Abstract
Traditional morphological identification of invertebrate marine species is limited in early life history stages for many taxa. In this study, we demonstrate, by example of Vestimentiferan tubeworms (Siboglinidae, Polychaeta), that the simultaneous fluorescence in situ hybridization (FISH) of both eukaryotic host and bacterial symbiont cells is possible on a single semi-thin (1 μm) section. This allows the identification of host specimens to species level as well as offering visualization of bacteria distributed within the host tissue. Previously published 18S rRNA host-specific oligonucleotide probes for Riftia pachyptila, Tevnia jerichonana and a newly designed Oasisia alvinae probe, as well as a 16S rRNA probe targeting symbionts found in all host species, were applied. A number of standard fixation and hybridization parameters were tested and optimized for the best possible signal intensity and cellular resolution. Ethanol conserved samples embedded in LR White low viscosity resin yielded the best results with regard to both signal intensity and resolution. We show that extended storage times of specimens does not affect the quality of signals attained by FISH and use our protocol to identify morphologically unidentifiable tubeworm individuals from a small data set, conforming to previous findings in succession studies of the Siboglinidae family.
Keywords: Vestimentifera, Polychaeta, Tubeworms, Fluorescence in situ hybridization, FISH, LR White
Introduction
The morphological identification of marine invertebrate species is traditionally conducted under a dissection microscope by analyzing species-specific characteristics. Apart from being time consuming, successful identification often needs an experienced expert. Moreover, in early life history stages species-specific characteristics may not always be present. Many invertebrates show identical, or nearly identical, early developmental stages until metamorphosis into the adult form takes place, making species identification impossible at this stage based on morphology alone. Assessment of early developmental stages in, for example, polychaete trochophores requires high-resolution techniques such as scanning electron microscopy (Jones and Gardiner, 1989) to reveal morphological details. This is ultimately a destructive method, however, as specimens cannot be used for further investigations such as proteinic or nucleic analysis post-identification. Morphological characteristics may show a certain degree of plasticity dependent on external factors such as availability of food, water temperature, or other environmental variables (Boidron-Metairon, 1988; Shirley et al., 1987; Strathmann et al., 1992). This also extends to adult forms, which may show highly plastic phenotypes in, for example, deep sea Vestimentifera (Polychaeta, Siboglinidae). Southward et al. (1995) showed, by comparing allozyme patterns, that several Ridgeia piscesae (Jones, 1985) specimens, originally described as separate species after original morphological analyses, were in fact the same species exhibiting several different morphotypes.
Nucleic assessment for species identification has several advantages. Taxonomic resolution is high enough to differentiate at a species level and variations in developmental stages, specimen age, or physiological states have no apparent effect. Polymerase chain reaction (PCR) and PCR-restriction fragment length polymorphism (PCR-RFLP) have in past studies been used to identify invertebrate larvae (Olson et al., 1991; Mullineaux et al., 2000; Hunt et al., 2003). The required extraction of DNA or RNA will partially damage or destroy, in the case of early developmental stages, sample material thus making potential histological or immunohistological studies of individual samples post-identification difficult. Furthermore, the accuracy of PCR-RFLP is not always given; in some cases only 60% of all samples could be identified positively (Hunt et al., 2003).
In areas of marine research, such as in the deep sea, non-destructive methods for molecular identification of organisms would be of great advantage due to the inaccessibility and high costs associated with sample collection. Species-specific signature molecules such as the bacterial and archaeal 16S rRNA or the eukaryotic 18S rRNA can be targeted with oligonucleotide probes designed for sequence regions that are conserved within a species, but not found in closely related ones. Often a single base pair mismatch can be sufficient to discriminate between species with nearly 100% efficiency (Pradillon et al., 2007). Fluorescence in situ hybridization (FISH), commonly used for the identification, visualization, enumeration and localization of bacteria or archaea based on 16S rRNA sequences (Amann et al., 1990), makes use of oligonucleotide probes coupled with a fluorochrome label. Several studies have in the past concentrated on the application of FISH to eukaryotic microorganisms such as yeast and phytoplankton (Lischewski et al., 1996; Simon et al., 1995).
Surprisingly only few studies have so far focused on the identification of metazoan marine organisms in early developmental stages (e.g., Goffredi et al., 2006). One of the reasons for this is possibly the strong autofluorescence observed in many species (Pradillon, 2002, cited in Pradillon et al., 2007). Pradillon et al. (2007) offered a novel in situ hybridization (ISH) method for identification of marine organisms using horseradish peroxidase (HRP) or digoxygenin (DIG) labelled oligonucleotide probes and successfully tested this method on eggs and larvae. Specimens were treated as a whole and successful oligonucleotide probe binding was revealed by enzymatic reactions with the respective catalysts. Although this approach is ideal for species identification, histological visualization of eukaryotic tissue and bacterial or archaeal cell localization is not possible using this method alone. This may however be required for analysis of endosymbiotic relationships in which both partners need to be localized and identified. Nevertheless, developmental questions have been answered in past studies such as Nussbaumer et al. (2006) using symbiont-specific probes and FISH on semi-thin (1 μm) sections, but without the capability of identifying the host to species level. The embedding in a hydrophilic resin, such as LR White, generally allows for greater histological detail than can be observed with the standard 4–5 μm paraffin wax embedded sections, often used in molecular studies (Nussbaumer et al., 2006; Gros and Maurin, 2008). By providing better support to specimen tissue, thinner sections of 1 μm can be attained. Additionally hydrophilic resins permit ultrastructural analyses as well as post-embedding treatments such as immunohistochemistry or nucleic hybridization (Pflugfelder et al., 2009; Nussbaumer et al., 2006).
Coupling the advantages of FISH such as the localization and enumeration of symbionts with the possibility for identifying eukaryotic hosts, one can work on several levels of data acquisition from the same specimen. This is of particular advantage for deep-sea organisms containing endosymbionts such as the Vestimentifera. Prior to the development of the bacteria-housing organ, the trophosome, larvae appear as trochophores dispersed in the pelagial. Metamorphosis into the early juvenile stage and the development of the trophosome occurs after settlement. However, individual morphological characteristics to differentiate between species have still not developed at this stage. Only in the late juvenile to early adult stage of individuals at least a few millimetres in length, can species-specific characters be detected (Bright and Lallier, 2010). All three Vestimentiferan species: Riftia pachyptila (Jones, 1981), Tevnia jerichonana (Jones, 1985), and Oasisia alvinae (Jones, 1985), co-occurring on the East Pacific Rise, share the same sulfide-oxidizing endosymbiont of the Gammaproteobacteria family, phylotype II based on 16S rRNA sequences (Feldman et al., 1997). The genome of R. pachyptila's endosymbiont has been sequenced and named Candidatus Endoriftia persephone (Robidart et al., 2008). It is therefore not possible to differentiate between host species using the symbiont alone. We present a protocol in which both host and symbiont can be made visible by simultaneous hybridization of 16S rRNA and 18S rRNA with fluorochrome labelled oligonucleotide probes. This allows identification of host species on semi-thin sections offering higher analytical resolution for studies such as Nussbaumer et al. (2006) as well as revealing symbiont localization in the host tissue. The protocol was tested using different standard fixation and embedding procedures with specimens stored up to nine years.
Materials and methods
Sample collection
Collections of vestimentiferan tubeworms were conducted on several sites in the 9°50′N East Pacific Rise region during cruises with R/V Atlantis and DSV Alvin between 2001 and 2009 as well as with R/V L’Atalante and DSV Nautile in 2010. Several sampling sites were selected during this time, on all of which tubeworm aggregations were dominant, namely TICA (9°50.447′N, 104°17.493′W, ∼2500 m depth), and the closely situated sites V-vent (9°47.266′N, 104°16.970′W, ∼2510 m depth), Bio 9 (9°50.305′N, 104°17.484′W, ∼2500 m depth) and East Wall (9°50.553′N; 104°17.514′W, ∼2530 m depth). A minimum of three specimens for each of the three species, identified by morphological criteria (Fig. 1) were used for three different fixation and embedding procedures analyzed in this study. Most specimens were large juveniles or adults, thus in the symbiotic stage of their life history, exhibiting a trophosome with intracellular endosymbionts. In addition, small individuals, unidentifiable through morphological characteristics, were collected and selected for molecular identification (Table 1).
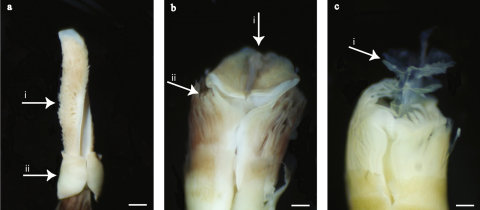
Fig. 1.
Light micrographs of the three Vestimentifera species used in this study. Arrows indicate characteristic morphological traits. (a) Riftia pachyptila specimen (# 1536-1), (i) plume with tentacular lamellae horizontal to axis, (ii) ventrally overlapping dorsolateral vestimentum flaps; (b) Tevnia jerichonana specimen (# 1536-2), (i) small vertical lamina on top of obturaculum, (ii) branchial filaments in concentric lamellae aligned around obturaculum; (c) Oasisia alvinae specimen (# 1536-3), (i) central axial rod carrying 2–3 translucent white saucer-like structures on top of obturaculum (Jones, 1981, 1985). Scale bars: a = 1 mm; b and c = 300 μm.
Table 1.
A minimum of three specimens for each species and each fixation/embedding procedure was used. Sample collections ranged from 2001 to 2010, however not all sampling years were represented in each fixation/sectioning type.
| Species | Specimen number | Year of collection | Submersible and dive number | Collection site | Sample origin | Fixation/embedding |
|---|---|---|---|---|---|---|
| Riftia pachyptila | 417 | 2001 | Alvin, 3725 | TICA | N/A | PFA/LR White |
| Riftia pachyptila | 612 | 2003 | Alvin, 3942 | TICA | Basalt | PFA/LR White |
| Riftia pachyptila | 1094 | 2006 | Alvin, 4293 | V-Vent | Basalt | PFA/LR White |
| Tevnia jerichonana | 420 | 2001 | Alvin, 3730 | East Wall | N/A | PFA/LR White |
| Tevnia jerichonana | 926 | 2004 | Alvin, 4062 | TICA | N/A | PFA/LR White |
| Tevnia jerichonana | 979 | 2004 | Alvin, 4067 | Bio 9 | N/A | PFA/LR White |
| Oasisia alvinae | 924 | 2004 | Alvin, 4062 | TICA | N/A | PFA/LR White |
| Oasisia alvinae | 925 | 2004 | Alvin, 4062 | TICA | N/A | PFA/LR White |
| Oasisia alvinae | 943 | 2004 | Alvin, 4066 | TICA | N/A | PFA/LR White |
| Riftia pachyptila | 1094 | 2006 | Alvin, 4293 | V-Vent | Basalt | PFA/Cryo |
| Riftia pachyptila | 1095 | 2006 | Alvin, 4293 | V-Vent | Basalt | PFA/Cryo |
| Tevnia jerichonana | 1052 | 2006 | Alvin 4260 | TICA | N/A | PFA/Cryo |
| Oasisia alvinae | 943 | 2004 | Alvin, 4066 | TICA | N/A | PFA/Cryo |
| Oasisia alvinae | 1214 | 2009 | Alvin, 4575 | TICA | Basalt | PFA/Cryo |
| Oasisia alvinae | 1422 | 2009 | Alvin, 4580 | TICA | TASC 98-2 | PFA/Cryo |
| Vestimentiferana | 1067-2 | 2006 | Alvin, 4260 | TICA | TASC 38 | PFA/Cryo |
| Vestimentiferana | 1067-3 | 2006 | Alvin, 4260 | TICA | TASC 38 | PFA/Cryo |
| Vestimentiferana | 1067-4 | 2006 | Alvin, 4260 | TICA | TASC 38 | PFA/Cryo |
| Vestimentiferana | 1067-6 | 2006 | Alvin, 4260 | TICA | TASC 38 | PFA/Cryo |
| Vestimentiferana | 1067-7 | 2006 | Alvin, 4260 | TICA | TASC 38 | PFA/Cryo |
| Vestimentiferana | 1080-1 | 2006 | Alvin, 4260 | TICA | TASC 38 | PFA/Cryo |
| Vestimentiferana | 1080-7 | 2006 | Alvin, 4260 | TICA | TASC 38 | PFA/Cryo |
| Riftia pachyptila | 612 | 2003 | Alvin, 3942 | TICA | Basalt | Ethanol/LR White |
| Riftia pachyptila | 1535-1 | 2010 | Nautile, 1734 | TICA | Basalt | Ethanol/LR White |
| Riftia pachyptila | 1536-1 | 2010 | Nautile, 1743 | TICA | Basalt | Ethanol/LR White |
| Tevnia jerichonana | 926 | 2004 | Alvin, 4062 | TICA | N/A | Ethanol/LR White |
| Tevnia jerichonana | 1536-2 | 2010 | Nautile, 1743 | TICA | Basalt | Ethanol/LR White |
| Tevnia jerichonana | 1536-4 | 2010 | Nautile, 1743 | TICA | Basalt | Ethanol/LR White |
| Oasisia alvinae | 924 | 2004 | Alvin, 4062 | TICA | N/A | Ethanol/LR White |
| Oasisia alvinae | 1535-2 | 2010 | Nautile, 1734 | TICA | Basalt | Ethanol/LR White |
| Oasisia alvinae | 1536-3 | 2010 | Nautile, 1743 | TICA | Basalt | Ethanol/LR White |
Vestimentiferan specimens not identified morphologically due to small size and lack of characteristics prior to hybridization.
Some of the tubeworms were sampled from natural surfaces, such as basalt, located underneath large tubeworm aggregations, or from the tubes of large specimens. Others were picked from tubeworm artificial settlement cubes (TASC's) after Nussbaumer et al. (2006). TASC's consist of several (8–12) H-PVC plates (5 cm × 5 cm × 0.5 cm), held together by a bolt, on which 20 grooves are cut into one side of each plate. The grooves from one plate and the backside of an adjoining plate create depressions into which endocryptic larvae can settle. Natural samples and TASC's were put into clean, isolated bioboxes for recovery. Tubeworms were picked by hand from basalt, large tubes or TASC's and photographed for reference using a Nikon Coolpix 990 camera attached to a BMS dissection microscope.
Fixation
Samples were fixed in: (1) 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS: 145 mM NaCl, 1.4 mM NaH2PO4, 8 mM Na2HPO4, pH 7.5), containing 10% (w/v) sucrose, at 4 °C for 12 h (Nussbaumer et al., 2006). Samples were rinsed in PBS several times, transferred into 30%, 50% and finally 70% ethanol and stored therein. (2) Alternatively, 100% ethanol was used as a conservation and storage medium for newly collected specimens.
Embedding
Roughly 1 mm long trunk pieces were cut from small specimens ensuring that both trophosome and skin were present. Two different embedding methods followed: (1) gradual dehydration in ethanol followed by embedding in medium grade LR White low viscosity resin (London Resin Company, Reading, Berkshire, UK) after Nussbaumer et al. (2006). Dehydration was done in steps of 70%, 80%, 90% and 100% ethanol for 10 min each, repeating the last step three times. Transfer into LR White resin, using a mixture of 100% ethanol to resin (50:50) for 60 min followed by eight transfers into fresh LR White resin for 30 min each, with the exception of the last change, being left overnight. The samples were oriented in LR White filled gelatin capsules, sealed so that no air was enclosed, and placed into a desiccator at 45 °C for a minimum of 48 h. (2) Infiltration with a mixture of polyvinyl pyrrolidone (PVP) and sucrose 1:4 (v/w) for 3 days prior to cryosectioning (Tokuyasu, 1980).
Sectioning
Semithin sections (1 μm) of LR White embedded specimens, were cut using a diamond knife on an ultramicrotome (Super cut S, Leica Microsystems, Wetzlar, Germany). For each slide four sections were stretched with chloroform vapor, and placed on a drop of 20% acetone on 0.2% poly-l-lysine coated glass slides. Slides were dried on a hotplate less than 50 °C overnight, in order to prevent RNA destruction. Cryosections (1 μm) were cut with a glass knife on a Reichert-Jung FC4D Ultracut E and arranged on slides as described above.
Probe design
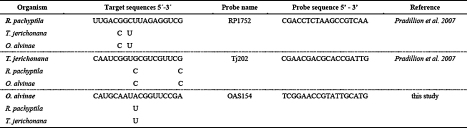
Oligonucleotide probes targeting the eukaryotic 18S rRNA gene sequences specific to Riftia pachyptila (Pradillon et al., 2007), Tevnia jerichonana (Pradillon et al., 2007) and Oasisia alvinae (this study) were used in this study (Table 3). To design a probe specific to O. alvinae the 18S rRNA partial sequence, totalling 1764 base pairs, was obtained from the European Bio Informatics online database (Acc. Number: AF168743.1). A ClustalW2 (European Bio Informatics) analysis, comparing the three 18S rRNA tubeworm sequences, always revealed at least one mismatch between species. Using the probe-design tool in the software package ARB (Ludwig et al., 2004) a oligonucleotide probe specific to O. alvinae was designed. All probes are 18 nucleotides long and contain at least 1 mismatch to the other two species. The probe was named after the first 3 letters of the genus followed by the position targeted on the 18S rRNA gene.
Table 3.
Oligonucleotide probe sequences for the three vestimentiferan species used in this study. All probes are Cy3 marked on the 5′ end and target the eukaryotic 18S rRNA. Mismatches to each of the other two species are depicted under the target sequence column.
Probes
All host-specific probes were labelled with a Cy3 dye. The probe Rif445 (Nussbaumer et al., 2006) targeting the 16S rRNA of the endosymbiotic sulfide-oxidising bacteria, found as the obligate symbiont in all three species was used with a 6-FAM (Fluos) label. The universal probe Univ1390 (Zheng et al., 1996), targeting eukaryotes, bacteria and archaea was also labelled with a Fluos dye and used as a positive control on all slides. As negative controls the nonsense probe NONEUB (Wallner et al., 1993) labelled in both Cy3 and Fluos, and the application of a non-target host-specific probe, revealing possible false hybridization on each hybridized slide, were applied (Table 2). Probe concentration was always 50 ng/μl working solution and all probes were labelled on the 5′-end with their respective fluorescein dyes. Probes were ordered from ThermoFisher Scientific (Waltham, MA, USA).
Table 2.
Positive (Univ1390) and negative (NONEUB) controls applied to each slide during all procedures as well as the sulfide-oxidising symbiont probe (Rif445), sequence of each probe and type of fluorescent labels are listed.
| Probe Name | Specificity | Target molecule | Sequence 5′–3′ | Label | Source |
|---|---|---|---|---|---|
| Univ1390 | All organisms | 16S rRNA | GACGGGCGGTGTGTACAA | 6-FAM (Fluos) | Zheng et al. (1996) |
| NONEUB | Complementary control for EUB338 | 16S rRNA | ACTCCTACGGGAGGCAGC | 6-FAM + Cy3 | Wallner et al. (1993) |
| Rif445 | R. pachyptila, T. jerichonana, O. alvinae symbiont | 16S rRNA | TCCTCAGGCTTTTCTTCC | 6-FAM | Nussbaumer et al. (2006) |
Fluorescence in situ hybridization
Of the 4 sections on each slide, three were used for host-specific probe application coupled with either the Univ1390 or the symbiont-specific Rif445 probe and one section acted as a negative control on which the NONEUB probe in both Cy3 and Fluos was added. By adding the probes for both target and non-target organisms to each slide specificity tests were carried out with each hybridisation. Around each section a ring was drawn with a liquid blocking PAP pen (Daido Sangyo Co. Ltd., Tokyo, Japan). The sections were soaked in PBS for 5 min and subsequently left to air dry at room temperature. Either 0.5 μg/ml or 0.1 mg/ml proteinase K (1 mg/ml, Roth) in PBT (PBS + 0.25% Triton X) digestion followed at 46 °C in a hybridization oven for 0, 5, 10 or 15 min and was stopped with 0.2 N HCl for 1 min at room temperature. Slides were then washed in either a 20 mM or 100 mM Tris/HCl (pH 8) solution at room temperature for 5 or 2 × 10 min respectively and left to air dry.
Hybridization chambers were prepared with a Kimwipe® (Kimberley–Clark) and 1.5 ml hybridization buffer (900 mM NaCl, 20 mM Tris/HCl, pH 8, 0.02% sodium dodecyl sulphate (SDS), 0–30% formamide deionized (Sigma–Aldrich, St. Louis, MO, USA)) and pre-heated in a hybridization oven at 46 °C. Of each 50 ng/ml probe working solution 3.6 μl were mixed with 30 μl hybridization buffer and pipetted onto each section. Slides were put into hybridization chambers and incubated in a hybridization oven at 46 °C for 3–24 h.
Stringent washing buffer (112–900 mM NaCl, 20 mM Tris/HCl, pH 8, 5 mM EDTA, pH 8 and 0.01% SDS at 48 °C for 10 min) was used to remove unspecific binding of probes. Stringency of washing buffer was set through regulating NaCl concentrations according to the formamide concentration in the hybridization buffer (Pernthaler et al., 2001). Slides were left to air dry at room temperature. 30 μl DAPI (1 μg/ml in PBS working solution) stained each section for 3 min, and was rinsed off with PBS. Slides were coated with CitiFluor™ mounting medium (CitiFluor Ltd., Leicester, UK) and a coverslip placed over the sections.
Imaging
Slides were analyzed on a Zeiss Imager A1 Axio epifluorescence microscope illuminated with a mercury lamp and connected to a Windows based PC running the analySIS Pro (version 3.2, Soft Imaging System, Munster, Germany) software package. Overlays of FISH images were done either directly within analySIS or in Adobe Photoshop CS4 (Adobe Systems Inc., San Jose, CA, USA).
Results
Probe design
The 18S rRNA gene sequences of the three studied species, Riftia pachyptila, Tevnia jerichonana and Oasisia alvinae, all co-occurring on hydrothermal vents of the East Pacific Rise, showed a high similarity of 99.1%. However, 18 nucleotide long sequence stretches, unique to each species, were identified. A corresponding host-specific probe for O. alvinae was designed, with only one unique base pair discriminating it from previously published host-specific probes of the other two species (Pradillon et al., 2007) (Table 3).
Fluorescence in situ hybridization
A total of 116 slides for all fixation/embedding procedures were hybridized in this study, of which 36 contained sections of R. pachyptila, 49 of T. jerichonana and 31 of O. alvinae. No spectrophotometric analysis was used to determine signal intensity, but rather subjective observation of the fluorescent signal, compared to the negative controls on each slide. Notation followed a four point system of no detectable signal, weak but still detectable signal, signal of medium intensity and a signal of strong intensity.
Ethanol fixation/LR White sections
To test this fixation and embedding procedure we hybridized slides of three specimens of each host species (Riftia pachyptila # 612, # 1535-1, # 1536-1; Tevnia jerichonana # 926, # 1536-2, # 1536-4; Oasisia alvinae # 924, # 1535-2, # 1536-3). A medium to strong signal was found for all specimens of each host species (Fig. 2). Formamide concentrations and hybridization times were optimal at 10% formamide and three hours for all three host-specific probes. There was no detectable difference between proteinase K concentrations of either 0.5 μg/ml or 0.1 mg/ml in sections of R. pachyptila and T. jerichonana. On O. alvinae sections, proteinase K concentrations resulted in a range of signal intensities. High concentrations of 0.1 mg/ml showed medium signal intensities (# 924), whereas low concentrations of 0.5 μg/ml showed both weak (# 1536-3) and very strong signals (# 924) depending on the specimen hybridized.
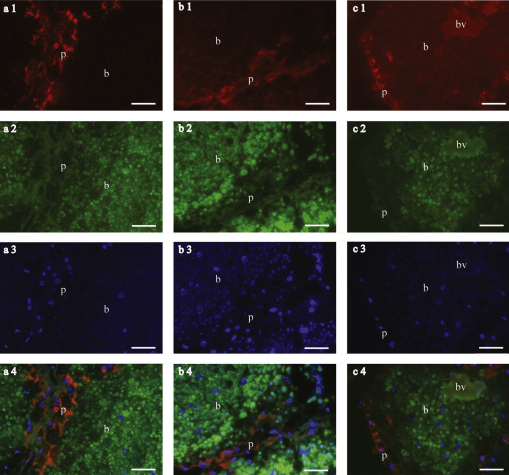
Fig. 2.
Epifluorescence images of trophosome sections showing both symbionts located in bacteriocytes (b, bacteria) and host tissue (p, peritoneum; bv, blood vessel). All specimens were conserved in ethanol and embedded in LR White. FISH was conducted with 10% formamide and 3 h hybridisation time. Host-specific probes in Cy3 of (a1) Rp1752 Riftia pachyptila (# 1536-1), (b1) Tj202 Tevnia jerichonana (# 1536-2) and (c1) Oas154 Oasisia alvinae (# 1536-3). (a2, b2 and c2) symbiont specific Probe Rif445 in Fluos, targeting bacteria found in all 3 species. (a3, b3 and c3) DAPI staining of DNA. (a4, b4 and c4) overlay of host-specific probe (red), symbiont-specific probe (green) and DAPI (blue). Scale bar = 20 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Consistency of hybridizations was tested by repeating the above procedure for each species on a second set of sections (# 612, # 926, # 924). The second run yielded similar results as the first confirming the consistency of this procedure.
Using specimens collected over a time span of seven years the effect of long storage times, in 100% ethanol, on hybridization signals was tested. Samples stored for short periods (one to two months: R. pachyptila # 1536-1, # 1536-2; T. jerichonana # 1536-2, # 1536-4; O. alvinae # 1536-2, # 1535-3) and long periods (seven years, R. pachyptila # 612; six years, T. jerichonana # 926; six years, O. alvinae # 924) showed no consistent differences in signal intensity for any of the studied species.
Both positive and negative controls using the general probe Univ1390 and the NONEUB probe showed expected hybridization signals on all slides. The symbiont-specific probe was additionally applied on all specimens of all three species and always yielded a positive signal of medium or high intensity (Fig. 2; a2, b2 and c2).
PFA fixation/cryosections
Paraformaldehyde fixed, cryosectioned specimens of all three species were hybridized (Riftia pachyptila # 1094, # 1095; Tevnia jerichonana # 1052; Oasisia alvinae # 943, # 1422, # 1214). Optimal hybridization conditions were given with 10% formamide at 3 hours incubation (Fig. 3). Signal intensity varied slightly between individuals within the three species, but also between different sections of the same individual according to proteinase K concentrations of 0.5 μg/ml or 0.1 mg/ml. All, however, resulted in positive signals. In all cases positive hybridization was noted for the probe Univ1390 and no signal for the probe NONEUB.
Fig. 3.
PFA fixed cryosectioned Riftia pachyptila specimen (# 1095). (a) Rp1752 probe in Cy3 targeting host 18S rRNA, (b) Univ1390 in Fluos (c) DAPI staining of DNA, (d) overlay of images a, b and c (b, bacteriocyte; p, peritoneum). Scale bar = 50 μm.
In addition to testing the protocol with large individuals, morphologically identified prior to hybridization, several small tubeworm specimens (Table 1), that could not be identified due to the lack of species-specific characters in their early developmental stages, were used. These were in the size range between 1200 μm and 3000 μm in length, similar to some specimens used in the study of Nussbaumer et al. (2006). Hybridization followed as above and individuals were identified as being R. pachyptila (#1063-3) and T. jerichonana (# 1067-2, # 1067-4, # 1067-6, # 1067-7, # 1080-1, # 1080-7).
PFA fixation/LR White sections
As with the previous two procedures, slides of at least three specimens per species were hybridized (Riftia pachyptila # 417, # 612, # 1094; Tevnia jerichonana # 420, # 979, # 926; Oasisia alvinae # 943, # 924, # 925). In contrast to above described treatments, positive and negative controls did not work consistently with PFA fixed, LR White embedded specimens. For both R. pachyptila and T. jerichonana, controls worked consistently for only one specimen out of the three. For the other two specimens of these species, and in all O. alvinae specimens, only between 54% and 77% of slides gave appropriate results on the control sections. Only these were used for further evaluation of this treatment.
Results of hybridization with the host-specific probe on R. pachyptila sections, in which positive and negative controls worked, were inconclusive. Host-specific signals were observed only eight out of 15 times. Out of these, 10% formamide, 19 h hybridization time and both tested proteinase K concentrations were optimal for all three specimens. Higher formamide concentrations resulted in loss of signal and shorter hybridization times resulted in weaker signals.
Hybridization of T. jerichonana with the host-specific probe was also inconclusive. In only three out of 25 slides, host-specific signals with the appropriate controls were observed. This was the case for specimens # 420 and # 979 for which 20% formamide and 19 h hybridization time resulted in a weak host-specific signal. Proteinase K concentrations made no difference in signal intensity.
Sections of O. alvinae showed no host-specific signal for any formamide concentration (0–30%), any proteinase K concentration (0.5 μg/ml or 0.1 mg/ml), and any incubation time (3–24 h) in any of the three specimens for any of the repeated runs.
Whereas host-specific probes applied on PFA-fixed, LR White embedded specimens gave unreliable results, the symbiont-specific probe always yielded positive results when it was applied. (Riftia pachyptila # 612, # 1094, Tevnia jerichonana # 979, # 926, Oasisia alvinae # 925, # 943, # 1422). At least for the symbiont, this method is applicable and results in positive signals for specimens, which have been stored in 70% ethanol after fixation, for up to eight years.
Discussion
In this study, simultaneous 16S rRNA hybridization using a symbiont-specific probe for the symbiont phylotype II, present in all three host species, and 18S rRNA host specific probes yielded positive results. This offers visualization for both host and symbiont in endosymbiotic relationships such as these (Fig. 4). Previous studies have focused on the identification of eggs and larvae or symbiont localization alone (e.g. Nussbaumer et al., 2006; Pradillon et al., 2007), the combination of both however offers a higher analytical resolution in a fast and easy to use protocol. Additionally, costs for the procedure are kept to a minimum and each specimen can be used to answer several analytical questions simultaneously.
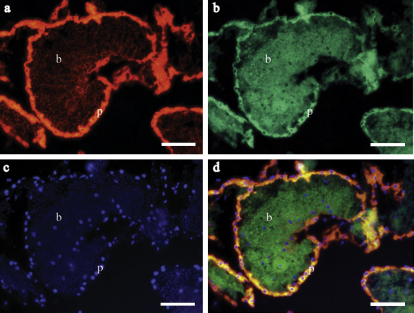
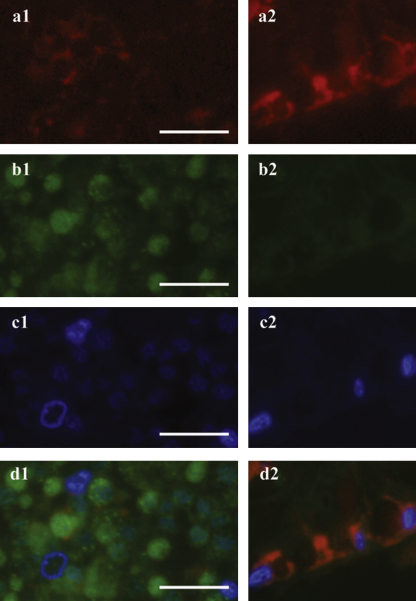
Fig. 4.
Ethanol conserved Oasisia alvinae (# 1536-3) epifluorescence images showing endosymbionts contained in bacteriocytes (a1–d1) and host peritoneum (a2–d2). Probe Rp1752 shows host tissue inside bacteriocytes (a1) and peritoneum (a2). Probe Rif445 reveals symbionts in bacteriocytes (b1). DAPI staining of both host and symbiont DNA is shown by c1 and c2. An overlay of all applied probes and DAPI is given by d1 and d2. Scale bar = 5 μm.
The three treatments in this study resulted in various signal qualities and intensities. A difference in attaining positive hybridization signals for both bacteria and eukaryotic tissue was noted for specimens within each of the three treatments. This, however, resulted in some limitations regarding the versatility of using hydrophilic resins to gain molecular information from a single sample.
Specimens treated with ethanol and embedded in LR White gave excellent results for both 18S rRNA host and 16S rRNA symbiont hybridizations with high signal intensity (Fig. 2). Simply using ethanol to conserve samples is fast, easy and inexpensive, however, overall shrinkage due to a fast dehydration process and decreased overall tissue fixation (i.e. lipids are not fixed well in ethanol) compared to, for example PFA fixation, have to be taken into account. It is thus strongly recommended to perform any size measurements or micrographs prior to conservation. Only in O. alvinae was a variation in signal intensity observed with different proteinase K treatments. High concentrations may cause soft samples to lose their target rRNA due to over permeabilization which ultimately lead to a loss of signal (Pradillon et al., 2007). We can verify this with the example of specimen # 924 for which high concentrations of 0.1 mg/ml resulted in a weaker signal than low concentrations of 0.5 μg/ml. Additionally the use of ethanol as an initial conservation medium also allows for the possibility to purify DNA and sequence 18S rRNA gene sequences of unknown species in order to design oligonucleotide probes using software packages such as ARB.
PFA fixed cryosectioned samples also yielded good host specific signals (Fig. 3), however the total signal intensity was not as strong as ethanol/LR White specimens. For fast species identification this seems ideal, however, there are several drawbacks with cryosections. The incubation in PVP takes three days, which is just as long as embedding in LR White, eliminating infiltration and polymerization work steps however. It is difficult to attain sections that show good histological detail using cryosectioning and the cutting process is coupled with a high risk of losing sample material due to frozen blocks breaking free from the fixation table making this method potentially far more destructive. Samples are not encased in a resin and therefore cannot be stored indefinitely post-sectioning. Furthermore, the structural integrity of the specimen may also be jeopardized through the freezing process in liquid nitrogen followed by subsequent thawing (Griffiths et al., 1984).
The fixation of samples with PFA followed by embedding in LR White gave unreliable results for host species identification. Paraformaldehyde cross-links free amino groups thus preserving cell structure and fixing a sample. It allows probe penetration and access to ribosomes within the cell as shown by example of cryosections. A polymer resin such as LR White also allows probe binding as shown by ethanol sections. The combination of both, PFA and LR White resin, however yielded positive signals only on a few occasions. There seems to be a restriction of probes to access ribosomes even if the cells have been permeabilized enough for the above mentioned protocols to work. Bacterial cells, however, showed positive signals for every application of the symbiont specific Rif445 probe, confirming the results of Nussbaumer et al. (2006), although much longer hybridization times to attain these signals were needed in our study.
There are two possible explanations for these differences between symbiont-specific and host-specific probe binding: (i) The rRNA content of symbionts may be higher than in hosts, therefore in the same given hybridization time more probes bind to symbiont ribosomes. (ii) The accessibility of ribosomes may be influenced by the cross-linking of amino groups during PFA fixation. Furthermore in combination with a resin this effect may be enhanced. Target molecules of prokaryotes and eukaryotes differ in length and thus in structure, which may influence the accessibility of certain regions on the target genes. A possible solution would be the application of HRP labelled probes targeting the 18S rRNA and catalysed fluorescence reporter deposition FISH (CARD-FISH) (Pernthaler et al., 2001, 2002) to attain hybridization signals 26–41 times higher than conventional fluorochrome labelled probes (Hoshino et al., 2008).
Collected specimens stretched over a time-span of seven years and no difference in signal quality was observed between these time periods. In fact the oldest specimen, R. pachyptila # 612, showed very strong signals for both host and symbiont on all tested slides. The ability to successfully conduct nucleic studies after long storage times has several advantages and specimens can be analyzed months or even years after collection.
This time frame allows for long-term projects such as colonization or succession studies in the future. As a first trial, seven morphologically unidentified, small tubeworm specimens were identified by application of the presented cryosectioning procedure. In contrast to similar studies using PCR-RFLP (Hunt et al., 2003), all tested specimens yielded positive hybridization results and could be identified successfully. Six out of seven were found to be Tevnia jerichonana, known as an early colonizer after disturbance events such as volcanic eruptions, and facilitator for the late settling Riftia pachyptila (Shank et al., 1998; Mullineaux et al., 1998, 2000), which was represented by a single specimen found from this limited data set. Considering the volcanic eruption of January 2006 in this region (Tolstoy et al., 2006) and the time of collection being only nine months post-eruption, at which point T. jerichonana tubeworm aggregations were observed, we have already yielded interesting results by use of this procedure, conforming to the findings of Shank et al. (1998) and Mullineaux et al. (2000).
In conclusion, we have described a fast, inexpensive procedure, allowing identification of individual animals to species level and offer a fluorescent visualization of both host and symbiont from a single specimen. We show that storage times of up to seven years still result in reliable hybridizations and we have shown the pros and cons of different fixation/embedding procedures. Furthermore LR White offers the potential for several further analytical methods making the use of this low viscosity resin ideal for future studies in this field.
Acknowledgments
We thank Daniela Gruber from the Department of Ultrastructure, University of Vienna, for help with Cryosectioning. We also thank Ulrich Dirks and Andrea Nussbaumer for helpful input into FISH procedures. This study was funded by the Austrian Science Foundation FWF grant numbers P20282-B17 (M.B.) and Y277-B03 (E.R.T.). Furthermore, we would like to thank the crews of the R/V Atlantis and DSV Alvin as well as the crews of the R/V L’Atalante and DSV Nautile for their skilful specimen retrieval.
References
- Amann R., Krumholz L., Stahl D. Fluorescent oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J Bacteriol. 1990;172:762–770. doi: 10.1128/jb.172.2.762-770.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boidron-Metairon I. Morphological plasticity in laboratory-reared echinoplutei of Dendraster excentricus (Eschscholtz) and Lytechinus variegatus (Lamarck) in response to food conditions. Mar Bio Ecol. 1988;119:31–41. [Google Scholar]
- Bright M., Lallier F. The biology of vestimentiferan tubeworms. Oceanogr Mar Biol Ann Rev. 2010;48:213–266. [Google Scholar]
- Feldman R.A., Black M.B., Cary C.S., Lutz R.A., Vrijenhoek R.C. Molecular phylogenetics of bacterial endosymbionts and their vestimeniferan hosts. Mol Mar Biol Biotechnol. 1997;6:268–277. [PubMed] [Google Scholar]
- Goffredi S., Jones W., Scholin C., Marin I.R., Vrijenhoek R. Molecular detection of marine invertebrate larvae. Mar Biotechnol. 2006;8:149–160. doi: 10.1007/s10126-005-5016-2. [DOI] [PubMed] [Google Scholar]
- Griffiths G., McDowall A., Back R., Dubochet J. On the preparation of cryosections for immunocytochemistry. J Ultrastruct Res. 1984;89:65–78. doi: 10.1016/s0022-5320(84)80024-6. [DOI] [PubMed] [Google Scholar]
- Gros O., Maurin L. Easy flat embedding of oriented samples in hydrophilic resin (LR White) under controlled atmosphere: application allowing both nucleic acid hybridizations (CARD-FISH) and ultrastructural observations. Acta Histochem. 2008;110:427–443. doi: 10.1016/j.acthis.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Hunt H.L., Metaxas A., Jennings R.M., Halanych K.M., Mullineaux L.S. Testing biological control of colonization by vestimentiferan tubeworms at deep-sea hydrothermal vents (East Pacific Rise, 9̊50′N) Deep-Sea Res I. 2003;51:225–234. [Google Scholar]
- Hoshino T., Yilmaz S., Noguera D.R., Daims H., Wagner M. Quantification of target molecules needed to detect microorganisms by fluorescence in situ hybridization (FISH) and catalyzed reporter deposition-FISH. Appl Environ Microb. 2008;74:5068–5077. doi: 10.1128/AEM.00208-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M.L. Riftia pachyptila Jones: observations on the vestimentiferan worm from the Galapagos Rift. Science. 1981;213:333–336. doi: 10.1126/science.213.4505.333. [DOI] [PubMed] [Google Scholar]
- Jones M.L. On the Vestimentifera, new phylum: six new species, and other taxa, from hydrothermal vents and elsewhere. Bull Biol Soc Wash. 1985;6:117–158. [Google Scholar]
- Jones M.L., Gardiner S.L. On the early development of the vestimentiferan tube worm Ridgeia sp. and observations on the nervous system and trophosome of Ridgeia sp. and Riftia pachyptila. Biol Bull. 1989;177:254–276. [Google Scholar]
- Lischewski A., Amann R., Harmsen D., Markert H., Hacker J., Morschhäuser J. Specific detection of Candida albicans and Candida tropicalis by fluorescence in situ hybridisation with an 1S rRNA-targeted oligonucleotide probe. Microbiology. 1996;142:2731–2740. doi: 10.1099/13500872-142-10-2731. [DOI] [PubMed] [Google Scholar]
- Ludwig W., Strunk O., Westram R., Richter L., Meier H., Yadhukumar ARB: a software environment for sequence data. Nucleic Acid Res. 2004;32:1363–1371. doi: 10.1093/nar/gkh293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullineaux L.S., Mills S.W., Goldman E. Recruitment variation during a pilot colonization study of hydrothermal vents (9̊50′N East Pacific Rise) Deep-Sea Res II. 1998;45:441–464. [Google Scholar]
- Mullineaux L.S., Fisher C.R., Peterson C.H., Schaeffer S.W. Tubeworm succession at hydrothermal vents: use of biogenic cues to reduce habitat selection error? Oecology. 2000;123:275–284. doi: 10.1007/s004420051014. [DOI] [PubMed] [Google Scholar]
- Nussbaumer A.D., Fisher C.R., Bright M. Horizontal endosymbiont transmission in hydrothermal vent tubeworms. Nature. 2006;441:345–348. doi: 10.1038/nature04793. [DOI] [PubMed] [Google Scholar]
- Olson R., Runstadler J., Kocher T. Whose larvae? Nature. 1991;351:357–358. doi: 10.1038/351357b0. [DOI] [PubMed] [Google Scholar]
- Pernthaler A., Preston C.M., Pernthaler J., DeLong E.F., Amann R. A Comparison of fluorescently labeled oligonucleotide and polynucleotide probes for the detection of pelagic marine bacteriaand archaea. Appl Environ Microbiol. 2002;68:661–667. doi: 10.1128/AEM.68.2.661-667.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernthaler J., Glöckner F.O., Schoenhuber W., Amann R Fluorescence in situ hybridization (FISH) with rRNA-targeted oligonucleotide probes. Methods Microbiol. 2001;30:207–226. [Google Scholar]
- Pflugfelder B., Cary C.C., Bright M. Dynamics of cell proliferation and apoptosis reflect different life strategies in hydrothermal vent and cold seep vestimentiferan tubeworms. Cell Tissue Res. 2009;337:149–165. doi: 10.1007/s00441-009-0811-0. [DOI] [PubMed] [Google Scholar]
- Pradillon F., Schmidt A., Peplies J., Dubilier N. Species identification of marine invertebrate early stages by whole-larvae in situ hybridisation of 18S ribosomal RNA. Mar Ecol Prog Ser. 2007;333:103–116. [Google Scholar]
- Robidart J.C., Bench S.R., Feldman R.A., Novoradovsky A., Podell S.B., Gaasterland T. Metabolic versatility of the Riftia pachyptila endosymbiont revealed through metagenomics. Environ Microbiol. 2008;10:727–737. doi: 10.1111/j.1462-2920.2007.01496.x. [DOI] [PubMed] [Google Scholar]
- Shank T.M., Fornari D.J., Von Damm K.L., Lilley M.D., Haymon R.M., Lutz R.A. Temporal and spatial patterns of biological community development at nascent deep-sea hydrothermal vents (9̊50′N East Pacific Rise) Deep-Sea Res II. 1998;45:465–515. [Google Scholar]
- Shirley S., Shirley T., Rice S. Latitudinal variation in the Dungeness crab Cancer magister: zoeal morphology explained by incubation temperature. Mar Biol. 1987;95:371–376. [Google Scholar]
- Simon N., LeBot N., Marie D., Partensky F., Vaulot D. Fluorescence in situ hybridization with rRNA-targeted oligonucleotide probes to identify small phytoplankton by flow cytometry. Appl Environm Microbiol. 1995;61:2506–2513. doi: 10.1128/aem.61.7.2506-2513.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southward E.C., Tunnicliffe V., Black M. Revision of the species of Ridgeia from northeast Pacific hydrothermal vents, with a redescription of Ridgeia picesae Jones (Pogonophora: Obturata = Vestimentifera) Can J Zool. 1995;73:282–295. [Google Scholar]
- Strathman R.R., Fenaux L., Strathmann M.F. Heterochronic developmental plasticity in larval sea urchins and its implications for evolution of non feeding larvae. Evolution. 1992;46:972–986. doi: 10.1111/j.1558-5646.1992.tb00613.x. [DOI] [PubMed] [Google Scholar]
- Tolstoy M., Cowen J.P., Baker E.T., Fornari D.J., Rubin K.H., Shank T.M. A seafloor spreading event captured by seismometers. Science. 2006;314:1920–1922. doi: 10.1126/science.1133950. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. Immunochemistry on ultrathin frozen sections. Histochem J. 1980;12:381–403. doi: 10.1007/BF01011956. [DOI] [PubMed] [Google Scholar]
- Wallner G., Amann R., Beisker W. Optimizing fluorescent in situ hybridization with rRNA-targeted oligonucleotide probes for flow cytometric identification of microorganisms. Cytometry. 1993;14:136–143. doi: 10.1002/cyto.990140205. [DOI] [PubMed] [Google Scholar]
- Zheng D., Alm E.W., Stahl D.A., Raskin L. Characterization of universal small-subunit rRNA hybridization probes for quantitative molecular microbial ecology studies. Appl Environ Microbiol. 1996;62:4504–4513. doi: 10.1128/aem.62.12.4504-4513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]