion in isolated rat heart. Cardiovasc Drugs Ther. 1990;4:791–795. doi: 10.1007/BF00051276. [DOI] [PubMed] [Google Scholar]
- McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16:R551–560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Merrill GF. Acetaminophen and low-flow myocardial ischemia: efficacy and antioxidant mechanisms. Am J Physiol Heart Circ Physiol. 2002;282:H1341–1349. doi: 10.1152/ajpheart.00716.2001. [DOI] [PubMed] [Google Scholar]
- Murphy E, Steenbergen C. Cardioprotection in females: a role for nitric oxide and altered gene expression. Heart Fail Rev. 2007;12:293–300. doi: 10.1007/s10741-007-9035-0. [DOI] [PubMed] [Google Scholar]
- Nadtochiy SM, Burwell LS, Brookes PS. Cardioprotection and mitochondrial S-nitrosation: effects of S-nitroso-2-mercaptopropionyl glycine (SNO-MPG) in cardiac ischemia-reperfusion injury. J Mol Cell Cardiol. 2007;42:812–825. doi: 10.1016/j.yjmcc.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadtochiy SM, Burwell LS, Ingraham CA, Spencer CM, Friedman AE, Pinkert CA, Brookes PS. In vivo cardioprotection by S-nitroso-2-mercaptopropionyl glycine. J Mol Cell Cardiol. 2009;46:960–968. doi: 10.1016/j.yjmcc.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negoro S, Kunisada K, Fujio Y, Funamoto M, Darville MI, Eizirik DL, Osugi T, Izumi M, Oshima Y, Nakaoka Y, Hirota H, Kishimoto T, Yamauchi-Takihara K. Activation of signal transducer and activator of transcription 3 protects cardiomyocytes from hypoxia/reoxygenation-induced oxidative stress through the upregulation of manganese superoxide dismutase. Circulation. 2001;104:979–981. doi: 10.1161/hc3401.095947. [DOI] [PubMed] [Google Scholar]
- O’Rourke B, Cortassa S, Aon MA. Mitochondrial ion channels: gatekeepers of life and death. Physiology (Bethesda) 2005;20:303–315. doi: 10.1152/physiol.00020.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi ST, Ohnishi T, Muranaka S, Fujita H, Kimura H, Uemura K, Yoshida K, Utsumi K. A possible site of superoxide generation in the complex I segment of rat heart mitochondria. J Bioenerg Biomembr. 2005;37:1–15. doi: 10.1007/s10863-005-4117-y. [DOI] [PubMed] [Google Scholar]
- Oshima Y, Fujio Y, Nakanishi T, Itoh N, Yamamoto Y, Negoro S, Tanaka K, Kishimoto T, Kawase I, Azuma J. STAT3 mediates cardioprotection against ischemia/reperfusion injury through metallothionein induction in the heart. Cardiovasc Res. 2005;65:428–435. doi: 10.1016/j.cardiores.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci U S A. 2002;99:1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillard M, Gomez L, Augeul L, Loufouat J, Lesnefsky EJ, Ovize M. Postconditioning inhibits mPTP opening independent of oxidative phosphorylation and membrane potential. J Mol Cell Cardiol. 2009;46:902–909. doi: 10.1016/j.yjmcc.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Paradies G, Ruggiero FM, Petrosillo G, Quagliariello E. Peroxidative damage to cardiac mitochondria: cytochrome oxidase and cardiolipin alterations. FEBS Lett. 1998;424:155–158. doi: 10.1016/s0014-5793(98)00161-6. [DOI] [PubMed] [Google Scholar]
- Park JW, Chun YS, Kim YH, Kim CH, Kim MS. Ischemic preconditioning reduces Op6 generation and prevents respiratory impairment in the mitochondria of post-ischemic reperfused heart of rat. Life Sci. 1997;60:2207–2219. doi: 10.1016/s0024-3205(97)00236-1. [DOI] [PubMed] [Google Scholar]
- Pasdois P, Beauvoit B, Costa AD, Vinassa B, Tariosse L, Bonoron-Adele S, Garlid KD, Dos Santos P. Sarcoplasmic ATP-sensitive potassium channel blocker HMR1098 protects the ischemic heart: implication of calcium, complex I, reactive oxygen species and mitochondrial ATP-sensitive potassium channel. J Mol Cell Cardiol. 2007;42:631–642. doi: 10.1016/j.yjmcc.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Phillips D, Aponte AM, Covian R, Balaban RS. Intrinsic protein kinase activity in mitochondrial oxidative phosphorylation complexes. Biochemistry. 2011;50:2515–2529. doi: 10.1021/bi101434x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D, Reilley MJ, Aponte AM, Wang G, Boja E, Gucek M, Balaban RS. Stoichiometry of STAT3 and mitochondrial proteins: Implications for the regulation of oxidative phosphorylation by protein-protein interactions. J Biol Chem. 2010;285:23532–23536. doi: 10.1074/jbc.C110.152652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietri S, Culcasi M, Cozzone PJ. Real-time continuous-flow spin trapping of hydroxyl free radical in the ischemic and post-ischemic myocardium. Eur J Biochem. 1989;186:163–173. doi: 10.1111/j.1432-1033.1989.tb15191.x. [DOI] [PubMed] [Google Scholar]
- Piper HM, Sezer O, Schleyer M, Schwartz P, Hutter JF, Spieckermann PG. Development of ischemia-induced damage in defined mitochondrial subpopulations. J Mol Cell Cardiol. 1985;17:885–896. doi: 10.1016/s0022-2828(85)80102-4. [DOI] [PubMed] [Google Scholar]
- Riess ML, Camara AK, Kevin LG, An J, Stowe DF. Reduced reactive O2 species formation and preserved mitochondrial NADH and [Ca2+] levels during short-term 17 degrees C ischemia in intact hearts. Cardiovasc Res. 2004;61:580–590. doi: 10.1016/j.cardiores.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Robinson NC, Strey F, Talbert L. Investigation of the essential boundary layer phospholipids of cytochrome c oxidase using Triton X-100 delipidation. Biochemistry. 1980;19:3656–3661. doi: 10.1021/bi00557a003. [DOI] [PubMed] [Google Scholar]
- Rouslin W. Mitochondrial complexes I, II, III, IV, and V in myocardial ischemia and autolysis. Am J Physiol. 1983;244:H743–748. doi: 10.1152/ajpheart.1983.244.6.H743. [DOI] [PubMed] [Google Scholar]
- Salloum FN, Chau VQ, Hoke NN, Abbate A, Varma A, Ockaili RA, Toldo S, Kukreja RC. Phosphodiesterase-5 inhibitor, tadalafil, protects against myocardial ischemia/reperfusion through protein-kinase g-dependent generation of hydrogen sulfide. Circulation. 2009;120:S31–36. doi: 10.1161/CIRCULATIONAHA.108.843979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafian TA, Montes C, Imura T, Qi J, Coppola G, Geschwind DH, Sofroniew MV. Disruption of astrocyte STAT3 signaling decreases mitochondrial function and increases oxidative stress in vitro. PLoS One. 2010;5:e9532. doi: 10.1371/journal.pone.0009532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauve AA, Wolberger C, Schramm VL, Boeke JD. The biochemistry of sirtuins. Annu Rev Biochem. 2006;75:435–465. doi: 10.1146/annurev.biochem.74.082803.133500. [DOI] [PubMed] [Google Scholar]
- Schulman D, Latchman DS, Yellon DM. Effect of aging on the ability of preconditioning to protect rat hearts from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2001;281:H1630–1636. doi: 10.1152/ajpheart.2001.281.4.H1630. [DOI] [PubMed] [Google Scholar]
- Shidoji Y, Hayashi K, Komura S, Ohishi N, Yagi K. Loss of molecular interaction between cytochrome c and cardiolipin due to lipid peroxidation. Biochem Biophys Res Commun. 1999;264:343–347. doi: 10.1006/bbrc.1999.1410. [DOI] [PubMed] [Google Scholar]
- Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RM, Suleman N, Lacerda L, Opie LH, Akira S, Chien KR, Sack MN. Genetic depletion of cardiac myocyte STAT-3 abolishes classical preconditioning. Cardiovasc Res. 2004;63:611–616. doi: 10.1016/j.cardiores.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Spiegel HE, Wainio WW. Some features of barbiturate interaction and inhibition of NADH-cytochrome C oxidoreductase in respiring systems. J Pharmacol Exp Ther. 1969;165:23–29. [PubMed] [Google Scholar]
- Stephanou A, Brar B, Heads R, Knight RD, Marber MS, Pennica D, Latchman DS. Cardiotrophin-1 induces heat shock protein accumulation in cultured cardiac cells and protects them from stressful stimuli. J Mol Cell Cardiol. 1998;30:849–855. doi: 10.1006/jmcc.1998.0651. [DOI] [PubMed] [Google Scholar]
- Stewart S, Lesnefsky EJ, Chen Q. Reversible blockade of electron transport with amobarbital at the onset of reperfusion attenuates cardiac injury. Transl Res. 2009;153:224–231. doi: 10.1016/j.trsl.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Stowe DF, Aldakkak M, Camara AK, Riess ML, Heinen A, Varadarajan SG, Jiang MT. Cardiac mitochondrial preconditioning by Big Ca2+-sensitive K+ channel opening requires superoxide radical generation. Am J Physiol Heart Circ Physiol. 2006;290:H434–440. doi: 10.1152/ajpheart.00763.2005. [DOI] [PubMed] [Google Scholar]
- Strub GM, Paillard M, Liang J, Gomez L, Allegood JC, Hait NC, Maceyka M, Price MM, Chen Q, Simpson DC, Kordula T, Milstien S, Lesnefsky EJ, Spiegel S. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J. 2010 doi: 10.1096/fj.10-167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanek K, Chen Q, Derecka M, Salloum FN, Zhang Q, Szelag M, Cichy J, Kukreja RC, Dulak J, Lesnefsky EJ, Larner AC. Mitochondrial-targeted signal transducer and activator of transcription (STAT3) protects against ischemia-induced changes in the electron transport chain and the generation of reactive oxygen species. J Biol Chem. 2011 doi: 10.1074/jbc.M111.226209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor ER, Hurrell F, Shannon RJ, Lin TK, Hirst J, Murphy MP. Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J Biol Chem. 2003;278:19603–19610. doi: 10.1074/jbc.M209359200. [DOI] [PubMed] [Google Scholar]
- Tompkins AJ, Burwell LS, Digerness SB, Zaragoza C, Holman WL, Brookes PS. Mitochondrial dysfunction in cardiac ischemia-reperfusion injury: ROS from complex I, without inhibition. Biochim Biophys Acta. 2006;1762:223–231. doi: 10.1016/j.bbadis.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Ueta H, Ogura R, Sugiyama M, Kagiyama A, Shin G. O2−· spin trapping on cardiac submitochondrial particles isolated from ischemic and non-ischemic myocardium. J Mol Cell Cardiol. 1990;22:893–899. doi: 10.1016/0022-2828(90)90120-q. [DOI] [PubMed] [Google Scholar]
- Uren RT, Dewson G, Chen L, Coyne SC, Huang DC, Adams JM, Kluck RM. Mitochondrial permeabilization relies on BH3 ligands engaging multiple prosurvival Bcl-2 relatives, not Bak. J Cell Biol. 2007;177:277–287. doi: 10.1083/jcb.200606065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Hoek TL, Li C, Shao Z, Schumacker PT, Becker LB. Significant levels of oxidants are generated by isolated cardiomyocytes during ischemia prior to reperfusion. J Mol Cell Cardiol. 1997;29:2571–2583. doi: 10.1006/jmcc.1997.0497. [DOI] [PubMed] [Google Scholar]
- Verdin E, Hirschey MD, Finley LW, Haigis MC. Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci. 2010;35:669–675. doi: 10.1016/j.tibs.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey DA, Kelley M, Li L, Huang Y. Sphingosine protects aging hearts from ischemia/reperfusion injury: Superiority to sphingosine 1-phosphate and ischemic pre- and postconditioning. Oxid Med Cell Longev. 2009;2:146–151. doi: 10.4161/oxim.2.3.8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey DA, Li L, Jin Z-Q, Kelley M, Honbo N, Zhang J, Karliner S. A Sphingosine Kinase Form 2 Knockout Sensitizes Mouse Myocardium to Ischemia/Reoxygenation Injury and Diminishes Responsiveness to Ischemic Preconditioning. Oxid Med Cell Longev. 2011:2011. doi: 10.1155/2011/961059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vik SB, Capaldi RA. Lipid requirements for cytochrome c oxidase activity. Biochemistry. 1977;16:5755–5759. doi: 10.1021/bi00645a016. [DOI] [PubMed] [Google Scholar]
- Vlasova II, Tyurin VA, Kapralov AA, Kurnikov IV, Osipov AN, Potapovich MV, Stoyanovsky DA, Kagan VE. Nitric oxide inhibits peroxidase activity of cytochrome c.cardiolipin complex and blocks cardiolipin oxidation. J Biol Chem. 2006;281:14554–14562. doi: 10.1074/jbc.M509507200. [DOI] [PubMed] [Google Scholar]
- Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, Koeck T, Derecka M, Szczepanek K, Szelag M, Gornicka A, Moh A, Moghaddas S, Chen Q, Bobbili S, Cichy J, Dulak J, Baker DP, Wolfman A, Stuehr D, Hassan MO, Fu XY, Avadhani N, Drake JI, Fawcett P, Lesnefsky EJ, Larner AC. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323:793–797. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol. 2005;17:617–625. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Liu X, Lee CP, Chua BH, Ho YS. Attenuation of doxorubicin-induced contractile and mitochondrial dysfunction in mouse heart by cellular glutathione peroxidase. Free Radic Biol Med. 2006;41:46–55. doi: 10.1016/j.freeradbiomed.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Zhu SG, Kukreja RC, Das A, Chen Q, Lesnefsky EJ, Xi L. Dietary nitrate supplementation protects against Doxorubicin-induced cardiomyopathy by improving mitochondrial function. J Am Coll Cardiol. 2011;57:2181–2189. doi: 10.1016/j.jacc.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]