Abstract
Adenosine released during myocardial ischemia mediates cardioprotective preconditioning. Multivalent drugs covalently bound to nanocarriers may differ greatly in chemical and biological properties from the corresponding monomeric agents. Here, we conjugated chemically functionalized nucleosides to poly(amidoamine) (PAMAM) dendrimeric polymers and investigated their effects in rat primary cardiac cell cultures and in the isolated heart. Three conjugates of A3 adenosine receptor (AR) agonists, chain-functionalized at the C2 or N6 position, were cardioprotective, with greater potency than monomeric agonist Cl-IB-MECA. Multivalent amide-linked MRS5216 was selective for A1 and A3ARs, and triazole-linked MRS5246 and MRS5539 (optionally containing fluorescent label) were A3AR-selective. The conjugates protected ischemic rat cardiomyocytes, an effect blocked by an A3AR antagonist MRS1523, and isolated hearts with significantly improved infarct size, rate of pressure product, and rate of contraction and relaxation. Thus, strategically derivatized nucleosides tethered to biocompatible polymeric carriers display enhanced cardioprotective potency via activation of A3AR on the cardiomyocyte surface.
Keywords: dendrimer, cardiomyocyte, adenosine receptor, ischemia, isolated heart, rat
1. Introduction
The nucleoside adenosine is released in large amounts during myocardial ischemia and plays a major role in mediating preconditioning and other cardioprotective effects in most animal species, including humans [1–3]. Four subtypes of adenosine receptors (ARs), which belong to the rhodopsin family of G protein-coupled receptors (GPCRs), are defined, but only three (A1, A2A and A3 ARs) are functionally expressed in cardiomyocytes [4]. Adenosine released during ischemia and ischemia/reperfusion is cardioprotective. Activation of cardiomyocyte A1 and A3ARs has been shown to effectively limit infarct size and reduce contractile dysfunction in various animal models of ischemia/reperfusion injury, and A2AAR agonists are cardioprotective via an antiinflammatory effect [5]. A3AR agonists have also displayed protective effects in ischemic models of the nervous system, lung and skeletal muscle [6–8]. Activation of this receptor preconditions cardiomyocytes in culture, in isolated hearts, and in vivo protects against the damaging effects of ischemia/reperfusion injury. A3AR agonists are attractive as cardioprotective agents because they do not alter systemic hemodynamic parameters in non-rodent species and are effective upon preischemic administration or only during reperfusion [8–13]. The prototypical A3AR agonists N6-(3-iodobenzyl)-adenosine-5′-N-methylcarboxamide (IB-MECA) and its 2-chloro derivative Cl-IB-MECA have been tested in experimental animal models of ischemia/reperfusion injury [14,15]. Knowing that the hypertrophied heart is more vulnerable to ischemic injury, we have found that selective activation of either A1AR by 2-chloro-N6-cyclopentyladenosine (CCPA) or A3AR by Cl-IB-MECA in the normal and hypertrophied heart was cardioprotective against ischemia reperfusion injury [15].
The structure activity relationship (SAR) of nucleoside derivatives as agonists at the ARs and their associated signaling pathways has been extensively studied [16,17], and we recently focused on extending this analysis to multivalent nucleoside conjugates of poly (amidoamine) (PAMAM) dendrimers. PAMAM dendrimers are treelike polymers with wide application in drug delivery in vivo [18]. PAMAM dendrimers are polyamide-like in structure and as such generally biocompatible. Assuming proper functionalization of a ligand for covalent conjugation, the resulting GPCR ligand-dendrimer (GLiDe) conjugates have displayed dramatically increased potency or selectivity in comparison to the monomeric, small molecular ligands [19–22]. The use of nanocarriers for stably conjugated drugs that act at the cell surface may provide pharmacokinetic and pharmacodynamic advantages, such as impeded metabolic degradation or the possibility of tissue selectivity [18, 22–24].
The aim of our study was to investigate the cardioprotective effects in neonatal rat primary cardiac cell cultures and in isolated heart models (12-week old rats) of two recently reported multivalent A3AR agonists [25], MRS5216 1 and MRS5246 2, and a newly synthesized conjugate MRS5539 6 (Figure 1), which allows for the incorporation of a fluorescent reporter group. These structural variations of GLiDe conjugates differed in the linkage method, attachment point on the adenosine pharmacophore, size of the PAMAM dendrimer carrier, and degree of substitution of peripheral functional groups. High A3AR affinity and potent cardioprotective activity was demonstrated for these three conjugates.
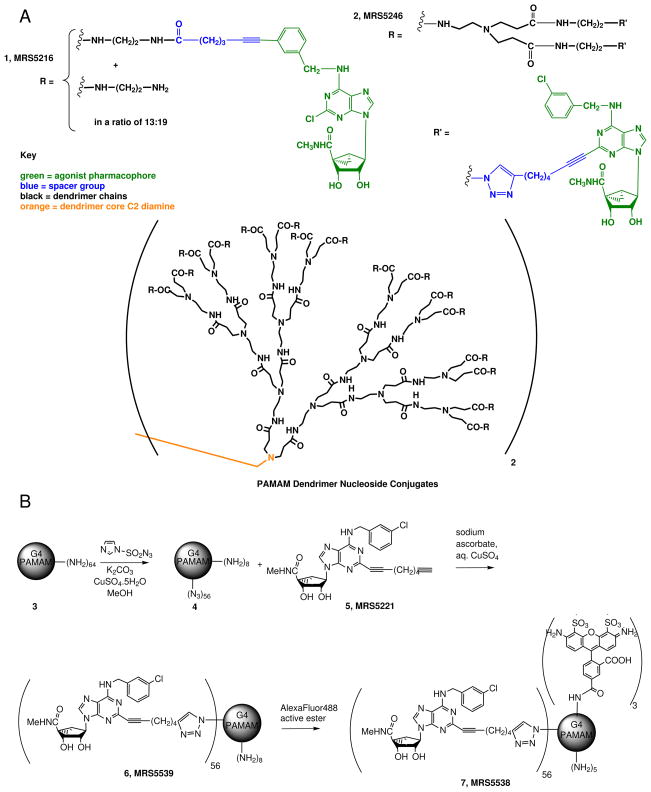
Figure 1.
The structures of the dendrimeric derivatives. A. Structures of 1, an amide-linked nucleoside conjugate of a G3 PAMAM dendrimer (compound 11 in Ref. 25) and 2, a recently synthesized nucleoside conjugate of a G4 PAMAM dendrimer (compound 6 in Ref. 23), containing a functionalized 2-alkynyl A3AR agonist. B. The synthesis of a novel dendrimer derivative 6 and its fluorescent analogue 7. Average stoichiometric ratios are shown.
2. Materials and Methods
2.1. Chemical synthesis materials and instrumentation
Dendrimer conjugates 1 and 2 and the monomeric nucleoside precursor 5 were prepared as reported [25,26]. Conjugation of the monomeric nucleoside to the dendrimer was through formation of an amide in 1 or a triazole using click chemistry [21] in 2. All reagents and solvents (regular and anhydrous) were of analytical grade and obtained from commercial suppliers and used without further purification. Aqueous dialysis following dendrimeric conjugation was done with Spectra/Por Dialysis membrane MWCO 3,500, diameter 11.5 mm (Spectrum Laboratories, Rancho Dominguez, CA, USA). 1H NMR ascertained sample purity and spectra were recorded with a Bruker 400 MHz NMR spectrometer. Chemical shifts are reported in parts per million (ppm) relative to tetramethylsilane or deuterated solvent as the internal standard (δH: CDCl3 7.26 ppm). ESI - High Resolution Mass Spectroscopic measurements were performed on a proteomics optimized Q-TOF-2 (Micromass-Waters) using external calibration with polyalanine. Observed mass accuracies are those expected on the basis of known performance of the instrument as well as the trends in masses of standard compounds observed at intervals during the series of measurements. Reported masses are observed masses uncorrected for this time-dependent drift in mass accuracy.
2.2. Synthesis of G4 PAMAM, conjugated with A3AR agonist 5 (6)
Freshly prepared aqueous sodium ascorbate (1 M solution, 0.82 mL) was added to a mixture of dendrimeric azide derivative 4 (51 mg, 3.21 μmol) [25] and dialkynyl nucleoside 5 (109 mg, 204 μmol) [26] in a mixture of t-butanol (4.4 mL) and water (4.4 mL), followed by addition of 7.5% aqueous cupric sulfate (1.36 mL, 410 μmol). The reaction mixture was stirred at room temperature overnight, and the product was purified by extensive dialysis in water for 2 days. The mixture was lyophilized to give compound 6 (118 mg, 81%) as a powder. 1H NMR (DMSO-d6, 400 MHz) δ 8.50 (bs), 8.15 (bs), 7.60 (s), 7.29–7.59 (m), 5.44 (s), 4.67–5.05 (m), 4.16 (s), 3.86 (s), 2.78 (s), 2.67 (s), 2.08–2.23 (m), 1.82 (s), 1.61 (s), 0.98–1.30 (m). ESI-MS: calcd. 44,727; found 44,795.
2.3. Synthesis of G4 PAMAM, conjugated with A3AR agonist 5 and AlexaFluor 488 (7)
Sodium tetraborate buffer (0.5 mL) was added to a solution of compound 6 (10 mg, 0.21 μmol) and AlexaFluor 488 N-hydroxysuccinimide-ester (Invitrogen Life Science, Carlsbad, CA, USA, 1 mg, 1.13 μmol) in N,N-dimethylformamide (1.5 mL) and stirred overnight at room temperature under light protected condition. The reaction mixture was purified by extensive dialysis in water for 2 days and the resulting solution lyophilized to give the AlexaFluor 488-conjugated compound 7 (7.0 mg, 68%) as an orange colored foamy solid. ESI-MS: calcd. 47,284; found 47,446.
The conjugate 6 was subjected to ultrafiltration and then both the high and low molecular weight (MW) fraction subjected to an A3AR binding assay. Dilute solutions (1 μM) of the dendrimer conjugates (following storage at −20°C for at least 6 months) were prepared in 50 mM Tris-HCl (pH 7.5) and 10 mM MgCl2 buffer. Wash buffer solutions whose concentrations of Tris-HCl, MgCl2, and DMSO matched that of the solutions of dendrimer conjugates were also prepared for rinsing the centrifugal filters before ultrafiltration. Centrifugal filters (Milipore Ltd., Amicon Ultra-0.5, 10,000 MW Cutoff) were rinsed with 400 μL of wash buffer for 5 min at 16,000 x g, after which the filters were placed into new microcentrifuge tubes. Ultrafiltration of the solutions of dendrimer conjugates was then performed by adding 400 μL of each solution to a filter and spinning the filters for 20 min at 16,000 x g. The remaining material in the filters after ultrafiltration was collected by inverting the filters, placing them into new microcentrifuge tubes, and spinning the filters for 2 min at 1000 x g. Once collected, the remaining material (approx. 20 μL) was resuspended in 400 μL of 50 mM Tris-HCl (pH 7.5) and 10 mM MgCl2 buffer. Finally, the fractions collected during the rinse and ultrafiltration steps and the resuspended material were tested in a radioligand binding assay using membranes derived from A3AR-expressing CHO cells. The high affinity binding activity was associated with a high MW fraction.
2.4. Effects on hypoxic cardiomyocytes
Cardiomyocyte cultures [27,28] were washed in serum- and glucose-free medium before being incubated in the presence of AR ligands, under hypoxic conditions. Exposure to argon (100%) in a hypoxic chamber in glucose-free media for 90 min was used to simulate ischemic conditions in the primary cardiac myocyte cultures. At the end of the hypoxic period, damage was evaluated, using morphological and biochemical assessments [29].
Cytotoxicity was assessed by spectrophotometric measurement of lactate dehydrogenase (LDH) released into the culture medium. Protein content and LDH activity were determined as previously described [28]. Briefly, 25 μl of the supernatant was transferred to a 96-well dish, and the LDH activities were determined by using LDH-L kits (Sigma, St. Louis, MO, USA), according to the manufacturer’s instructions. The results are expressed in relation to the controls of the same experiment. Experiments were performed in four to eight replicates and were repeated at least six times [11].
The cell viability assay is based on binding of propidium iodide (PI) to the nuclei of cells whose plasma membranes have become permeable due to cell damage. Cell viability was determined by PI fluorometry using a multi-well plate reader (TECAN SpectraFluor Plus, Männedorf, Switzerland), as described previously [29]. Briefly, cardiomyocytes following hypoxia received PBS containing glucose and 5 μM PI and were incubated for 30 min at 37°C. Fluorescence (A) from each plate/well was measured at excitation and emission wavelengths of 540 and 630 nm, respectively. The background fluorescence (B) was assessed with an unstained plate. Experiments were terminated by permeabilizing plasma membranes with digitonin (300 μM) to label all nuclei with PI. A final fluorescence (C) was measured 30 min after digitonin treatment. The percentage of viable cells (V) was calculated as V=100(A–B)/(C–B) [29].
2.5. Staining of cardiomyocytes with conjugate 7
Cardiac cells were treated with cytosine arabinoside (10 μM) one day after plating for 72 h (to eliminate multiplying cells) and then stained with the fluorescent dendrimer 7 (10 pM) for 20 min at room temperature. The cells were washed 3–4 times with PBS and photographed using a confocal microscope.
2.6. Isolated heart model
All experiments were performed on 12 week-old male Sprague Dawley rats, n=7 in each group. Heparin (100 units/rat) was administered intraperitoneally. The isolated heart was perfused with oxygenated Krebs Henseleit solution. Retrograde aortic perfusion was initiated at a perfusion pressure of 90 cm H2O with modified Krebs-Henseleit buffer solution (KH), which contained 118 mM NaCl, 25 mM NaHCO3, 1.2 mM KH2PO4, 1.2 mM MgSO4.7H2O, 2.5 mM CaCl2, 4.7 mM KCl, 11.1 mM glucose, bubbled with a mixture of O2 95%/CO2 5%, resulting in a pH of 7.35–7.40. PO2 and PCO2 values in the perfusion medium were 550–650 and 25–30 mm Hg, respectively. The pulmonary artery was incised to facilitate drainage. The isolated heart was perfused with KH for a 20 min stabilization period, treated with the dendrimer derivatives (MRS5216 or MRS5246, each at 10 or 100 pM) through a side arm perfusion during the last 5 min of the stabilization period, after which it was kept on the Langendorff system for another 30 min in ischemic conditions and 30 min of reperfusion (Figure S2, Supporting Information). Temperature was maintained at 37±0.5 °C, by placing a thermostatic water jacket around the perfusate reservoir and the isolated heart. The temperature of the heart was monitored with a micro thermocouple in the right ventricle connected to a digital thermometer (Webster Laboratories, Altadena, CA, USA). A fluid-filled latex balloon was inserted via the left atrium into the left ventricle and inflated to set a left-ventricular end-diastolic pressure (LVEDP) between 5–10 mmHg. Coronary flow was collected every 10 min, left ventricular developed pressure (LVP) was continuously recorded and the derivatives ±dP/dtmax were calculated by the CODAS data acquisition system (San Diego, CA, USA) [13]. Hearts were excluded from further data analysis as a result of one of the following undesirable situations: 1. A time delay in the aortic cannulation (>3 min); 2. Damage of the aorta during the cannulation; 3. Inability to maintain a constant coronary flow rate at the end of stabilization; 4. Poor temperature control of the water bath throughout the experiment protocol.
2.7. Infarct size
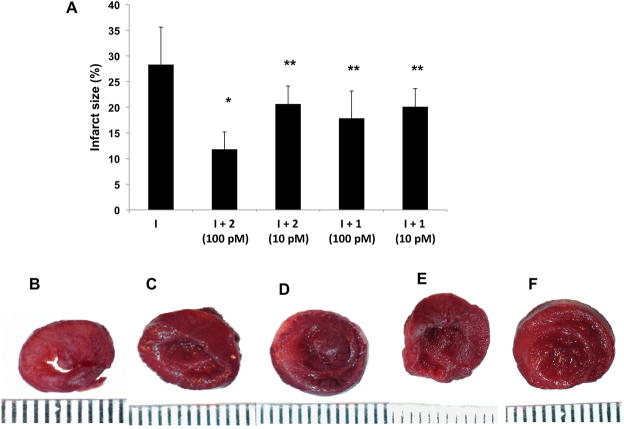
At the end of experiment, the heart was removed from the apparatus, weighed, and sliced in the ventricular area. The sections were incubated in 1% triphenyltetrazolium chloride (TTC) for 30 min (37°C) and then in 4% formaldehyde for ~24 h. Ischemic myocardium, which is still viable, stains red with TTC, whereas the necrotic myocardium does not stain and appears pale white. TTC reacts with dehydrogenase enzyme in the presence of cofactor NADH to form formazon pigment in viable cells, which become brick red in color. The infarcted cells that have lost dehydrogenase enzyme remain unstained. Thus, the infarcted portion of the myocardium remains unstained, while the normal viable myocardium is stained brick red with TTC. The infarct area – IA (white) and the area at risk – AAR (red and white) from each section were measured using an image analyzer. Ratios of IA/AAR of each section were multiplied by its appropriate slice weight to calculate each parameter weight then summed and expressed as a percentage of AAR [30].
2.8. Statistical analysis
All results were expressed as mean ± SE. Values of the stabilization period were considered as 100%. ANOVA was used to compare groups; the Bonferroni test was used to compare differences between the groups at every checked point. Significance was accepted at P<0.05.
3. Results
3.1. Structures and synthesis of PAMAM dendrimer conjugates of AR agonists
Previously, a GLiDe conjugate of an AR agonist that was only moderately potent (μM) but not subtype-selective in activating the A3AR was reported to protect A3AR-expressing HL-1 mouse cardiac cells against apoptosis induced by hydrogen peroxide. That conjugate displayed greater protective potency than monomeric nucleoside agonists [20]. In the present study, we have improved both the pharmacological properties of the conjugates and used much more indicative measures of cardioprotection. We examined the anti-ischemic effects of three multivalent A3AR agonists of greatly enhanced A3AR affinity, amide conjugate 1 and triazole-linked conjugates 2 and 6 (Figure 1). These macromolecular conjugates were applied under conditions of hypoxia/ischemia in neonatal cultured rat cardiomyocytes and in isolated hearts using the Langendorff system.
The synthesis and initial pharmacological characterization of 1 and 2 were recently reported [25]. Conjugate 2 was derived from a fully substituted generation 4 (G4) PAMAM dendrimer, linked by click chemistry (specifcally, a 2+3 cycloaddition reaction) [21,31] to an alkyne-functionalized adenosine derivative, and was selective for the A3AR with a Ki value of 0.14 nM [25]. Conjugate 1 was derived through the amide linkage of a carboxy-functionalized N6 substituent of the adenine moiety to a G3 PAMAM dendrimer. Both adenosine analogues contained a (N)-methanocarba modification that substituted the ribose moiety with a rigid bicyclo[3.1.0] hexane ring system to maintain an A3AR-preferred conformation.
Another dendrimeric conjugate prepared here, 6, was structurally related to 2, except not fully substituted and retaining eight of the original 64 amino groups of the G4 PAMAM dendrimer (Figure 1B). The presence of these amino groups permitted the subsequent introduction of a fluorescent label, AlexaFluor488, through amide formation to yield fluorescent conjugate 7.
3.2. Pharmacological characterization of PAMAM dendrimer conjugates in AR binding and functional assays
A sub-nM affinity of 2 at the human A3AR was previously reported [25]. Conjugate 1 bound more weakly than 2 to the ARs, but with mixed selectivity for the A1 and the A3ARs with Ki values of 7.1 and 17.6 nM, respectively [25]. The binding affinities of conjugates 6 and 7 were determined in AR binding assays (Table 1). The presence of the fluorescent group did not appreciably alter the observed afffinity. In each case, the AR affinity of the triazole-linked multivalent conjugate greatly exceeded the affinity of the corresponding monomer, 5 [26]. An affinity enhancement in the multivalent ligand compared to monomer was also observed previously for the amide-linked conjugate 1 [25]. Conjugates 2, 6, and 7 were subjected to centrifugal ultrafiltration with a cutoff of 3000 D to remove any residual small MW impurities followed by radioligand binding experiments at the A3AR to confirm that the binding was associated with the high MW fraction containing the polymer. Also, the fluorescence associated with conjugate 7 was retained with this high MW fraction.
Table 1.
Potency of nucleoside-dendrimer conjugates at three subtypes of human ARs.a
| Compound | Ki, hA1 (nM) | Ki, hA2A (nM) | Ki, hA3 (nM) |
|---|---|---|---|
| Monomers | |||
| Cl-IB-MECAb | 260 ± 60 | 2300 ± 100 | 0.29 ± 0.04 |
| 5b | >10,000 | 7040 ± 1430 | 29.4 ± 9.8 |
| Dendrimer derivatives | |||
| 1b,c | 7.1 | 5750 ± 1600 | 17.6 ± 2.8 |
| 2b,c | 22.6 ± 6.9 | 32.2 ± 6.4 | 0.14 ± 0.09 |
| 4d | NA | NA | NA |
| 6d | 240 ± 70 | 220 ± 40 | 2.11 ± 0.51 |
| 7 | 450 | 190 ± 30 | 2.57 ± 0.29 |
All experiments were done on CHO or HEK293 (A2A only) cells stably expressing one of four subtypes of human ARs. The binding affinity for A1, A2A, and A3ARs was expressed as Ki values (n = 3–5) and was determined by using agonist radioligands ([3H]R-PIA, [3H]CGS21680, or [125I]I-AB-MECA, respectively), unless noted. A percentage in parentheses refers to inhibition of radioligand binding at the indicated concentration. The concentrations of the dendrimer-ligand complexes were measured by the concentration of the dendrimer, not the ligand. Therefore, all binding Ki values of dendrimers are expressed as Kiapp values.
1, MRS5216; 2, MRS5246; 6, MRS5539; 7, MRS5538.
Compound 4 is the dendrimer precursor of 6. Compound 6 is the precursor of 7. NA – not active in inhibition of radioligand binding (<10%) at 1 μM.
Preliminary to the characterization of these conjugates in cardioprotection, we wished to confirm that high functional potency is also present at the rat A3AR, in light of the fact that AR affinity of certain ligand has displayed variation between species [16]. Therefore, 2 was evaluated in the stimulation of hexosaminidase release in RBL (rat basophilic leukemia)-2H3 cells, an effect known to reflect activation of the rat A3AR, which is highly expressed in that cell line [32]. In enhancing hexosaminidase release (Figure S1, Supporting Information), conjugate 2 was two orders of magnitude more potent (pEC50 = 10.43 ± 0.20) but equiefficacious to the selective small molecular A3AR agonist Cl-IB-MECA (pEC50 = 8.38 ± 0.27). This confirmed that this conjugate was highly potent at the rat A3AR, similar to its enhanced potency at the human A3AR. It is to be noted that while A3AR agonists release inflammatory mediators including histamine from murine mast cells, other species, notably human, lack this A3AR response.
3.3. Pharmacological characterization of dendrimer conjugates in cardiomyocytes
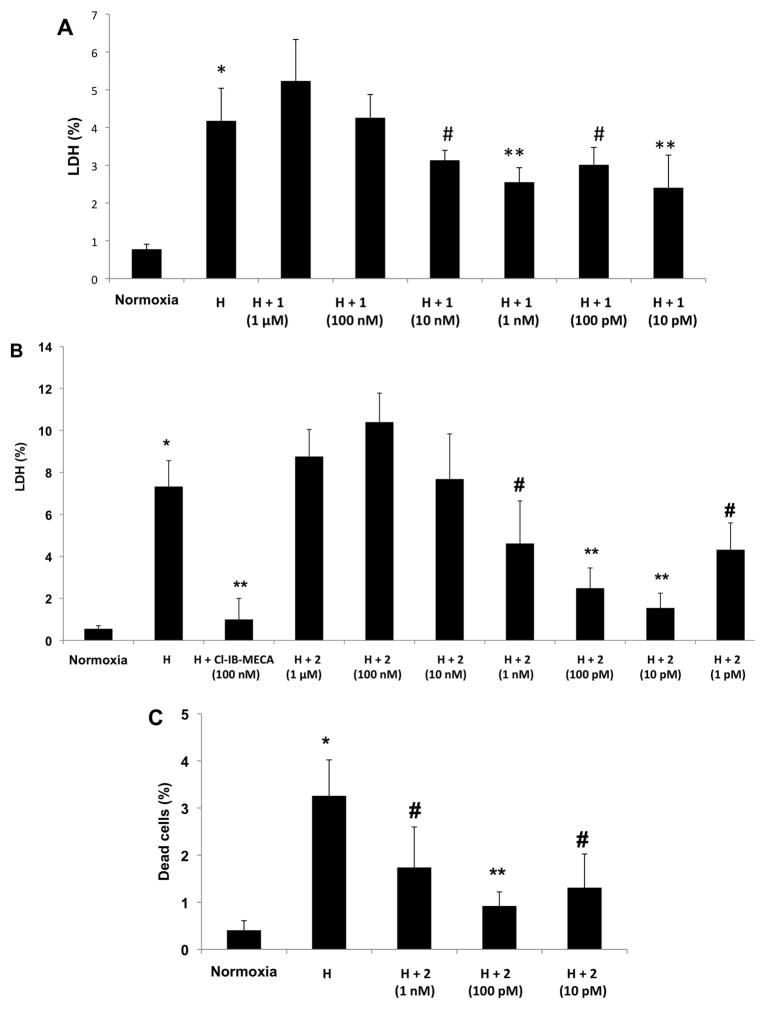
In cultured neonatal rat cardiomyocytes, cell damage following hypoxia was demonstrated by measurement of LDH released into the culture medium. The monomeric, selective A3AR agonist Cl-IB-MECA provided cardioprotection at a concentration of 100 nM, as shown in previous studies of cultured neonatal rat cardiomyocytes [14]. Treatment of the cardiomyocytes with 1 at a high concentration of 1 μM, and then exposure to hypoxia was more injurious to the cells than hypoxia alone (Figure 2A). Thus, higher concentrations of 1 revealed greater cell damage, while lower concentrations reduced hypoxic injury. Treatment with concentrations of 1 and 10 pM significantly protected the cells against hypoxic stress.
Figure 2.
A. Effects of conjugate 1 on cardiomyocytes subjected to hypoxia. Cardiomyocytes were treated with 1 at concentrations of 1 μM, 100 nM, 10 nM, 1 nM, 100 pM, 10 pM, and exposed to hypoxia (H). LDH released to the medium was determined immediately after hypoxia. 100% activity was the total LDH activity in homogenate of untreated cells. * P<0.001 compared to normoxia. ** P<0.001 compared to hypoxia; # P<0.05 compared to hypoxia. B., C. Effects of conjugate 2 on cardiomyocytes subjected to hypoxia. Cardiomyocytes were treated with 2 at concentrations of 1 μM, 100 nM, 10 nM, 1 nM, 100 pM, 10 pM, 1 pM, and exposed to hypoxia (H). (B) LDH released to the medium was determined immediately after hypoxia. 100% activity was the total LDH activity in homogenate of untreated cells. (C) Percent of dead cells determined by PI staining. 100% is the number of cells in each well. * P<0.001 compared to normoxia. ** P<0.001 compared to hypoxia. # P<0.05 compared to hypoxia.
Treatment with the multivalent A3AR agonist 2 at a high concentration of 1 μM, and then exposure to hypoxia was also injurious to the cells (Figure 2B,C), but lower concentrations (1 pM to 1 nM) protected the cardiomyocytes against hypoxia. Nucleoside conjugate 2 at 10 and 100 pM provided the most effective and significant protection against hypoxic stress, as indicated by either LDH release or PI staining of the cardiomyocytes. Thus, both multivalent A3AR agonists were demonstrated to protect the cardiac cells in culture.
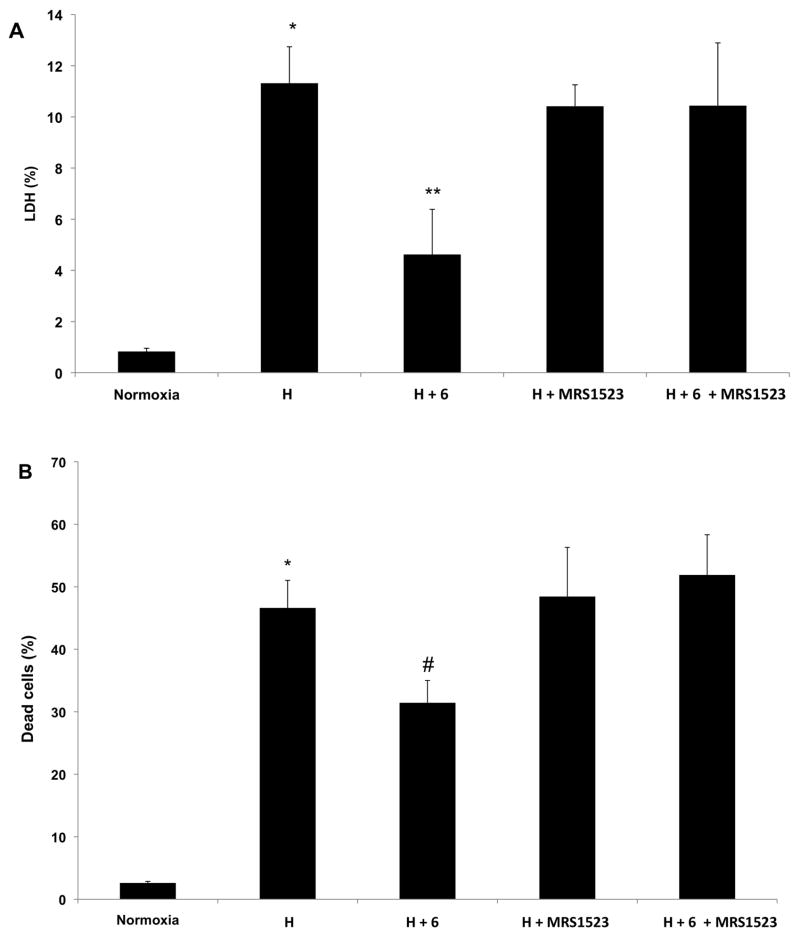
Treatment with the conjugate 6 at 1 nM prior to exposure to hypoxia (Figure 3) also protected the cardiomyocytes against hypoxia. The protective effect was completely reversed by the selective A3AR antagonist MRS1523 at 1 μM. This antagonism was evident from either LDH release or PI staining of the cardiomyocytes. Thus, this multivalent A3AR agonist was demonstrated to protect the cardiac cells via A3AR activation.
Figure 3.
Antagonism by MRS1523 of the effects of 6 on cardiomyocytes subjected to hypoxia. Cardiomyocytes were treated with 6 (1 nM) and exposed to hypoxia (H). (A) LDH released to the medium was determined immediately after hypoxia. 100% activity was the total LDH activity in homogenate of untreated cells. (B) Percent of dead cells determined by PI staining. 100% is the number of cells in each well. *P<0.001 compared to normoxia. **P<0.001 compared to hypoxia. #P<0.05 compared to hypoxia.
The cell injury encountered with dendrimers 1 and 2 at high concentration was not antagonized by MRS1523 (1 μM) and therefore not dependent on the A3AR. As a control in the absence of hypoxia, the azide-derivatized dendrimer 4 at 10 and 100 nM had no effect on LDH release in the cardiomyocyte cultures after 2 h or 4 h exposure (3 groups of cells, each containing 3 wells). Therefore, this unconjugated dendrimer, i.e. the synthetic precursor of 6, was not toxic to the cells.
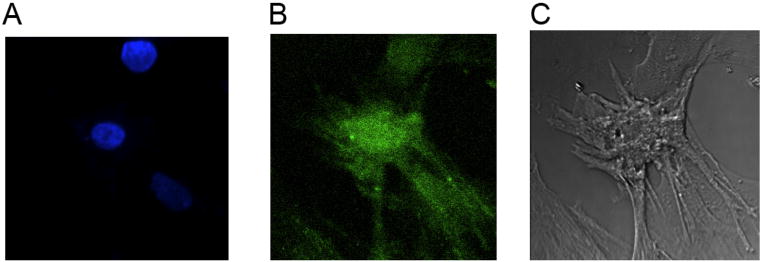
The fluorescent AlexaFluor 488 label contained in dendrimer derivative 7 was used to trace the localization of this dendrimer within the cardiomyocytes. When applied at 10 pM to the cultured cells for 20 min, fluorescence was concentrated around the nucleus apparently on the cell surface with homogeneous staining, indicating a uniform distribution of A3AR on the cardiomyocytes (Figure 4). More prolonged staining or at temperatures higher than 24 °C induced internalization of the dendrimer (not shown).
Figure 4.
Staining of cardiac cells with 7. Cardiac cells treated with cytosine arabinoside (10 μM) one day after plating for 72 h and then stained with the fluorescent dendrimer derivative 7 (10 pM) for 20 min at room temperature. For counterstaining we used Hoechst 33342 (10 uM), which stains the nuclei of the cells. The cells were washed 3–4 times with PBS and photographed using a confocal microscope microscope at 405/450 nm for Hoechst and 488/520 nm for 7. A. Hoechst 33342 stain, B. AlexaFluor fluorescence, C. Phase contrast.
3.4. Pharmacological characterization of dendrimer conjugates in the isolated heart
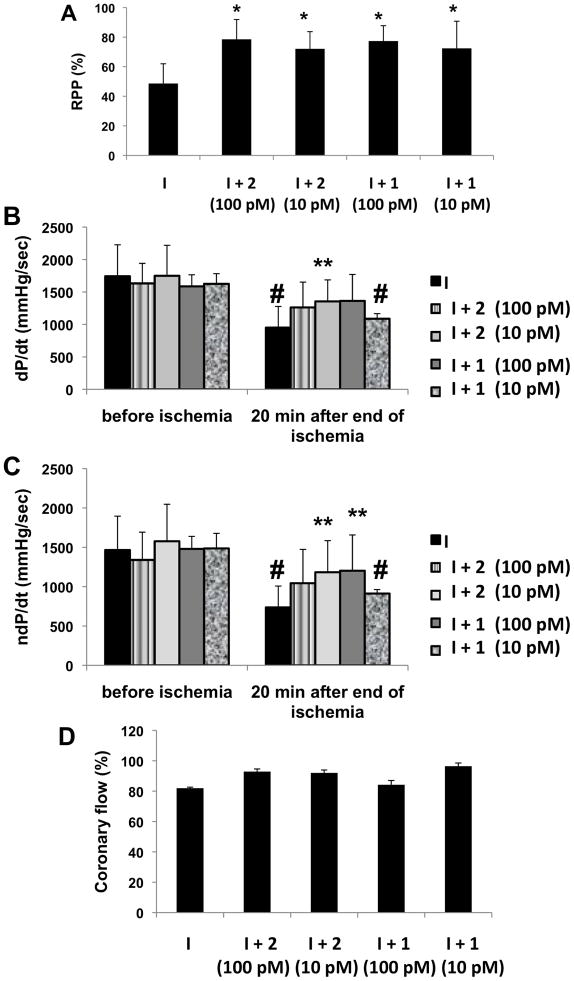
We extended these results from cell culture to the whole heart to show that the GLiDe conjugates act similarly in anti-ischemic protection. In the light of the results in cultures, concentrations of 10 or 100 pM of conjugates 1 and 2 were chosen for treating the isolated hearts in the Langendorff system, and these pM concentrations were cardioprotective (Figures 5 and 6). The results were illustrated by a smaller infarct size in the treated hearts compared to the untreated controls, especially by 2 at a concentration of 100 pM, which from ~28% to ~13%, which represents a >50% reduction. Recovery of rate pressure product (RPP, Figure 6A) was calculated by multiplication of developed pressure and heart rate. The results indicated a ~ 25% improvement of the RPP in hearts exposed to either dendrimer at both concentrations compared to untreated ischemic hearts. The rate of myocardial contraction and relaxation, particularly in the hearts treated with 2 (10 pM), was also improved in comparison to the control group at the end of reperfusion. An approximately ~ 25% improvement in positive and negative dP/dtmax was demonstrated (Figure 6B). Coronary flow was similar in all groups (Figure 6D). The coronary flow rate at the stabilization period was 9.93 ± 0.93 ml/min with approximately 10 – 15 % decline at 30 min of reperfusion.
Figure 5.
Infarct size limiting effects of dendrimers on isolated hearts in the Langendorff system subjected to ischemia. Isolated rat hearts were treated with 1 or 2 at concentrations of 10 or 100 pM, and exposed to ischemia (I). A. Damaged area calculated by Image Pro 5.1 Plus of infarct size of slices of hearts stained by TTC and exposed to: ischemia (B), 2 (100 pM) before the ischemia (C), 2 (10 pM) before the ischemia (D), 1 (100 pM) before the ischemia (E), 1 (10 pM) before the ischemia (F). *P<0.001 compared to ischemia. **P<0.05 compared to ischemia. The scale below the heart is marked in mm units.
Figure 6.
Physiological effects of dendrimers on isolated hearts in the Langendorff system subjected to ischemia. Isolated rat hearts were treated with 1 or 2 at concentrations of 10 or 100 pM, and exposed to ischemia (I). A. Percent of rate pressure product (RPP) after the ischemia. Preischemic RPP is considered as 100%. (B) Positive dP/dtmax (dP/dt) and (C) negative (ndP/dtmax) ndP/dt before and after the ischemia. Coronary flow (D) is expressed as % of the preischemic value.
*P<0.05 compared to ischemia. **P<0.05 compared to non-treated hearts 30 min after the end of ischemia. #P<0.05 compared to before ischemia.
4. Discussion
The heart is subjected to oxidative stress during various pathological conditions, such as ischemic heart disease, an acute myocardial infarction, and during acute coronary interventions such as percutaneous coronary intervention (angioplasty), thrombolysis or coronary artery bypass graft surgery [33–35].
Ischemic injury to myocardium in response to coronary occlusion remains the leading cause of death in Western countries, raising interest in mechanisms capable of limiting myocardial damage. Adenosine released in large amounts during myocardial ischemia plays a major role in mediating preconditioning and other cardioprotective effects in most animal species studied, including humans. Since ischemic preconditioning of the heart is not applicable in a clinical setting, variant preconditioning stimuli have received increasing attention. Ischemic preconditioning can be elicited via the native agonist adenosine [3] and by the synthetic nucleoside derivatives Cl-IB-MECA, an A3AR agonist, and CCPA, an A1AR agonist [12]. These agonists activate GPCRs to trigger both acute and delayed ischemic preconditioning [1,17,36]. A3AR agonists, including Cl-IB-MECA, have entered clinical trials for cancer and inflammatory diseases [37], but the cardioprotective properties of these agonists have not yet been tested clinically. The cardioprotective action of A3AR agonists has been established using an A3AR knockout mouse [38].
In this study, we examined the cardioprotective properties of three PAMAM dendrimer conjugates of AR agonists, 1, 2, and 6. Compound 2, with a sub-nM Ki value, is a more potent and selective agonist of the A3AR than 1, but both act as full agonists in inhibition of adenylate cyclase mediated by the human A3AR [25]. Compound 1 is a smaller multivalent conjugate (MW 13.7 KD) and less substituted (13 nucleoside moieties out of a total 32 terminal positions) compared to the fully substituted 2 (MW 50 KD), which contains 64 nucleoside moieties on each dendrimer. Another structural difference that contributes to the variation in AR binding affinity and selectivity is the linkage between the pharmacophore and the dendrimeric carrier. Conjugate 2 is linked through a chemically stable triazole moiety, which is formed by a facile and orthogonal click reaction of an azide-derivatized dendrimer with an alkyne-derivatized nucleoside [25]. Compound 6 (MW 45 KD) is close in structure to multivalent conjugate 2. For use as pharmacological probes of ARs, the A3AR-selective 2 and 6 are preferred over 1 that also binds to the A1AR, which has its own distinct cardiovascular effects [14,15,27,36]. Also, the 2-alkynyl group present in 2 and 6 was previously associated with improved consistency of A3AR affinity between species [26].
Three dendrimer derivatives of A3AR agonists, similarly to a monomeric agonist of the A3AR, demonstrated anti-hypoxic effects on neonatal rat cardiomyocyte cultures and isolated hearts. However, the dendrimer conjugates had signi cantly greater potency (10–100 pM) than an A3AR-selective monomeric nucleoside Cl-IB-MECA, which protected in the 100 nM range. Protection or greater injury of the neonatal rat cultured cardiomyocytes was dependent on dendrimer concentration. Curiously, while nM and pM concentrations provided protection to the cultured cells against hypoxia, μM concentrations were injurious. The mechanism of this injury was not explored, but it might be a function of greater internalization. The lower, protective conjugate concentrations had no effect on the heart when given without hypoxia. As in previous studies, the concentration of the multivalent dendrimeric derivative, rather than the concentration of individual pharmacophore moieties was used for pharmacological characterization. A selective A3AR antagonist blocked the protection in cardiomyocytes induced by one of the conjugates, indicating action dependent on A3AR.
Dendrimeric conjugation of GPCR ligands is a means of modulating their pharmacokinetic and pharmacodynamic characteristics [19,39], assuming that the linking chemistry is done in a way that preserves or enhances the pharmacological properties of the ligand. In general, the chemical and biological properties of multivalent drugs bound to nanocarriers may differ greatly from those of the corresponding monomeric agents [18,40]. This approach provides an opportunity to tune the pharmacokinetics and pharmacodynamics in an otherwise unattainable manner and to introduce reporter or targeting moieties [22]. In the present study, unusually high potencies in cardioprotection have been observed. Given the presence of multiple sites for covalent derivatization of the biologically active GLiDe conjugate without losing receptor affinity, it might be possible to target these macromolecular drugs to the desired site of action, i.e. the heart. Furthermore, a possibility exists to introduce reporter groups such as fluorescent moieties without losing receptor affinity, as we have shown here, to trace the drug in the body. It is also conceivable that the nucleoside bound to a polymeric carrier would have a reduced rate of metabolism in vivo [41], while preserving or enhancing the potency and selectivity.
We have previously shown that the monomeric A3AR agonist Cl-IB-MECA, by activating the A3AR, stimulates Ca-ATPase of the sarcoplasmic reticulum and thereby protects the cardiomyocytes from Ca2+ overload [42]. This protection is unique to the activation of A3AR and not associated with the A1AR. Thus, these two ARs are distinct in effects on Ca2+ homeostasis but merge mechanistically in protection against hypoxia via the activation of p38 MAPK [29]. It is worth mentioning that Wan et al. conclude that activation of A3AR protects from myocardial ischemic injury via the activation of sarcolemmal KATP channels, and not via mitochondrial KATP channels [43]. The phosphorylation of p38 MAPK is a crucial intracellular signaling step in cardioprotection by A1 and A3AR agonists. Here we have shown that A3AR activation following pre-hypoxic administration of dendrimeric agonists significantly protected the cardiomyocytes and the isolated heart with significant reduction in all measured parameters. Several GLiDe conjugates reduced biochemical indicators of injury (released enzymes), decreased infarct size (tetrazolium staining), and improved contractile parameters following ischemia and reperfusion.
Polymeric drug conjugates, in general, are being explored for their pharmacological advantages over monomeric drugs. One unique characteristic of these multivalent ligands as AR agonists is that they are theoretically capable of interacting with multiple receptor molecules on the cell surface, which could have an amplifying effect on the biological activity [22]. Further studies are needed to determine the precise mechanism of the dendrimer conjugate-induced cardioprotection and if simultaneous binding of multiple A3AR protomers is the basis for the increased potency in cell culture and in the isolated heart preparation. Also, the pharmacodynamic and pharmacokinetic characteristics of the multivalent adenosine agonists in an animal model of ischemic reperfusion remain to be determined. A given GPCR ligand bound covalently to a polymeric carrier may have a reduced rate of metabolism, although this remains to be examined for the present derivatives. It is to be emphasized that the current strategy, which preserves or enhances the AR potency and selectivity in the intact conjugates, requires neither cellular internalization nor cleavage of the drug moiety for the desired biological activity. We previously demonstrated that we could minimize the internalization in platelets of structurally related AR agonist-PAMAM conjugates by reducing the number of free amino groups on the dendrimer periphery [39]. The protective action of these highly potent A3 agonist conjugates in the intact heart suggests that the vascular endothelium does not serve as a barrier to these polymeric derivatives, perhaps because of leakage during ischemia.
In conclusion, both the isolated heart and the cardiomyocyte cell culture experiments support the beneficial effects of low concentrations of conjugates 1 and 2 and of a novel derivative 6 that permitted incorporation of a fluorescent label. The three dendrimers, similarly to the monomeric agonist of the A3AR, protected rat cardiomyocyte cultures and isolated hearts. The ability of A3AR agonists to precondition the heart against hypoxia is a known phenomenon, and the monomeric potent agonist reached a maximal effect was generally equiefficacious to the conjugates. However, the high potencies observed with the nanoconjugates were unprecedented, with the difference in concentration of roughly 4 orders of magnitude. Thus, this research provides the groundwork for the potential use of multivalent drugs in treating cardiac diseases, and in vivo studies are in progress.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NIDDK. This work was also in part supported by The Adar Program for the Advancement of Research in Heart Function and the Horowitz Foundation at Bar-Ilan University.
Abbreviations
- AAR
area at risk
- AB-MECA
N6-(4-aminobenzyl)-5′-N-methylcarboxamidoadenosine
- AR
adenosine receptor
- CCPA
2-chloro-N6-cyclopentyladenosine
- DMSO
dimethylsulfoxide
- GLiDe
GPCR-ligand dendrimer
- GPCR
G protein coupled receptor
- CGS21680
2-[p-(2-carboxyethyl)phenyl-ethylamino]-5′-N-ethylcarboxamidoadenosine
- Cl-IB-MECA
2-chloro-N6-(3-iodobenzyl)-5′-N-methylcarboxamidoadenosine
- IB-MECA
N6-(3-iodobenzyl)-5′-N-methylcarboxamidoadenosine
- LDH
lactate dehydrogenase
- MRS1523
5-propyl-2-ethyl-4-propyl-3-(ethylsulfanylcarbonyl)-6-phenylpyridine-5-carboxylate
- MW
molecular weight
- R-PIA
R-(−)-N6-2-phenylisopropyl adenosine
- PAMAM
poly(amidoamine)
- RPP
rate pressure product
- TTC
triphenyltetrazolium chloride
- LVP
left ventricular developed pressure
- PI
propidium iodide
Footnotes
Supporting information Available: Procedures for cell culture, binding assays, time protocol for perfused heart experiments, and RBL-2H3 cell assay (and results) are provided.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cohen MV, Downey JM. Adenosine: trigger and mediator of cardioprotection. Basic Res Cardiol. 2008;103:203–15. doi: 10.1007/s00395-007-0687-7. [DOI] [PubMed] [Google Scholar]
- 2.Liang BT, Jacobson KA. A physiological role of adenosine A3 receptor: sustained cardioprotection. Proc Natl Acad Sci USA. 1998;95:6995–99. doi: 10.1073/pnas.95.12.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mubagwa K, Flameng W. Adenosine, adenosine receptors and myocardial protection: an updated overview. Cardiovasc Res. 2001;2:25–39. doi: 10.1016/s0008-6363(01)00358-3. [DOI] [PubMed] [Google Scholar]
- 4.Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, Freeman TC. Tissue distribution of adenosine receptor mRNAs in the rat. Br J Pharmacol. 1996;118:1461–8. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel RAG, Glover DK, Broisat A, Kabul HK, Ruiz M, Goodman NC, Kramer CM, Meerdink DJ, Linden J, Beller GA. Reduction in myocardial infarct size at 48 hours after brief intravenous infusion of ATL-146e, a highly selective adenosine A2A receptor agonist. Am J Physiol Heart Circ Physiol. 2009;297:H637–42. doi: 10.1152/ajpheart.00705.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Lubitz DKJE, Lin RC-S, Popik P, Carter MF, Jacobson KA. Adenosine A3 receptor stimulation and cerebral ischemia. Eur J Pharmacol. 1994;263:59–67. doi: 10.1016/0014-2999(94)90523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matot I, Weininger CF, Zeira E, Galun E, Joshi BV, Jacobson KA. A3 Adenosine receptors and mitogen activated protein kinases in lung injury following in-vivo reperfusion. Critical Care. 2006;10:R65. doi: 10.1186/cc4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng J, Wang R, Zambraski E, Wu D, Jacobson KA, Liang BT. Protective roles of adenosine A1, A2A, and A3 receptors in skeletal muscle ischemia and reperfusion injury. Am J Physiol Heart. 2007;293:H3685–91. doi: 10.1152/ajpheart.00819.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cross HR, Murphy E, Black RG, Auchampach J, Steenbergen C. Overexpression of A3 adenosine receptors decreases heart rate, preserves energetics, and protects ischemic hearts. Am J Physiol Heart Circ Physiol. 2002;283:H1562–8. doi: 10.1152/ajpheart.00335.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Headrick JP, Peart J. A3 adenosine receptor-mediated protection of the ischemic heart. Vascul Pharmacol. 2005;42:271–9. doi: 10.1016/j.vph.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Shneyvays V, Leshem D, Zinman T, Mamedova LK, Jacobson KA, Shainberg A. Role of adenosine A1 and A3 receptors in regulation of cardiomyocyte homeostasis after mitochondrial respiratory chain injury. Am J Physiol. 2005;288:H2792–801. doi: 10.1152/ajpheart.01157.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auchampach JA, Rizvi A, Qiu Y, Tang XL, Maldonado C, Teschner S, Bolli R. Selective activation of A3 adenosine receptors with N6-(3-iodobenzyl)adenosine-5′-N-methyluronamide protects against myocardial stunning and infarction without hemodynamic changes in conscious rabbits. Circ Res. 1997;80:800–9. doi: 10.1161/01.res.80.6.800. [DOI] [PubMed] [Google Scholar]
- 13.Hochhauser H, Kaminski O, Shalom H, Leshem D, Shneyvays V, Shainberg A, Vidne BA. Role of adenosine receptor activation in antioxidant enzyme regulation during ischemia-reperfusion in the isolated rat heart. Antioxid Redox Signal. 2004;6:335–44. doi: 10.1089/152308604322899404. [DOI] [PubMed] [Google Scholar]
- 14.Safran N, Shneyvays V, Balas N, Jacobson KA, Nawrath H, Shainberg A. Cardioprotective effects of adenosine A1 and A3 receptor activation during hypoxia in isolated rat cardiac myocytes. Mol Cell Biochem. 2001;217:143–52. doi: 10.1023/a:1007209321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hochhauser E, Leshem D, Kaminski O, Cheporko Y, Vidne BA, Shainberg A. The protective effect of prior ischemia reperfusion adenosine A1 and A3 receptor activation in the normal and hypertrophied heart. Interact Cardiovasc Thorac Surg. 2007;6:363–8. doi: 10.1510/icvts.2006.136317. [DOI] [PubMed] [Google Scholar]
- 16.Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller C. Nomenclature and classification of adenosine receptors – An update. Pharmacol Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klinger M, Freissmuth M, Nanoff C. Adenosine receptors: G protein-mediated signaling and the role of accessory proteins. Cell Signal. 2002;14:99–108. doi: 10.1016/s0898-6568(01)00235-2. [DOI] [PubMed] [Google Scholar]
- 18.Menjoge AR, Kannan RM, Tomalia DA. Dendrimer-based drugs and imaging conjugates: design considerations for nanomedical applications. Drug Discov Today. 2010;15:171–85. doi: 10.1016/j.drudis.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y, Hechler B, Klutz A, Gachet C, Jacobson KA. Toward multivalent signaling across G protein–coupled receptors from poly(amidoamine) dendrimers. Bioconjugate Chem. 2008;19:406–11. doi: 10.1021/bc700327u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keene AM, Balasubramanian R, Lloyd J, Shainberg A, Jacobson KA. Multivalent dendrimeric and monomeric adenosine agonists attenuate cell death in HL-1 mouse cardiomyocytes expressing the A3 receptor. Biochem Pharmacol. 2010;80:188–196. doi: 10.1016/j.bcp.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nwe K, Brechbiel MW. Growing applications of “click chemistry” for bioconjugation in contemporary biomedical research. Cancer Biother Radiopharm. 2009;24:289–302. doi: 10.1089/cbr.2008.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobson KA. GPCR ligand-dendrimer (GLiDe) conjugates: future smart drugs? Trends Pharmacol Sci. 2010;31:575–9. doi: 10.1016/j.tips.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villaraza AJL, Bumb A, Brechbiel MW. Macromolecules, dendrimers, and nanomaterials in magnetic resonance imaging: the interplay between size, function, and pharmacokinetics. Chem Rev. 2010;110:2921–59. doi: 10.1021/cr900232t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bi X, Shi X, Baker JR. Synthesis, characterization and stability of a luteinizing hormone-releasing hormone (LHRH)-functionalized poly(amidoamine) dendrimer conjugate. J Biomater Sci Polym Ed. 2008;19:131–42. doi: 10.1163/156856208783227686. [DOI] [PubMed] [Google Scholar]
- 25.Tosh DK, Yoo LS, Chinn M, Hong KI, Kilbey SM, Barrett MO, Fricks IP, Harden TK, Gao ZG, Jacobson KA. Polyamidoamine (PAMAM) dendrimer conjugates of “clickable” agonists of the A3 adenosine receptor and coactivation of the P2Y14 receptor by a tethered nucleotide. Bioconjug Chem. 2010;21:372–84. doi: 10.1021/bc900473v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tosh DK, Chinn M, Yoo LS, Kang DW, Luecke H, Gao ZG, Jacobson KA. 2-Dialkynyl derivatives of (N)-methanocarba nucleosides: “Clickable” A3 adenosine receptor-selective agonists. Bioorg Med Chem. 2010;18:508–17. doi: 10.1016/j.bmc.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shneyvays V, Leshem D, Mamedova LK, Shainberg A. Activation of adenosine A1 and A3 receptors protects mitochondria during hypoxia in cardiomyocytes by distinct mechanisms. In: Dhalla NS, Takeda N, Singh M, Lukas A, editors. Myocardial Ischemia and Preconditioning. Boston: Kluwer Academic Publishers; 2003. pp. 347–64. [Google Scholar]
- 28.Shneyvays V, Jacobson KA, Li AH, Nawrath H, Zinman T, Isaac A, Shainberg A. Induction of apoptosis in rat cardiomyocytes by A3 adenosine receptor activation and its suppression by isoproterenol. Exp Cell Res. 2000;25:111–26. doi: 10.1006/excr.2000.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leshem-Lev D, Hochhauser E, Chanyshev B, Isak A, Shainberg A. Adenosine A1 and A3 receptor agonists reduce hypoxic injury through the involvement of P38 MAPK. Mol Cell Biochem. 2010;345:153–60. doi: 10.1007/s11010-010-0568-5. [DOI] [PubMed] [Google Scholar]
- 30.Yitzhaki S, Shainberg A, Cheporko Y, Vidne BA, Sagie A, Jacobson KA, Hochhauser E. Uridine-5′-triphosphate (UTP) reduces infarct size and improves rat heart function after myocardial infarct. Biochem Pharmacol. 2006;72:949–55. doi: 10.1016/j.bcp.2006.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Best MD. Click chemistry and bioorthogonal reactions: Unprecedented selectivity in the labeling of biological molecules. Biochemistry. 2009;48:6571–84. doi: 10.1021/bi9007726. [DOI] [PubMed] [Google Scholar]
- 32.Ramkumar V, Stiles GL, Beaven MA, Ali H. The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J Biol Chem. 1993;268:16887–90. [PubMed] [Google Scholar]
- 33.Morin D, Hauet T, Spedding M, Tillment JP. Mitochondria as target for antiischemic drugs. Adv Drug Deliv. 2001;49:151–74. doi: 10.1016/s0169-409x(01)00132-6. [DOI] [PubMed] [Google Scholar]
- 34.Michiels C. Physiological and pathological responses to hypoxia. Am J Pathol. 2004;164:1875–82. doi: 10.1016/S0002-9440(10)63747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simoons ML, Serruys PW, van den Brand M, Res J, Verheugt FW, Krauss XH, Remme WJ, Bär F, de Zwaan C, van der Laarse A, Vermeer F, Lubsen J, et al. Early thrombolysis in acute myocardial infarction: limitation of infarct size and improved survival. J Am Coll Cardiol. 1986;7:717–28. doi: 10.1016/s0735-1097(86)80329-1. [DOI] [PubMed] [Google Scholar]
- 36.Peart JN, Headrick JP. Adenosinergic cardioprotection: Multiple receptors, multiple pathways. Pharmacol Ther. 2007;114:208–21. doi: 10.1016/j.pharmthera.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Ochaion A, Bar-Yehuda S, Cohen S, Barer F, Patoka R, Amital H, Reitblat T, Reitblat A, Ophir J, Konfino I, et al. The anti-inflammatory target A3 adenosine receptor is over-expressed in rheumatoid arthritis, psoriasis and Crohn’s disease. Cell Immunol. 2009;258:115–22. doi: 10.1016/j.cellimm.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 38.Ge ZD, Peart JN, Kreckler LM, Wan TC, Jacobson MA, Gross GJ, Auchampach JA. Cl-IB-MECA [2-chloro-N6-(3-iodobenzyl)adenosine-5′-N-methylcarboxamide] reduces ischemia/reperfusion injury in mice by activating the A3 adenosine receptor. J Pharmacol Exp Therap. 2006;319:1200–10. doi: 10.1124/jpet.106.111351. [DOI] [PubMed] [Google Scholar]
- 39.Kim Y, Hechler B, Gao ZG, Gachet C, Jacobson KA. PEGylated dendritic unimolecular micelles as versatile carriers for ligands of G protein-coupled receptors. Bioconjug Chem. 2009;20:1888–98. doi: 10.1021/bc9001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomalia DA. In quest of a systematic framework for unifying and de ning nanoscience. J Nanoparticle Res. 2009;11:1251–310. doi: 10.1007/s11051-009-9632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee CC, MacKay JA, Frechet JM, Szoka FC. Designing dendrimers for biological applications. Nat Biotechnol. 2005;23:1517–26. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 42.Shneyvays V, Zinman T, Shainberg A. Analysis of calcium responses mediated by the A3 adenosine receptor in cultured newborn rat cardiac myocytes. Cell Calcium. 2004;36:387–96. doi: 10.1016/j.ceca.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Wan TC, Ge ZD, Tampo A, Mio Y, Bienengraeber MW, Tracey WR, Gross GJ, Kwok WM, Auchampach JA. The A3 adenosine receptor agonist CP-532,903 [N6- (2,5-dichlorobenzyl)-3′-aminoadenosine-5′-N-methylcarbox-amide] protects against myocardial ischemia/reperfusion injury via the sarcolemmal ATP-sensitive potassium channel. J Pharmacol Exp Ther. 2008;324:234–43. doi: 10.1124/jpet.107.127480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.