Abstract
Alzheimer's disease (AD) is an incurable and progressive neurodegenerative senile disorder associated with the brain accumulation of Aβ plaques. Although vaccines that reduce Aβ plaques can control AD, the rationale for their use at the onset of the disease remains debatable. Old humans and mice usually respond poorly to vaccines due to presumably age-related immunological impairments. Here, we report that by modifying vaccines, the poor responsiveness of old mice can be reversed. Unlike the Aβ peptide vaccine, DNA immunizations with the amino-terminal Aβ(1-11) fragment exposed on the surface of HBsAg particles elicit high levels of anti-Aβ antibody both in young and old mice. Importantly, in AD model 3xTgAD mice, the vaccine reduced Aβ plaques, ameliorated cognitive impairments and, surprisingly, significantly increased life span. Hence, we propose that vaccines targeting Aβ(1-11) can efficiently combat AD-induced pathological alterations and provide survival benefit in patients with AD.
Keywords: Aβ, Alzheimer's disease vaccine, old age, life span
INTRODUCTION
Alzheimer's disease (AD) is a common disorder of the elderly that currently affects over 5 million Americans. Although the primary cause(s) of AD is unknown, the disease process involves the progressive extracellular accumulation of aggregated forms of amyloid β-peptide (Aβ) and associated intracellular deposits of hyperphosphorylated tau protein and brain atrophy [1, 2]. AD patients exhibit progressive cognitive and emotional/behavioral impairments as a result of synaptic dysfunction and neuronal degeneration in multiple interconnected brain regions including the hippocampus, frontal cortex and amygdala [1, 2]. Studies of experimental cell culture and animal models of AD suggest that the neurotoxic activity of Aβ occurs when Aβ is in an oligomeric form and involves membrane-associated oxidative stress that impairs synaptic plasticity and memory and causes neuritic and tau hyperphosphorylation [3]. The accumulation of Aβ can also trigger the induction of harmful inflammatory responses that involve the activation of microglia and astrocytes and cause infiltration of Aβ-specific T cells into the brain [4], and may eventually lead to patho-neurophysiological impairments and death [5]. Preclinical studies demonstrated that Aβ deposits can be reduced by passive administration of Aβ-specific antibody [6-8] or by active immunization with Aβ(1-42) [9-11].
In a clinical trial of active Aβ immunization in AD patients, the generation of antibody to Aβ was associated with a slower rate of decline of cognitive functions [12-15]. Unfortunately, the latter trial was halted because a subset of patients developed meningoencephalitis due to the induction and infiltration of Aβ-specific T cells [13, 16]. In retrospect, the induction of cellular responses could be expected, as immunizations with a full-length Aβ(1-42) peptide elicit T cell responses to epitopes primarily localized within Aβ(15-42) both in transgenic mice and humans [4, 17], despite initial findings that indicated the inability to induce T cells due to immune tolerance to Aβ [18]. Thus, new approaches for the generation of AD vaccines should be aimed to generate antibody responses without activating Aβ-specific T cells that may already be induced in old people and AD patients [4]. For example, this might be accomplished by targeting the amino-terminal Aβ(1-11) fragment which does not contain known T cell epitopes, only immunodominant B cell epitopes [19, 20] which comprise the majority of antibody generated in AD patients immunized with Aβ(1-42) [21]. Importantly, vaccines targeting the amino-terminus of Aβ(1-11) could successfully reduce Aβ plaques and AD symptoms in transgenic mice immunized at a young age [11, 19]. However, it remains unknown whether AD vaccine would retain its efficacy when people acquired old age-related decline of immunity [22, 23] and/or antibody hyporesponsiveness due to chronic exposure to Aβ [18]. This may also explain a relatively poor response rate (up to 77% of responders) in a recent clinical trial in AD patients immunized with Aβ(1-42) peptide vaccine (AN1792) [24].
Here, to develop an AD vaccine specifically tailored for the elderly, we generated a DNA based formulation (Aβ-coreS) expressing Aβ(1-11) fused to HBsAg, a primary component of hepatitis B virus (HBV) vaccine. We chose HBsAg based on its ability to self-assemble into 24-nm particles and to enhance immunogenicity of foreign antigens by repetitively exposing them on its surface [25]. To provide additional T cell help, the construct also contained a strong T helper epitope of HBcAg from a capsid antigen of HBV [26]. As a result, the vaccine, designated Aβ-CoreS, generated high titers of antibody to Aβ both in young and old mice, while a control construct expressing HBsAg alone only worked well in young mice. Unlike mice immunized with a full-length Aβ(1-42) peptide that elicited readily detectable T cell responses, Aβ-CoreS – immunized mice failed to induce Aβ-specific T cells. For the first time we provide proof that immunizations of old AD mice early in the disease process can not only improve AD-associated cognitive impairments, but also significantly extend life span.
MATERIALS AND METHODS
Reagents, cells, DNA constructs and mice
BALB/C and C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME). Triple transgenic (3xTgAD) AD mice were originally generated in a hybrid 129/C57BL/6 genetic background [27], and were then backcrossed onto a pure C57BL/6 genetic background for 8 generations; Aβ and tau pathologies and learning and memory deficits in these mice have been reported previously [28]. All mice were bred and housed in a pathogen-free environment at the National Institute on Aging Animal Facility, Baltimore, MD. DNA fragments encoding the T helper epitope of HBcAg (residues 70-85) and Aβ(1-11) were fused in frame with the S gene of HBV (HBsAg, subtype ayw) and cloned into a mammalian pVAX1 vector (Invitrogen). Control constructs expressed HBsAg alone (S) or HBsAg fused with MC148 of the poxvirus molluscum contagiosum [29]. All constructs were verified by DNA sequencing (Keck DNA Sequencing Lab, New Haven, CT).The peptides MOPC-315 Ig (91-101) (ALWFRNHFVFGGGTK) [30] were all synthesized by Peptide Technologies (Washington, DC) to a purity >99% by HPLC. Aβ(1-42) was purchased from Bachem (Torrance, CA).
In vivo manipulations
Animal care was provided in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 86-23, 1985), and all animal procedures were approved by the Animal Care and Use Committee of the National Institute on Aging Intramural Research Program. Three different groups of mice were immunized three-four times (as indicated in the text) every two weeks starting when mice were 4 months old (young mice), 1 year old, or 1.5 years old. To do this, mice were electroporated with 25 μg DNA in 50 μl endotoxin-free water intradermally in the base of the tail using a parallel row needle array (with two rows of four needles/row, a 1 mm gap between needles within a row, a 4 mm gap between rows and a needle length of 3 mm) connected to a PA-4000 electroporation system (Cyto Pulse Sciences, Inc., Glen Burnie, MD). A Pulse AgileR electroporation protocol was used consisting of two pulses of 450 V (1125 V/cm), 0.125 S pulse interval and 0.05 mS pulse duration followed by eight pulses of 110 V (275 V/cm) 0.125 S pulse interval and 10 mS pulse duration. Control mice were subcutaneously immunized with 10 μg Aβ(1-42) (or mock with 100 μl PBS) emulsified in 100 μl incomplete Freund's adjuvant (IFA) or coupled with alum (Sigma). Date of death was recorded and differences in survival between groups were determined by non-parametric logrank test (BMDP statistical software, Los Angeles).
T cell activation assay
To assess T cell proliferation, splenocytes from immunized and mock-treated mice were first labeled with carboxyfluorescein succinimidyl ester (CFSE, Invitrogen, Carlsbad, CA). The cells then were stimulated with either 1 μg/ml of Aβ(1-42) or 10 μg/ml of HBsAg (#R86870, Biodesign, Saco, ME) in RPMI1640 medium containing 10% fetal bovine serum and antibiotics and 50 U/ml of IL-2. Control CFSE-labeled cells (1 × 105) were stimulated with anti-CD3/CD28-coupled beads or mouse-gp10025-33 (EGSRNQDWL) [31]. After 7 days incubation, the cells were stained with anti-CD4 and anti-CD8 antibodies (BD Pharmingen) to quantify the proportion (%) of proliferated (CFSE-diluted) cells which was calculated as: (Experimental – target spontaneous)/(target maximum after anti-CD3/CD28 Aβ treatment – target spontaneous) x100. For 3xTgAD splenocytes, the cells were also re-stimulated for another 7 days with irradiated autologous BMDCs [31] pulsed with 1 μg/ml Aβ(1-42) peptide or HBsAg. Serum levels of antibody to Aβ and HBsAg were measured in 96-well ELISA plates coated with 3 μg/ml Aβ(1-42) or HBsAg (ayw, Biodesign) using goat anti-mouse IgG-HRPO (Jackson).
Elevated Plus Maze
The elevated plus maze test was conducted under a controlled light intensity of 1,300 lux. The apparatus consisted of a plus-shaped maze elevated 60 cm from the floor. Two open arms, each 25 cm x 5 cm with a 1 cm high edge, were crossed by two arms of the same dimensions, but enclosed by 30 cm high walls. Each animal was placed in the middle of the maze, facing an open arm, and allowed to explore the apparatus for 5 min. Following testing, mice were returned to their home cages and the apparatus was cleaned with 70% ethanol to remove any odor cues. Activity was monitored and analyzed using ANY-maze tracking software (Stoeltling Co., Wood Dale, IL). Entrance to an arm was considered when all 4 paws entered the arm.
Fear Conditioning
Fear conditioning tests were conducted in sound attenuating boxes (model ENV-022V; Med associates, Inc., St. Albans, VT). During the training session, mice were placed in a contextual conditioning chamber (model MED-VFC-NIR-M; Med Associates) and baseline freezing activity was recorded for 2 minutes. After 2 minutes, mice were subjected to 3 pairings of audio tones and foot shocks, each separated by thirty seconds without stimuli for a total of 5 minutes. Each 30 second audio tone (5 kHz, 70 dB) was immediately followed by a 2 second foot shock (0.5 mA) from an exposed electrified metal floor grid. The percent time spent freezing was used as a measure of the strength of the conditioned response. Following training (as well as following the next phases of this test as described below) mice were returned to their home cages and all equipment was cleaned with water and 70% ethanol to remove any odor cues. The next day, the mice were returned to the testing chamber for the contextual fear session. Activity in the chamber was monitored for 5 minutes without any tones or shock. The percentage of time spent freezing was used to as a measure of contextual fear conditioning. Following the contextual testing, mice were returned to their home cages and all equipment was cleaned with 70% ethanol. Three hours after the contextual fear test, the mice were placed in the same test chambers, but with an altered floor and wall context. Animals were observed for 5 minutes of baseline activity followed by 5 audio tones identical to those presented in the training phase (5 kHz, 70 dB). Each 30 second tone was separated by 30 seconds of silence for a total of 10 minutes spent in the test chamber. The percentage of time spent freezing during the baseline phase and during the tone phase was used as a measure of the cued fear conditioning response.
Detection of Aβ and Immunohistochemistry
Hippocampi were homogenized in modified RIPA buffer with protease inhibitor on ice. Protein concentrations were determined using the Bradford assay. Immunoblots were performed using 45μg of total protein extract separated on 4-12% SDS-PAGE gels and then transferred electrophoretically to a 22 μm nitrocellulose membrane. Membranes were blocked with 5% milk in TBS-t, washed in TBS-t and incubated overnight at 4° in a primary 6E10 antibody (1:1000, Santa Cruz Biotechnology, CA) or actin (Sigma-Aldrich; St. Louis, MO). Membranes were then incubated in a secondary antibody solution for 30 minutes (1:5000, then in the presence of horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG) for 30 minutes. The optical density of immunoreactive bands was detected and quantified by chemiluminescence using the ECL system.
Immunohistochemical analyses were carried out on sections (~5 μm thick) of paraffin-embedded mouse brains. In brief, the sections were deparaffinized before being exposed to citrate buffer (0.01 M, pH 6.0) by heating in a microwave for 3 min and treated with 0.3% hydrogen peroxide in methyl alcohol for 20 min to block endogenous peroxidase activity. Aβ plaques were stained by incubating overnight at 4 °C with Aβ antibody #2454 or control isotype-matched antibody (Cell Signaling technology, Danvers, MA), and detected with anti-rabbit biotinylated secondary antibody and avidin–biotin peroxidase complex (Vector Elite Kit; Vector Laboratories, Burlingame, CA, USA), and diaminobenzidine (DAB) using a peroxidase substrate kit (Vector Laboratories). Before being mounted, the sections were counterstained with hematoxylin. For quantification, digitized images of immunostained sections were obtained with a Qimaging Retiga 2000 SVGA FAST 1394 cooled digital camera system with 1600×1200 pixel array with 12-bit, 20MHz digitization camera mounted on a NIKON 80i Research Upright Microscope using IP lab software (BD Biosciences-Bio-imaging).
Negative stain immunoelectron microscopy
(IEM) was performed using 1μl of samples placed on a carbon-coated Collodion-filmed nickel grid and allowed to air dry. The samples were blocked using a goat blocking buffer and incubated with 6E10 antibody. After incubation with anti-mouse immunogold secondary antibody, samples were washed with 50mMTris (pH 7.2) containing 125mMNaCl, 0.1% (W/V) BSA, and Tween-20 (0.05%). The samples were stained by uranyl acetate (0.5% aqueous solution) and examined in the electron microscope (Hitachi H7600, Tokyo, Japan). The images were captured by a digital CCD camera and analyzed with AMT software (Danvers, MA). Immunogold secondary antibody, colloidon, uranyl acetate, and blocking buffer were from Electron Microscope Sciences (Hatfield, PA).
Statistical Analysis
Results are presented as the mean of triplicates ± SEM of at least three experiments. Differences were tested using Student's t test and a 2 sided p-value less than 0.05 was considered statistically significant.
RESULTS
Aβ-coreS is a potent immunogen in young and old mice
It remains poorly understood whether AD can be combated by vaccine administration at the onset of the disease, as vaccines can lose their efficacy due to tolerance to Aβ [18] and old age-associated immunological impairments [22, 23]. To test this, we DNA immunized mice of various ages ranging from 8 week-old (young) to 15 month-old. As shown in Fig.1A, while three immunizations with HBsAg-expressing DNA vaccine generated significant humoral responses in 8 week-old mice, practically no vaccine-specific (anti-S) antibody was detected in 15 month-old C57BL/6 mice in side-by-side experiments. Additional experiments indicated that the vaccine lost efficacy starting from 12 months of age regardless of the mouse strain used (BALB/C, C57BL/6 and AKR/J mice; data not shown). Moreover, we could not improve the immunogenicity of HBsAg in old mice even after fusing it with a viral antigen MC148 (MC148-S, Fig.1A), a strategy shown to be effective for the generation of anti-tumor immune responses [32]. Of note, comparable anti-HBsAg antibody can be induced in old mice after five or more immunizations (data not shown). Thus, as in old humans [22], old mice respond poorly to vaccines.
FIGURE 1.
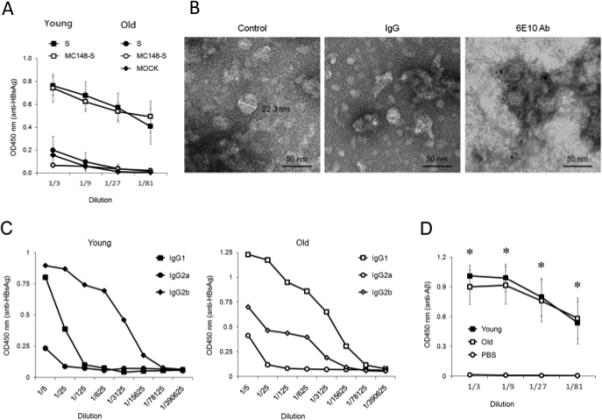
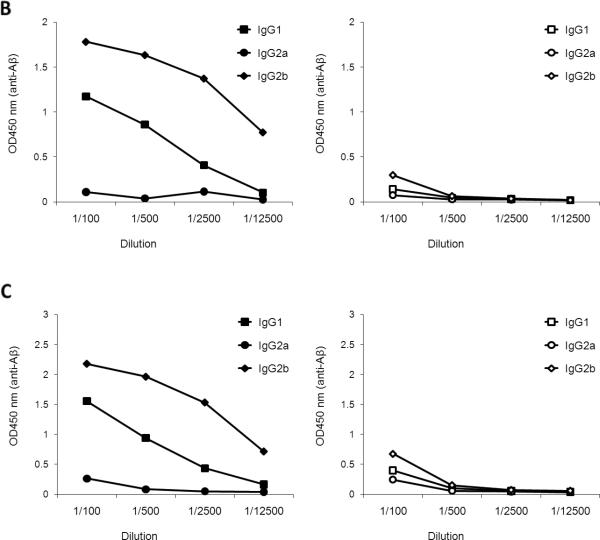
(A) DNA immunizations with HBsAg (S) or HBsAg fused with MC148 (MC148-S) only induce high levels of anti-HBsAg antibody in young, but not old, C57BL/6 mice. (B) Immunogold-electron microscopy images of Aβ-CoreS show that Aβ is exposed on the surface of 24-36-nm particles. Aβ-CoreS expressed in yeast was partially purified by silica gel absorption as described elsewhere [49]. The samples were untreated (control) or stained with control antibody (IgG) or Aβ-specific antibody 6E10. Bar indicates 50 nm. (C) Immunizations with Aβ-CoreS generated comparably high levels of antibody to HBsAg in both young and old C57BL/6 mice in a side-by-side experiment. Shown, a representative ELISA result of major immunoglobulin types recognizing HBsAg. (D) Aβ-CoreS vaccine also generated comparably levels of anti-Aβ antibody in young and old C57BL/6 mice in a side-by-side experiment. Y-axis shows relative concentration (OD450) of antibody in sera of mice DNA immunized three times, as detected by ELISA. X-axis shows serum titration. All data were reproduced at least three times using 4-5 mice per group experiments. From here on, *P<0.05.
Next, to develop an AD vaccine that circumvents poor vaccine responses in old age, we generated a chimeric HBsAg gene expressing Aβ(1-11) fragment and a strong Th epitope of capsid antigen of HBV [26]. The immunogenicity of foreign antigens can be enhanced by their repeated expression on the surface of self-assembled HBsAg particles [33], a primary component of a widely used HBV vaccine. The resulting construct (designated Aβ-CoreS) formed 24-36 – nm particles with Aβ(1-11) exposed on their surface, as revealed by ELISA (data not shown) and electron microscopy immune-gold analysis using 6E10 antibody that recognizes the amino-terminus of Aβ (Fig. 1B). Unlike the HBsAg vaccine (Fig.1A), immunizations with Aβ-CoreS generated high and comparable levels antibody to HBsAg in young and 15 month-old C57BL/6 mice (Fig.1C), indicating that the age-associated poor humoral responses can be reversed. Importantly, the Aβ-CoreS vaccine also generated comparable levels of anti-Aβ antibody in both ages of WT C57BL/6 (Fig.1D) and 3xTgAD (Fig.2A) mice. Aβ-CoreS generated Aβ-specific antibody predominantly represented by IgG2b (titers >1:6000 after three immunizations) and to a lesser degree IgG1, without detectable IgG2a or IgG3 (Fig.2B,C). Moreover, confirming a recent report from others on the inability of Aβ(1-42) peptide immunizations to overcome tolerance to Aβ [18], immunizations with Aβ(1-42) peptide failed to generate Aβ-specific antibody in 12 month 3xTgAD mice in side-by-side experiments regardless of an adjuvant use (Alum, Fig.2A; and IFA, data not shown). Hence, despite the old age- and potential Aβ tolerance –associated issues, the Aβ-CoreS vaccine induces potent anti-Aβ humoral responses in 3xTgAD mice.
FIGURE 2.
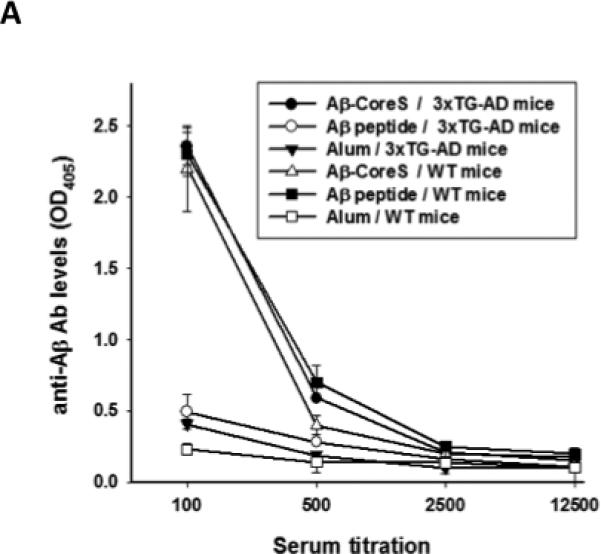
(A) Aβ-CoreS generated high levels of anti-Aβ antibody in both WT and 3xTgAD mice, when immunizations started at 12 month-old age. In contrast, the ability of Aβ(1-42) peptide to generate anti-Aβ antibody in WT was lost in 12 month-old 3xTgAD in a side-by-side experiment (A). The majority of antibody generated in Aβ-CoreS immune young (B) and old (C)3xTgAD mice was IgG2b (data of a side-by-side experiment). Control immunizations with S antigen (right panel) did not generate Aβ-specific antibody (B, C). Y-axis shows relative concentration (OD450) of antibody serially diluted sera (X-axis) of at least four per group mice DNA immunized three times, as detected by ELISA.
Aβ-CoreS does not induce T cell responses to Aβ
A recent clinical trial using a full-length Aβ(1-42) was halted due to the induction of harmful T cell responses [13, 16]. To circumvent this, Aβ-CoreS expresses Aβ(1-11), a fragment which only encodes a B cell epitope that cannot induce potentially harmful Aβ-specific T cell responses [11, 19]. To confirm this prediction, WT (C57BL/6) mice were DNA immunized with Aβ-CoreS or with a full-length Aβ(1-42) peptide emulsified in IFA. Ten days after the third immunization, splenic T cells were ex vivo labeled with CFSE and stimulated with antigen-presenting cells (APCs) pulsed for five days with Aβ(1-42) or control irrelevant peptide (Mock, [31]). The cells were then stained with anti-CD4 and CD8 antibodies to quantify the proportion of CD4+ and CD8+ T cells that diluted CFSE (proliferated T cells). While both CD4+ and CD8+ T cells from mice immunized with full-length Aβ(1-42) readily proliferated (Fig. 3A,B), we failed to detect Aβ-specific T cell activity in splenocytes from Aβ-CoreS-immunized mice (Fig. 3A,B). As in young mice (Fig.3A,B), Aβ-CoreS immunizations of old mice also did not activate Aβ-specific CD4+ and CD8+ T cells (Fig.3C,D). Aβ-CoreS did not elicit Aβ-specific T cell responses even if the immunizations started at a young age (8 weeks old) and then the mice were booster-immunized at 12 months (data not shown). This is not due to the inability of mice to recognize Aβ, as significant T cell proliferation was detected in young and old mice immunized with Aβ(1-42) peptide (Fig.3B,D). Thus, Aβ-CoreS cannot induce T cell responses in WT mice despite the generation of antibody responses to Aβ (Fig.1D). Similarly, immunizations with Aβ-CoreS also failed to induce proliferation of T cells from 3xTgAD mice (data not shown), although a high rate of spontaneous proliferation of T cells from mock -treated 3xTgAD mice did not allow us to quantify it. Taken together and in concordance with the use of Aβ(1-11) fragment [11, 19], the Aβ-CoreS formulation predominantly generates anti-Aβ antibody without eliciting Aβ-specific cellular responses.
FIGURE 3.
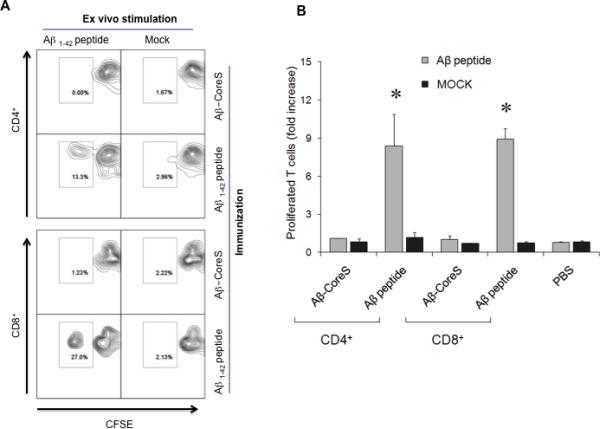
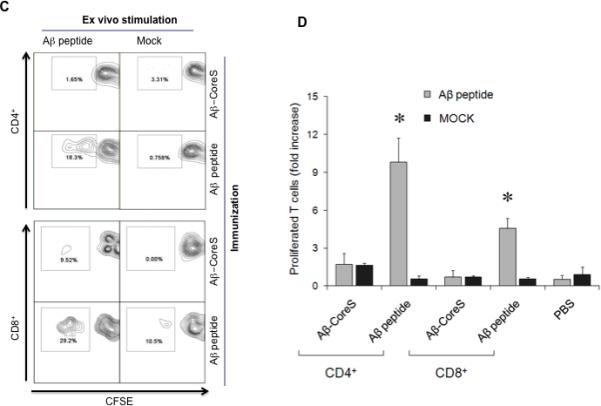
Immunizations with Aβ-CoreS did induce Aβ-specific T cell responses in young (A,B) and old (C, D) C57BL/6 mice. In contrast, T cells from mice immunized with a full-length Aβ(1-42) peptide readily proliferated upon incubation with Aβ peptide (Aβ1-42 peptide in A and C, and grey bars in B and D). CFSE -labeled splenocytes were stimulated with 2 μg Aβ(1-42) peptide or control g100 peptide (Mock) for 7 days. Numbers in the dot histograms of CFSE dilution assay (A, C) show a representative proportion of proliferated T cells of a summary graph (B, D). Y-axis shows T cell proliferation, presented as a fold increase compared with untreated controls (B, D) of CFSE diluted cells ± SEM of triplicate experiments. The experiments were replicated at least three times. No specific T cell responses were detected in control mice injected with PBS.
Aβ-CoreS vaccine can reduce Aβ plaques, improve cognition and extend survival of 3xTgAD mice
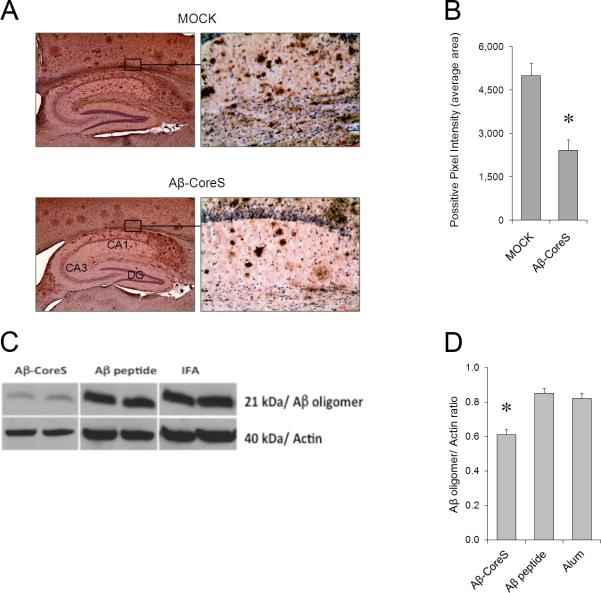
Antibody to Aβ can reduce the accumulation of Aβ plaques in hippocampus and prevent the loss of cognitive functions in several different transgenic mouse models of AD [9-11]. In concordance, compared with the mock-treated group, immunizations with Aβ-CoreS started in 12 month-old 3xTgAD mice significantly reduced the accumulation of Aβ plaques in the hippocampus of 15 month-old mice, as revealed by immunohistochemical analysis (Fig. 4A,B) and western blot hybridization (Fig. 4C,D). In contrast, immunizations with Aβ(1-42) peptide failed to reduce Aβ plaques in 3xTgAD mice, correlating with its inability to induce humoral responses (Fig.2A). Importantly, compared with control treatment, 3xTgAD mice immunized with Aβ-CoreS exhibited enhanced hippocampus-dependent cognitive learning, as they exhibited enhanced freezing behavior in the contextual fear conditioning task (Fig.5A). Neuropsychiatric symptoms and anxiety impairments in particular are common in patients with mild cognitive impairments and are significant clinical predictors of earlier conversion to AD [34]. The elevated plus maze is the gold-standard test to assess anxiety in mice [35]. In this task, lower anxiety levels result in higher amounts of time spent in the open arms of the elevated plus maze, rather than in the relative safety of the closed arms. Compared with mock -immunized mice, Aβ-CoreS -immunized mice spent less time in the open arm of the elevated plus maze (Fig.5B), suggesting that Aβ-CoreS also modified behaviors other than cognitive function in the 3xTgAD mice.
FIGURE 4.
Aβ-CoreS immunization reduced Aβ plaques in hippocampus of 3xTgAD mice, as shown by immunohistochemistry staining (A,B) or western blot hybridization (C,D) with 6E10 antibody. A and C show a representative picture of average levels of Aβ plaques ± SEM in five (B) and two individual mouse samples each (C), respectively. Data are from 15 month-old 3xTgAD mice i.d. immunized three times with 25 μg DNA encoding Aβ-CoreS starting at 12 months of age. Control mice were immunized with 25 μg DNA encoding HBsAg (Mock), or s.c. 10 μg Aβ(1-42) peptide in IFA (C) or in alum (D, Aβ peptide), or adjuvant alone, IFA (C) and alum (D). Note: ELISA results of the experiment depicted in (D, four mice per group) are shown in Fig.2A.
FIGURE 5.
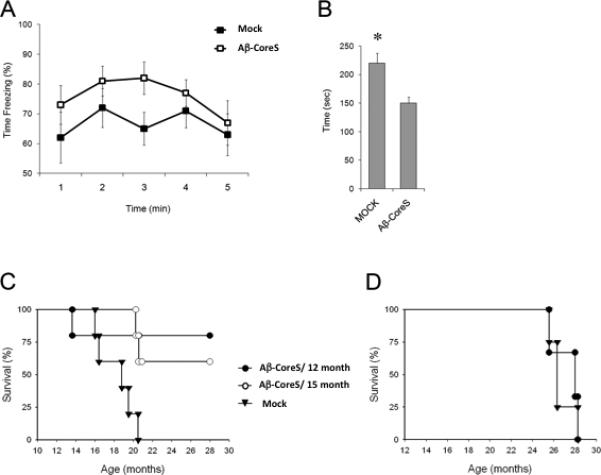
The reduction of Aβ plaques is associated with enhanced freezing behavior in the hippocampus-dependent contextual fear conditioning task (A); and decreased anxiety of 3xTgAD mice (as evident by reduced time spent in the open arm of the elevated plus maze; seconds, B). The relatively short life span of 3xTgAD mice was significantly enhanced by immunizing with Aβ-CoreS (C). The mice were mock treated or immunized with Aβ-CoreS when they were 1 and 1.5 years old, respectively, and observed until they reached 28 months old (C). Survival benefit of the Aβ-CoreS vaccine was lost when immunizations started at 22 months of age in surviving 3xTgAD mice (D). Shown, average ± SEM of 8 mice (A-C, P<0.05) and 4 mice (D) per group experiments comparing Aβ-CoreS and mock immunizations.
3xTgAD mice have a relatively short life span, with less than 25% of the mice surviving until 20 months of age after mock-treatment or DNA immunizations with S antigen (Fig.5C and data not shown). In contrast, more than 70% of the 3xTgAD mice immunized with Aβ-CoreS when they were 12 month-old were alive by 28 months of age when the experiment was terminated (Fig.5C). Importantly, Aβ-CoreS immunizations initiated at 15 months of age also significantly prolonged survival (Fig. 5C). However, the survival benefit of the vaccine was lost in the few naturally surviving 3xTgAD mice that were immunized at 20 month of age (Fig. 5D), presumably due to the advanced stage of the disease.
DISCUSSION
A recent Aβ(1-42) peptide vaccine trial in AD patients (AN1792) had to be prematurely halted due to the induction of T cell-mediated meningoencephalitis [16, 17, 36]. In retrospect, this could be expected, as Aβ contains a number of human and mouse T cell epitopes in its 15-42 portion [4, 17]. To circumvent this problem, a current trend is to utilize the amino terminal Aβ(1-11) fragment that primarily encodes B cell epitopes. For example, vaccine formulations containing this region primarily induce humoral responses and reduce Aβ -associated neurotoxicity without activating T cells [11, 19-21, 37]. Confirming the absence of Aβ-specific T cell epitopes, our Aβ-CoreS vaccine also failed to elicit T cell responses to Aβ in non-transgenic WT mice, which should not be hyporesponsive or affected by a chronic Aβ exposure. Since no known human T cell epitopes were shown in Aβ(1-11) [4, 17], the use of Aβ-CoreS in AD patients may also be safe. However, the risk of the induction of T cells in patients with certain genetic backgrounds or antibody-induced brain inflammation cannot be ruled out.
The need for AD vaccines that work and are designed for middle to old -aged individuals is high [41]. The safety concerns may preclude the feasibility of AD immunizations in apparently healthy people prior to diagnosis/onset of AD and development of age-associated immunological impairments [22, 23]. Moreover, the chronic exposure to Aβ may also induce AD vaccine hyporesponsiveness [18]. This may explain our inability to generate antibody in 3xTgAD mice immunized with Aβ(1-42) peptide and a relatively poor response in AD patients (AN1792 clinical trial) which only yielded about 70% antibody responders despite repeated immunizations [24]. Although ant-Aβ humoral responses could be enhanced in young mice, for example, by utilizing a prime-boost immunization approach [38] or various virus-like particle -based vaccines [39, 40], it remains unknown whether these vaccine strategies would also overcome Aβ- and old age-associated hyporesponsiveness. In contrast, we show that Aβ-CoreS retains its efficacy when administered in relatively aged subjects early in the disease process (<15 month old). The vaccine generated high levels of antibody both in young and old non-transgenic and 3xTgAD mice. As a result, the immunized 3xTgAD mice exhibited reduced Aβ plaques in the hippocampus and improved performance in hippocampal-dependent contextual fear memory. Others have also seen a similar reduction in the hippocampal Aβ56 and the monomeric Aβ oligomer and reversal of cognitive decay in a different model, orally AAV/Aβ immunized 10 month-old Tg2576 mice [42]. Thus, the old age-associated antibody hyporesponsiveness is reversible by administering strongly immunogenic vaccines like Aβ-CoreS. Although it is a focus of a different study, the enhanced immunogenicity of Aβ-CoreS may be a result of the unique composition of our vaccine, i.e. the presence of strong heterologous T helper epitopes of HBcAg [26] and the use of empty self-assembled HBsAg particles that allows a repeated exposure of Aβ(1-11) on its surface. We previously reported that the exposure of peptides on the surface of HBsAg particles drastically enhanced their immunogenicity [33]. Interestingly, this is despite the fact that, unlike a chemokine-based Aβ vaccine which had mostly IgG1 [11], the Aβ-CoreS-immunized 3xTgAD mice mostly generated IgG2b, an Fc-receptor low affinity binder that would be expected to be less effective in the protection from AD [19]. Similar preferential IgG2b production was also observed when Aβ peptide was used with monophosphoryl lipid A adjuvant [43]. Thus, it raises an interesting possibility that the IgG2b antibody cleared Aβ plaques in a similar fashion as F(ab)2 antibody that solubilizes plaques without involving FcR binding [44]. Aβ plaque solubilization was previously observed both in AD patients and transgenic AD mice, which was thought to be a cause of the redistribution of the Congophilic material to the vasculature and induction of a transient cerebral amyloid angiopathy [45, 46].
Taken together, we provide proof-of-principle that an Aβ vaccine retains its potency in old mice and induces therapeutic benefit when administered at a time point early in the disease process in 3xTgAD mice. Interestingly, immunizations with Aβ-CoreS not only reduced Aβ plaques and enhanced cognitive functions, but also extended the survival of 3xTgAD mice, significantly prolonging their overall survival. The 20 month average life span of 3xTgAD mice is shorter than the average life span (25 months) of the background wild type strain (C57BL/6) of mice [47]. Dementia is shown to be associated with an enhanced mortality in humans [5], raising the possibility of premature death in AD patients too. However, long-term survival or clinical outcome in AD patients was not improved by immunization with a full-length Aβ(1-42) in a follow-up study of phase I trial [6]. This might be due to either a poor immunogenicity of the vaccine, or the small number of people tested and the induction of harmful pro-inflammatory Th1 responses in the CNS [36]. Although the mechanism of the survival benefit of our vaccine is unknown and merits further study, it is tempting to speculate that this is due to the inhibition of Aβ -associated pathologies or through the induction of antibody -mediated homeostasis of Aβ in the CNS as in case of Caenorhabditis elegans [48]. Taken together, we propose that vaccines that target Aβ(1-11) can be also safely used in AD patients to promote extended survival by reducing Aβ-induced pathology and dementia.
Highlights.
Can vaccine for Alzheimer's disease (AD) be effective at the onset of the disease?
Vaccine use in old mice ameliorates cognitive AD impairments.
Vaccine provides survival benefit and prolongs life span of AD mice.
Acknowledgements
We are grateful to Ana Lustig (NIA/NIH) for proofreading and helpful comments. This project has been funded in whole or in part by the Intramural Research Program of the National Institute on Aging, NIH, and contract HHSN26120080001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest disclosure: The authors work for the US government and declare no competing financial interests.
REFERENCES
- 1.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004 Aug 5;430(7000):631–9. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golde TE, Dickson D, Hutton M. Filling the gaps in the abeta cascade hypothesis of Alzheimer's disease. Curr Alzheimer Res. 2006 Dec;3(5):421–30. doi: 10.2174/156720506779025189. [DOI] [PubMed] [Google Scholar]
- 3.Jin M, Shepardson N, Yang T, Chen G, Walsh D, Selkoe DJ. Soluble amyloid beta-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2011 Apr 5;108(14):5819–24. doi: 10.1073/pnas.1017033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monsonego A, Zota V, Karni A, Krieger JI, Bar-Or A, Bitan G, et al. Increased T cell reactivity to amyloid beta protein in older humans and patients with Alzheimer disease. The Journal of clinical investigation. 2003 Aug;112(3):415–22. doi: 10.1172/JCI18104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie J, Brayne C, Matthews FE. Survival times in people with dementia: analysis from population based cohort study with 14 year follow-up. Bmj. 2008 Feb 2;336(7638):258–62. doi: 10.1136/bmj.39433.616678.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, et al. Long-term effects of Abeta42 immunisation in Alzheimer's disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008 Jul 19;372(9634):216–23. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 7.Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nature Medicine. 2000 Aug;6(8):916–9. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 8.Ryan DA, Mastrangelo MA, Narrow WC, Sullivan MA, Federoff HJ, Bowers WJ. Abeta-directed single-chain antibody delivery via a serotype-1 AAV vector improves learning behavior and pathology in Alzheimer's disease mice. Mol Ther. 2010 Aug;18(8):1471–81. doi: 10.1038/mt.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, et al. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000 Dec 21-28;408(6815):982–5. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 10.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999 Jul 8;400(6740):173–7. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 11.Movsesyan N, Ghochikyan A, Mkrtichyan M, Petrushina I, Davtyan H, Olkhanud PB, et al. Reducing AD-like pathology in 3xTg-AD mouse model by DNA epitope vaccine - a novel immunotherapeutic strategy. PLoSONE. 2008;3(5):e2124. doi: 10.1371/journal.pone.0002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bombois S, Maurage CA, Gompel M, Deramecourt V, Mackowiak-Cordoliani MA, Black RS, et al. Absence of beta-amyloid deposits after immunization in Alzheimer disease with Lewy body dementia. Arch Neurol. 2007 Apr;64(4):583–7. doi: 10.1001/archneur.64.4.583. [DOI] [PubMed] [Google Scholar]
- 13.Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005 May 10;64(9):1553–62. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 14.Castellino F, Boucher PE, Eichelberg K, Mayhew M, Rothman JE, Houghton AN, et al. Receptor-mediated uptake of antigen/heat shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct processing pathways. J Exp Med. 2000 Jun 5;191(11):1957–64. doi: 10.1084/jem.191.11.1957. 191(11):1957-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hock C, Konietzko U, Papassotiropoulos A, Wollmer A, Streffer J, von Rotz RC, et al. Generation of antibodies specific for beta-amyloid by vaccination of patients with Alzheimer disease. Nature Medicine. 2002 Nov;8(11):1270–5. doi: 10.1038/nm783. [DOI] [PubMed] [Google Scholar]
- 16.Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003 Jul 8;61(1):46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 17.Monsonego A, Imitola J, Petrovic S, Zota V, Nemirovsky A, Baron R, et al. Abeta-induced meningoencephalitis is IFN-gamma-dependent and is associated with T cell-dependent clearance of Abeta in a mouse model of Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2006 Mar 28;103(13):5048–53. doi: 10.1073/pnas.0506209103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monsonego A, Maron R, Zota V, Selkoe DJ, Weiner HL. Immune hyporesponsiveness to amyloid beta-peptide in amyloid precursor protein transgenic mice: implications for the pathogenesis and treatment of Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2001 Aug 28;98(18):10273–8. doi: 10.1073/pnas.191118298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bard F, Barbour R, Cannon C, Carretto R, Fox M, Games D, et al. Epitope and isotype specificities of antibodies to beta -amyloid peptide for protection against Alzheimer's disease-like neuropathology. Proceedings of the National Academy of Sciences of the United States of America. 2003 Feb 18;100(4):2023–8. doi: 10.1073/pnas.0436286100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee M, Bard F, Johnson-Wood K, Lee C, Hu K, Griffith SG, et al. Abeta42 immunization in Alzheimer's disease generates Abeta N-terminal antibodies. Ann Neurol. 2005 Sep;58(3):430–5. doi: 10.1002/ana.20592. [DOI] [PubMed] [Google Scholar]
- 21.Nicoll JA, Barton E, Boche D, Neal JW, Ferrer I, Thompson P, et al. Abeta species removal after abeta42 immunization. J Neuropathol Exp Neurol. 2006 Nov;65(11):1040–8. doi: 10.1097/01.jnen.0000240466.10758.ce. [DOI] [PubMed] [Google Scholar]
- 22.Kumar R, Burns EA. Age-related decline in immunity: implications for vaccine responsiveness. Expert Rev Vaccines. 2008 May;7(4):467–79. doi: 10.1586/14760584.7.4.467. [DOI] [PubMed] [Google Scholar]
- 23.Webster RG. Immunity to influenza in the elderly. Vaccine. 2000 Feb 25;18(16):1686–9. doi: 10.1016/s0264-410x(99)00507-1. [DOI] [PubMed] [Google Scholar]
- 24.Bayer AJ, Bullock R, Jones RW, Wilkinson D, Paterson KR, Jenkins L, et al. Evaluation of the safety and immunogenicity of synthetic Abeta42 (AN1792) in patients with AD. Neurology. 2005 Jan 11;64(1):94–101. doi: 10.1212/01.WNL.0000148604.77591.67. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Q, Wang Y, Freed D, Fu TM, Gimenez JA, Sitrin RD, et al. Maturation of recombinant hepatitis B virus surface antigen particles. Hum Vaccin. 2006 Jul-Aug;2(4):174–80. doi: 10.4161/hv.2.4.3015. [DOI] [PubMed] [Google Scholar]
- 26.Milich DR, Peterson DL, Schodel F, Jones JE, Hughes JL. Preferential recognition of hepatitis B nucleocapsid antigens by Th1 or Th2 cells is epitope and major histocompatibility complex dependent. J Virol. 1995 May;69(5):2776–85. doi: 10.1128/jvi.69.5.2776-2785.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003 Jul 31;39(3):409–21. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 28.Liu D, Pitta M, Lee JH, Ray B, Lahiri DK, Furukawa K, et al. The KATP channel activator diazoxide ameliorates amyloid-beta and tau pathologies and improves memory in the 3xTgAD mouse model of Alzheimer's disease. J Alzheimers Dis. 2010;22(2):443–57. doi: 10.3233/JAD-2010-101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruffini PA, Neelapu SS, Kwak LW, Biragyn A. Idiotypic vaccination for B-cell malignancies as a model for therapeutic cancer vaccines: from prototype protein to second generation vaccines. Haematologica. 2002;87(9):989–1001. [PubMed] [Google Scholar]
- 30.Bogen B, Lambris JD. Minimum length of an idiotypic peptide and a model for its binding to a major histocompatibility complex class II molecule. EMBO J. 1989;8:1947–52. doi: 10.1002/j.1460-2075.1989.tb03599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schiavo R, Baatar D, Olkhanud P, Indig FE, Restifo N, Taub D, et al. Chemokine receptor targeting efficiently directs antigens to MHC class I pathways and elicits antigen-specific CD8+ T-cell responses. Blood. 2006;107(12):4597–605. doi: 10.1182/blood-2005-08-3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruffini PA, Biragyn A, Coscia M, Harvey LK, Cha SC, Bogen B, et al. Genetic fusions with viral chemokines target delivery of nonimmunogenic antigen to trigger antitumor immunity independent of chemotaxis. JLeukocBiol. 2004;76(1):77–85. doi: 10.1189/jlb.1003481. [DOI] [PubMed] [Google Scholar]
- 33.Borisova G, Arya B, Dislers A, Borschukova O, Tsibinogin V, Skrastina D, et al. Hybrid hepatitis B virus nucleocapsid bearing an immunodominant region from hepatitis B virus surface antigen. J Virol. 1993 Jun;67(6):3696–701. doi: 10.1128/jvi.67.6.3696-3701.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallagher D, Coen R, Kilroy D, Belinski K, Bruce I, Coakley D, et al. Anxiety and behavioural disturbance as markers of prodromal Alzheimer's disease in patients with mild cognitive impairment. Int J Geriatr Psychiatry. 2011 Feb;26(2):166–72. doi: 10.1002/gps.2509. [DOI] [PubMed] [Google Scholar]
- 35.Dawson GR, Tricklebank MD. Use of the elevated plus maze in the search for novel anxiolytic agents. Trends Pharmacol Sci. 1995 Feb;16(2):33–6. doi: 10.1016/s0165-6147(00)88973-7. [DOI] [PubMed] [Google Scholar]
- 36.Monsonego A, Imitola J, Zota V, Oida T, Weiner HL. Microglia-mediated nitric oxide cytotoxicity of T cells following amyloid beta-peptide presentation to Th1 cells. Journal of immunology. 2003 Sep 1;171(5):2216–24. doi: 10.4049/jimmunol.171.5.2216. [DOI] [PubMed] [Google Scholar]
- 37.Ghochikyan A. Rationale for peptide and DNA based epitope vaccines for Alzheimer's disease immunotherapy. CNS Neurol Disord Drug Targets. 2009 Apr;8(2):128–43. doi: 10.2174/187152709787847298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davtyan H, Mkrtichyan M, Movsesyan N, Petrushina I, Mamikonyan G, Cribbs DH, et al. DNA prime-protein boost increased the titer, avidity and persistence of anti-Abeta antibodies in wild-type mice. Gene Ther. 2010 Feb;17(2):261–71. doi: 10.1038/gt.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bach P, Tschape JA, Kopietz F, Braun G, Baade JK, Wiederhold KH, et al. Vaccination with Abeta-displaying virus-like particles reduces soluble and insoluble cerebral Abeta and lowers plaque burden in APP transgenic mice. Journal of immunology. 2009 Jun 15;182(12):7613–24. doi: 10.4049/jimmunol.0803366. [DOI] [PubMed] [Google Scholar]
- 40.Chackerian B, Rangel M, Hunter Z, Peabody DS. Virus and virus-like particle-based immunogens for Alzheimer's disease induce antibody responses against amyloid-beta without concomitant T cell responses. Vaccine. 2006 Sep 11;24(37-39):6321–31. doi: 10.1016/j.vaccine.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 41.Lemere CA, Masliah E. Can Alzheimer disease be prevented by amyloid-beta immunotherapy? Nat Rev Neurol. 2010 Feb;6(2):108–19. doi: 10.1038/nrneurol.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mouri A, Noda Y, Hara H, Mizoguchi H, Tabira T, Nabeshima T. Oral vaccination with a viral vector containing Abeta cDNA attenuates age-related Abeta accumulation and memory deficits without causing inflammation in a mouse Alzheimer model. Faseb J. 2007 Jul;21(9):2135–48. doi: 10.1096/fj.06-7685com. [DOI] [PubMed] [Google Scholar]
- 43.Maier M, Seabrook TJ, Lemere CA. Modulation of the humoral and cellular immune response in Abeta immunotherapy by the adjuvants monophosphoryl lipid A (MPL), cholera toxin B subunit (CTB) and E. coli enterotoxin LT(R192G). Vaccine. 2005 Oct 25;23(44):5149–59. doi: 10.1016/j.vaccine.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 44.Bacskai BJ, Kajdasz ST, McLellan ME, Games D, Seubert P, Schenk D, et al. Non-Fc-mediated mechanisms are involved in clearance of amyloid-beta in vivo by immunotherapy. J Neurosci. 2002 Sep 15;22(18):7873–8. doi: 10.1523/JNEUROSCI.22-18-07873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boche D, Zotova E, Weller RO, Love S, Neal JW, Pickering RM, et al. Consequence of Abeta immunization on the vasculature of human Alzheimer's disease brain. Brain. 2008 Dec;131(Pt 12):3299–310. doi: 10.1093/brain/awn261. [DOI] [PubMed] [Google Scholar]
- 46.Wilcock DM, Rojiani A, Rosenthal A, Levkowitz G, Subbarao S, Alamed J, et al. Passive amyloid immunotherapy clears amyloid and transiently activates microglia in a transgenic mouse model of amyloid deposition. J Neurosci. 2004 Jul 7;24(27):6144–51. doi: 10.1523/JNEUROSCI.1090-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Forster MJ, Morris P, Sohal RS. Genotype and age influence the effect of caloric intake on mortality in mice. Faseb J. 2003 Apr;17(6):690–2. doi: 10.1096/fj.02-0533fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alavez S, Vantipalli MC, Zucker DJ, Klang IM, Lithgow GJ. Amyloid-binding compounds maintain protein homeostasis during ageing and extend lifespan. Nature. 2011 Apr 14;472(7342):226–9. doi: 10.1038/nature09873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wampler DE, Lehman ED, Boger J, McAleer WJ, Scolnick EM. Multiple chemical forms of hepatitis B surface antigen produced in yeast. Proceedings of the National Academy of Sciences of the United States of America. 1985 Oct;82(20):6830–4. doi: 10.1073/pnas.82.20.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]