Abstract
Variation in response styles in the hypothalamic-pituitary-adrenal (HPA) axis are known to be predictors of short- and long-term health outcomes. The nature of HPA responses to stressors changes with developmental stage, and some components of the stress response exhibit long-term individual consistency (i.e., are trait-like) while others are transient or variable (i.e., state-like). Here we evaluated the response of marmoset monkeys (Callithrix geoffroyi) to a standardized social stressor (social separation and exposure to a novel environment) at three different stages of development: juvenile, subadult, and young adult). We monitored levels of urinary cortisol (CORT), and derived multiple measures of HPA activity: Baseline CORT, CORT reactivity, CORT Area Under the Curve (AUC), and CORT regulation. Juvenile marmosets exhibited the most dramatic stress response, had higher AUCs, and tended to show poorer regulation. While baseline CORT and CORT regulation were not consistent within an individual across age, CORT reactivity and measures of AUC were highly correlated across time; i.e., individuals with high stress reactivity and AUC as juveniles also had high measures as subadults and adults, and vice-versa. Marmoset co-twins did not exhibit similar patterns of stress reactivity. These data suggest that regardless of the source of variation in stress response styles in marmosets, individually-distinctive patterns are established by six months of age, and persist for at least a year throughout different phases of marmoset life history.
Keywords: Stress reactivity, Trait, urinary cortisol, stability, marmoset, Area under the curve
The neuroendocrine stress response mediated by the hypothalamic-pituitary-adrenal (HPA) axis is critical for a variety of adaptive physiological and psychological processes, including energy regulation, coping with short-term stressors, and mediating psychosocial responses to these stressors. Long-term exposure to elevated glucocorticoid (GC) hormones, the end-product of the HPA response, can be associated with a variety of deleterious psychological, physiological, and immune states (Sapolsky et al., 2000; Tasker, 2006). There is considerable individual variation in the magnitude, timing, and duration of the HPA response to stressors, suggesting that individuals may differ in their susceptibility to the negative consequences of long-term exposure to stressors, and that individuals that exhibit atypically high levels of GC release in response to moderate or even mild stressors may also be at risk for negative health consequences (DeVries et al., 2007).
A central question in examining individual differences in the nature of HPA responses to stressors is the degree to which individuals show consistency in the “style” of responding to stressors across time and across contexts (i.e., do individual stress responses show stable, trait-like properties in individuals?). Most, but not all, studies on human participants show moderate to relatively high intra-individiual consistency among multiple measures of HPA function, including baseline cortisol (CORT; Flinn, 2009; Hamer et al., 2006), stress reactivity to physical or psychological stressors (Berger et al., 1987; Cohen et al., 2000; Leung et al.; PrÈville et al., 2008), responses to dexamethasone/corticotropin releasing hormone (DEX/CRH) challenge (Modell et al., 1998; Watson et al., 2005), and the cortisol awakening response (CAR; Hellhammer et al., 2007; Thorn et al., 2009). However, several studies report a lack of significant individual stability in multiple components of the HPA response, including baseline CORT in samples collected six weeks apart (Kirschbaum et al., 1990), CORT reactivity to two public speaking stressors separated by 4 weeks (Hamer et al., 2006), and DEX/CRH responding in high-risk psychiatric patients tested at a 4 year interval (Modell et al., 1998). Thus, there is considerable evidence (with obvious caveats) that many parameters of HPA function remain stable over repeated testing sessions. However, a serious limitation of these studies is the restricted time scale across which multiple measures are taken. Most of the studies assess HPA function across days, weeks or months, and only a few carry measures over the course of years, and hence are limited to a one or two stages of life history (e.g., infancy and childhood, young adult, elderly), and it is clear that average HPA function varies significantly across life history stages (e.g., prepubertal to postpubertal: Gunnar et al., 2009; early postnatal to later postnatal: Sapolsky and Meaney, 1986; Pryce et al., 2002).
In nonhuman primates, there is mixed evidence for individual stability in HPA function. Higley et al., 1992) compared CORT responses during separation tests in mother- vs. peer-reared rhesus macaque (Macaca mulatta) infants and juveniles at 6- and 18-months-of-age. Baseline CORT was similar between the two rearing conditions, but peer-reared juveniles showed higher CORT responses to separation than mother-reared infants. However, regardless of rearing condition, baseline CORT and CORT during early, but not late stages of separation, were highly consistent across the one-year period. Likewise, Capitanio et al., 1998) showed that CORT responses to three separate conditions (baseline, physical restraint stress, and DEX challenge were highly stable among individual rhesus macaques at four different sampling points throughout a one-year period. Interestingly, correlations of CORT responses by individuals across stressor conditions were not significantly correlated (e.g., an individual’s restraint stress CORT levels were not significantly associated with their response to DEX challenge or to their baseline CORT. Finally, Lilly et al., 1999) assessed immune function and HPA activity in female macaques in three contexts: upon capture from a free-ranging colony, during solitary housing, and during a transition to group living. Immune parameters exhibited significant individual stability over these three contexts, and mean CORT levels varied among conditions, but individual differences in CORT levels were not consistent across the three conditions. Similar to the findings on human stress responses, the data on stability in stress profiles are mixed in nonhuman primates.
Marmoset monkeys (genus Callithrix) represent an interesting case study for evaluating individual consistency in stress responses. Chronic physical and psychogenic stressors can produce states that are similar to clinical symptoms observed in human populations, including reduced reproductive function (Johnson et al., 1991), increased anxiety-like behavior, HPA axis dysfunction, and altered social competency (Johnson et al., 1996; Smith et al., in press). Marmosets live in family-like social units, and relationships within these groups can be both a source of buffering of the HPA system against psychosocial stressors (Rukstalis and French, 2005; Smith et al., 1998) and a potent activator of the stress response as a consequence of intrafamily conflict (Smith and French, 1997b). In addition, since marmosets routinely give birth to fraternal, or dizygotic twins (Benirschke, 1995), it is possible to contrast the combined effects of shared genes and common intrauterine environments with differences in postnatal environments in shaping stress-related phenotypes. In contrast to human and other nonhuman primates (e.g., macaques), however, marmosets exhibit exceedingly high circulating levels of glucocorticoids (Coe et al., 1992 and have correspondingly low sensitivity in glucocorticoid receptors (Chrousos et al., 1982).
One previous study has examined age-related changes in stress reactivity in common marmosets (C. jacchus). Pryce et al., 2002) studied changes in basal HPA activity from one week of age through adulthood, and stress-induced changes in HPA function at two, six, and 12 months of age. Yamamoto, 2003) has defined major age classes for marmosets as infant (0 – 5 months), juvenile (5 – 10 months), subadult (10 – 15 months), and young adult (> 15 months). Unlike rodents, which have a distinct hyporesponsive HPA system in the postnatal period (Sapolsky and Meaney, 1986; Walker et al., 1986), one-week old marmosets have significantly elevated basal plasma ACTH and CORT. Pryce and colleagues (2002) noted that basal ACTH and CORT levels reached adult-like levels by four months of age. Furthermore, when exposed to capture and isolation from the natal family group, two-month old marmosets had significantly higher stress reactivity (both ACTH and CORT) than six month and 12-month old marmosets. Because Pryce et al.’s (2002) study was a combination of longitudinal and cross-sectional designs, however, the authors were not able to track individual stability across age in either basal or stress-induced neuroendocrine function.
To address the question of stability in individual stress response styles, we exposed white-faced marmosets (C. geoffroyi) to a psychosocial stressor (separation from their natal family group and housing for 8 hours in a novel environment) at 6-, 12-, and 18-months of age. By six months of age, infants are independent of carriers but are the youngest offspring in the family group. By 12 months of age, marmosets are approaching adult body weight, and serving as caregivers for younger siblings, but are typically prepubertal in the natal family group. By 18 months, both males (Baker et al., 1999; Birnie et al.; Birnie et al., 2011) and females (Saltzman et al., 1997; Smith et al., 1997; Filippini & French, in prep) have typically reached puberty (as indexed by elevated testosterone and the appearance of normative ovulatory cycles). In this study, we addressed three questions regarding the HPA stress response. First, we inquired whether there were age-related changes in the response characteristics of the HPA system (baseline CORT, maximum CORT response to the stressor, AUC, and regulation of the HPA axis after cessation of the stressor). Secondly, we tested the degree to which individuals maintained consistent HPA response styles across three distinctly separate phases of marmoset life history. Finally, we assessed whether fraternal co-twins displayed concordant stress response styles. To the extent that shared alleles and common intrauterine environments shape stress response styles, we predicted that twin littermates would exhibit common patterns of stress reactivity.
Methods
Subjects
The subjects in this experiment were 28 white-faced marmoset offspring (Callithrix geoffroyi) born to breeding pairs in the Callitrichid Research Center at the University of Nebraska at Omaha. Six breeding pairs produced an average of three litters for the study (range one to five litters; see Table 1 for details on litter size and sex ratios). All subjects lived in intact family groups with their mother, father, and older and younger siblings. Sixteen of the marmosets recruited into the study were male, and 12 were female. Family groups were housed in large breeding enclosures (minimum of 0.8 m3 per individual) that contained natural branches, rope vines, sleeping enclosures, and a variety of enrichment and foraging devices. The marmosets were fed a diet of commercially-prepared marmoset diet (Zu-Preme©) at 0800 h, and fresh fruit, eggs, and invertebrate protein at 1400 h. Fresh water was available at all times. Additional details on husbandry and management can be found in (Schaffner et al., 1995). All animal procedures were approved by the UNMC/UNO Institutional Animal Care and Use Committee, and the CRC is a USDA-licensed and AAALAC-accredited facility.
Table 1.
Source and sex ratios of marmosets utilized in the study
| Breeding Pair | Number of Litters | Litter Size at Testing and Sex Ratio* |
|---|---|---|
| S—R | 5 | 1.1, 1.0, 1.1, 0.1, 1.1 |
| P—A | 2 | 1.0, 2.0 |
| B—E/S | 2 | 1.0, 1.0 |
| D—S | 4 | 0.1, 1.1, 1.1, 1.1 |
| L—M | 4 | 1.1, 1.0, 0.1, 0.2 |
| U—A | 1 | 2.0 |
For each litter, numbers represent male.female
Standardized Psychosocial Stressor Procedure
Each of the offspring in the experiment was exposed to a standardized psychosocial stressor at three time points in development: 6, 12, and 18 months of age. At each developmental stage, we collected urine samples on three successive days. We utilized a noninvasive sampling procedure that involves training marmosets to urinate into aluminum pans in exchange for a food reward (see French et al., 1996 for details). First-void urine samples were collected between 0600 and 0800 h on the day prior to, the day of, and the day after the stressor. On the day of the stressor, the marmoset was removed from its natal family group by capture with a net at approximately 0900 h, and was transferred to a large, novel transport cage (50 × 50 × 50 cm) located in a room some distance from the colony room that contained the family’s enclosure. The stressor therefore involved both a social component (social separation from the family members) and a novelty component (housing in an unfamiliar cage in an unfamiliar room). We utilized this manipulation since it is known to reliably produce elevated cortisol in adult marmosets (Rukstalis and French, 2005; Smith et al., 1998). The marmoset remained in this environment for 8 hours, and was returned to its home cage at 1700 h. During the separation phase of the experiment, plastic sheets were placed under the transport cage, and urine on these sheets was checked and collected once per hour. On the third day of the procedure, an additional first-void sample was collected using our reward paradigm. If twin siblings were tested on the same day, they were placed in different novel cages/rooms with no visual contact, and only limited acoustic and olfactory contact.
After collection, urine samples were pipetted into 1.5 ml microcentrifuge tubes, spun briefly in a centrifuge at 2500 rpm to remove detritus, then transferred to a clean vial and stored at −20°C until assay. Because not all marmosets urinated at every hourly collection time, data were collapsed into four two-hour blocks for analysis (e.g., 0900–1100 h, 1100 – 1300 h, etc.). Five marmosets did not provide a Day 3 first-void urine sample, and as a consequence were not used in analysis for some of the derived stress measures. This yielded a total of 15 males and eight females.
Cortisol Assay
We used a cortisol enzyme immunoassay that has been developed and validated for marmosets (Smith and French, 1997b). Briefly, well plates were coated with rabbit anticortisol antibody, and 50 µl of standard and urine sample (diluted 1:6,400 in double-distilled H2O) was added. Standards were serially diluted in distilled and deionized H2O, and ranged from 1,000 – 7.8 pg/well. Labeled cortisol (horseradish peroxidase, HRP) was added to each well, and the plates incubated for 2 h. After separating free from bound hormone in each well by washing the plate four times, we added 100 µl of substrate (ABTS and H2O2) and allowed the plate to develop until the B0 wells reached an optical density of approximately 1.0. The sample concentration was determined with a four-parameter sigmoidal fit function. Two quality control urine pools, one that averaged approximately 70% binding (Low QC) and one that averaged 30% binding (High QC) were assayed on every plate to insure reliability and stability. Cortisol assays were conducted over a period of eight years, and intra-assay coefficients of variation (CV) for high and low pools ranged from 3.7 – 7.3% and 3.3 – 6.1%, respectively. Interassay CVs for the high and low pools ranges from 8.7 – 14.7%, and 13.3 – 18.2%, respectively. To minimize procedural variability, all samples for a single individual (i.e., at all three time points) were assayed at the same time whenever possible. Samples that were above or below the endpoints on the standard curve were rediluted and reassayed. To control for variable fluid intake and output, creatinine concentrations were determined with a modified Jaffe reaction colorimetric assay, and the concentration of hormone in the urine sample (in µg/ml) was divided by the creatinine concentration (in mg/ml) to yield values of cortisol in µg Cortisol/mg Creatinine.
Determinants of Stress Reactivity and Analysis
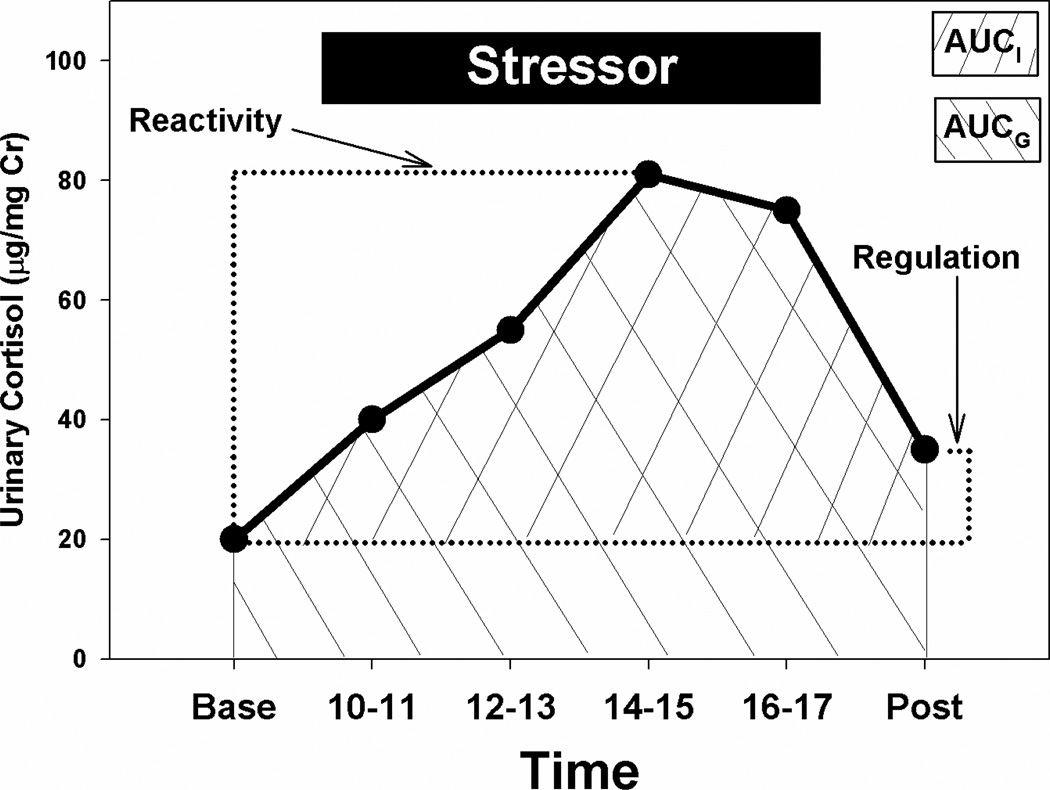
Figure 1 depicts a typical pattern of baseline cortisol, response to social separation/novelty exposure, and the return of cortisol to near baseline levels on the following day. We extracted four measures from these glucocorticoid response curves. First, stress reactivity was defined as the maximum cortisol concentration during the stressor, relative to the initial baseline levels (expressed as change in cortisol levels). Second, the regulation of the stress response was assessed by comparing the average of the first two first-void baseline concentrations with the levels noted on the third day’s baseline first-void sample (Day 3 cortisol - average Day 1 + 2 cortisol). A large number indicates poor regulation, since levels on Day 3 remain elevated even though the stressor has been terminated for at least 15 hours, while a smaller number or negative value indicates a well-regulated stress response, since the Day 3 baseline has returned to or near the pre-stressor baseline samples. Finally, we calculated the area under the curve (AUC) for the stress response in two different ways. In the first measure (AUC-ground, AUCG), the area under the cortisol response curve with respect to the zero point was calculated using the trapezoidal method. In the second (AUC-increase, AUCI), the area under the curve was calculated relative to the baseline value. Each provides slightly different information about the integration of the magnitude of the cortisol response and the duration of the response (increase over baseline in the case of AUCI, and an estimate of total exposure to glucocorticoids in the case of AUCG; Pruessner et al., 2003), and it is recommended that both be evaluated in studies of responses to temporally well-defined stressors.
Figure 1.
Schematic of basic and derived measures of the stress response to a standardized psychosocial stressor in marmosets. Baseline = average of CORT concentrations in two successive collections of first-void urine; Reactivity = highest CORT concentration during stressor minus the Baseline value; Regulation = CORT concentration in post-sressor first-void sample minus mean Baseline value; Area Under Curve – Ground = area under curve with respect to zero, and AUC – Increase = area under curve with respect to baseline.
As is the convention in related studies (Dettling et al., 2002a, Dettling et al., 2007, Law et al., 2008), we treated individual marmosets as independent subjects in the analyses. Changes across age in the stress response were analyzed with repeated measures ANOVAs (three-way repeated measures: age (6, 12, and 18 months of age), sex (male vs. female), and time of sample collection (baseline, 1100 h, 1300 h, 1500 h, 1700 h, post-separation). Alpha was set at the conventional 0.05 level. The degree of consistency and stability in the parameters of baseline cortisol, stress reactivity, stress recovery, and AUCG and AUCI were evaluated with Pearson’s correlation, as was the similarity in these measures between co-twins from a common litter. To control for inflation in experiment-wide alpha error for correlations, we set at p < 0.01.
Results
Age differences in stress reactivity to social separation/novelty exposure
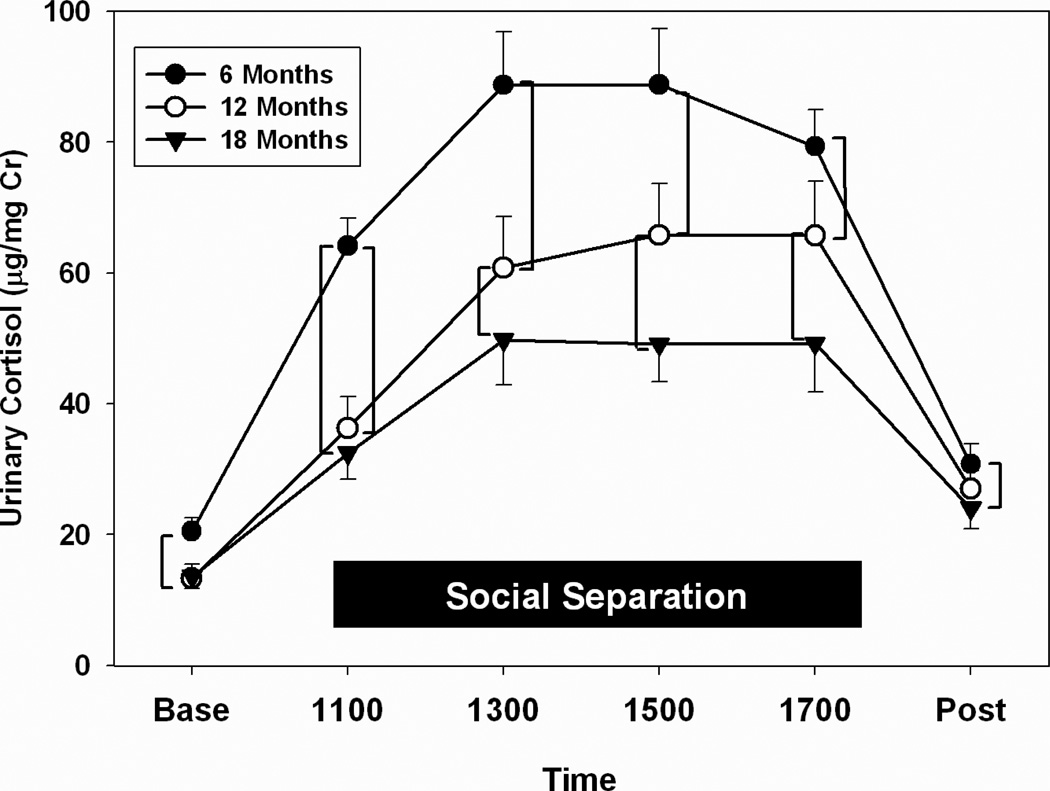
Regardless of age, marmosets responded with increases in urinary cortisol to separation from their natal family group and being housed in a novel environment (main effect of time: F(5,105) = 45.30, p < 0.001; Fig. 2). We noted clear age-related changes in the dynamics of cortisol production and excretion as a function of the separation/novelty stress (interaction of age and time: F(10,210) = 7.63, p < 0.001). The magnitude of the stress response was significantly higher in 6-month-old marmosets, and became attenuated as marmosets passed through 12 and 18 months of age. Post-hoc comparisons (highlighted by brackets in Fig. 2) revealed that, regardless of age, levels of urinary cortisol were significantly elevated over Baseline for all subsequent samples, including the Post-separation value (p’s ≤ 0.001). Six-month-old marmosets had a dramatic increase in urinary cortisol in the first samples collected after separation/novelty, and their mean cortisol levels were significantly higher than those in 12- and 18-month-old marmosets for the duration of the stressor. They also showed significantly elevated urinary cortisol, relative to 18-month-old marmosets, at the Post-stress time point (p’s < 0.01). Twelve- and 18-month-old marmosets exhibited slower elevations in urinary cortisol immediately after exposure to the stressor (1100 h), but the 12-month-old marmosets exhibited significantly higher levels of urinary cortisol at 1300 h, 1500 h, and 1700 h, relative to the older marmosets (p’s ≤ 0.02).
Figure 2.
Stress response curves in marmoset at 6, 12, and 18 months of age. Values here and in all figures represent means ± SE. Baseline and Post-stressor CORT values represent first void urine, and CORT values during social separation represent the average of two once-hourly collections (e.g., 1100 = mean CORT of samples collected between 0900 and 1100h, 1300 = average CORT of samples collected between 1100 and 1300h, etc.). Means that are connected by brackets are significantly different by post-hoc tests.
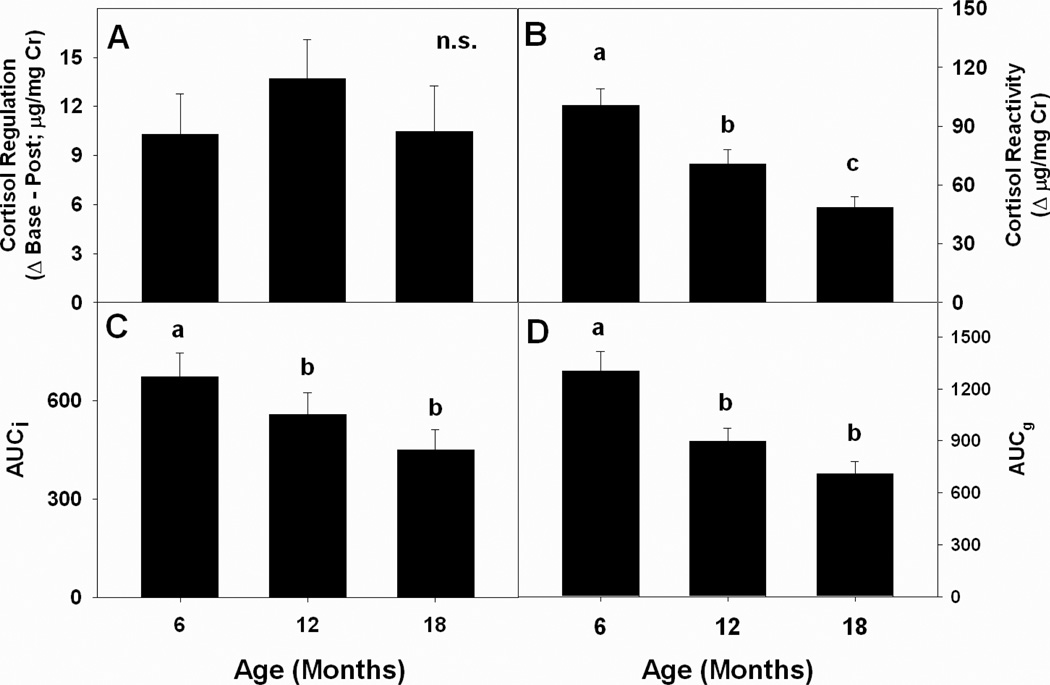
Derived components of the stress response also showed significant changes as a function of age. Cortisol reactivity during the stressor followed an age-graded pattern, with youngest marmosets exhibiting the highest maximum urinary cortisol levels during the stressor and oldest marmosets exhibiting the lowest levels (F(2,52) = 35.50, p < 0.01; Fig. 3B). Both measures of Area Under the Curve followed similar patterns, and 6-month-old marmosets had significantly higher mean AUCI and AUCG than 12- and 18-month-old marmosets, who did not differ from each other (AUCI: F(2,44) = 4.57, p < 0.016, Fig. 3C; AUCG: F(2,44) = 20.52, p < 0.001; Fig. 3D). Differences across age in the regulation of urinary cortisol back to baseline after reunion with the natal family group were not significantly different (Fig. 3A), and all means are positive, indicating that marmosets at all three developmental stages had persistent elevation of HPA function, even after cessation of the separation/novelty stressor.
Figure 3.
Mean values for components of the stress response across the three ages of sampling: (A) mean regulation of the CORT response (Post-stress value minus Baseline); (B) mean CORT reactivity; (C) Area Under the Curve – Increase (elevated CORT relative to Baseline); (D) Area Under the Curve – Ground (elevated CORT relative to 0 µg/mg Cr).
Consistency in patterns of stress reactivity during development
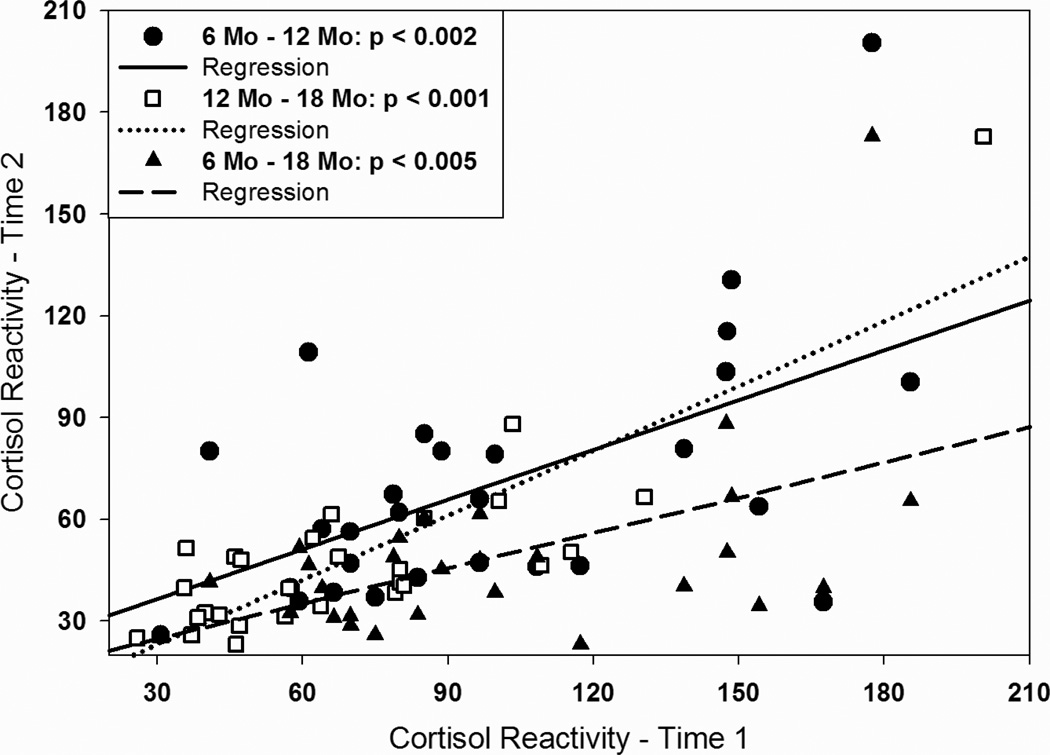
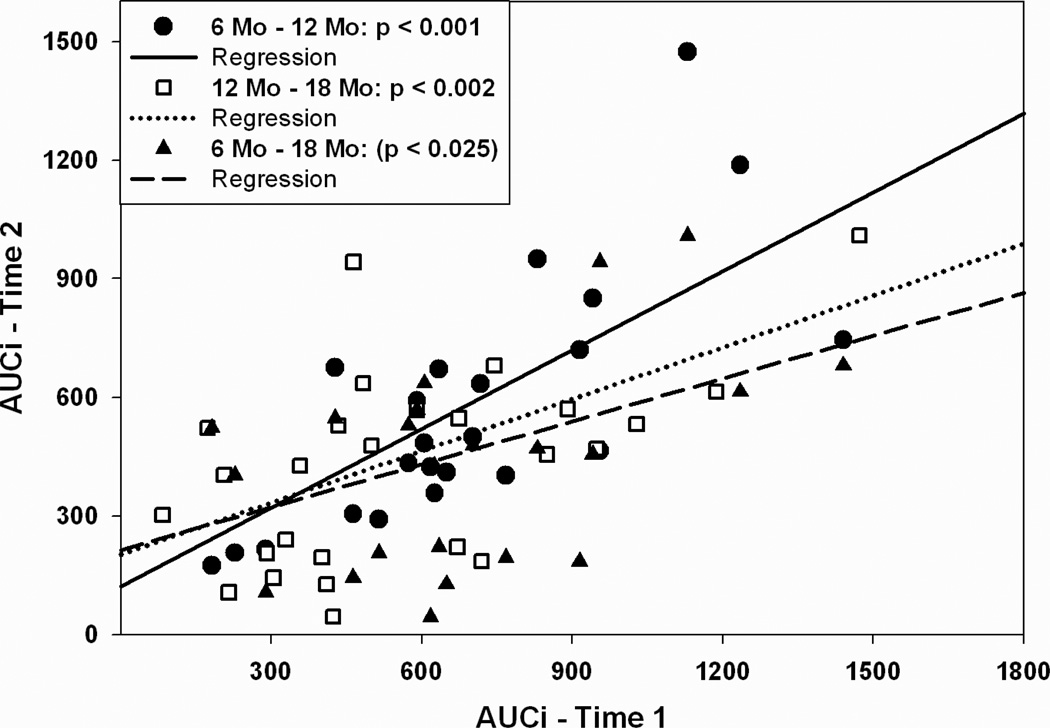
Some, but not all, aspects of the stress response to separation/novelty in marmosets were remarkably consistent within individuals throughout development. Table 2 presents bivariate Pearson correlations between stress measures at all time points. Baseline urinary cortisol and the regulation of the cortisol response to the stressor were not consistent among individuals over age. However, an individual’s cortisol reactivity at six-months of age was highly predictive of cortisol reactivity at 12 and 18 months of age (Fig. 4). Likewise, AUCG and AUCI at six-months of age was significantly correlated with the same measures at 12-months of age, and AUCI at six-months also tended to predict AUCI at 18 months of age (p < 0.05; Fig. 5). As with changes in cortisol with age, there were no sex differences in the patterns of correlations among stress response parameters across age between male and female marmosets.
Table 2.
Correlations (Pearson’s r) among parameters of the stress response for different developmental stages
| Stress Measure | 6 Mo – 12 Mo | 12 Mo – 18 Mo | 6 Mo – 18 Mo |
|---|---|---|---|
| Baseline CORT | 0.25 | 0.28 | 0.28 |
| CORT Reactivity | 0.56** | 0.83** | 0.52** |
| CORT Regulation | 0.26 | 0.07 | 0.14 |
| AUCg | 0.45† | 0.78** | 0.18 |
| AUCi | 0.73** | 0.56** | 0.43† |
p < 0.05;
p < 0.01;
p < 0.005
Figure 4.
Scatter plots showing relationship between individual differences in CORT reactivity (maximum CORT during stressor minus Baseline) for marmosets at different ages. X-axis values represent CORT reactivity at 6 or 12 months of age, and y-axis values represent CORT reactivity for the same individual marmosets at 12 or 18 months of age. Lines indicate best-fitting regression line for the various age comparisons.
Figure 5.
Scatter plots depicting Area Under the Curve – Increase for marmosets at different ages. See Fig. 4 for a description of the axes of the figure.
Correlation Among Stress Response Patterns in Co-twins
There was a remarkable lack of similarity in CORT stress response parameters among co-twins produced in a single litter. The correlations between the ten twin pairs for our stress measures are shown in Table 3. Only one r-value (AUCG at six months of age) reaches conventional levels of statistical significance (p < 0.05) and did not meet our enhanced criterion of p < 0.01. Four of the 15 correlations are negative, and six of 15 correlations are ≤ [0.10].
Table 3.
Pearson correlations among co-twins (n = 10 twin pairs) for stress-related cortisol measures at different ages
| Measure | Six Months | Twelve Months | Eighteen Months |
|---|---|---|---|
| Baseline CORT | −0.37 | 0.16 | 0.62 |
| CORT Reactivity | 0.01 | 0.02 | 0.08 |
| CORT Regulation | 0.06 | −0.56 | 0.12 |
| AUCG | 0.78* | 0.16 | −0.05 |
| AUCI | 0.24 | −0.53 | 0.10 |
(p < 0.05)
Discussion
Stress reactivity has been demonstrated to be a significant component of adaptation to environmental stimuli, and variation in the nature of the stress response has been linked to a variety of health outcomes. The data in this paper highlight two important sources of variation in HPA function in marmoset monkeys. First, we demonstrated that there are significant age-related differences in responses to a standard psychosocial stressor. Six-month-old marmosets showed higher maximal CORT responses, showed higher AUC responses, and had persistently elevated post-stressor CORT, relative to 12- and 18-month old marmosets. Second, we highlighted significant consistency in some components of the stress response, including maximal reactivity, and two indices of AUC. In contrast, baseline CORT and the regulation of CORT levels after cessation of the stressor did not show consistency across the three time points. Finally, our data are very clear on the point that co-twins do not necessarily exhibit similar patterns of HPA responses to a standardized psychosocial stressor.
When we compare our results directly with those of Pryce et al., 2002), we note that neither study detected significant sex difference in any parameter of stress reactivity at any age. The two studies differ, however, some important and interesting aspects. Pryce et al., 2002) reported that mean baseline CORT was similar from 6 months of age to adulthood, while we show that baseline CORT was significantly higher in 6-month-old than in 12-month-old marmosets, and tended to be higher than in 18-month-old marmosets (Fig. 2). There were also differences in the age at which dynamic components of the stress response in developing marmosets became stabilized. Pryce et al., 2002) reported that plasma ACTH and CORT became dampened in 6- and 12-month old marmosets, relative to the responses in 2-month old subjects, but that the response had stabilized by 6-months of age and was not different from the pattern at 12-months. In contrast, our data show that there is a reduction in HPA responses to the stressor between 6 and 12 months of age, and this reduction in the magnitude of the CORT response continues between 12 and 18 months of age. There are some methodological differences that could account for this discrepancy. First, Pryce et al., 2002) assessed HPA response via plasma samples, while we utilized excreted CORT in urine. This is not likely to be an important difference, however, since we have previously provided considerable biological validation that urinary CORT accurately reflects circulating levels of plasma CORT. We demonstrated that urinary CORT in marmosets: (i) tracks the well-documented circadian pattern in plasma (Smith and French, 1997b), (ii) responds within 30 to 60 min to both physical and social stressors (Smith and French, 1997b); and (iii) parallels the expected increases in circulating CORT during pregnancy (Smith and French, 1997a). Another likely interpretation of the differences in the studies deals with the fact that Pryce et al., 2002) included only three sample times (immediately prestressor baseline, immediate following the stressor, and 120 min following cessation of the stressor. We collected multiple samples throughout the duration of the stressor, and may have been able to characterize the dynamics of the stress response in a more finely detailed manner. Nonetheless, both studies highlight important developmental changes in the way in which marmosets of different ages respond to standardized stressors.
Our data revealed clear developmental differences in the magnitude and dynamics of the CORT response to a standardized social stressor. Relative to older marmosets, younger marmosets tended to have higher basal CORT values, had significantly elevated CORT during the stress period, and showed higher AUC measures. Studies on age-related variation in human and animal stress profiles tend to concentrate either on discrete changes in early infancy (Sapolsky and Meaney, 1986; Walker et al., 1986; review of human studies in Gunnar and Donzella, 2002) or changes associated with healthy and pathological aging (review in Kudielka and Kirschbaum, 2005). Two cross-sectional studies in human infants, children, and adolescents suggest a U-shaped function between these age ranges (which were the targeted life stages studied here) and stress reactivity. In infants and toddlers, the stress associated with healthy baby physicals (including handling and inoculations) was highest at 2 months of age, and dropped systematically at each subsequent physical at 6, 12, and 24 months of age (Davis and Granger, 2009). In a public speaking stress induction protocol, 9- and 11-year-old boys and girls showed little elevation in CORT, while 15-year-olds of both sexes displayed significant elevations of CORT when exposed to the stress procedure. At 13 years of age, there was a sexually dimorphic response: girls responded to the stress protocol with elevated CORT, while boys failed to respond (Gunnar et al., 2009). There is a methodological limitation to the data associated with the Davis and Granger, 2009) study, since there was a reduction in the number of inoculations the participants received that also correlated with increased age. Thus, reduced stress reactivity in toddlers could simply be explained by the fact that the examination was, in fact, less stressful, as opposed to a development change in CRH or ACTH secretion, changes in GC production, or changes in negative feedback. There is a conceptual limitation to the study on older children. In public speaking tasks, it is clear that the social/evaluative component is the most important variable associated with stress reactivity (Dickerson and Kemeny, 2004), and it could be that younger adolescents find the evaluative threat associated with public speaking to, in fact, constitutes less of a stressor than older adolescents.
Our data clearly portray a negative monotonic relationship between age and stress reactivity in marmosets – marmosets recruit a less extreme stress response to a standardized stressor as they age from 6- to 18-months of age. One explanation for this result is that the maturing HPA axis becomes more sensitive to negative feedback, and older marmosets are better able to maintain lower levels of CORT in the face of the stressor. It is well-established that both mineralocorticoid receptors (MR) and glucocorticoid receptors (GR) in the hippocampus play a crucial role in regulating negative feedback in the HPA axis (De Kloet et al., 1998), and thus changes in MR and GR expression and density could account for age-related differences in the magnitude of stress responses. However, in common marmosets, the expression of GR mRNA in the hippocampus is stable from birth throughout ontogeny (Pryce et al., 2005). MR receptor mRNA increases early in infancy in marmosets, is stable by 4 months of age, and does not change from 4 months through adulthood (Pryce et al., 2005). Thus, it is unlikely that GR and/or MR changes account for the differences in stress responses in marmosets of different ages. The other alternative is that younger marmosets process the contextual stimuli associated with separation from the family and exposure to novel environments differently from older marmosets, appraise this environment as a greater threat to homeostasis, and therefore recruit a larger HPA response.
While the dynamic phases of the CORT stress response were individually idiosyncratic across age, baseline CORT measures (average of two samples from ‘undisturbed’ marmosets) were not consistent for individuals across time. It is likely that baseline cortisol is highly labile, sensitive to a number of factors that would be expected to differ from day-to-day and month-to-month. Among these factors may be the amount of food consumed on the previous day, variation in activity/wakefulness during the previous evening (Fite et al., 2003), presence or absence of intragroup conflict (Smith and French, 1997b). The unpredictability of these and others as yet unidentified factors that alter baseline CORT could certainly account for the lack of temporal stability in this component of stress reactivity in marmosets.
What are the possible causal factors that produce and maintain consistent individual differences in HPA response styles, such as the ones we have demonstrated in the present paper? There are certainly strong genetic determinants of HPA responsivity, as evidenced from three lines of empirical evidence. First, in nonhuman animals selective breeding for high and low stress responsivity lines or strains can produce significant divergence in CORT responses to stress in as little as a single generation (mice: Touma et al., 2008; rats: Roman et al., 2004; birds: Carere et al., 2003; Evans et al., 2006; fish: Fevolden et al., 1991; Overli et al., 2005). Secondly, twin studies have indicated a strong hereditary component to account for variation in stress reactivity. Relative to dizygotic (DZ) twins, monozygotic (MZ) twins have more similar baseline CORT (Steptoe et al., 2009), more similar responses to a public speaking task (particularly CORT reactitivity; Federenko et al., 2004), have more similar CARs (Wust et al., 2000), and more similar CORT reactivity in response to playing an arousing computer game (Steptoe et al., 2009). [It is interesting in this light that we fail to see commonality in patterns of stress responses in dizygotic twin pairs in marmosets.] Finally, there is a growing list of candidate genes that show polymorphisms that map onto differences in stress reactivity, including genes that directly affect HPA function (e.g., glucocorticoid and mineralocorticoid receptor genes; reviewed in DeRijk et al., 2011) and those associated with other neurotransmitter systems (GABA: Uhart et al., 2004; monoamines: Jabbi et al., 2007). To date no systematic investigation of the genetic basis underlying individual variation in marmoset stress reactivity has been published, but it would seem fruitful to explore this possibility.
In addition to strict genetic influences on stress reactivity, variation in early environments (both pre- and postnatal) could influence stress response styles. It is becoming increasingly apparent from studies of rodents (Diorio and Meaney, 2007), nonhuman primates (Parker and Maestripieri, 2011), and humans (Gunnar and Quevedo, 2008; Ellis et al., 2006 Flinn et al., 2011) that the quality of early social environments exerts a profound influence on lifetime patterns of stress response styles. This notion has also been explored in common marmosets via experimental manipulations of early life experiences in infants. Infant marmosets that received early parental deprivation (a duration of 30 to 120 min per day from postnatal day 2 – 28) exhibited elevated basal CORT early during a social separation, but blunted CORT levels by the end of the parental deprivation phase, relative to normally-reared infants (Dettling et al., 2002a). When tested with a psychosocial stressor at 18–20 weeks of age (approximately the age of the youngest marmosets in our study), the early-deprived marmosets again exhibited blunted baseline CORT, but their stress-induced changes in CORT did not differ from nondeprived control marmosets (Dettling et al., 2002b). Further, experimental manipulation of early experience in marmosets (again, early parental deprivation) resulted in differential gene expression associated with synaptic function and plasticity in areas of the brain involved in emotional processing and stress coordination, particularly the hippocampus (Law et al., 2008). These results suggest that early variation in the quality of postnatal life can produce long-term changes in HPA function and the brain areas involved in the regulation of this system in the marmoset.
It should also come as no surprise that there is growing evidence that genetics and early environments interact to uniquely determine stress response styles (Boyce and Ellis, 2005). For instance, Ouellet-Morin et al., 2008) reported that under conditions of low early family adversity, MZ twin toddlers had more similar salivary CORT responses to a mild stressor than DZ twins. However, in instances where twins were reared in a home characterized by high family adversity, there was no difference in stress response styles between MZ and DZ twins. In rhesus macaques, infants that carry at least one short allele of the serontonin transporter gene (rh5HTTLPR) exhibit altered HPA function in response to a separation stressor, and the magnitude of changed HPA function is modulated by differences in rearing conditions (Barr et al., 2004). Recently, McCormack et al., 2009) demonstrated that infants with the “at-risk” short allele for serotonin transporter who are not abused as infants exhibit lower CORT reactivity than those that are abused, and infants who lack the short allele but who are abused as infants exhibit higher levels of CORT during a stressor than monkeys of a similar genotype that are not abused. These finding suggests that allelic variants that influences stress reactivity are more influential in shaping stress phenotype in some early environments than in others, and that research strategies that evaluate gene-environment interactions will yield valuable insights. Once allelic variation in genes that regulate stress reactivity are identified in marmosets, this information will allow us to track the ways in which early variation in offspring care interact with this genetic variation to shape long-term patterns of stress reactivity.
In sum, our results provide the first demonstration that multiple components of the HPA response change significantly across developmental stages in marmosets. However, in spite of the ontogenetic changes, HPA responsiveness remains remarkably consistent within an individual across multiple phases of life history in marmoset monkeys. Our initial evaluation of concordance of stress reactivity in co-twins suggests that shared alleles among twins and a common intrauterine environment may not be major contributors to individual differences in shaping HPA function. We are currently exploring whether normative and natural variation in the quality and quantity of offspring care received early in life can account for individual variation in HPA function (Burrell et al., in prep.), and it my be that variation in early experience may contribute to important and persistent individual differences in stress response style. However, regardless of the origin of these individual differences, HPA response styles are set by six months of age in marmosets, and persist for at least a year.
Highlights.
Stress reactivity decreases from juvenile to young adult stages of marmoset life
Stress reactivity is highly stable within individual marmosets over time
Marmoset co-twins do not display similar stress response profiles
Acknowledgements
We thank Heather Jensen and her dedicated student staff and volunteers for their excellence in care of the marmoset colony. Shelton Hendricks and Jon-Ryan Cavanaugh provided useful comments on a previous version of this manuscript. Jeffrey E. Fite was the inspiration behind the program of research that led to this study, and we acknowledge his continuing legacy to our research program. We thank the University of Nebraska at Omaha for partial support for the marmoset colony, and the NIH (HD 042882) for support of the research program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker JV, Abbott DH, Saltzman W. Social determinants of reproductive failure in male common marmosets housed with their natal family. Anim. Behav. 1999;58(3):501–513. doi: 10.1006/anbe.1999.1200. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Schwandt M, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol. Psychiatry. 2004;55(7):733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Benirschke K. The biology of the twinning process: how placentation influences outcome. Semin. Perinatol. 1995;19(5):342–350. doi: 10.1016/s0146-0005(05)80012-6. [DOI] [PubMed] [Google Scholar]
- Berger M, Bossert S, Krieg JC, Dirlich G, Ettmeier W, Schreiber W, von Zerssen D. Interindividual differences in the susceptibility of the cortisol system: An important factor for the degree of hypercortisolism in stress situations? Biol. Psychiatry. 1987;22(11):1327–1339. doi: 10.1016/0006-3223(87)90067-9. [DOI] [PubMed] [Google Scholar]
- Birnie AK, Smith AS, Nali C, French JA. Social and developmental influences on urinary androgen levels in young male white-faced marmosets (Callithrix geoffroyi) Am. J. Primatol. 2011;73(4):378–385. doi: 10.1002/ajp.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev. Psychopathol. 2005;17(2):271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Lerche NW. Individual differences in peripheral blood immunological and hormonal measures in adult male rhesus macaques (Macaca mulatta): evidence for temporal and situational consistency. Am. J. Primatol. 1998;44(1):29–41. doi: 10.1002/(SICI)1098-2345(1998)44:1<29::AID-AJP3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Carere C, Groothuis T, Mˆstl E, Daan S, Koolhaas J. Fecal corticosteroids in a territorial bird selected for different personalities: daily rhythm and the response to social stress. Horm. Behav. 2003;43(5):540–548. doi: 10.1016/s0018-506x(03)00065-5. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Renquist D, Brandon D, Eil C, Pugeat M, Vigersky R, Cutler GB, Jr, Loriaux DL, Lipsett MB. Glucocorticoid hormone resistance during primate evolution: receptor-mediated mechanisms. Proc. Natl. Acad. Sci. U. S. A. 1982;79(6):2036–2040. doi: 10.1073/pnas.79.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Savage A, Bromley LJ. Phylogenetic influences on hormone levels across the primate order. Am. J. Primatol. 1992;28(2):81–100. doi: 10.1002/ajp.1350280202. [DOI] [PubMed] [Google Scholar]
- Cohen S, Hamrick NMS, Rodriguez MS, Feldman PJ, Rabin BS, Manuck SB. The stability of and intercorrelations among cardiovascular, immune, endocrine, and psychological reactivity. Ann. Behav. Med. 2000;22(3):171–179. doi: 10.1007/BF02895111. [DOI] [PubMed] [Google Scholar]
- Davis EP, Granger DA. Developmental differences in infant salivary alpha-amylase and cortisol responses to stress. Psychoneuroendocrinology. 2009;34(6):795–804. doi: 10.1016/j.psyneuen.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 1998;19(3):269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- DeRijk RH, de Kloet ER, Zitman FG, van Leeuwen N. Mineralocorticoid receptor gene variants as determinants of HPA axis regulation and behavior. Endocr Dev. 2011;20:137–148. doi: 10.1159/000321235. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Feldon J, Pryce CR. Repeated parental deprivation in the infant common marmoset (Callithrix jacchusprimates) and analysis of its effects on early development. Biol. Psychiatry. 2002a;52(11):1037–1046. doi: 10.1016/s0006-3223(02)01460-9. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Feldon J, Pryce CR. Early deprivation and behavioral and physiological responses to social separation/novelty in the marmoset. Pharmacol. Biochem. Behav. 2002b;73(1):259–269. doi: 10.1016/s0091-3057(02)00785-2. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Schnell CR, Maier C, Feldon J, Pryce CR. Behavioral and physiological effects of an infant-neglect manipulation in a bi-parental, twinning primate: impact is dependent on familial factors. Psychoneuroendocrinology. 2007;32(4):331–349. doi: 10.1016/j.psyneuen.2007.01.005. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Craft TK, Glasper ER, Neigh GN, Alexander JK. 2006 Curt P. Richter award winner: Social influences on stress responses and health. Psychoneuroendocrinology. 2007;32(6):587–603. doi: 10.1016/j.psyneuen.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Diorio J, Meaney MJ. Maternal programming of defensive responses through sustained effects on gene expression. J. Psychiatry Neurosci. 2007;32(4):275–284. [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Jackson JJ, Boyce WT. The stress response systems: Universality and adaptive individual differences. Dev. Rev. 2006;26(2):175–212. [Google Scholar]
- Evans MR, Roberts ML, Buchanan KL, Goldsmith AR. Heritability of corticosterone response and changes in life history traits during selection in the zebra finch. Journal of Evolutionary Biology. 2006;19(2):343–352. doi: 10.1111/j.1420-9101.2005.01034.x. [DOI] [PubMed] [Google Scholar]
- Federenko IS, Nagamine M, Hellhammer DH, Wadhwa PD, W¸st S. The heritability of hypothalamus pituitary adrenal axis responses to psychosocial stress is context dependent. J. Clin. Endocrinol. Metab. 2004;89(12):6244. doi: 10.1210/jc.2004-0981. [DOI] [PubMed] [Google Scholar]
- Fevolden SE, Refstie T, Roed KH. Selection for high and low cortisol stress response in Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss) Aquaculture. 1991;95(1–2):53–65. [Google Scholar]
- Fite JE, French JA, Patera KJ, Hopkins EC, Rukstalis M, Jensen HA, Ross CN. Nighttime wakefulness associated with infant rearing inCallithrix kuhlii. International journal of primatology. 2003;24(6):1267–1280. [Google Scholar]
- Flinn MV. Are cortisol profiles a stable trait during child development? American Journal of Human Biology. 2009;21(6):769–771. doi: 10.1002/ajhb.20981. [DOI] [PubMed] [Google Scholar]
- Flinn MV, Nepomnaschy PA, Muehlenbein MP, Ponzi D. Evolutionary functions of early social modulation of hypothalamic-pituitary-adrenal axis development in humans. Neurosci. Biobehav. Rev. 2011;35(7):1611–1629. doi: 10.1016/j.neubiorev.2011.01.005. [DOI] [PubMed] [Google Scholar]
- French JA, Brewer KJ, Schaffner CM, Schalley J, Hightower Merritt D, Smith TE, Bell SM. Urinary steroid and gonadotropin excretion across the reproductive cycle in female Wied's black tufted ear marmosets (Callithrix kuhli) Am. J. Primatol. 1996;40(3):231–245. doi: 10.1002/(SICI)1098-2345(1996)40:3<231::AID-AJP2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27(1–2):199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Quevedo KM. Early care experiences and HPA axis regulation in children: a mechanism for later trauma vulnerability. Prog. Brain Res. 2008;167:137–149. doi: 10.1016/S0079-6123(07)67010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamusñpituitaryñadrenal activity over the transition to adolescence: Normative changes and associations with puberty. Dev. Psychopathol. 2009;21(01):69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M, Gibson EL, Vuononvirta R, Williams E, Steptoe A. Inflammatory and hemostatic responses to repeated mental stress: individual stability and habituation over time. Brain. Behav. Immun. 2006;20(5):456–459. doi: 10.1016/j.bbi.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Hellhammer J, Fries E, Schweisthal O, Schlotz W, Stone A, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: state-and trait components. Psychoneuroendocrinology. 2007;32(1):80–86. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biol. Psychiatry. 1992;32(2):127–145. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Korf J, Kema I, Hartman C, Van der Pompe G, Minderaa R, Ormel J, Den Boer J. Convergent genetic modulation of the endocrine stress response involves polymorphic variations of 5-HTT, COMT and MAOA. Mol. Psychiatry. 2007;12(5):483–490. doi: 10.1038/sj.mp.4001975. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Kamilaris TC, Carter S, Gold PW, Chrousos GP. ìEnvironmental stressî and reproductive success in the common marmoset (Callithrix jacchus jacchus) Am. J. Primatol. 1991;25(3):191–201. doi: 10.1002/ajp.1350250306. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Kamilaris TC, Carter CS, Calogero AE, Gold PW, Chrousos GP. The biobehavioral consequences of psychogenic stress in a small, social primate (Callithrix jacchus jacchus) Biol. Psychiatry. 1996;40(5):317–337. doi: 10.1016/0006-3223(95)00397-5. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Steyer R, Eid M, Patalla U, Schwenkmezger P, Hellhammer D. Cortisol and behavior: 2. Application of a latent state-trait model to salivary cortisol. Psychoneuroendocrinology. 1990;15(4):297–307. doi: 10.1016/0306-4530(90)90080-s. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol. Psychol. 2005;69(1):113. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Law AJ, Pei Q, Walker M, Gordon-Andrews H, Weickert CS, Feldon J, Pryce CR, Harrison PJ. Early parental deprivation in the marmoset monkey produces long-term changes in hippocampal expression of genes involved in synaptic plasticity and implicated in mood disorder. Neuropsychopharmacology. 2008;34(6):1381–1394. doi: 10.1038/npp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung E, Tasker SL, Atkinson L, Vaillancourt T, Schulkin J, Schmidt LA. Perceived maternal stress during pregnancy and its relation to infant stress reactivity at 2 days and 10 months of postnatal life. Clin. Pediatr. (Phila) 2010;49(2):158. doi: 10.1177/0009922809346570. [DOI] [PubMed] [Google Scholar]
- Lilly AA, Mehlman PT, Higley JD. Trait like immunological and hematological measures in female rhesus across varied environmental conditions. Am. J. Primatol. 1999;48(3):197–223. doi: 10.1002/(SICI)1098-2345(1999)48:3<197::AID-AJP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- McCormack K, Newman TK, Higley JD, Maestripieri D, Sanchez MM. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Horm. Behav. 2009;55(4):538–547. doi: 10.1016/j.yhbeh.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modell S, Lauer CJ, Schreiber W, Huber J, Krieg JC, Holsboer F. Hormonal response pattern in the combined DEX-CRH test is stable over time in subjects at high familial risk for affective disorders. Neuropsychopharmacology. 1998;18(4):253–262. doi: 10.1016/S0893-133X(97)00144-9. [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I, Boivin M, Dionne G, Lupien SJ, Arsenault L, Barr RG, PÈrusse D, Tremblay RE. Variations in heritability of cortisol reactivity to stress as a function of early familial adversity among 19-month-old twins. Arch. Gen. Psychiatry. 2008;65(2):211. doi: 10.1001/archgenpsychiatry.2007.27. [DOI] [PubMed] [Google Scholar]
- Overli O, Winberg S, Pottinger TG. Behavioral and Neuroendocrine Correlates of Selection for Stress Responsiveness in Rainbow Trout--a Review. Integr Comp Biol. 2005;45(3):463–474. doi: 10.1093/icb/45.3.463. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Maestripieri D. Identifying key features of early stressful experiences that produce stress vulnerability and resilience in primates. Neurosci. Biobehav. Rev. 2011;35(7):1466–1483. doi: 10.1016/j.neubiorev.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PrÈville M, Zarit S, Susman E, Boulenger P, Lehoux R. Response variability of salivary cortisol among older adults under psychological stress. Aging & Mental Health. 2008 doi: 10.1080/13607860701616499. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Palme R, Feldon J. Development of pituitary-adrenal endocrine function in the marmoset monkey: infant hypercortisolism is the norm. J. Clin. Endocrinol. Metab. 2002;87(2):691. doi: 10.1210/jcem.87.2.8244. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Feldon J, Fuchs E, Knuesel I, Oertle T, Sengstag C, Spengler M, Weber E, Weston A, Jongen-Relo A. Postnatal ontogeny of hippocampal expression of the mineralocorticoid and glucocorticoid receptors in the common marmoset monkey. Eur. J. Neurosci. 2005;21(6):1521–1535. doi: 10.1111/j.1460-9568.2005.04003.x. [DOI] [PubMed] [Google Scholar]
- Roman O, Seres J, Pometlova M, Jurcovicova J. Neuroendocrine or behavioral effects of acute or chronic emotional stress in Wistar Kyoto (WKY) and spontaneously hypertensive (SHR) rats. Endocr. Regul. 2004;38(4):151–155. [PubMed] [Google Scholar]
- Rukstalis M, French JA. Vocal buffering of the stress response: exposure to conspecific vocalizations moderates urinary cortisol excretion in isolated marmosets. Horm. Behav. 2005;47(1):1–7. doi: 10.1016/j.yhbeh.2004.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman W, Schultz Darken NJ, Abbott DH. Familial influences on ovulatory function in common marmosets (Callithrix jacchus) Am. J. Primatol. 1997;41(3):159–177. doi: 10.1002/(SICI)1098-2345(1997)41:3<159::AID-AJP1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Research Reviews. 1986;11(1):65–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schaffner CM, Shepherd RE, Santos CV, French JA. Development of heterosexual relationships in wied's black tufted ear marmosets (Callithrix kuhli) Am. J. Primatol. 1995;36(3):185–200. doi: 10.1002/ajp.1350360303. [DOI] [PubMed] [Google Scholar]
- Smith AS, Birnie AK, French JA. Social isolation affects partner-directed social behavior and cortisol during pair formation in marmosets, Callithrix geoffroyi. Physiol. Behav. doi: 10.1016/j.physbeh.2011.06.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TE, Schaffner CM, French JA. Social and developmental influences on reproductive function in female Wied's black tufted-ear marmosets (Callithrix kuhli) Horm. Behav. 1997;31(2):159–168. doi: 10.1006/hbeh.1997.1380. [DOI] [PubMed] [Google Scholar]
- Smith TE, French JA. Social and reproductive conditions modulate urinary cortisol excretion in black tufted ear marmosets (Callithrix kuhli) Am. J. Primatol. 1997a;42(4):253–267. doi: 10.1002/(SICI)1098-2345(1997)42:4<253::AID-AJP1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Smith TE, French JA. Psychosocial stress and urinary cortisol excretion in marmoset monkeys. Physiol. Behav. 1997b;62(2):225–232. doi: 10.1016/s0031-9384(97)00103-0. [DOI] [PubMed] [Google Scholar]
- Smith TE, McGreer-Whitworth B, French JA. Close proximity of the heterosexual partner reduces the physiological and behavioral consequences of novel-cage housing in black tufted-ear marmosets (Callithrix kuhli) Horm. Behav. 1998;34(3):211–222. doi: 10.1006/hbeh.1998.1469. [DOI] [PubMed] [Google Scholar]
- Steptoe A, van Jaarsveld CHM, Semmler C, Plomin R, Wardle J. Heritability of daytime cortisol levels and cortisol reactivity in children. Psychoneuroendocrinology. 2009;34(2):273–280. doi: 10.1016/j.psyneuen.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker JG. Rapid glucocorticoid actions in the hypothalamus as a mechanism of homeostatic integration. Obesity (Silver Spring) 2006;14 Suppl 5:259S–265S. doi: 10.1038/oby.2006.320. [DOI] [PubMed] [Google Scholar]
- Thorn L, Hucklebridge F, Evans P, Clow A. The cortisol awakening response, seasonality, stress and arousal: A study of trait and state influences. Psychoneuroendocrinology. 2009;34(3):299–306. doi: 10.1016/j.psyneuen.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Touma C, Bunck M, Glasl L, Nussbaumer M, Palme R, Stein H, Wolferstatter M, Zeh R, Zimbelmann M, Holsboer F, Landgraf R. Mice selected for high versus low stress reactivity: a new animal model for affective disorders. Psychoneuroendocrinology. 2008;33(6):839–862. doi: 10.1016/j.psyneuen.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Uhart M, McCaul M, Oswald L, Choi L, Wand G. GABRA6 gene polymorphism and an attenuated stress response. Mol. Psychiatry. 2004;9(11):998–1006. doi: 10.1038/sj.mp.4001535. [DOI] [PubMed] [Google Scholar]
- Walker CD, Perrin M, Vale W, Rivier C. Ontogeny of the stress response in the rat: role of the pituitary and the hypothalamus. Endocrinology. 1986;118(4):1445. doi: 10.1210/endo-118-4-1445. [DOI] [PubMed] [Google Scholar]
- Watson S, Thompson JM, Malik N, Nicol Ferrier I, Young AH. Temporal stability of the dex/CRH test in patients with rapid cycling bipolar I disorder: a pilot study. Aust. N. Z. J. Psychiatry. 2005;39(4):244–248. doi: 10.1080/j.1440-1614.2005.01560.x. [DOI] [PubMed] [Google Scholar]
- Wust S, Federenko I, Hellhammer DH, Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000;25(7):707–720. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto ME. From dependence to sexual maturity: the behavioural ontogeny of Callitrichidae. In: Rylands AB, editor. Marmosets and tamarins: systematics, behaviour and ecology. Oxford: Oxford University Press; 2003. pp. 235–250. [Google Scholar]