Abstract
Ribosomal protein S1 covalently reacts with approximately one equivalent of iodoacetylethylenediamine (1,5-napthol sulfonate (IAEDANS) or iodoacetylaminofluorescein (IAAF). The product AEDANS-S1 can bind to 30S ribosomal subunits lacking S1 as shown by polyacrylamide-agarose gel electrophoresis AEDANS-S1 and AAF-S1 when added back to S1-depleted 30S subunits modulate poly(U)-dependent polyphenylalanine synthesis in the presence of IF3 in a very similar way to unmodified S1. AEDANS-S1 also stimulates RI7-dependent fMet-tRNA binding to 1.0M NH4C1 washed ribosomes whereas AAF-S1 does not. Both static and nanosecond fluorescence polarization techniques were used to study the rotational motions of AEDANS-S1. Several previous studies had indicated that S1 is a highly extended protein which can be modeled by a prolate ellipsoid with an axial ratio of 10 to 1. However, the rotational correlation time we find is about half that expected for such a particle. This suggests that S1 is a flexible protein with at least two domains that can rotate independently.
Full text
PDF







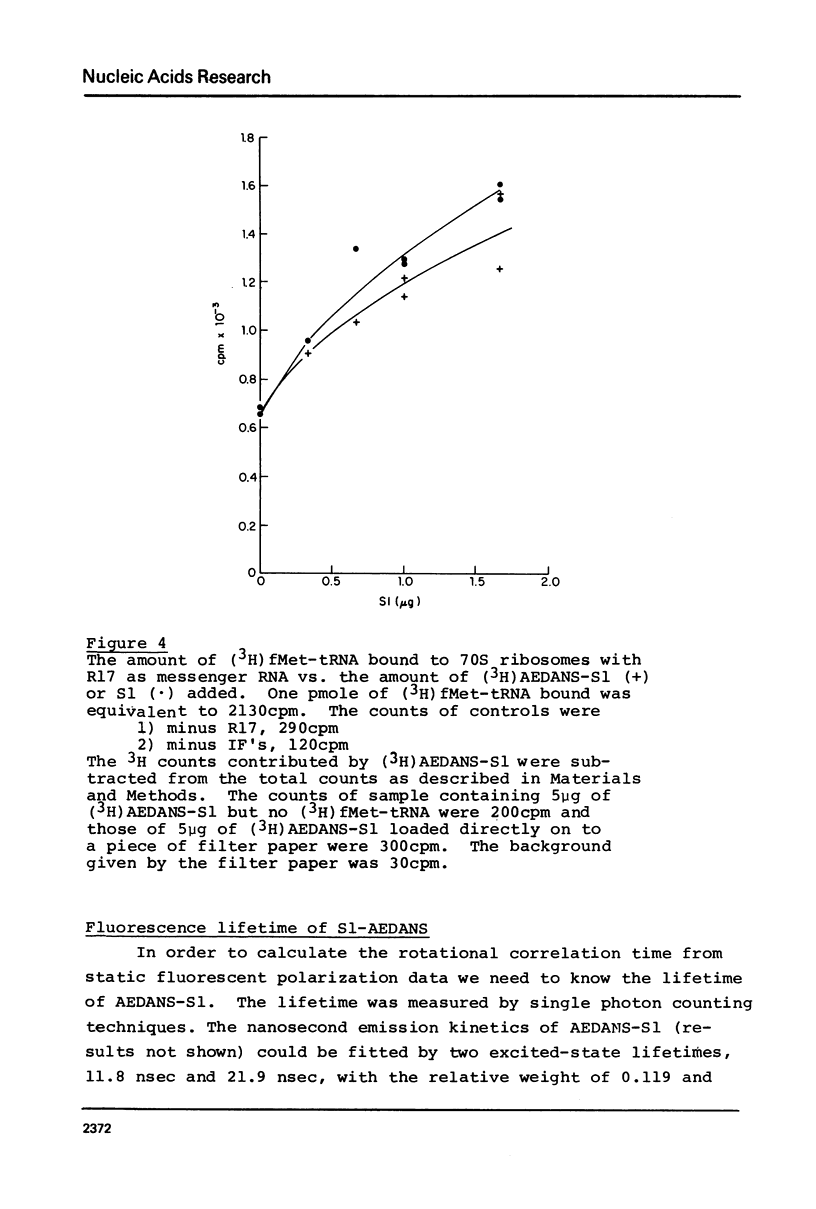
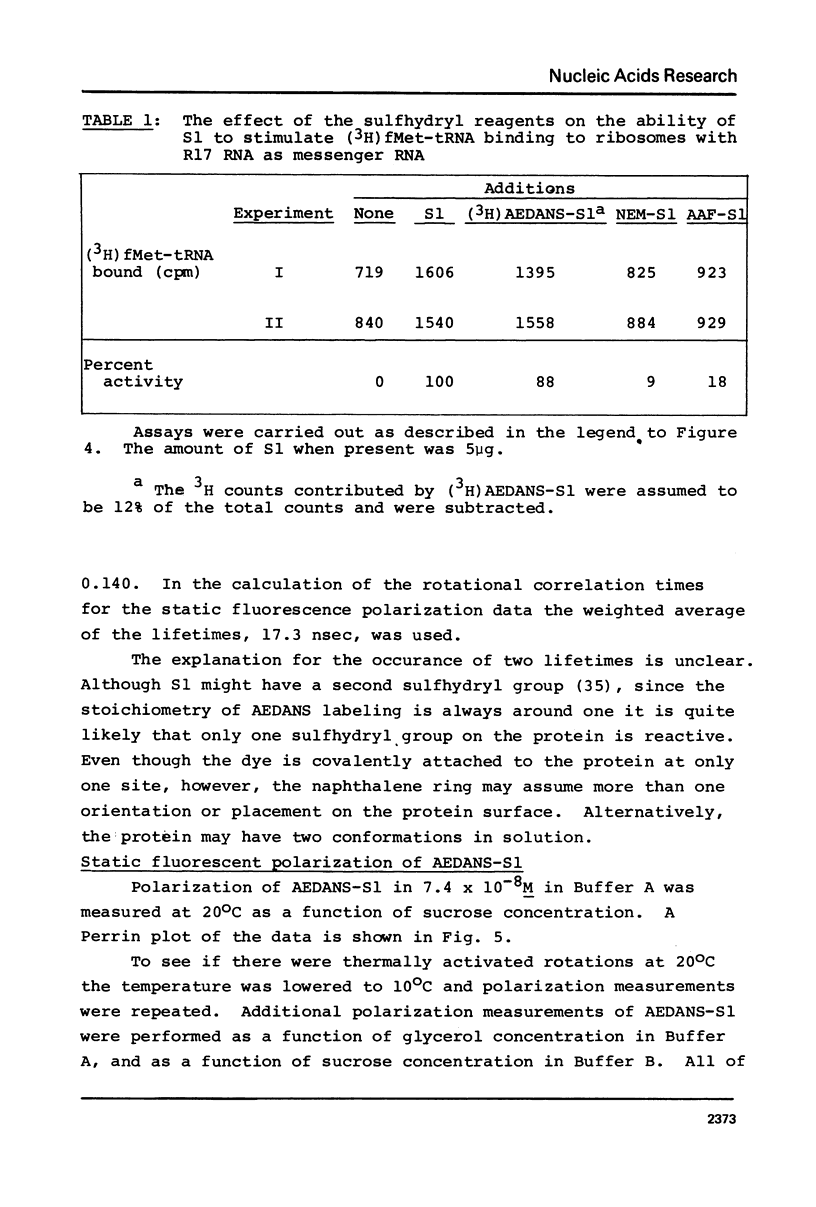
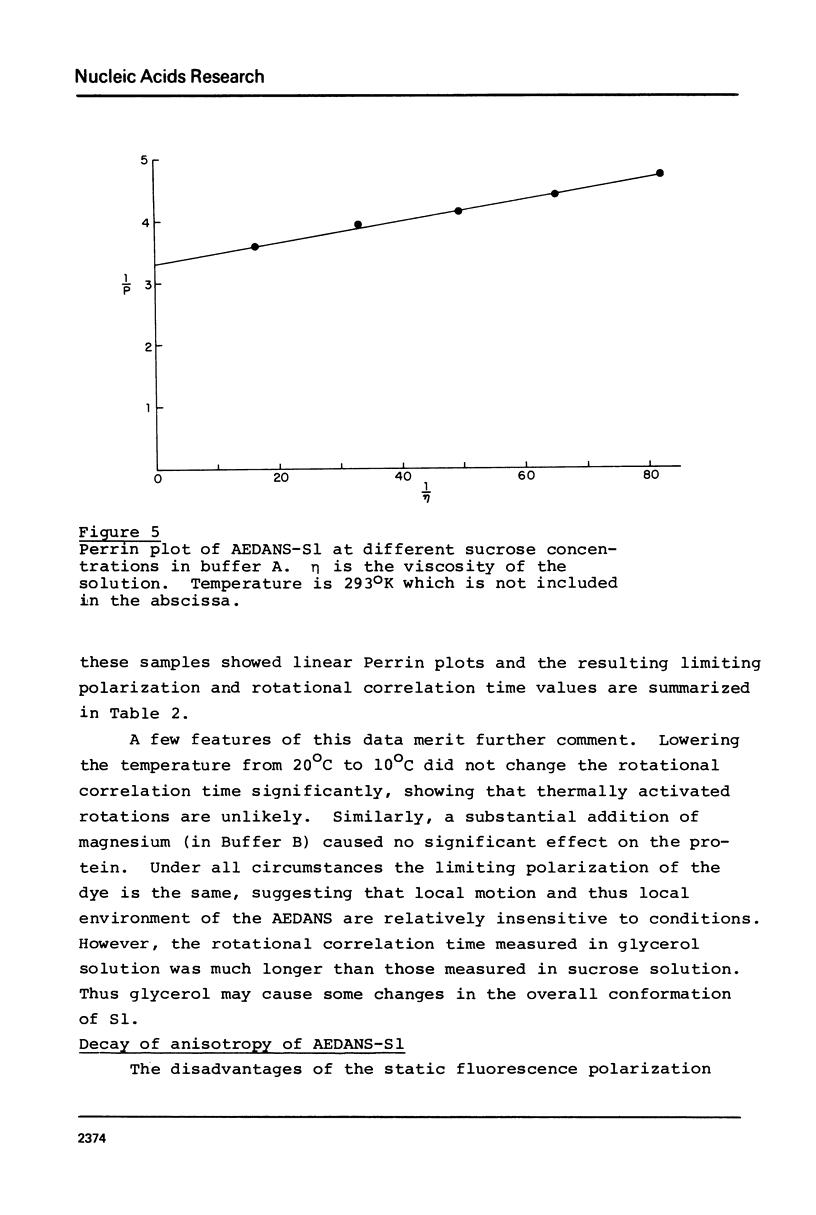
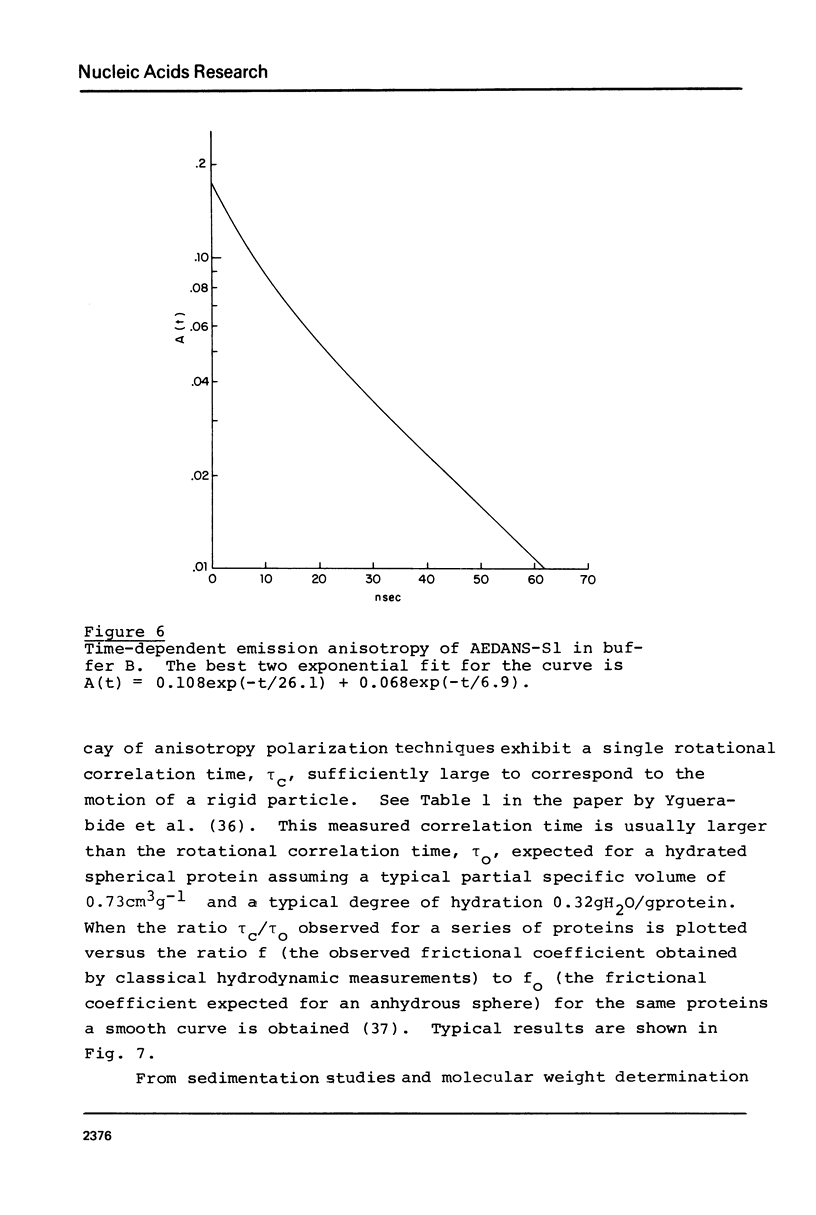
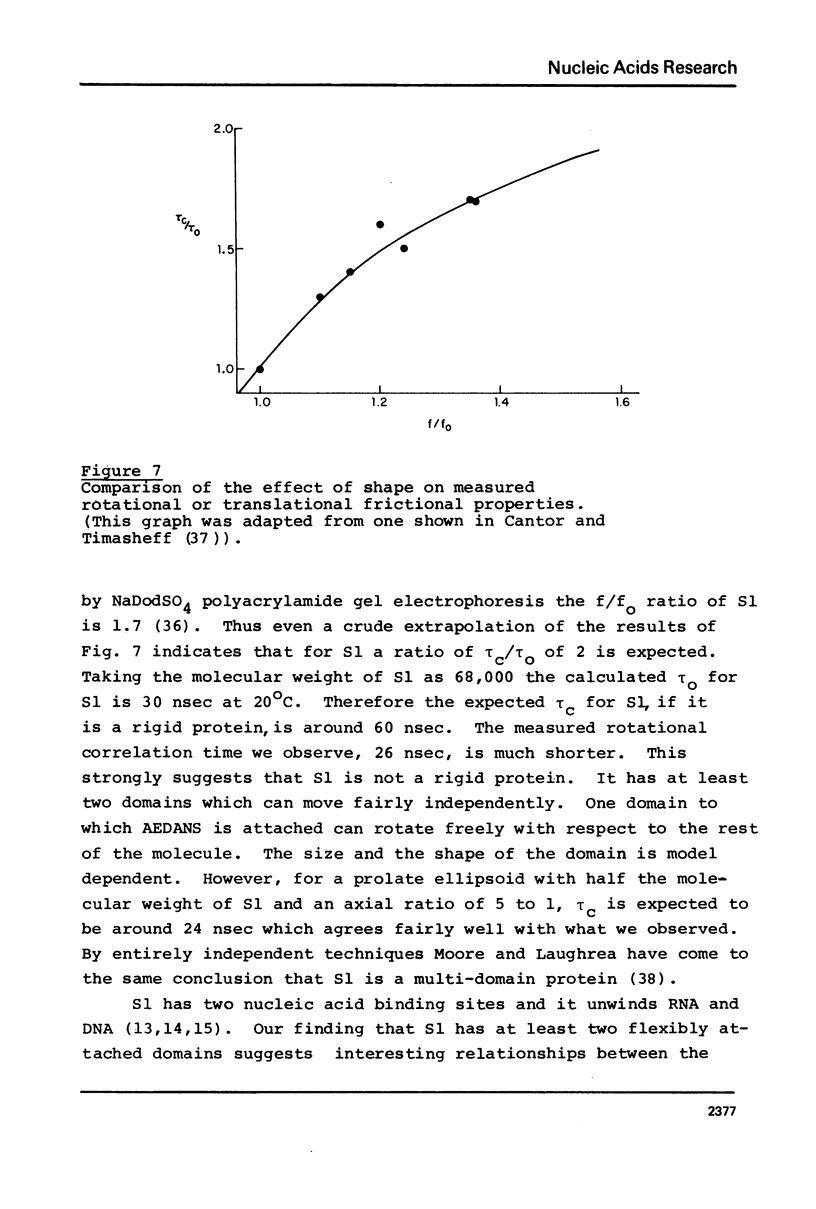


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acharya A. S., Moore P. B. Reaction of ribosomal sulfhydryl groups with 5,5'-dithiobis(2-nitrobenzoic acid). J Mol Biol. 1973 May 15;76(2):207–221. doi: 10.1016/0022-2836(73)90385-9. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay P. K., Wu C. W. Fluorescence and chemical studies on the interaction of Escherichia coli DNA-binding protein with single-stranded DNA. Biochemistry. 1978 Sep 19;17(19):4078–4085. doi: 10.1021/bi00612a032. [DOI] [PubMed] [Google Scholar]
- Bear D. G., Ng R., Van Derveer D., Johnson N. P., Thomas G., Schleich T., Noller H. F. Alteration of polynucleotide secondary structure by ribosomal protein S1. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1824–1828. doi: 10.1073/pnas.73.6.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochon J. C., Wahl P., Charlier M., Maurizot J. C., Hélène C. Time resolved spectroscopy of the tryptophyl fluorescence of the E. coli LAC repressor. Biochem Biophys Res Commun. 1977 Dec 21;79(4):1261–1271. doi: 10.1016/0006-291x(77)91142-1. [DOI] [PubMed] [Google Scholar]
- Dahlberg A. E. Two forms of the 30 S ribosomal subunit of Escherichia coli. J Biol Chem. 1974 Dec 10;249(23):7673–7678. [PubMed] [Google Scholar]
- Draper D. E., von Hippel P. H. Nucleic acid binding properties of Escherichia coli ribosomal protein S1. I. Structure and interactions of binding site I. J Mol Biol. 1978 Jul 5;122(3):321–338. doi: 10.1016/0022-2836(78)90193-6. [DOI] [PubMed] [Google Scholar]
- Eikenberry E. F., Bickle T. A., Traut R. R., Price C. A. Separation of large quantities of ribosomal subunits by zonal ultracentrifugation. Eur J Biochem. 1970 Jan;12(1):113–116. doi: 10.1111/j.1432-1033.1970.tb00827.x. [DOI] [PubMed] [Google Scholar]
- Fiser I., Scheit K. H., Stöffler G., Kuechler E. Identification of protein S 1 at the messenger RNA binding site of the Escherichia coli ribosome. Biochem Biophys Res Commun. 1974 Oct 8;60(3):1112–1118. doi: 10.1016/0006-291x(74)90427-6. [DOI] [PubMed] [Google Scholar]
- Fiser I., Scheit K. H., Stöffler G., Kuechler E. Proteins at the mRNA binding site of the Escherichia coli ribosome. FEBS Lett. 1975 Aug 15;56(2):226–229. doi: 10.1016/0014-5793(75)81097-0. [DOI] [PubMed] [Google Scholar]
- Giri L., Subramanian A. R. Hydrodynamic properties of protein S1 from Escherichia coli ribosome. FEBS Lett. 1977 Sep 1;81(1):199–203. doi: 10.1016/0014-5793(77)80958-7. [DOI] [PubMed] [Google Scholar]
- Huang K. H., Cantor C. R. Studies of 30 S Escherichia coli ribosome reassembly using individual proteins labeled with an environmentally sensitive fluorescent prode. J Mol Biol. 1975 Oct 5;97(4):423–441. doi: 10.1016/s0022-2836(75)80052-0. [DOI] [PubMed] [Google Scholar]
- Huang K. H., Fairclough R. H., Cantor C. R. Singlet energy transfer studies of the arrangement of proteins in the 30 S Escherichia coli ribosome. J Mol Biol. 1975 Oct 5;97(4):443–470. doi: 10.1016/s0022-2836(75)80053-2. [DOI] [PubMed] [Google Scholar]
- Inouye H., Pollack Y., Petre J. Physical and functional homology between ribosomal protein S1 and interference factor i. Eur J Biochem. 1974 Jun 1;45(1):109–117. doi: 10.1111/j.1432-1033.1974.tb03535.x. [DOI] [PubMed] [Google Scholar]
- Kolb A., Hermoso J. M., Thomas J. O., Szer W. Nucleic acid helix-unwinding properties of ribosomal protein S1 and the role of S1 in mRNA binding to ribosomes. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2379–2383. doi: 10.1073/pnas.74.6.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laughrea M., Moore P. B. Physical properties of ribosomal protein S1 and its interaction with the 30 S ribosomal subunit of Escherichia coli. J Mol Biol. 1977 May 25;112(3):399–421. doi: 10.1016/s0022-2836(77)80189-7. [DOI] [PubMed] [Google Scholar]
- Lührmann R., Gassen H. G. Identification of the 30-S ribosomal proteins at the decoding site by affinity labelling with a reactive oligonucleotide. Eur J Biochem. 1976 Jun 15;66(1):1–9. doi: 10.1111/j.1432-1033.1976.tb10419.x. [DOI] [PubMed] [Google Scholar]
- Moore P. B. Reaction of N-ethyl maleimide with the ribosomes of Escherichia coli. J Mol Biol. 1971 Aug 28;60(1):169–184. doi: 10.1016/0022-2836(71)90456-6. [DOI] [PubMed] [Google Scholar]
- Moore P. B. The distribution of half-cystine in the ribosomal proteins of Escherichia coli. J Mol Biol. 1975 Oct 25;98(2):439–444. doi: 10.1016/s0022-2836(75)80129-x. [DOI] [PubMed] [Google Scholar]
- Paoletti J., Le Pecq J. B. Corrections for instrumental errors in measurement of fluorescence and polarization of fluorescence. Anal Biochem. 1969 Oct 1;31(1):33–41. doi: 10.1016/0003-2697(69)90238-3. [DOI] [PubMed] [Google Scholar]
- Pongs O., Stöffler G., Bald R. W. Location of protein S1 of Escherichia coli ribosomes at the 'A'-site of the codon binding site. Affinity labeling studies with a 3'-modified A-U-G analog. Nucleic Acids Res. 1976 Jul;3(7):1635–1646. doi: 10.1093/nar/3.7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobura J. E., Chowdhury M. R., Hawley D. A., Wahba A. J. Requirement of chain initiation factor 3 and ribosomal protein S1 in translation of synthetic and natural messenger RNA. Nucleic Acids Res. 1977 Jan;4(1):17–29. doi: 10.1093/nar/4.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szer W., Hermoso J. M., Boublik M. Destabilization of the secondary structure of RNA by ribosomal protein S1 from Escherichia coli. Biochem Biophys Res Commun. 1976 Jun 7;70(3):957–964. doi: 10.1016/0006-291x(76)90685-9. [DOI] [PubMed] [Google Scholar]
- Tal M., Aviram M., Kanarek A., Weiss A. Polyuridylic acid binding and translating by Escherichia coli ribosomes: stimulation by protein I, inhibition by aurintricarboxylic acid. Biochim Biophys Acta. 1972 Oct 27;281(3):381–392. doi: 10.1016/0005-2787(72)90452-2. [DOI] [PubMed] [Google Scholar]
- Thammana P., Cantor C. R. Studies on ribosome structure and interactions near the m62Am62A sequence. Nucleic Acids Res. 1978 Mar;5(3):805–823. doi: 10.1093/nar/5.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. O., Kolb A., Szer W. Structure of single-stranded nucleic acids in the presence of ribosomal protein S1. J Mol Biol. 1978 Aug 5;123(2):163–176. doi: 10.1016/0022-2836(78)90319-4. [DOI] [PubMed] [Google Scholar]
- Van Dieijen G., Van Der Laken C. J., Van Knippenberg P. H., Van Duin J. Function of Escherichia coli ribosomal protein S1 in translation of natural and synthetic messenger RNA. J Mol Biol. 1975 Apr 15;93(3):351–366. doi: 10.1016/0022-2836(75)90282-x. [DOI] [PubMed] [Google Scholar]
- Wahba A. J., Miller M. J., Niveleau A., Landers T. A., Carmichael G. G., Weber K., Hawley D. A., Slobin L. I. Subunit I of G beta replicase and 30 S ribosomal protein S1 of Escherichia coli. Evidence for the identity of the two proteins. J Biol Chem. 1974 May 25;249(10):3314–3316. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yguerabide J., Epstein H. F., Stryer L. Segmental flexibility in an antibody molecule. J Mol Biol. 1970 Aug;51(3):573–590. doi: 10.1016/0022-2836(70)90009-4. [DOI] [PubMed] [Google Scholar]
- Yguerabide J. Nanosecond fluorescence spectroscopy of macromolecules. Methods Enzymol. 1972;26:498–578. doi: 10.1016/s0076-6879(72)26026-8. [DOI] [PubMed] [Google Scholar]




