Abstract
p53 is a crucial tumor suppressor that is mutated or deleted in a majority of cancers. Exactly how p53 prevents tumor progression has proved elusive for many years; however, this information is crucial to define targets for chemotherapeutic development that can effectively restore p53 function. Bioactive sphingolipids have recently emerged as important regulators of proliferative, apoptotic and senescent cellular processes. In this study, we demonstrate that the enzyme sphingosine kinase 1 (SK1), a critical enzyme in the regulation of the key bioactive sphingolipids ceramide, sphingosine and sphingosine-1-phosphate (S1P), serves as a key downstream target for p53 action. Our results show that SK1 is proteolysed in response to genotoxic stress in a p53-dependent manner. p53 null mice display elevation of SK1 levels and a tumor-promoting dysregulation of bioactive sphingolipids in which the anti-growth sphingolipid ceramide is decreased and the pro-growth sphingolipid S1P is increased. Importantly, deletion of SK1 in p53 null mice completely abrogated thymic lymphomas in these mice and prolonged their life span by ~30%. Deletion of SK1 also significantly attenuated the formation of other cancers in p53 heterozygote mice. The mechanism of p53 tumor suppression by loss of SK1 is mediated by elevations of sphingosine and ceramide, which in turn were accompanied by increased expression of cell cycle inhibitors and tumor cell senescence. Thus, targeting SK1 may restore sphingolipid homeostasis in p53-dependent tumors and provide insights into novel therapeutic approaches to cancer.
Keywords: Ceramide, sphingosine-1-phosphate, p53, sphingosine kinase, cancer, senescence
Introduction
Cancer is a complex process in which aberrant growth signals lead to the expansion of damaged cells that would normally not proliferate. Moreover, discerning the intricate molecular mechanisms that lead to tumor growth should enable the development of more effective cancer therapies that target them (Kang et al., 2005; Lees and Weinberg, 1999). One class of molecules that have emerged as critical regulators of several cellular processes of direct relevance to cancer, including growth, differentiation, apoptosis, and senescence are the bioactive sphingolipids (Hannun and Obeid, 2008; Pruett et al., 2008; Pyne and Pyne, 2010). Dysregulation of sphingolipid metabolic pathways has been demonstrated in several cancers, suggesting that alterations in sphingolipid metabolism may represent an important step in tumorigenesis (Cuvillier and Levade, 2003; Long et al., 2010; Ryland et al., 2011; Watson et al., 2010).
SK1 holds a crucial position in sphingolipid metabolism with its function required for homeostatic processing of the pro-apoptotic sphingolipids, ceramide and sphingosine. Moreover, the product of SK1, sphingosine-1-phosphate (S1P), is not only a metabolic intermediate required for exit from the sphingolipid metabolic pathway, but has been shown to act as a pro-growth signal in stark contrast to the functions of its upstream sphingolipid family members (Pitson, 2010). Thus, it appears that SK1 acts as a balance between pro-death and pro-growth bioactive signaling lipids and, intuitively, alterations in this enzyme’s activity could be involved in carcinogenesis. Indeed, previous work from our lab showed that SK1 message levels are universally increased in various types of human cancers (Johnson et al., 2005). Furthermore, multiple independent studies have shown that the sphingosine kinase 1 (SK1)/sphingosine-1-phosphate (S1P) pathway plays a role in cancer cell growth and viability and in the resistance of tumors to chemotherapy (Akao et al., 2006; Bayerl et al., 2008; Li et al., 2009; Long et al., 2010; Pchejetski et al., 2008; Watson et al., 2010).
Similar oncogenic properties have long been attributed to the loss of the tumor suppressor p53 (also known as TP53), which has been the most studied tumor suppressor protein due to its involvement in more than 50% of human cancers (Soussi, 2007; Vogelstein B, 2000; Weisz et al., 2007). Since alteration of p53 signaling has been recognized as a central component of human carcinogenesis, nearly 20 different types of genetically modified mice targeting p53 in various ways have been made over the past twenty years to aid in the study of this medically relevant tumor suppressor (Soussi, 2007; Zilfou and Lowe, 2009). The most common phenotype observed in mice which lack both p53 alleles is decreased survival due to their development of thymic lymphoma, whereas in mice which lack only one p53 allele, osteosarcomas and soft tissue sarcomas predominate, and animal survival is not as dramatically decreased as in complete knockout (KO) animals (Donehower et al., 1992; Jacks et al., 1994; Lozano). Thus, p53 deficient mice can serve as an appropriate in vivo model for the study of carcinogenesis in multiple tissues.
Interestingly, some studies have suggested a link between the p53 tumor suppressor protein and bioactive sphingolipids, especially ceramide and sphingosine 1-phosphate (S1P) (Lopez-Marure et al., 2000; Oskouian et al., 2006; Pruschy et al., 1999) as reviewed in (Heffernan-Stroud and Obeid, In press). Genotoxic stress has been shown to induce the pro-apoptotic sphingolipid, ceramide in a p53-dependent manner (Dbaibo et al., 1998), and, indeed, several inducers of apoptosis have been shown to increase ceramide in cells concomitant with p53 activation (El-Assaad et al., 2003; Kim et al., 2002; Sawada et al., 2004). Moreover, we found that treatment of Molt-4 T-cell leukemia cells with the DNA damaging agent Actinomycin D results in degradation of SK1, the key enzyme that regulates the balance between ceramides, sphingosine, and S1P, and these effects were not observed in cells overexpressing the papilloma virus E6 protein which targets p53 to degradation, suggesting a role for p53 in this process (Taha et al., 2004). Furthermore, Oskouian et al. showed that overexpression of the enzyme that breaks down S1P, S1P lyase, can potentiate an apoptotic response to DNA damage in a p53-dependent manner (Oskouian et al., 2006). These studies suggest that some of the enzymes controlling ceramide and S1P levels in cells may act as effectors/modulators of the p53 tumor suppressor pathway; however, the mechanisms and significance of these relations to sphingolipids are not known.
Because SK1 message levels are universally increased in various types of cancers (Johnson et al., 2005) and p53 may regulate SK1 in response to genotoxic stress in leukemia cells, we hypothesized that dysregulation of the SK1/S1P pathway could be involved in p53-altered cancers. To test this hypothesis, we evaluated the p53-dependent regulation of SK1 in mouse non-cancer cells as well as SK1 expression in p53 null tumors. Next we evaluated the effect of genetic ablation of SK1 in p53 knockout (KO) mice on spontaneous tumor development and survival to determine if restoration of the sphingolipid profile could offer protective tumor cell senescence. Finally, we expanded these studies to include other tissue/tumor types by crossing SK1 knockout (KO) mice with p53 heterozygote mice and assessing the effects of SK1 gene dosage on tumorigenesis and survival.
Results
p53 negatively regulates SK1
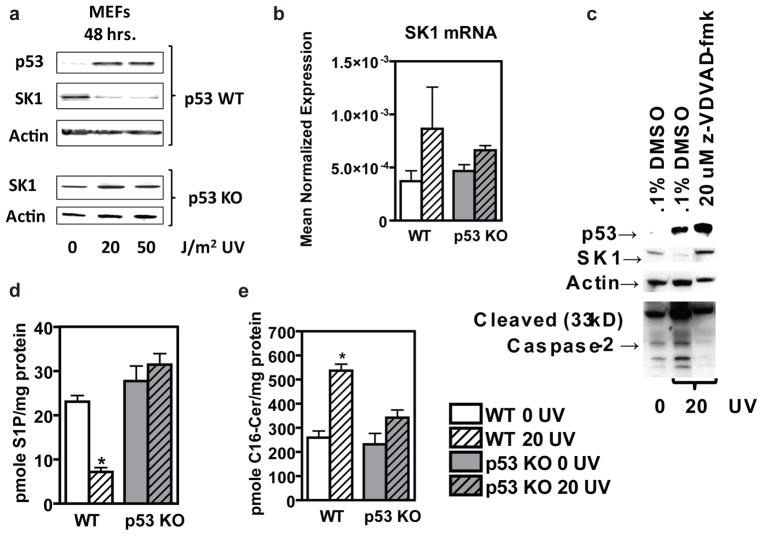
First, we set out to investigate a mechanistic connection among genotoxic stress, p53, and SK1. To this end, we utilized mouse embryonic fibroblasts (MEFs) from WT and p53 KO mice and evaluated the effects of UV irradiation on SK1. We found that induction of p53 was required for a reduction in SK1 protein to occur following genotoxic stress (Figure 1a), thus, establishing p53 as an upstream regulator of sphingolipid metabolism. These studies also revealed that p53-dependent downregulation of SK1 was occurring at the protein, rather than message level (Figure 1a,b). Additionally, we were able to inhibit the effects of p53-mediated genotoxic stress on SK1 with a caspase-2 inhibitor, suggesting proteolytic degradation as the mechanism of p53-dependent SK1 regulation (Figure 1c) as is consistent with previous studies suggesting regulation of SK1 through proteolysis (Loveridge et al., 2010; Taha et al., 2005). This finding is also in keeping with several reports showing p53-dependent activation of caspase-2 (Baptiste-Okoh et al., 2008; Cuenin et al., 2008; Ho et al., 2009; Vakifahmetoglu et al., 2008). We found that genotoxic stress led to p53-dependent downregulation of SK1, which, in turn, decreased the pro-growth signaling product of SK1, S1P (Figure 1d). Importantly, this downregulation of SK1 also led to the accumulation of the upstream, anti-growth signaling sphingolipid, ceramide (Figure 1d). This regulation of SK1 and these potentially tumor-suppressive changes in sphingolipid signaling molecules required p53 (Figure 1d), and could constitute an important component of the p53 tumor suppressor pathway.
Figure 1.
Primary mouse cell lines respond to genotoxic stress through p53-dependent, caspase-2 mediated proteolysis of SK1 to alter the balance of pro- and anti-growth sphingolipids. (a) Mouse embryonic fibroblasts (MEFs) expressing WT p53 or with p53 knocked out, were treated with indicated doses of UV radiation (J/m2), cultured for 48 hours, and then whole cell lysate was examined by (a) Western blotting with antibodies to the indicated proteins, or, (b) harvested in RLT buffer, prepared for qRT-PCR, with enzyme expression normalized to β-actin expression for each reaction performed in triplicate, or, (c) WT MEFs were pre-treated with 20 uM z-VDVAD-fmk (caspase-2 inhibitor) dissolved in DMSO or DMSO control in fresh media and incubated for one hour prior to treatment with 0 or 20 J/m2 UV radiation, incubated for 48 hours before being harvested by scraping on ice using Tris-Triton lysis buffer supplemented with protease and phosphatase inhibitors from Sigma for western blotting with antibodies to the indicated proteins (n=3), or (d) harvested for sphingolipidomic analysis to measure S1P and C16-ceramide by LC/MS, expressed as pmol lipid per mg protein ± SEM (n=3, and *p < 0.05 by Student’s t test relative to WT 0 UV).
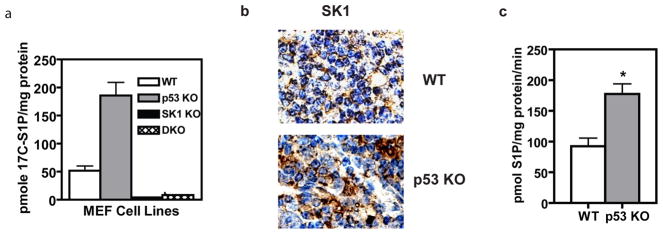
In light of these results, we hypothesized that cells lacking p53 would lack proper regulation of SK1. Indeed, treating WT, p53 KO, SK1 KO, and DKO MEFs with labeled substrate for SK1, C17-sphingosine, showed increased production of C17-S1P in the p53 KO cells (Figure 2a). These studies also confirmed that the main enzyme responsible for S1P production in these cells is SK1 rather than SK2, since very little S1P was produced in cells lacking SK1 (Figure 2a). Next, we investigated whether p53 was required for the regulation of SK1 in vivo. Because p53 KO mice develop thymic lymphomas (Donehower et al., 1992; Jacks et al., 1994), we compared SK1 expression and activity in the thymus glands of WT and p53 KO mice at 60 days of age, a time during which there is negative selection and significant cell turnover in the developing murine thymus. Consistent with our cellular studies, we observed no differences in SK1 message levels between WT and p53 KO thymus glands (Supplementary Figure 1). In contrast, using immunohistochemistry, we observed more intense staining for SK1 protein in tissues lacking regulatory p53 when compared to those with WT p53 (Figure 2b). In addition, SK1 activity was significantly increased in tissues lacking p53 (Figure 2c), demonstrating that SK1 is deregulated which can lead to changes in sphingolipid metabolism that signal for tumor-promotion in the thymic tissue of p53 KO mice.
Figure 2.
SK1 activity is increased in the absence of its regulator, p53. (a) MEFs (passage 4–7) of the indicated genotype were treated with 1 nM 17C-labeled sphingosine and following a 30 minute incubation, cells were harvested for sphingolipidomic analysis and 17C-containing S1P was normalized to the amount of protein submitted for each sample (n=4 and p< 0.01 by One-way ANOVA with Dunnett’s post test). (b) Immunohistochemistry for SK1 on formalin-fixed thymic sections from WT or p53 KO mice (pictures representative of n=3). (c) Homogenates of WT thymus and p53 KO thymic lymphoma from 4 month-old mice were incubated with sphingosine and 32P labeled ATP to determine SK1 activity (n=3, and *p < 0.05 by Student’s t test).
Spontaneous tumor formation in p53 KO mice requires SK1
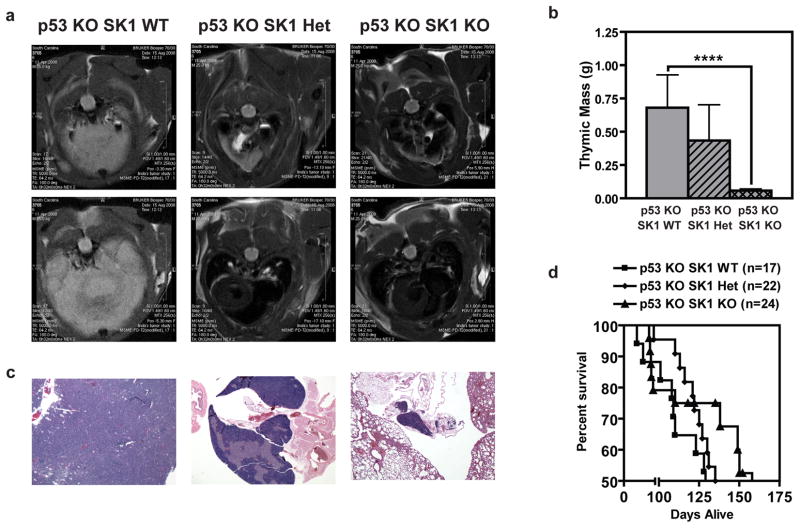
Next, we investigated the functional consequences of p53 effects on SK1. Ceramide exerts apoptotic and growth-suppressive effects (Jayadev et al., 1995; Kolesnick and Fuks, 2003; Obeid et al., 1993), whereas S1P exerts opposing anti-apoptotic and pro-growth effects (Lee et al., 1998; Olivera et al., 1999; Olivera and Spiegel, 1993); indeed, ceramide has been proposed as a tumor suppressor lipid and S1P as a tumor-promoting lipid (Cuvillier et al., 1996; Hannun and Obeid, 2008). Therefore, to determine if SK1 deregulation in p53 KO mice contributes to tumor development, we crossed p53 KO mice (Jacks et al., 1994) with SK1 KO mice (Allende et al., 2004) to ascertain whether enforced SK1 downregulation was protective against carcinogenesis. We initially focused on thymic lymphoma as the major and most consistent tumor in p53 KO mice (Donehower et al., 1992; Jacks et al., 1994). The results revealed a significant and gene-dosage-dependent effect in which tumor burden was decreased in p53 KO mice lacking a single SK1 allele. Remarkably, p53 KO mice lacking both SK1 alleles were almost totally protected from thymic lymphoma development, compared to p53 KO mice expressing WT SK1 (Figure 3a). In necropsy studies, thymic mass decreased with loss of SK1 expression in p53 KO mice (Figure 3b), and histological evaluation confirmed protection from thymic lymphoma in the DKO mice (Figure 3c). Such protection from carcinogenesis in the DKO mice led to increases in median survival by more than 36%, compared to p53 KO mice expressing both SK1 alleles (Figure 3d). These results suggest that deregulation of SK1 in p53 KO mice may contribute to early tumor development and that SK1 knockout could protect these mice from thymic lymphoma development thereby increasing survival.
Figure 3.
KO of SK1 protects p53 KO mice from thymic lymphoma. (a) Representative MRI of chest cavity from a mouse of each genotype. Post-imaging, necropsy, and histology confirmed that the large white mass observed was thymic lymphoma, the characteristic lesion observed in p53 KO mice, with a smaller mass observed in SK1 Het mice and a normal thymus and clear chest cavity in the DKO mouse. (b) Weight of thymi dissected from male mice immediately after euthanization at 120 days-of-age. Data are averages of thymic weight from 5–9 mice of each genotype ± SEM. Asterisks indicate statistically significant differences (P < 0.02 by Student’s t test). (c) H & E stain of thymi with histology representative of what was found for each genotype. (d) Kaplan-Meier survival analysis of p53 KO mice with different SK1 genotypes. Each point represents a mouse that died naturally or was sacrificed due to tumor burden or unthriftiness, whereas the n listed includes mice that are still living, all of which contribute to the survival estimate curve. Differences between the survival curves are statistically significant (by log-rank comparison P < 0.04).
Decreased tumor burden in p53-SK1 double KO mice via induction of cellular senescence
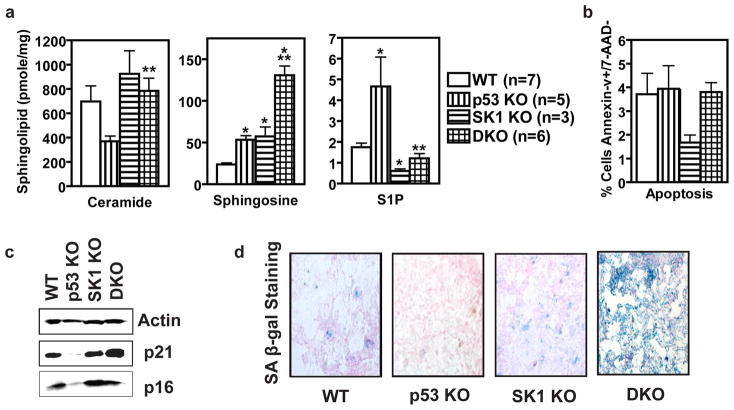
To investigate how knockout of SK1 protects p53 KO mice from the development of thymic lymphoma, we first measured sphingolipids in thymus glands from mice with varied p53 and SK1 expression including p53 WT SK1 WT (WT), p53KO SK1 WT (p53 KO), p53 WT SK1 KO (SK1 KO), and p53 KO SK1 KO (DKO). Consistent with deregulated SK1 activity (Figure 2c), thymic tissue from p53 KO mice had decreased upstream sphingolipid ceramide and increased S1P, the product of SK1 (Figure 4a). The p53 KO thymus glands also had increased sphingosine (Figure 4a), which could indicate decreased activity of a ceramide synthase, but most likely indicates increased ceramide breakdown by a ceramidase (Supplementary Figure 1). As expected, thymus glands from SK1 KO mice contained significantly less S1P and more sphingosine than those from WT mice (Figure 4a). Of note, knockout of SK1 suppressed the pro-growth signaling sphingolipid, S1P, in the p53 KO mice to that of WT levels (Figure 4a). Likewise, knockout of SK1 also restored the levels of the anti-growth ceramide to WT levels in the thymic tissue of DKO mice (Figure 4a), demonstrating a role for SK1 in maintaining ceramide homeostasis. Taken together, these results demonstrate significant effects of p53 (and its loss) on the levels of bioactive sphingolipids. The results also implicate SK1 in mediating these changes.
Figure 4.
Loss of SK1 leads to tumor cell senescence in p53 KO thymus. (a) Thymi were harvested from mice of the indicated genotypes, homogenized, and submitted to lipid analysis. C16-ceramide, sphingosine, and S1P were measured by LC/MS. Data are averages of lipid levels determined for thymi from three or more mice and are expressed as pmol lipid per mg protein ± SEM. Asterisks indicate statistically significant differences (P < 0.05 by Student’s t test relative to WT, *, or DKO relative to p53 KO, **). (b) Thymi were harvested from mice of the indicated genotypes, processed into a single cell suspension, stained and analyzed via flow cytometry. Data are averages ± SEM of the percentages of Annexin-V positive, 7-AAD negative-staining cells in thymuses from three or more mice. (c) Protein markers of cell cycle arrest and senescence. Thymi from mice of the indicated genotypes were homogenized, and equal amounts were examined by Western blotting. (d) Senescence associated β-gal staining of thymuses from age-matched mice of the indicated genotypes.
Similar changes were also observed for sphingolipids in the blood of these mice (Supplementary Figure 2). Thus, knockout of SK1 seemed to function to re-balance these two bioactive sphingolipids to WT levels in p53 KO tissues. However, sphingosine was significantly increased in DKO tissue compared to either KO mouse alone (Figure 4a middle panel). Again these changes are consistent with decreased phosphorylation of sphingosine with knockout of SK1, but also suggest an increase in ceramide catabolism with knockout of p53. Overall, our data suggest that knockout of SK1 protects p53 KO mice from tumor development by restoring dysregulated ceramide and S1P in these tissues to those of WT levels.
To determine the pathophysiologic significance of bioactive sphingolipid dysregulation, we analyzed thymic tissue from mice of the four genotypes for apoptosis and senescence, functions known to be reciprocally regulated by ceramide and S1P (Hannun and Obeid, 2008; Taha et al., 2006). First, we suspected that a decrease in ceramide and the increase in S1P in p53 KO thymus glands may predispose these mice to carcinogenesis as a result of an inadequate apoptotic response. However, we did not find a significant difference between genotypes in the percentage of apoptotic cells present in the thymus glands (Figure 4b). When split into T-cell subtypes, p53 KO thymi had decreased apoptosis of double-positive T cells, but this was offset by increased apoptosis of double-negative and single-positive CD8+ cells (Supplementary Figure 3). However, such differences in apoptosis among the cell subtypes appeared as minor differences in overall cell population between genotypes rather than differential susceptibility to apoptosis (Supplementary Figure 3). Therefore, we concluded that a decrease in apoptosis was not responsible for the increased thymus size observed in p53 KO mice. This conclusion is further supported by an elegant study in which an apoptosis-deficient p53 mutant mouse that retained p53-dependent cell cycle arrest and senescence was still protected from thymic lymphoma development, thus ruling out a role for apoptosis in the tumor suppressive action of p53 (Liu et al., 2004). In contrast, evaluation of expression of p16 and p21, regulators of cell cycle arrest and senescence (Alesse et al., 1998; Bringold and Serrano, 2000; el-Deiry et al., 1994; Lukas et al., 1995) revealed differential expression of p21 and p16 in the thymi of the various genotypes (Figure 4c). These proteins are known to be regulated by sphingolipids such that ceramide induces their expression (Lee et al., 2000; Venable and Obeid, 1999). Quantitatively, levels of p21, and to a slightly lesser extent p16, closely correlated with the corresponding changes in both ceramide and S1P observed in these tissues (Supplementary Figure 4), thus, expression of p16 and p21 was decreased in the p53 KO, increased in the SK1 KO, and reverted to near WT levels in the DKO model. These results suggest that the predominant effect of SK1 loss is to restore ceramide and S1P levels and consequently the expression of p16 and p21, which are suppressed in tissues lacking p53.
Because both p16 and p21 are important mediators of cell senescence (Jung et al.), we next evaluated thymus senescence. The results showed that staining for senescence-associated β-galactosidase was profoundly more intense in the DKO tissues than what was observed in the p53 KO tissues (Figure 4d). These results clearly suggest that induction of senescence is a major response of the DKO cells. Interestingly, the β-galactosidase staining correlated best with the levels of sphingosine (Figure 4a), which has previously been associated with senescence pathways (Chao et al., 1992; Debacq-Chainiaux et al., 2010; Yoon et al., 2009). Collectively, these results suggest that by correcting ceramide and S1P levels, and possibly increasing sphingosine, knockout of SK1 rectifies the expression of cell cycle inhibitors and induces a strong cellular senescence response to prevent tumor development in p53 KO tissues.
Multiple p53-dependent tumor types are reliant on SK1
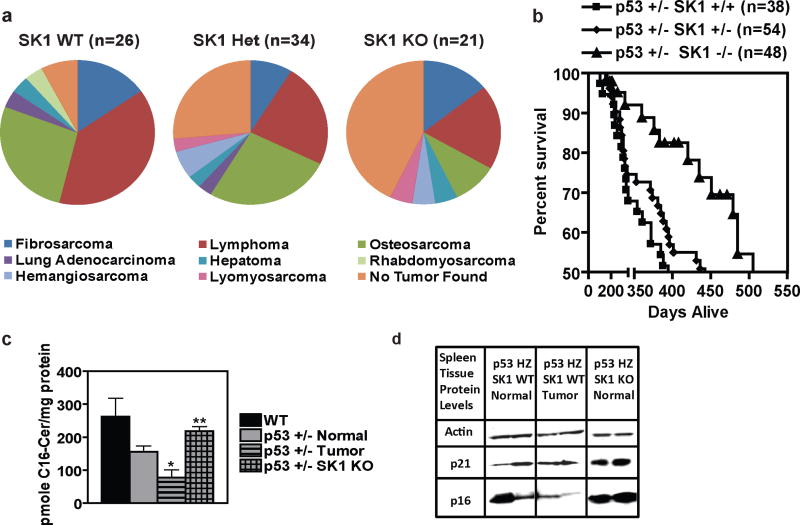
Next, we wanted to investigate whether the protective effect of SK1 KO extended to other p53-dependent tumor types in addition to thymic lymphoma. Unlike the aggressive thymic lymphomas observed in p53 KO mice, p53 heterozygote mice tend to develop sarcomas and splenic lymphomas and survive longer than p53 KO mice (Jacks et al., 1994). Therefore, we crossed p53 heterozygote mice with SK1 KO mice to create p53 +/− SK1 +/+, p53 +/− SK1 +/−, and p53 +/− SK1 −/− mice and monitored them for tumor development. The results showed that decreased expression of SK1 protected against tumor development in p53 heterozygote mice (Figure 5a), with knockout of SK1 increasing the percent of p53 heterozygote mice surviving tumor-free to 42.9% (compared to 7.7% for those expressing both SK1 alleles). Knockout of SK1 offered substantial protection from the development of lymphoma, osteosarcoma, lung adenocarcinoma, and rhabdomyosarcoma in the p53 heterozygote mice (Figure 5a). Importantly, this decreased tumor burden observed in p53 heterozygote mice lacking SK1 resulted in a 27% increase in their median survival when compared to those with WT SK1 (Figure 5b). These results indicate a more generalized role for SK1 in p53-induced carcinogenesis that applies to several tissues and types of tumors.
Figure 5.
KO of SK1 protects p53 heterozygote mice from tumor development. (a) Pie chart representation of tumor burden found during necropsy and confirmed via histology of p53 heterozygote mice with the indicated SK1 genotype. (b) Kaplan-Meier survival analysis of p53 heterozygote mice with different SK1 genotypes. Each point represents a mouse that died naturally or was sacrificed due to tumor burden or unthriftiness, whereas the n listed includes mice that are still living, all of which contribute to the survival estimate curve. Differences between the survival curves are statistically significant (by log-rank comparison P < 0.02). (c) Spleens were harvested from mice of the indicated genotypes and phenotypes, homogenized, and submitted to lipid analysis. C16-ceramide was measured by LC/MS. Data are averages of lipid levels determined for thymuses from three mice and are expressed as pmole lipid per mg protein ± SEM. Asterisk indicates a statistically significant difference (P < 0.05 by Student’s t test relative to the other groups). (d) Protein markers of cell cycle arrest and senescence. Spleens from mice of the indicated genotypes and phenotypes were homogenized, and equal amounts were examined by Western blotting.
Interestingly, analysis of spleens from WT mice and p53 +/− SK1 WT mice with and without tumors, and spleens from p53 +/− SK1 KO mice revealed that ceramide levels best matched the expression of cell cycle inhibitors. Whereas normal spleens from p53 heterozygous mice contained less ceramide than WT spleens, this decrease was more profound in tumor tissues from afflicted spleens in the p53 heterozygous mice (Figure 5c). Importantly, knockout of SK1 in p53 heterozygote mice restored ceramide to near WT levels (Figure 5c). These differences in ceramide, in turn, closely matched the expression of the cell cycle inhibitors p21 and p16 (Figure 5d), suggesting that knockout of SK1 protects p53 heterozygote mice from tumors through a mechanism similar to that within p53 KO mice. These data suggest that restoring the downregulation of SK1, as seen with WT p53, can, in turn, restore ceramide and cell cycle inhibitor expression to protect p53-deficient mice from the development of various types of tumors, including osteosarcoma and splenic lymphoma as well as thymic lymphoma.
Discussion
This work has identified a novel connection between the p53 pathway and its regulation of sphingolipid metabolism. The results show that p53, through its activation of caspase-2, negatively regulates SK1 in response to genotoxic stress, and that loss of p53 results in significant changes in bioactive sphingolipids through SK1 leading to increased S1P levels and decreased ceramide. Functionally, knockout of SK1 significantly increased survival and decreased tumor burden in both p53 KO and p53 heterozygote mice, demonstrating that p53-dependent regulation of SK1 is essential for its tumor suppressive effects. This improved survival in p53-deficient mice with knockout of SK1 was associated with restoration of sphingolipid homeostasis (decreased S1P, increased ceramide) and induction of cellular senescence in the thymuses of these mice.
There is an evolving body of literature supporting a role for SK1 in cancer pathogenesis (Bergelin et al., 2009; Pitson et al., 2005; Vadas et al., 2008; Xia et al., 2000). SK1 message level is increased in several tumor types (Akao et al., 2006; Bayerl et al., 2008; Johnson et al., 2005; Kawamori et al., 2006), and knock down of SK1 was shown to be protective from the development of colon cancer in an experimental model of azoxymethane-induced colon carcinogenesis (Kawamori et al., 2009). Moreover, it has also been suggested that knockdown of SK2 can slow the growth of breast cancer xenografts through an immune-mediated process (Weigert et al., 2009). Therefore several groups are currently working on the production and characterization of selective SK1 and SK2 inhibitors so that their specific roles in anti-cancer therapies may be better understood (Hengst et al., 2010; Mathews et al., 2010). Recent studies investigating the application of SK1 inhibitors as anti-cancer agents have proved promising (Paugh et al., 2008). Although not yet commercially available, the same group found that treatment with their SK1 inhibitor could slow the growth of glioblastoma xenografts through the inhibition of Akt (Kapitonov et al., 2009). Interestingly, some recently developed SK1 inhibitors work through proteasomal degradation of SK1 (Loveridge et al., 2010), further supporting that proteolysis may be an important mechanism of SK1 regulation as suggested by our study. As selective inhibitors are developed and made commercially available, their in vivo optimization will allow for investigation as to whether the tumor-protective effect of SK1 knockout found in this study can in fact be mimicked pharmacologically.
Finally, in this report, we uncover tumor cell senescence as a novel mechanism by which loss of SK1 is protective from tumor development. Moreover, the associated changes in sphingolipid profile and protein expression could mechanistically explain the increased proliferation in p53 KO tumors and the protective senescence response in DKO mice. The results clearly demonstrate significant derangement in ceramide, sphingosine, and S1P levels in p53 knock out cells and tissues and in p53 KO-induced tumors. These changes occur in directions known to promote carcinogenesis (decreased ceramide and increased S1P) (Hannun and Obeid, 2008; Ogretmen and Hannun, 2004; Taha et al., 2006). Together with previous studies demonstrating the p53-dependent effects on ceramide in human leukemia and neuroblastoma cell lines as well as in mouse fibroblasts (Dbaibo et al., 1998; Kim et al., 2002), our data suggest that the major effect of p53 on sphingolipid metabolism is to induce proteolysis of SK1 (Taha et al., 2004), which subsequently leads to ceramide accumulation and loss of S1P. Because SK1 knockout could potentially be mimicked by treatment with a small molecule inhibitor, this method of inducing tumor cell senescence may offer a promising therapeutic avenue for patients with altered p53 status, which has proved to be an elusive aspect of cancer for many years.
Materials and Methods
Reagents
Actinomycin D and Etoposide were purchased from Sigma. For western blots (specific procedures detailed in SI Materials and Methods) we used complete-mini protease inhibitor tablets (Roche, Indianapolis, IN, USA), ripa buffer (Boston Bioproducts, Ashland, MA, USA), BCA protein assay (Pierce, Rockford, IL, USA), and precast 10% Tris-HCl gels (BioRad, Hercules, CA, USA). SA-β-galactosidase staining kits were purchased from Cell Signaling (Danvers, MA, USA). SK1 primary antibodies were custom made for our laboratory either at MUSC (human) or by BioSource/Fisher (mouse). Other primary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). HRP-conjugated secondary antibodies were obtained from Jackson Labs (Bar Harbor, ME, USA), except for rabbit secondary which was purchased from Santa Cruz Biotechnology.
Cell Culture
Following harvest, mouse embryonic fibroblasts (MEFs) were maintained in Dulbecco’s Modified Eagles Medium (DMEM) containing high glucose (Gibco), supplemented with L-glutamine, 10% (v/v) fetal bovine serum (Gibco or Hyclone) and 1% (v/v) antimycotic-antibiotic solution (Gibco). Cells were kept in a humidified incubator at 37 °C with 5% CO2 Where indicated, UV irradiation of cells was performed using a Biorad GS Gene Linker cabinet that emits UVC light (λ = 253.7 nm).
Animals
Under our protocol, ARC# 2690, which was approved by the MUSC IACUC, we created a breeding colony of C57BL/6 mice with varying p53 and SK1 status. The SK1 KO mice were originally obtained from Dr. Rick Proia (Allende et al., 2004) crossbred with WT C57BL/6 mice in the DLAR at MUSC and maintained as an SK1 KO colony. Two male p53 heterozygote mice were obtained from the laboratory of Dr. C. Schweinfest and bred as previously described (Jacks et al., 1994) and additional p53 heterozygote breeders were purchased from Jackson Labs (stock number 002101). Genotyping of mice was performed using a Qiagen (Valencia, CA, USA) DNAeasy Blood and Tissue kit and specific primers from IDT (Coralville, IA, USA. Mating pairs of p53 +/− SK1+/− or WT or SK1 KO mice were set up and MEFs were harvested and genotyped between embryonic day 12.5–14.5 of gestation. Further breeding and genotyping information and MEF collection details are provided in the SI
Assessing Survival
Mice were monitored daily for tumor development and general health by an unbiased caretaker. Mice that appeared to be suffering or in pain, unable to eat, or immobile were sacrificed and necropsied. Survival was measured by the number of days between each mouse’s date of birth and date of death, or date of sacrifice due to humane reasons. Runts and mice with genetic issues common to the C57BL/6 background, namely blindness and malocclusion, were sacrificed and not included in the survival study.
Assessing Tumor Burden
At 4 months-of-age, p53 KO mice harboring each possible SK1 genotype: WT, HZ, or KO, were sacrificed and necropsied with the assistance of a veterinary pathologist (KLH). Each organ was inspected and weighed, and then representative sections were fixed for histological evaluation. Thymic mass was the primary indicator of tumor burden in the p53 KO mice, whereas, p53 HZ mice suffered a more diverse range of tumors with more varied latency. Thus, p53 HZ mice were sacrificed and necropsied at different ages due to individual unthriftiness, or they were necropsied after they were found dead to determine tumor burden. Each organ was inspected and any abnormal tissues or masses were fixed for histological evaluation by KLH.
Animal Imaging
Micro-magnetic resonance imaging of mice was performed by Dr. P. Chou under the guidance of Dr. B. Memet in the MUSC Animal Imaging Core Facility with a 7 T MR Bruker Biospec USR (Bruker Inc., Billerica, MA, USA) actively shielded scanner with a 30-cm horizontal bore and Paravision software.
Tissue Homogenization
Following tissue collection as described in SI Materials and Methods, tissues were minced with dissecting scissors in 350 μL of SK1 lysis buffer or RLT buffer (Qiagen) in a 2-mL microcentrifuge tube before being homogenized on ice with a handheld Tissue Tearor (Biospec, Bartlesville, OK, USA) set at level 25 for 1 min. Samples were then transferred to a clean microcentrifuge tube and sonicated for 10 sec to ensure homogeneity before being aliquoted for protein or other analysis.
Immunohistochemistry
Formalin-fixed, paraffin-embedded sections were hydrated through histoclear and an ethanol gradient, slides were boiled in citrate buffer for antigen retrieval, and they were immersed in 10% H2O2 in methanol to quench endogenous peroxide activity. Sections were then blocked with normal serum and incubated overnight at 4 °C with rabbit anti-SK1 primary antibody followed by a 30-min incubation with biotinylated anti-rabbit IgG, and then ABC-HRP (Vector Laboratories, Burlingame, CA, USA). The slides were then developed with DAB/H2O2 (Thermo Scientific, Hudson, NH, USA), counterstained with hematoxylin, and dehydrated with an ethanol gradient and Histoclear.
Senescence-associated β-galactosidase (SA-β-gal) Staining
Frozen 10-mm sections of thymi were fixed in 0.2% glutaraldehyde/PBS for 10 min at 4 °C, washed with PBS (pH 6.0), stained with the SA-β-gal staining kit (Cell Signaling) overnight at 37 °C, washed with H2O, counterstained with eosin, and dehydrated in an ethanol gradient with histoclear as previously described (Sun et al., 2007).
SK1 Activity Assay
After tissue homogenization in SK1 lysis buffer, either an aliquot of protein (30 μg) from each homogenized tissue sample, or 30 μL of sample was normalized to its protein concentration and assayed for sphingosine kinase activity in the presence of 32P-ATP and sphingosine for 30 min at 37 ºC. At the end of the reaction, lipids were extracted and subjected to TLC. 32P-sphingosine-1-phosphate was measured using autoradiography. Quantification of S1P-specific radioactivity was performed by scraping the S1P-specific bands into scintillation vials that had their contents quantified via scintillation counting.
Sphingolipidomic Analysis
Cell samples were prepared as detailed in SI Materials and Methods. Each sample was vortexexed and then 250 uL was aliquoted into a labeled 15-mL conical flask. This and the remaining sample in a 1.5-mL microcentrifuge tube were then snap frozen in a dry ice/methanol bath and frozen at −80 °C prior to sphingolipidomic analysis or BCA protein assay, respectively. Tissue samples were homogenized as described above and, after protein analysis, an aliquot of homogenized sample (1 mg protein) was transferred to a 15-mL conical flask and frozen at −80 °C until sphingolipidomic analysis. Alternatively, 100 μL of homogenized sample was submitted and lipids were subsequently corrected to the sample protein concentration. Sphingolipid masses were determined by ESI/MS/MS. Analysis of ceramides, sphingosine, and sphinosine-1-phosphate was performed on a Thermo Finnigan TSQ 7000 triple quadrupole mass spectrometer operating in a multiple reaction-monitoring positive ionization mode, as described (Bielawski et al., 2009; Bielawski et al., 2006).
Statistical Analysis
Data are represented as mean ± SEM, unless otherwise indicated. Unpaired student’s t-test, One-way ANOVA with Dunnett’s post-test, or logrank test statistical analyses were performed using Prism/GraphPad software (La Jolla, CA, USA).
Supplementary Material
Acknowledgments
Support for this work
This work was supported, in part, by Award Number I01BX000156 from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development, NIH/NIGMS R01 GM062887, NIH/NCI P01 CA097132 - project 3 (to LMO). NIH/NCI P01 CA097132 - project 1 (to YAH). American Heart Association Pre-Doctoral Fellowship (AHA 081509E – to RWJ), NIH MSTP Training Grant (GM08716 – to RWJ, AMDC, LAHS ), MUSC Hollings Cancer Center Abney Foundation Scholarship (to RWJ, LAHS), NIH/NIEHS TG T32 ES012878, and NIH/NRSA F30ES017379 (to LAHS).
LC-MS/MS analysis of sphingolipids was performed by Lipidomics Shared Resource, MUSC (Methods 2006, 39: 82–91) supported by NCI Grants: IPO1CA097132 and P30 CA 138313 and NIH/NCRR SC COBRE Grant P20 RR017677. Laboratory space in the CRI building of MUSC was supported by the NIH, Grant C06 RR018823 from the Extramural Research Facilities Program of the National Center for Research Resources.
This work was supported by various grants from the NIH and VA as well as the AHA as detailed in SI. LC-MS/MS analysis of sphingolipids was performed by Lipidomics Shared Resource, MUSC (Methods 2006, 39: 82–91) supported by grants from NCI and NIH as specified in SI. Refer to SI for individual acknowledgements and the VA disclaimer.
Footnotes
Conflict of Interest The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
References
- Akao Y, Banno Y, Nakagawa Y, Hasegawa N, Kim TJ, Murate T, et al. High expression of sphingosine kinase 1 and S1P receptors in chemotherapy-resistant prostate cancer PC3 cells and their camptothecin-induced up-regulation. Biochem Biophys Res Commun. 2006;342:1284–90. doi: 10.1016/j.bbrc.2006.02.070. [DOI] [PubMed] [Google Scholar]
- Alesse E, Zazzeroni F, Angelucci A, Giannini G, Di Marcotullio L, Gulino A. The growth arrest and downregulation of c-myc transcription induced by ceramide are related events dependent on p21 induction, Rb underphosphorylation and E2F sequestering. Cell Death Differ. 1998;5:381–9. doi: 10.1038/sj.cdd.4400358. [DOI] [PubMed] [Google Scholar]
- Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, et al. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem. 2004;279:52487–92. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- Baptiste-Okoh N, Barsotti AM, Prives C. Caspase 2 is both required for p53-mediated apoptosis and downregulated by p53 in a p21-dependent manner. Cell Cycle. 2008;7:1133–8. doi: 10.4161/cc.7.9.5805. [DOI] [PubMed] [Google Scholar]
- Bayerl MG, Bruggeman RD, Conroy EJ, Hengst JA, King TS, Jimenez M, et al. Sphingosine kinase 1 protein and mRNA are overexpressed in non-Hodgkin lymphomas and are attractive targets for novel pharmacological interventions. Leuk Lymphoma. 2008;49:948–54. doi: 10.1080/10428190801911654. [DOI] [PubMed] [Google Scholar]
- Bergelin N, Blom T, Heikkila J, Lof C, Alam C, Balthasar S, et al. Sphingosine kinase as an oncogene: autocrine sphingosine 1-phosphate modulates ML-1 thyroid carcinoma cell migration by a mechanism dependent on protein kinase C-alpha and ERK1/2. Endocrinology. 2009;150:2055–63. doi: 10.1210/en.2008-0625. [DOI] [PubMed] [Google Scholar]
- Bielawski J, Pierce JS, Snider J, Rembiesa B, Szulc ZM, Bielawska A. Comprehensive quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods Mol Biol. 2009;579:443–67. doi: 10.1007/978-1-60761-322-0_22. [DOI] [PubMed] [Google Scholar]
- Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods. 2006;39:82–91. doi: 10.1016/j.ymeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Bringold F, Serrano M. Tumor suppressors and oncogenes in cellular senescence. Exp Gerontol. 2000;35:317–29. doi: 10.1016/s0531-5565(00)00083-8. [DOI] [PubMed] [Google Scholar]
- Chao R, Khan W, Hannun YA. Retinoblastoma protein dephosphorylation induced by D-erythro-sphingosine. J Biol Chem. 1992;267:23459–62. [PubMed] [Google Scholar]
- Cuenin S, Tinel A, Janssens S, Tschopp J. p53-induced protein with a death domain (PIDD) isoforms differentially activate nuclear factor-kappaB and caspase-2 in response to genotoxic stress. Oncogene. 2008;27:387–96. doi: 10.1038/sj.onc.1210635. [DOI] [PubMed] [Google Scholar]
- Cuvillier O, Levade T. Enzymes of sphingosine metabolism as potential pharmacological targets for therapeutic intervention in cancer. Pharmacol Res. 2003;47:439–45. doi: 10.1016/s1043-6618(03)00053-7. [DOI] [PubMed] [Google Scholar]
- Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–3. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- Dbaibo GS, Pushkareva MY, Rachid RA, Alter N, Smyth MJ, Obeid LM, et al. p53-dependent ceramide response to genotoxic stress. J Clin Invest. 1998;102:329–39. doi: 10.1172/JCI1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debacq-Chainiaux F, Boilan E, Dedessus Le Moutier J, Weemaels G, Toussaint O. p38(MAPK) in the senescence of human and murine fibroblasts. Adv Exp Med Biol. 2010;694:126–37. doi: 10.1007/978-1-4419-7002-2_10. [DOI] [PubMed] [Google Scholar]
- Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–21. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- El-Assaad W, Kozhaya L, Araysi S, Panjarian S, Bitar FF, Baz E, et al. Ceramide and glutathione define two independently regulated pathways of cell death initiated by p53 in Molt-4 leukaemia cells. Biochem J. 2003;376:725–32. doi: 10.1042/BJ20030888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Deiry WS, Harper JW, O’Connor PM, Velculescu VE, Canman CE, Jackman J, et al. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994;54:1169–74. [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- Heffernan-Stroud LA, Obeid LM. p53 and regulation of bioactive sphingolipids. Adv Enzyme Regul. doi: 10.1016/j.advenzreg.2010.10.003. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengst JA, Wang X, Sk UH, Sharma AK, Amin S, Yun JK. Development of a sphingosine kinase 1 specific small-molecule inhibitor. Bioorg Med Chem Lett. 2010;20:7498–502. doi: 10.1016/j.bmcl.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Ho LH, Taylor R, Dorstyn L, Cakouros D, Bouillet P, Kumar S. A tumor suppressor function for caspase-2. Proc Natl Acad Sci U S A. 2009;106:5336–41. doi: 10.1073/pnas.0811928106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, et al. Tumor spectrum analysis in p53-mutant mice. Current Biology. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- Jayadev S, Liu B, Bielawska AE, Lee JY, Nazaire F, Pushkareva M, et al. Role for ceramide in cell cycle arrest. J Biol Chem. 1995;270:2047–52. doi: 10.1074/jbc.270.5.2047. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Johnson KY, Crellin HG, Ogretmen B, Boylan AM, Harley RA, et al. Immunohistochemical distribution of sphingosine kinase 1 in normal and tumor lung tissue. J Histochem Cytochem. 2005;53:1159–66. doi: 10.1369/jhc.4A6606.2005. [DOI] [PubMed] [Google Scholar]
- Jung YS, Qian Y, Chen X. Examination of the expanding pathways for the regulation of p21 expression and activity. Cell Signal. 22:1003–12. doi: 10.1016/j.cellsig.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Bader AG, Zhao L, Vogt PK. Mutated PI 3-kinases: cancer targets on a silver platter. Cell Cycle. 2005;4:578–81. doi: 10.4161/cc.4.4.1586. [DOI] [PubMed] [Google Scholar]
- Kapitonov D, Allegood JC, Mitchell C, Hait NC, Almenara JA, Adams JK, et al. Targeting Sphingosine Kinase 1 Inhibits Akt Signaling, Induces Apoptosis, and Suppresses Growth of Human Glioblastoma Cells and Xenografts. Cancer Research. 2009;69:6915–6923. doi: 10.1158/0008-5472.CAN-09-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamori T, Kaneshiro T, Okumura M, Maalouf S, Uflacker A, Bielawski J, et al. Role for sphingosine kinase 1 in colon carcinogenesis. Faseb J. 2009;23:405–14. doi: 10.1096/fj.08-117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamori T, Osta W, Johnson KR, Pettus BJ, Bielawski J, Tanaka T, et al. Sphingosine kinase 1 is up-regulated in colon carcinogenesis. Faseb J. 2006;20:386–8. doi: 10.1096/fj.05-4331fje. [DOI] [PubMed] [Google Scholar]
- Kim SS, Chae HS, Bach JH, Lee MW, Kim KY, Lee WB, et al. P53 mediates ceramide-induced apoptosis in SKN-SH cells. Oncogene. 2002;21:2020–8. doi: 10.1038/sj.onc.1205037. [DOI] [PubMed] [Google Scholar]
- Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene. 2003;22:5897–906. doi: 10.1038/sj.onc.1206702. [DOI] [PubMed] [Google Scholar]
- Lee JY, Bielawska AE, Obeid LM. Regulation of cyclin-dependent kinase 2 activity by ceramide. Exp Cell Res. 2000;261:303–11. doi: 10.1006/excr.2000.5028. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, et al. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–5. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- Lees JA, Weinberg RA. Tossing monkey wrenches into the clock: new ways of treating cancer. Proc Natl Acad Sci U S A. 1999;96:4221–3. doi: 10.1073/pnas.96.8.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Yu CP, Xia JT, Zhang L, Weng GX, Zheng HQ, et al. Sphingosine kinase 1 is associated with gastric cancer progression and poor survival of patients. Clin Cancer Res. 2009;15:1393–9. doi: 10.1158/1078-0432.CCR-08-1158. [DOI] [PubMed] [Google Scholar]
- Liu G, Parant JM, Lang G, Chau P, Chavez-Reyes A, El-Naggar AK, et al. Chromosome stability, in the absence of apoptosis, is critical for suppression of tumorigenesis in Trp53 mutant mice. Nat Genet. 2004;36:63–8. doi: 10.1038/ng1282. [DOI] [PubMed] [Google Scholar]
- Long JS, Edwards J, Watson C, Tovey S, Mair KM, Schiff R, et al. Sphingosine kinase 1 induces tolerance to human epidermal growth factor receptor 2 and prevents formation of a migratory phenotype in response to sphingosine 1-phosphate in estrogen receptor-positive breast cancer cells. Mol Cell Biol. 2010;30:3827–41. doi: 10.1128/MCB.01133-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Marure R, Ventura JL, Sanchez L, Montano LF, Zentella A. Ceramide mimics tumour necrosis factor-alpha in the induction of cell cycle arrest in endothelial cells. Induction of the tumour suppressor p53 with decrease in retinoblastoma/protein levels. Eur J Biochem. 2000;267:4325–33. doi: 10.1046/j.1432-1327.2000.01436.x. [DOI] [PubMed] [Google Scholar]
- Loveridge C, Tonelli F, Leclercq T, Lim KG, Long JS, Berdyshev E, et al. The sphingosine kinase 1 inhibitor 2-(p-hydroxyanilino)-4-(p-chlorophenyl)thiazole induces proteasomal degradation of sphingosine kinase 1 in mammalian cells. J Biol Chem. 2010;285:38841–52. doi: 10.1074/jbc.M110.127993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano G. Mouse models of p53 functions. Cold Spring Harb Perspect Biol. 2:a001115. doi: 10.1101/cshperspect.a001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas J, Parry D, Aagaard L, Mann DJ, Bartkova J, Strauss M, et al. Retinoblastoma-protein-dependent cell-cycle inhibition by the tumour suppressor p16. Nature. 1995;375:503–6. doi: 10.1038/375503a0. [DOI] [PubMed] [Google Scholar]
- Mathews TP, Kennedy AJ, Kharel Y, Kennedy PC, Nicoara O, Sunkara M, et al. Discovery, biological evaluation, and structure-activity relationship of amidine based sphingosine kinase inhibitors. J Med Chem. 2010;53:2766–78. doi: 10.1021/jm901860h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259:1769–71. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- Olivera A, Kohama T, Edsall L, Nava V, Cuvillier O, Poulton S, et al. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J Cell Biol. 1999;147:545–58. doi: 10.1083/jcb.147.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera A, Spiegel S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- Oskouian B, Sooriyakumaran P, Borowsky AD, Crans A, Dillard-Telm L, Tam YY, et al. Sphingosine-1-phosphate lyase potentiates apoptosis via p53- and p38-dependent pathways and is down-regulated in colon cancer. Proc Natl Acad Sci U S A. 2006;103:17384–9. doi: 10.1073/pnas.0600050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paugh SW, Paugh BS, Rahmani M, Kapitonov D, Almenara JA, Kordula T, et al. A selective sphingosine kinase 1 inhibitor integrates multiple molecular therapeutic targets in human leukemia. Blood. 2008;112:1382–91. doi: 10.1182/blood-2008-02-138958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pchejetski D, Doumerc N, Golzio M, Naymark M, Teissie J, Kohama T, et al. Chemosensitizing effects of sphingosine kinase-1 inhibition in prostate cancer cell and animal models. Mol Cancer Ther. 2008;7:1836–45. doi: 10.1158/1535-7163.MCT-07-2322. [DOI] [PubMed] [Google Scholar]
- Pitson SM. Regulation of sphingosine kinase and sphingolipid signaling. Trends Biochem Sci. 2010 doi: 10.1016/j.tibs.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Pitson SM, Xia P, Leclercq TM, Moretti PA, Zebol JR, Lynn HE, et al. Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. J Exp Med. 2005;201:49–54. doi: 10.1084/jem.20040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett ST, Bushnev A, Hagedorn K, Adiga M, Haynes CA, Sullards MC, et al. Biodiversity of sphingoid bases ("sphingosines") and related amino alcohols. J Lipid Res. 2008;49:1621–39. doi: 10.1194/jlr.R800012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruschy M, Resch H, Shi YQ, Aalame N, Glanzmann C, Bodis S. Ceramide triggers p53-dependent apoptosis in genetically defined fibrosarcoma tumour cells. Br J Cancer. 1999;80:693–8. doi: 10.1038/sj.bjc.6690411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- Ryland LK, Fox TE, Liu X, Loughran TP, Kester M. Dysregulation of sphingolipid metabolism in cancer. Cancer Biol Ther. 2011:11. doi: 10.4161/cbt.11.2.14624. [DOI] [PubMed] [Google Scholar]
- Sawada M, Kiyono T, Nakashima S, Shinoda J, Naganawa T, Hara S, et al. Molecular mechanisms of TNF-alpha-induced ceramide formation in human glioma cells: P53-mediated oxidant stress-dependent and -independent pathways. Cell Death Differ. 2004;11:997–1008. doi: 10.1038/sj.cdd.4401438. [DOI] [PubMed] [Google Scholar]
- Soussi T. p53 alterations in human cancer: more questions than answers. Oncogene. 2007;26:2145–2156. doi: 10.1038/sj.onc.1210280. [DOI] [PubMed] [Google Scholar]
- Sun P, Yoshizuka N, New L, Moser BA, Li Y, Liao R, et al. PRAK is essential for ras-induced senescence and tumor suppression. Cell. 2007;128:295–308. doi: 10.1016/j.cell.2006.11.050. [DOI] [PubMed] [Google Scholar]
- Taha TA, Kitatani K, Bielawski J, Cho W, Hannun YA, Obeid LM. Tumor necrosis factor induces the loss of sphingosine kinase-1 by a cathepsin B-dependent mechanism. J Biol Chem. 2005;280:17196–202. doi: 10.1074/jbc.M413744200. [DOI] [PubMed] [Google Scholar]
- Taha TA, Mullen TD, Obeid LM. A house divided: Ceramide, sphingosine, and sphingosine-1-phosphate in programmed cell death. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2006;1758:2027–2036. doi: 10.1016/j.bbamem.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha TA, Osta W, Kozhaya L, Bielawski J, Johnson KR, Gillanders WE, et al. Down-regulation of sphingosine kinase-1 by DNA damage: dependence on proteases and p53. J Biol Chem. 2004;279:20546–54. doi: 10.1074/jbc.M401259200. [DOI] [PubMed] [Google Scholar]
- Vadas M, Xia P, McCaughan G, Gamble J. The role of sphingosine kinase 1 in cancer: oncogene or non-oncogene addiction? Biochim Biophys Acta. 2008;1781:442–7. doi: 10.1016/j.bbalip.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Vakifahmetoglu H, Olsson M, Tamm C, Heidari N, Orrenius S, Zhivotovsky B. DNA damage induces two distinct modes of cell death in ovarian carcinomas. Cell Death Differ. 2008;15:555–66. doi: 10.1038/sj.cdd.4402286. [DOI] [PubMed] [Google Scholar]
- Venable ME, Obeid LM. Phospholipase D in cellular senescence. Biochim Biophys Acta. 1999;1439:291–8. doi: 10.1016/s1388-1981(99)00101-8. [DOI] [PubMed] [Google Scholar]
- Vogelstein BLD, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- Watson C, Long JS, Orange C, Tannahill CL, Mallon E, McGlynn LM, et al. High expression of sphingosine 1-phosphate receptors, S1P1 and S1P3, sphingosine kinase 1, and extracellular signal-regulated kinase-1/2 is associated with development of tamoxifen resistance in estrogen receptor-positive breast cancer patients. Am J Pathol. 2010;177:2205–15. doi: 10.2353/ajpath.2010.100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert A, Schiffmann S, Sekar D, Ley S, Menrad H, Werno C, et al. Sphingosine kinase 2 deficient tumor xenografts show impaired growth and fail to polarize macrophages towards an anti-inflammatory phenotype. Int J Cancer. 2009;125:2114–21. doi: 10.1002/ijc.24594. [DOI] [PubMed] [Google Scholar]
- Weisz L, Oren M, Rotter V. Transcription regulation by mutant p53. Oncogene. 2007;26:2202–11. doi: 10.1038/sj.onc.1210294. [DOI] [PubMed] [Google Scholar]
- Xia P, Gamble JR, Wang L, Pitson SM, Moretti PA, Wattenberg BW, et al. An oncogenic role of sphingosine kinase. Curr Biol. 2000;10:1527–30. doi: 10.1016/s0960-9822(00)00834-4. [DOI] [PubMed] [Google Scholar]
- Yoon CH, Kim MJ, Park MT, Byun JY, Choi YH, Yoo HS, et al. Activation of p38 mitogen-activated protein kinase is required for death receptor-independent caspase-8 activation and cell death in response to sphingosine. Mol Cancer Res. 2009;7:361–70. doi: 10.1158/1541-7786.MCR-08-0069. [DOI] [PubMed] [Google Scholar]
- Zilfou JT, Lowe SW. Tumor suppressive functions of p53. Cold Spring Harb Perspect Biol. 2009;1:a001883. doi: 10.1101/cshperspect.a001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.