Abstract
Pancreatic adenocarcinoma remains among the most lethal of human malignancies. Overall 5- year survival is less than 5%, and only 20% of patients presenting with localized disease amenable to surgical resection. Even in patients who undergo resection, long-term survival remains extremely poor. A major contributor to the aggressiveness of multiple cancers and pancreatic cancer in particular is the process of epithelial-to-mesenchymal transition (EMT). This review highlights the growing evidence of EMT in pancreatic cancer progression, focusing on the contribution of EMT to the development of cancer stem cells and on interaction of EMT with other pathways central to cancer progression such as Hedgehog signaling, the K-ras oncogene, and transforming growth factor-β (TGF-β). We will also discuss EMT-targeting agents currently in development and in clinical trials that may help to reduce the morbidity and mortality associated with pancreatic cancer.
Keywords: pancreatic cancer, epithelial-mesenchymal transition, microRNA, stem cells, drug resistance
EMT and Cancer Progression
Epithelial-to-mesenchymal transition (EMT) is a process controlled by a family of transcription factors that leads epithelial cells to undergo a phenotypic shift from cells with tight cell-cell junctions, clear basal and apical polarity, and sheet-like growth architecture into spindle-like, fusiform, motile cells that express distinct mesenchymal markers such as vimentin, fibronectin, and N-cadherin (1–3). While EMT is an embryologic process essential to normal development, it is also allows epithelial cancer cells to take on invasive properties and form distant metastases (1, 3–5). The process in tumors, while utilizing the same transcription factors and responsive to the same pathways as embryogensis, is qualitatively different, given the large degree of genetic abnormality and instability inherent within the cancer cells (3). Numerous studies have shown that cells harboring a mesenchymal phenotype demonstrate biology associated with cancer progression. This includes increased cell invasion, angiogenesis, chemotherapy resistance, increased tumorigenicity, and the formation of side populations of cancer stem cells (6–12). Metastatic foci in several cancers show evidence of EMT and elevated stem cell markers, which correlate with worse clinical outcomes and response to standard chemotherapy (13, 14). This has been seen in multiple epithelial cancers including breast, prostate, colorectal, lung ovarian, head and neck, and pancreatic cancers (10–22).
EMT is a dynamic process; however, by their nature, the in vivo studies from human cancer specimens that show a link between EMT and prognosis are static snapshots of tumors. It has been argued that what is thought to be EMT in vivo, simply reflects a shift in cell population resulting from a combination of epithelial apoptosis and mesenchymal proliferation, rather than a dynamic transition (23). More recently, transgenic mouse models of breast cancer and intestinal fibrosis have clearly demonstrated the role of dynamic EMT occurring in these settings (24, 25) and provide strong evidence for EMT as an active biological process within human cancers.
Pancreatic Cancer
In 2010, there were an estimated 43,000 new cases along with over 36,000 deaths from pancreatic cancer in the United States, making it the 4th leading cause of cancer death (26). Pancreatic ductal adenocarcinoma (PDAC) remains an extremely lethal disease, with a median survival of only 6 months and a dismal 5 year overall survival of less than 5% (27). Only 20% of patients present with disease amenable to resection, and even in these patients 5-year overall survival is still only 10%–20% (28–30). While surgical morbidity and mortality have improved significantly, long-term outcomes have remained relatively unchanged over the past 30 years (30, 31). The reasons for the extreme difficulty in treating PDAC are both anatomic and biologic. Given, the retroperitoneal location of the pancreas the disease frequently remains relatively asymptomatic in its early stages. Yet even when patients present with localized disease amenable to resection the biology of the disease is such that it is resistant to standard chemotherapies. Histologically, the tumors are encased within a dense fibrotic stroma, known as the desmoplastic reaction, which has been shown to limit chemotherapy delivery, increase the innate chemo-resistance of tumor cells, and promote a more aggressive cancer cell biology (6, 8, 11, 30–33). Given this, an active area of investigation focuses on how malignant ductal cells transform and then invade through this stroma with such efficiency and the cross-talk between the fibrous integument and the epithelial elements. A better understanding of the underlying biology and the interactions and signaling processes within cancer cells is needed in order to make meaningful progress in the treatment of this uniquely lethal disease.
EMT and PDAC
EMT, with its contribution to invasion, metastasis, chemo-resistance and the propagation of cancer stem cells, plays an especially important role in PDAC. EMT has been demonstrated in resected PDAC specimens and is a prominent feature of both in vivo and in vitro models of the disease (13, 34–37). Mesenchymal cells, identified by increased fibronectin and vimentin staining, along with decreased E-cadherin staining, show a positive correlation with high grade tumors (34). These changes have been linked to prognosis, with more mesenchymal tumors having worse survival and an increased number of metastases (13, 34).
EMT is controlled by a group of zinc finger transcription factors such as the Snail family (Snail and Slug), Zeb1, and Twist (1, 4, 5). In pancreatic cancer, Snail and Zeb1 are the most studied and are correlated with tumor grade and survival in vivo and with invasion and chemoresistance in several in vitro models (35–38). Over 80% of resected PDAC specimens have moderate to strong Snail expression, which was significantly more than either Slug or Twist. Snail expression, along with Zeb1, has been correlated with decreased E-cadherin levels and worse tumor grade and a poorer prognosis (35–38). Modulation of EMT pathways in vitro, Zeb1 in particular, leads to a reversal of EMT in pancreatic cancer cells and a restoration of chemosensitivity in previously resistant, mesenchymal cell lines (37, 39, 40).
EMT occurs in response to several distinct pathways, most notably and important to cancer include WNT, Notch, several receptor tyrosine kinase pathways, and transforming growth factor-β (TGF-β) (3). TGF–β plays an important and heterogeneous role in pancreatic cancer and is an essential driver of EMT (1, 32, 41–43). TGF-β ligands cause dimerization of the membrane bound TβRI and TβRII receptors, which leads to signal propagation through Smad dependent pathways (43, 44). Activated Smad-2 or Smad-3 localize to the nucleus with Smad-4 to serve as a transcriptional regulator (45). Mutations in TGF-β receptors and in Smad signaling are contributors to PDAC progression (44, 46). Over 50% of PDAC tumors have loss of Smad4; mouse models that replicate this show increased growth of pancreatic lesions owing to loss of TGF–β growth inhibition (46–48). While this suggests a tumor suppressive role for TGF–β, these tumors remain well differentiated. TGF–β responsive tumors (those with intact Smad signaling), conversely, show poor differentiation owing to an increase in EMT (48). Collagen, the primary component of the desmoplastic reaction, has been shown to increase Snail expression in pancreatic cancer cells through a TGF–β dependent process. This in turn, leads to an increase in membrane type 1-matrix metalloproteinase (MT1-MMP, a.k.a. MMP-14) expression and increased cell invasion (6). Matrix metalloproteinases, specifically MT1-MMP, represent novel therapeutic targets; silencing upstream TGF–β mediated EMT could decrease the progression of pancreatic cancer owed to MT1-MMP expression, though this remains to be verified (49). Additionally, tumors showing TGF–β driven EMT also show loss of oncogene dependent growth (50). Ablation of the oncogenic mutant K-ras leads to increased apoptosis in several cancer cell lines. Cells that underwent TGF–β induced EMT lose this K-ras dependence, which could be restored by targeting Zeb1 expression (50). This has important implications for future drug development, as molecules targeted against these growth pathways may be less effective in cells that have undergone EMT.
The WNT and Notch pathways are also important contributors to pancreatic cancer progression and regulators of both EMT (51, 52). Notch ligand Jagged1 downregulates E-cadherin via increased Slug, while Zeb1 expression increases Notch signaling (51, 53). Notch is also associated with gemcitabine resistance in pancreatic cancer cells (54). Blocking γ-secretase activity, essential for Notch mediated signaling, slowed tumor progression in mice (55). The WNT pathway has a strong association with cancer stem cells and the development of the stem cell-like phenotype (56). It can induce EMT directly, or through cross-talk with other pathways, including TGF-β, across several cancers, including PDAC (7, 57).
EMT, Cancer Stem Cells and PDAC
Populations of cells have been identified within a variety of cancers that possess properties such as self-renewal, tumor initiation, and differentiation. These cancer stem cells, originally identified in hematopoietic malignancies, have now been identified in numerous solid tumors and are associated with disease recurrence, metastases, and chemo-resistance (9, 14, 15, 58–61). It is becoming increasingly evident that non-stem cell populations within tumors can transform into stem cells (9–11). In breast cancer forced expression of a mutant K-ras in MCF-10 mammary cells leads to the development of a highly enriched population of CD44+/CD24− (90% of K-ras expressing cells vs. 1% of control cells) cells, which are the markers for breast cancer stem cells (11, 59). This transformation was associated with an increase in EMT markers, was potentiated by exogenous TGF-β treatment, and led to an increase in stem cell like behavior and drug-resistance (9–11, 62, 63).
Stem cell populations have also been identified in pancreatic cancer and their impact on disease progression continues to be elucidated (13, 22, 64, 65). Initial work utilized resected human PDAC specimens to generate xenograft models in nude mice. Within these tumor specimens, a small subpopulation of less than 1% of the total cell population possessed stem cell like properties. These cells, CD44+/CD24+/ESA+(epithelial specific antigen) showed tumorigenicity, the ability to differentiate into a heterogeneous tumor cell population, and maintenance of a self-renewing stem cell population (64). The triple positive cells were 100-fold more tumorigenic than unsorted cells. CD44 haw long been associated with pancreatic cancer progression. Different processing of CD44, known as splice variants, are associated with various cancer phenotypes (66–69). Splice variant V6 is increased in metastatic pancreatic cancer relative to the primary tumor, while other splice variants have recently been linked to EMT in breast cancer (66, 69).
Recently, using aldehyde dehydrogenase (ALDH) activity as a more specific marker of cancer stem cells, it was shown that ALDH-high cells comprise an even more select subpopulation of cells in human pancreatic cancers that are tumorigenic and capable of producing tumors at very low numbers (13). These ALDH-high cells have evidence of EMT and are increased within metastatic pancreatic cancer lesions. Patients with ALDH-positive tumors had increased metastases and worse survival (13).
In studying signaling pathways that may be utilized or differentially regulated by these cancer stem cells, both the Hedgehog signaling pathway and EMT associated gene expression were found to play a role. In the CD44+/CD24+/ESA+ cells, Hedgehog signaling was upregulated ten-fold compared to unsorted cells and to non-stem cell cancer cells, and when comparing ALDH-high to ALDH-low tumors, those with increased ALDH-high cells had increased Slug expression and decreased E-cadherin (13, 64).
Hedgehog signaling, EMT and cancer stem cells
Hedgehog signaling is an embryologic pathway that has been strongly implicated progression of several gastrointestinal malignancies and pancreatic cancer in particular (70, 71). The secreted ligand Sonic Hedgehog is increased in not only pancreatic cancer but precursor PanIN lesions as well (70). Forced hedgehog expression in mouse pancreas leads to the development of PanIN-like lesions that also possess mutant K-ras, and inhibiting Hedgehog signaling in vitro promotes apoptosis and limits proliferation (70). Within the surrounding stroma, Hedgehog signaling has been shown to be important for tumor growth, and targeting Hedgehog signaling can improve the delivery of gemcitabine in pancreatic cancer in vivo (33, 72). Hedgehog signaling is also involved in effecting EMT in a variety of other pathologic processes and malignancies (7, 18, 57, 73–78). In the development of hepatic fibrosis, quiescent hepatic stellate cells undergo EMT to become activated myofibroblasts in response to hedgehog signaling (73, 76). Whether hepatocytes, and not merely ductal cells in culture, or quiescent stellate cells, can undergo EMT and produce fibrosis is more controversial. In genetic labeling studies, mouse hepatocytes did not appear to be the source of type-I collagen producing cells (79, 80). However, while hepatocytes may themselves not undergo full EMT to contribute to fibrosis, they have been shown to be involved, upregulating their own expression of Snail and contributing the overall degree of liver fibrosis (81). In the kidney, tubular epithelial cells have been shown to undergo EMT and produce renal fibrosis (82). In non-small cell lung cancer, Hedgehog signaling mediates TGF–β induced EMT, and targeting Hedgehog pathways restored an epithelial phenotype and decreased invasion and tumorigenicity (77). Cross-talk between Hedgehog signaling and EMT pathways is also implicated in tumor progression in colon, esophageal, gastric, hepatocellular and pancreatic cancer (18, 22, 74, 75, 83).
The interaction between Hedgehog and EMT pathways leads to tumor progression through increased invasion, proliferation, and the induction and promotion of cancer stem cells (7, 18, 22, 84). In one set of experiments, pancreatic cancer cells selected based on slow cycling time, a feature of stem cell populations, demonstrated the traditional stem cell properties of tumorigenicity, the ability to differentiate, and self-renewal (84). Gene expression analysis of this sub-population demonstrated increased expression of Sonic Hedgehog and EMT associated genes. Furthermore, the cells had a mesenchymal morphology and showed increased invasion. Additional evidence of the link between EMT, Hedgehog Signaling, stem cells comes from work that sought to selectively target the Hedgehog pathway in a mouse model of pancreatic cancer (22). Hedgehog signaling was blocked in pancreatic cancer cells using the compound Cyclopamine, which led to the restoration of E-cadherin and reduction in cancer cell invasion. Treatment of a nude mouse model of pancreatic cancer led to a reduction of metastases and had a synergistic effect with gemcitabine (22). The authors also looked at the effect on stem cell populations and found that targeting Hedgehog signaling led to a reduction in the percentage of cells expressing the stem cell marker ALDH (22).
MicroRNA, EMT and Cancer Stem Cells
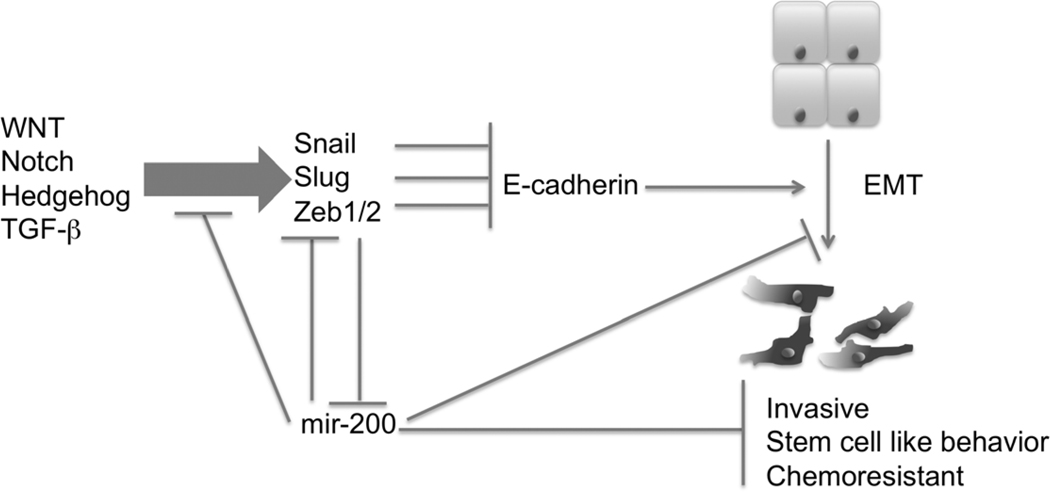
MicroRNAs (miRNAs), small, non-coding RNAs that affect a wide range of cell functions, are increasingly recognized to play a large role in many cancers (85–88). miRNAs primarily affect cell function by increasing or decreasing the stability of mRNA (85, 87). In cancer, they serve as both tumor promoters and suppressors and have been implicated in proliferation, apoptosis, and invasion (85–89). They have been shown to affect tumor progression through modulating both EMT and stem cell pathways (20, 89–93) (Figure 1). Work based on screening of over sixty cancer cell lines showed that the miR200 family of miRNAs are repressors of Zeb1 and Zeb2, thereby increasing E-cadherin expression and the epithelial phenotype, while conversely Zeb1 inhibits miR200 (20, 94). Additional work has shown that that forced expression of the entire miR200 family (miR-200a, b, c, miR-141 and miR-429) and miR205 can completely block TGF–β induced EMT (95).
Figure 1.
Schematic representation of the bidirectional feedback and regulation between EMT associated genes and the miR200 family of microRNAs. The miR200 family plays a central role in EMT and cancer progression, regressing the EMT associated gene Zeb1, TGF-β induced EMT, and the development of cancer stem cells. miR200 expression is in turn downregulated by Zeb1.
In pancreatic cancer, specific miRNA expression in vivo has been correlated with patient outcomes and has been shown to contribute to tumor cell invasion and metastatic ability (89, 96). Tissue microarrays of pancreatic cancer and multiple cell line analysis demonstrate increased miR200 expression is associated with increased E-cadherin expression and that patients with higher miR200 have improved survival over patients with low miR200 expression (89, 97). In contrast, miR21 expression, which is overexpressed in pancreatic cancer compared with normal pancreas, correlates with a poorer survival in patients with node-negative disease (27.7 months versus 15.2 months for patients with weak vs. strong miR21 expression) (96).
MicroRNAs also show an effect at the interplay between EMT and cancer stem cells, as several miRNAs effecting both EMT and stemness. In breast cancer, stem cell populations showed downregulation of the miR200 family, while increased miR200 expression abrogated stem cell colony growth and tumor formation (92). In pancreatic cancer specifically, EMT, miRNA and stemness correlated with gemcitabine resistance (90). The well differentiated epithelial pancreatic cancer line BxPC3 was treated with gemcitabine to select out a gemcitabine resistant cell population. This chemo-resistant line was significantly more mesenchymal in phenotype that the parental cells, with significantly increased Zeb1 expression, decreased E-cadherin and miR203, and an enhanced colony forming ability (90). Separate analysis of multiple pancreatic cancer cells lines showed that cell lines that are gemcitabine resistant were more mesenchymal and had decreased miR200 and let-7 expression compared with sensitive cell lines (93). Treatment with natural compounds 3,3'-diindolylmethane or Isoflavone, or forced expression of miR200 or let-7, led to a decrease in EMT markers Slug and vimentin, along with restoring gemcitabine sensitivity in previously resistant cells (93). Interestingly, let-7 is also repressed by type I collagen through a TGF–β and MT1-MMP dependent process (98). In the same cell line, collagen promoted Snail expression through TGF–β and subsequent MT1-MMP mediated invasion (6), further highlighting the interplay between the tumor microenvironment, TGF–β, miRNA, and EMT.
Targeting EMT and Future Directions
Given its role invasion, chemo-resistance, and the development of cancer stem cells, EMT and its pathways are intriguing targets for new therapies to combat pancreatic cancer. Specific targets include modulation of microRNAs involved in EMT, such as miR-200, directly targeting EMT transcription factors, or inhibiting related pathways like Hedgehog. The difficulty in developing strategies targeting EMT has been primarily related to technical difficulties in translating the methods developed in vitro into vehicles suitable for clinical application (99, 100). An alternative to siRNA and cellular transfection is finding chemical and biologic compounds that can affect these pathways. Examples with in vitro efficacy include CDF, a Circumin analogue, which increased miR-200 in pancreatic cancer to restore gemcitabine sensitivity; Silibinin, a natural flavonoid, directly inhibited Zeb1 in prostate cancer, reversing EMT; even the oral antiglycimic metformin has been shown to specifically target stem-cell populations by attenuating EMT (39, 101, 102). A separate compound, Salinomycin, discovered as part of a drug screen designed to find compounds effective against EMT, not only was specifically cytotoxic to cells that had undergone EMT, but also reduced the population of cancer stem cells within breast cancer cells (58).
Several clinical trials targeting EMT and related pathways in pancreatic are underway, but they remain early as either phase I or II trials. They include targeting TGF–β, Hedgehog inhibitors, and two trials looking to target Notch signal (Table 1). As these pathways are all involved with stemness and tumor survival, it is hoped that targeting them may sensitize tumors to other chemo and radiation therapy. As such, they may play a particularly valuable role in the neoadjuvant setting, trials of which are underway. The results of existing and future trials will be instrumental in determining whether targeting EMT and of stromal targeting more broadly, can be translated into meaningful improvement in outcomes for patients suffering from cancer.
Table 1.
Active Clinical Trials Targeting EMT and related Pathways in Pancreatic Cancer
| Pathway Targeted | Phase | Disease | Notable Information |
|---|---|---|---|
| Cancer Stem Cells(OMP21M18) | I | Unresectable Pancreatic Cancer | Monoclonal antibody against stem cells http://clinicaltrials.gov/ct2/show/NCT01189929 |
| Hedgehog (GDC-0449) | II | Metastatic Pancreatic Cancer | Combination with or without Gemcitabine http://clinicaltrials.gov/ct2/show/NCT01064622 |
| Hedgehog (GDC-0449) | II | Metastatic Pancreatic Cancer | Combination with Gemcitabine, Nab-Paxitacel, GDC-0499, http://clinicaltrials.gov/ct2/show/NCT01088815 |
| Hedgehog (GDC-0449) | II | Resectable Pancreatic Cancer – Neoadjuvant Therapy | http://clinicaltrials.gov/ct2/show/NCT01096732 |
| Hedgehog (IPI-926) | I/II | Untreated Metastatic Pancreatic Cancer | http://clinicaltrials.gov/ct2/show/NCT01130142 |
| Hedgehog (GDC-0449) | I | Metastatic Pancreatic Cancer | Combination with Erlotinib and with or without Gemcitabine http://clinicaltrials.gov/ct2/show/NCT00878163 |
| Hedgehog (GDC-0449) | 0 | Pancreatic Cancer – Cancer Stem cells | http://clinicaltrials.gov/ct2/show/NCT01195415 |
| Notch (MK0752) | I/II | Unresectable Pancreatic Cancer | Combination Therapy with Gemcitabine http://clinicaltrials.gov/ct2/show/NCT01098344 |
| Notch (RO4929097) | II | Previously Treated Metastatic Pancreatic Cancer | Outcomes – Survival and correlation with Stem Cell Markers http://clinicaltrials.gov/ct2/show/NCT01232829 |
| Notch (RO4929097) | I | Multiple Solid Tumors, including Pancreatic Cancer | Combination therapy with cediranib http://clinicaltrials.gov/ct2/show/NCT01131234 |
| Notch (RO4929097) | I | Pancreatic Cancer – Neoadjuvant therapy | Outcomes – Notch Inhibition and Cancer Stem Cell population changes http://clinicaltrials.gov/ct2/show/NCT01192763 |
| Notch (RO4929097) | I | Locally Advanced Pancreatic Cancer | Combination with Gemcitabine http://clinicaltrials.gov/ct2/show/NCT01145456 |
| WNT (PRI-724) | I | Advanced Solid Tumors (Unresectable Pancreatic Cancer) | http://clinicaltrials.gov/ct2/show/NCT01302405 |
Conclusion
EMT plays a central role in cancer progression. Being able to simultaneously affect invasion, chemo-resistance, and cancer stem cells makes EMT an immensely attractive target for developing new treatments. In pancreatic cancer especially, with its unique lethality and its multicellular dense fibrous stroma, a product of mesenchymal fibroblasts and EMT, halting this process is an extremely promising treatment avenue. Various existing compounds have been identified that can modulate several different aspects of EMT and its pathways, some of which are already being translated into clinical treatments, trials of which are currently underway. While the benefits are still uncertain, increasing our understanding of EMT, and its contribution to multiple components of tumor progression, will hopefully help identify additional targets and new therapies, and ultimately improve outcomes for patients with pancreatic cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Yang AD, Camp ER, Fan F, Shen L, Gray MJ, et al. Vascular endothelial growth factor receptor-1 activation mediates epithelial to mesenchymal transition in human pancreatic carcinoma cells. Cancer Research. 2006;66:46–51. doi: 10.1158/0008-5472.CAN-05-3086. [DOI] [PubMed] [Google Scholar]
- 3.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 4.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. The Journal of clinical investigation. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shields MA, Dangi-Garimella S, Krantz SB, Bentrem DJ, Munshi HG. Pancreatic cancer cells respond to type I collagen by inducing Snail expression to promote membrane type 1- matrix metalloproteinase dependent collagen invasion. J Biol Chem. 2011 doi: 10.1074/jbc.M110.195628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuxe J, Vincent T, de Herreros AG. Transcriptional crosstalk between TGFbeta and stem cell pathways in tumor cell invasion: Role of EMT promoting Smad complexes. Cell Cycle. 2010;9 doi: 10.4161/cc.9.12.12050. [DOI] [PubMed] [Google Scholar]
- 8.Dangi-Garimella S, Krantz SB, Barron MR, Shields MA, Heiferman MJ, et al. Three-Dimensional Collagen I Promotes Gemcitabine Resistance in Pancreatic Cancer through MT1-MMP-Mediated Expression of HMGA2. Cancer research. 2011 doi: 10.1158/0008-5472.CAN-10-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santisteban M, Reiman JM, Asiedu MK, Behrens MD, Nassar A, et al. Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Research. 2009;69:2887–2895. doi: 10.1158/0008-5472.CAN-08-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mani SA, Guo W, Liao M-J, Eaton EN, Ayyanan A, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morel A-P, Lièvre M, Thomas C, Hinkal G, Ansieau S, et al. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0002888. e2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanagawa J, Walser TC, Zhu LX, Hong L, Fishbein MC, et al. Snail promotes CXCR2 ligand-dependent tumor progression in non-small cell lung carcinoma. Clin Cancer Res. 2009;15:6820–6829. doi: 10.1158/1078-0432.CCR-09-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rasheed ZA, Yang J, Wang Q, Kowalski J, Freed I, et al. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J Natl Cancer Inst. 2010;102:340–351. doi: 10.1093/jnci/djp535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Hoogen C, van der Horst G, Cheung H, Buijs JT, Lippitt JM, et al. High aldehyde dehydrogenase activity identifies tumor-initiating and metastasis-initiating cells in human prostate cancer. Cancer Research. 2010;70:5163–5173. doi: 10.1158/0008-5472.CAN-09-3806. [DOI] [PubMed] [Google Scholar]
- 15.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer research. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 16.Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, et al. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Research. 2010;70:6945–6956. doi: 10.1158/0008-5472.CAN-10-0785. [DOI] [PubMed] [Google Scholar]
- 17.Carpentino JE, Hynes MJ, Appelman HD, Zheng T, Steindler DA, et al. Aldehyde dehydrogenase-expressing colon stem cells contribute to tumorigenesis in the transition from colitis to cancer. Cancer research. 2009;69:8208–8215. doi: 10.1158/0008-5472.CAN-09-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varnat F, Duquet A, Malerba M, Zbinden M, Mas C, et al. Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. EMBO Mol Med. 2009;1:338–351. doi: 10.1002/emmm.200900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, et al. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells. 2009;27:2059–2068. doi: 10.1002/stem.154. [DOI] [PubMed] [Google Scholar]
- 20.Park S-M, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldmann G, Dhara S, Fendrich V, Bedja D, Beaty R, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Research. 2007;67:2187–2196. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarin D, Thompson EW, Newgreen DF. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Research. 2005;65:5996–6000. doi: 10.1158/0008-5472.CAN-05-0699. discussion 6000-5991. [DOI] [PubMed] [Google Scholar]
- 24.Trimboli AJ, Fukino K, de Bruin A, Wei G, Shen L, et al. Direct evidence for epithelial-mesenchymal transitions in breast cancer. Cancer Research. 2008;68:937–945. doi: 10.1158/0008-5472.CAN-07-2148. [DOI] [PubMed] [Google Scholar]
- 25.Flier SN, Tanjore H, Kokkotou EG, Sugimoto H, Zeisberg M, et al. Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J Biol Chem. 2010;285:20202–20212. doi: 10.1074/jbc.M110.102012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 27.Bilimoria KY, Bentrem DJ, Ko CY, Ritchey J, Stewart AK, et al. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer. 2007;110:738–744. doi: 10.1002/cncr.22852. [DOI] [PubMed] [Google Scholar]
- 28.Altekruse S, Kosary C, Krapcho M, Neyman N, Aminou R, et al. SEER Cancer Statistics Review, 1975–2007. NCI. 2010;2010 [Google Scholar]
- 29.Stojadinovic A, Hoos A, Brennan MF. Conlon KCP Randomized clinical trials in pancreatic cancer. Surg Oncol Clin N Am. 2002;11:207–229. doi: 10.1016/s1055-3207(03)00082-6. x. [DOI] [PubMed] [Google Scholar]
- 30.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. discussion 1210-1191. [DOI] [PubMed] [Google Scholar]
- 31.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 32.Ottaviano AJ, Sun L, Ananthanarayanan V, Munshi HG. Extracellular matrix-mediated membrane-type 1 matrix metalloproteinase expression in pancreatic ductal cells is regulated by transforming growth factor-beta1. Cancer Research. 2006;66:7032–7040. doi: 10.1158/0008-5472.CAN-05-4421. [DOI] [PubMed] [Google Scholar]
- 33.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Javle MM, Gibbs JF, Iwata KK, Pak Y, Rutledge P, et al. Epithelial-mesenchymal transition (EMT) and activated extracellular signal-regulated kinase (p-Erk) in surgically resected pancreatic cancer. Annals of Surgical Oncology. 2007;14:3527–3533. doi: 10.1245/s10434-007-9540-3. [DOI] [PubMed] [Google Scholar]
- 35.Hotz B, Arndt M, Dullat S, Bhargava S, Buhr H-J, et al. Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer. Clin Cancer Res. 2007;13:4769–4776. doi: 10.1158/1078-0432.CCR-06-2926. [DOI] [PubMed] [Google Scholar]
- 36.Buck E, Eyzaguirre A, Barr S, Thompson S, Sennello R, et al. Loss of homotypic cell adhesion by epithelial-mesenchymal transition or mutation limits sensitivity to epidermal growth factor receptor inhibition. Mol Cancer Ther. 2007;6:532–541. doi: 10.1158/1535-7163.MCT-06-0462. [DOI] [PubMed] [Google Scholar]
- 37.Arumugam T, Ramachandran V, Fournier KF, Wang H, Marquis L, et al. Epithelial to Mesenchymal Transition Contributes to Drug Resistance in Pancreatic Cancer. Cancer Research. 2009;69:5820–5828. doi: 10.1158/0008-5472.CAN-08-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maier HJ, Schmidt-Strassburger U, Huber MA, Wiedemann EM, Beug H, et al. NF-kappaB promotes epithelial-mesenchymal transition, migration and invasion of pancreatic carcinoma cells. Cancer Lett. 2010;295:214–228. doi: 10.1016/j.canlet.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Ali S, Ahmad A, Banerjee S, Padhye S, Dominiak K, et al. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Research. 2010;70:3606–3617. doi: 10.1158/0008-5472.CAN-09-4598. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Wang Z, Li Y, Kong D, Banerjee S, Ahmad A, et al. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Research. 2009;69:2400–2407. doi: 10.1158/0008-5472.CAN-08-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 42.Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 43.Massagué J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 44.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 45.Brown KA, Pietenpol JA, Moses HL. A tale of two proteins: differential roles and regulation of Smad2 and Smad3 in TGF-beta signaling. J Cell Biochem. 2007;101:9–33. doi: 10.1002/jcb.21255. [DOI] [PubMed] [Google Scholar]
- 46.Schutte M, Hruban RH, Hedrick L, Cho KR, Nadasdy GM, et al. DPC4 gene in various tumor types. Cancer research. 1996;56:2527–2530. [PubMed] [Google Scholar]
- 47.Izeradjene K, Combs C, Best M, Gopinathan A, Wagner A, et al. Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer Cell. 2007;11:229–243. doi: 10.1016/j.ccr.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 48.Bardeesy N, Cheng K-H, Berger JH, Chu GC, Pahler J, et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 2006;20:3130–3146. doi: 10.1101/gad.1478706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Devy L, Huang L, Naa L, Yanamandra N, Pieters H, et al. Selective inhibition of matrix metalloproteinase-14 blocks tumor growth, invasion, and angiogenesis. Cancer Research. 2009;69:1517–1526. doi: 10.1158/0008-5472.CAN-08-3255. [DOI] [PubMed] [Google Scholar]
- 50.Singh A, Greninger P, Rhodes D, Koopman L, Violette S, et al. A gene expression signature associated with "K-Ras addiction" reveals regulators of EMT and tumor cell survival. Cancer Cell. 2009;15:489–500. doi: 10.1016/j.ccr.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leong KG, Niessen K, Kulic I, Raouf A, Eaves C, et al. Jagged1-mediated Notch activation induces epithelial-to-mesenchymal transition through Slug-induced repression of E-cadherin. J Exp Med. 2007;204:2935–2948. doi: 10.1084/jem.20071082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6392–6397. doi: 10.1073/pnas.0802047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brabletz S, Bajdak K, Meidhof S, Burk U, Niedermann G, et al. The ZEB1/miR-200 feedback loop controls Notch signalling in cancer cells. EMBO J. 2011 doi: 10.1038/emboj.2010.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Z, Li Y, Kong D, Banerjee S, Ahmad A, et al. Acquisition of Epithelial-Mesenchymal Transition Phenotype of Gemcitabine-Resistant Pancreatic Cancer Cells Is Linked with Activation of the Notch Signaling Pathway. Cancer research. 2009;69:2400–2407. doi: 10.1158/0008-5472.CAN-08-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Plentz R, Park J-S, Rhim AD, Abravanel D, Hezel AF, et al. Inhibition of gamma-secretase activity inhibits tumor progression in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology. 2009;136:1741–1749. doi: 10.1053/j.gastro.2009.01.008. e1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katoh M. Network of WNT and Other Regulatory Signaling Cascades in Pluripotent Stem Cells and Cancer Stem Cells. Curr Pharm Biotechnol. 2011;12:160–170. doi: 10.2174/138920111794295710. [DOI] [PubMed] [Google Scholar]
- 57.Zhou BP, Hung M-C. Wnt, hedgehog and snail: sister pathways that control by GSK-3beta and beta-Trcp in the regulation of metastasis. Cell Cycle. 2005;4:772–776. doi: 10.4161/cc.4.6.1744. [DOI] [PubMed] [Google Scholar]
- 58.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Korkaya H, Wicha MS. Cancer stem cells: nature versus nurture. Nat Cell Biol. 2010;12:419–421. doi: 10.1038/ncb0510-419. [DOI] [PubMed] [Google Scholar]
- 61.Riethdorf S, Pantel K. Disseminated tumor cells in bone marrow and circulating tumor cells in blood of breast cancer patients: current state of detection and characterization. Pathobiology. 2008;75:140–148. doi: 10.1159/000123852. [DOI] [PubMed] [Google Scholar]
- 62.Neth P, Ries C, Karow M, Egea V, Ilmer M, et al. The Wnt signal transduction pathway in stem cells and cancer cells: influence on cellular invasion. Stem Cell Rev. 2007;3:18–29. doi: 10.1007/s12015-007-0001-y. [DOI] [PubMed] [Google Scholar]
- 63.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010 doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, et al. Identification of pancreatic cancer stem cells. Cancer Research. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 65.Hong SP, Wen J, Bang S, Park S, Song SY. CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int J Cancer. 2009;125:2323–2331. doi: 10.1002/ijc.24573. [DOI] [PubMed] [Google Scholar]
- 66.Brown RL, Reinke LM, Damerow MS, Perez D, Chodosh LA, et al. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. The Journal of clinical investigation. 2011;121:1064–1074. doi: 10.1172/JCI44540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rudy W, Hofmann M, Schwartz-Albiez R, Zöller M, Heider KH, et al. The two major CD44 proteins expressed on a metastatic rat tumor cell line are derived from different splice variants: each one individually suffices to confer metastatic behavior. Cancer research. 1993;53:1262–1268. [PubMed] [Google Scholar]
- 68.Hofmann M, Rudy W, Günthert U, Zimmer SG, Zawadzki V, et al. A link between ras and metastatic behavior of tumor cells: ras induces CD44 promoter activity and leads to low-level expression of metastasis-specific variants of CD44 in CREF cells. Cancer research. 1993;53:1516–1521. [PubMed] [Google Scholar]
- 69.Gansauge F, Gansauge S, Zobywalski A, Scharnweber C, Link KH, et al. Differential expression of CD44 splice variants in human pancreatic adenocarcinoma and in normal pancreas. Cancer research. 1995;55:5499–5503. [PubMed] [Google Scholar]
- 70.Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–851. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 72.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 73.Syn W-K, Jung Y, Omenetti A, Abdelmalek M, Guy CD, et al. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology. 2009;137:1478–1488. doi: 10.1053/j.gastro.2009.06.051. e1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Isohata N, Aoyagi K, Mabuchi T, Daiko H, Fukaya M, et al. Hedgehog and epithelial-mesenchymal transition signaling in normal and malignant epithelial cells of the esophagus. Int J Cancer. 2009;125:1212–1221. doi: 10.1002/ijc.24400. [DOI] [PubMed] [Google Scholar]
- 75.Ohta H, Aoyagi K, Fukaya M, Danjoh I, Ohta A, et al. Cross talk between hedgehog and epithelial-mesenchymal transition pathways in gastric pit cells and in diffuse-type gastric cancers. Br J Cancer. 2009;100:389–398. doi: 10.1038/sj.bjc.6604846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choi SS, Syn W-K, Karaca GF, Omenetti A, Moylan CA, et al. Leptin promotes the myofibroblastic phenotype in hepatic stellate cells by activating the hedgehog pathway. J Biol Chem. 2010;285:36551–36560. doi: 10.1074/jbc.M110.168542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maitah MiY, Ali S, Ahmad A, Gadgeel S, Sarkar FH. Up-regulation of sonic hedgehog contributes to TGF-β1-induced epithelial to mesenchymal transition in NSCLC cells. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0016068. e16068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Katoh M, Katoh M. Integrative genomic analyses of ZEB2: Transcriptional regulation of ZEB2 based on SMADs, ETS1, HIF1alpha, POU/OCT, and NF-kappaB. Int J Oncol. 2009;34:1737–1742. doi: 10.3892/ijo_00000304. [DOI] [PubMed] [Google Scholar]
- 79.Taura K, Miura K, Iwaisako K, Osterreicher CH, Kodama Y, et al. Hepatocytes do not undergo epithelial-mesenchymal transition in liver fibrosis in mice. Hepatology. 2010;51:1027–1036. doi: 10.1002/hep.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scholten D, Osterreicher CH, Scholten A, Iwaisako K, Gu G, et al. Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice. Gastroenterology. 2010;139:987–998. doi: 10.1053/j.gastro.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rowe RG, Lin Y, Shimizu-Hirota R, Hanada S, Neilson EG, et al. Hepatocyte-derived snail1 propagates liver fibrosis progression. Mol Cell Biol. 2011;31:2392–2403. doi: 10.1128/MCB.01218-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boutet A, De Frutos CA, Maxwell PH, Mayol MJ, Romero J, et al. Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J. 2006;25:5603–5613. doi: 10.1038/sj.emboj.7601421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen X, Lingala S, Khoobyari S, Nolta J, Zern MA, et al. Epithelial Mesenchymal Transition and Hedgehog Signaling Activation are Associated with Chemoresistance and Invasion of Hepatoma Subpopulations. Journal of hepatology. 2011 doi: 10.1016/j.jhep.2010.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dembinski JL, Krauss S. Characterization and functional analysis of a slow cycling stem cell-like subpopulation in pancreas adenocarcinoma. Clin Exp Metastasis. 2009;26:611–623. doi: 10.1007/s10585-009-9260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dillhoff M, Wojcik SE, Bloomston M. MicroRNAs in solid tumors. J Surg Res. 2009;154:349–354. doi: 10.1016/j.jss.2008.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 87.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 88.McManus MT, Sharp PA. Gene silencing in mammals by small interfering RNAs. Nat Rev Genet. 2002;3:737–747. doi: 10.1038/nrg908. [DOI] [PubMed] [Google Scholar]
- 89.Yu J, Ohuchida K, Mizumoto K, Sato N, Kayashima T, et al. MicroRNA, hsa-miR-200c, is an independent prognostic factor in pancreatic cancer and its upregulation inhibits pancreatic cancer invasion but increases cell proliferation. Mol Cancer. 2010;9:169. doi: 10.1186/1476-4598-9-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 91.Peter ME. Let-7 and miR-200 microRNAs: guardians against pluripotency and cancer progression. Cell Cycle. 2009;8:843–852. doi: 10.4161/cc.8.6.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li Y, VandenBoom TG, Kong D, Wang Z, Ali S, et al. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Research. 2009;69:6704–6712. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, et al. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 96.Dillhoff M, Liu J, Frankel W, Croce C, Bloomston M. MicroRNA-21 is overexpressed in pancreatic cancer and a potential predictor of survival. J Gastrointest Surg. 2008;12:2171–2176. doi: 10.1007/s11605-008-0584-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kent OA, Mullendore M, Wentzel EA, López-Romero P, Tan AC, et al. A resource for analysis of microRNA expression and function in pancreatic ductal adenocarcinoma cells. Cancer biology & therapy. 2009;8:2013–2024. doi: 10.4161/cbt.8.21.9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dangi-Garimella S, Strouch MJ, Grippo PJ, Bentrem DJ, Munshi HG. Collagen regulation of let-7 in pancreatic cancer involves TGF-β1-mediated membrane type 1-matrix metalloproteinase expression. Oncogene. 2011;30:1002–1008. doi: 10.1038/onc.2010.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brown BD, Naldini L. Exploiting and antagonizing microRNA regulation for therapeutic and experimental applications. Nat Rev Genet. 2009;10:578–585. doi: 10.1038/nrg2628. [DOI] [PubMed] [Google Scholar]
- 100.Jackson AL, Linsley PS. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat Rev Drug Discov. 2010;9:57–67. doi: 10.1038/nrd3010. [DOI] [PubMed] [Google Scholar]
- 101.Wu K, Zeng J, Li L, Fan J, Zhang D, et al. Silibinin reverses epithelial-to-mesenchymal transition in metastatic prostate cancer cells by targeting transcription factors. Oncol Rep. 2010;23:1545–1552. [PubMed] [Google Scholar]
- 102.Vazquez-Martin A, Oliveras-Ferraros C, Cufí S, Del Barco S, Martin-Castillo B, et al. Metformin regulates breast cancer stem cell ontogeny by transcriptional regulation of the epithelial-mesenchymal transition (EMT) status. Cell cycle (Georgetown, Tex) 2010;9 [PubMed] [Google Scholar]