Summary
The hypothalamic melanocortin system, which includes neurons that produce proopiomelanocortin (POMC)-derived peptides, is a major negative regulator of energy balance. POMC neurons begin to acquire their unique properties during neonatal life. The formation of functional neural systems requires massive cytoplasmic remodeling that may involve autophagy, an important intracellular mechanism for the degradation of damaged proteins and organelles. Here we investigated the functional and structural effects of the deletion of an essential autophagy gene, Atg7, in POMC neurons. Lack of Atg7 in POMC neurons caused higher post-weaning body weight, increased adiposity, and glucose intolerance. These metabolic impairments were associated with an age-dependant accumulation of ubiquitin/p62-positive aggregates in the hypothalamus and a disruption in the maturation of POMC-containing axonal projections. Together, these data provide direct genetic evidence that Atg7 in POMC neurons is required for normal metabolic regulation and neural development, and they implicate hypothalamic autophagy deficiency in the pathogenesis of obesity.
Introduction
Appetite, energy balance, and metabolism are carefully regulated by the central nervous system (CNS) (see Elmquist et al., 2005; Gao and Horvath, 2007; Sawchenko, 1998 for reviews). The important components of this neural network include neurons located in the arcuate nucleus of the hypothalamus (ARH), particularly neurons producing proopiomelanocortin (POMC). POMC neurons reduce food intake and increase energy expenditure by releasing α-melanocyte-stimulating hormone (aMSH), a product of POMC processing, which activates melanocortin-4 receptors (MC4R) (Cone, 2006). More recent targeted deletion studies have specifically shown the importance of POMC neurons in mediating the physiological actions of metabolic hormones, such as leptin and insulin (Belgardt and Brüning, 2010; Elmquist et al., 2005). POMC neurons provide extensive projections to other parts of the hypothalamus, including the paraventricular (PVH) and dorsomedial (DMH) nuclei of the hypothalamus and the lateral hypothalamic area (LHA), to exert their anorectic effects. Each of these target nuclei also express MC4R (Cone, 2006). Similar to many other functional neural systems, POMC neurons begin to acquire their unique properties during neonatal life. In rodents, arcuate POMC neural circuits develop primarily during the first three weeks of postnatal life, under the influence of both intrinsic and extrinsic cues (Bouret, 2010; Levin, 2006; Sullivan and Grove, 2010). The process of developing highly specialized cellular structures, such as POMC neurons, also requires massive cytoplasmic remodeling. However, the cellular and molecular mechanisms underlying this remodeling process are largely unknown, and its functional relevance remains equally undetermined.
Autophagy is a major cellular degradation process in which parts of the cytoplasm and intracellular organelles are engulfed within double-membraned vesicles, known as autophagosomes, in preparation for the turnover and recycling of these cellular constituents (Klionsky, 2007). Another important function of autophagy is in the supply of nutrients for survival. It also plays an important role in cell growth, development, and homeostasis, where it helps to maintain a balance between the synthesis, degradation, and the subsequent recycling of cellular components (Cecconi and Levine, 2008; Maiuri et al., 2007). In particular, low levels of basal autophagy are important for maintaining the quality of proteins and organelles and are therefore important for the maintenance of cell function and growth. Recent genetic studies have also highlighted the importance of autophagy in physiological regulations. For example, the targeted deletion of essential autophagy genes have revealed that constitutive autophagy contributes to energy homeostasis (including body weight regulation, adiposity, and glucose homeostasis) by acting on liver physiology (Komatsu et al., 2007a), pancreatic morphology and function (Ebato et al., 2008; Jung et al., 2008), and adipocyte differentiation (Singh et al., 2009; Zhang et al., 2009). In addition, deficiency of autophagy in AgRP neurons causes leanness in adult mice (Kaushik et al., 2011), showing the involvement of hypothalamic autophagy in the control of energy balance.
Although recent advances have indicated that constitutive autophagy contributes to the central control of energy homeostasis, the structural and functional importance of autophagy in hypothalamic anorexigenic neural circuits remains unknown. In the present study, we generated mice deficient in Atg7, an essential autophagy gene, specifically in POMC neurons to determine the role of autophagy in this neuronal population. The results indicate that autophagy is important for normal maturation of POMC axonal projections and that the absence of autophagy in POMC neurons results in lifelong metabolic disturbances.
Results
Autophagy occurs constitutively in hypothalamic POMC processes
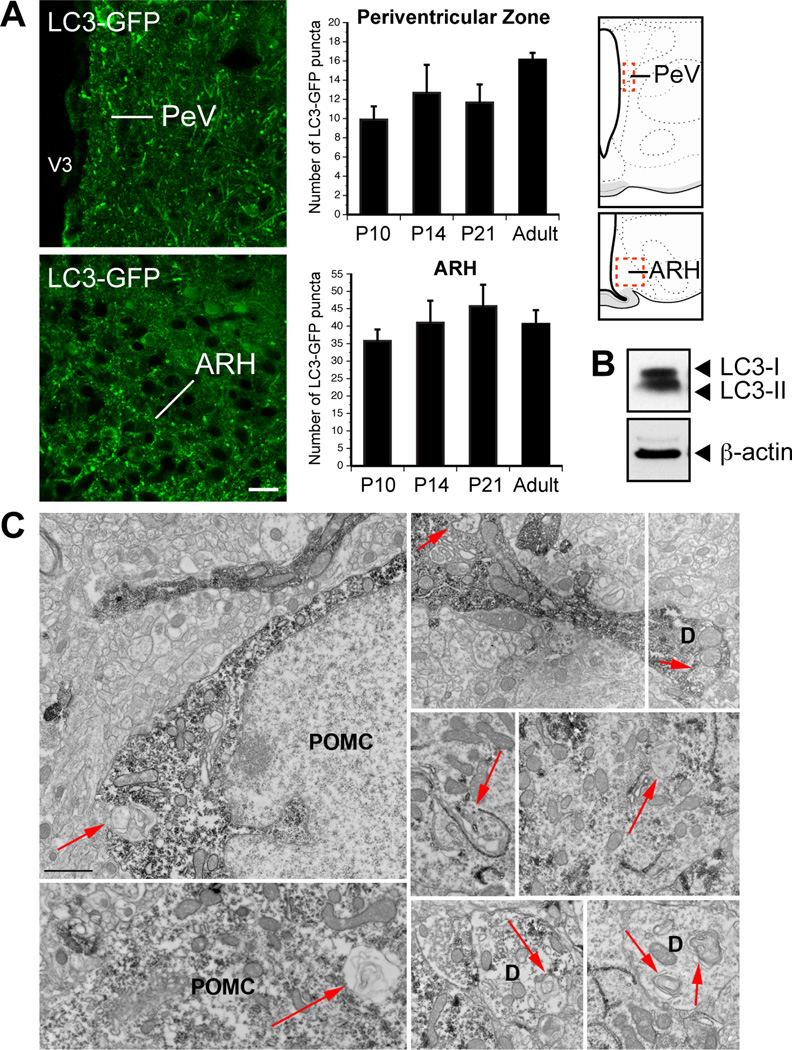
To investigate whether autophagy occurs in the postnatal hypothalamus under basal conditions, we used a transgenic mouse in which microtubule-associated protein 1 light chain 3 (LC3) has been fused to the green fluorescent protein (GFP). Because LC3 is a reliable marker of autophagosomes, LC3-GFP mice are widely used to monitor autophagy in vivo (Mizushima et al., 2004). LC3-GFP puncta were readily detectable in various regions of the CNS in basal conditions (Figure S1), including in the ARH (Figure 1A). LC3-GFP puncta were found in the ARH as early as postnatal day 10, and the presence of autophagosomes persisted at weaning and into adulthood (Figure1A). The density of LC3-GFP puncta displayed no significant variations across the ages studied (Figure 1A), and puncta were detected in both perikaryons and processes (Figures 1A). The LC3-GFP signal was not restricted to the ARH; it was also observed in processes located in the periventricular zone of the hypothalamus, which appears to be the major route for ascending ARH efferent connections (Bouret et al., 2004a, b) (Figures 1A). The presence of constitutive autophagy in the hypothalamus was further confirmed by the presence of both LC3 I (soluble form) and LC3 II (membrane-bound form) immunoreactivity in the adult hypothalamus under basal conditions (Figure 1B).
Figure 1.
Identification of autophagy in hypothalamic POMC processes. (A) Representative images and quantification of LC3-GFP puncta in the arcuate nucleus (ARH) and the hypothalamic periventricular zone (PeV) of P10, P14, P21, and adult (8- to 9-week-old) mice (n = 4–5 per group). Dashed boxes in the schematics represent the approximate borders of the areas used for quantification. (B) Immunoblot analysis of LC3 (LC3-I, 18 kDa; LC3-II, 16 kDa) and b-actin (as a loading control) from hypothalami derived from adult mice. (C) Representative electron micrographs showing autophagosomes (arrows) in POMC-immunolabeled perikarya and processes of P24 wild-type mice. D, dendrites, V3, third ventricle. Values are shown as mean ± SEM. *P < 0.05 versus Atg7loxP/loxP. Scale bar, 15 um (A) and 1 um (C).
Because POMC is expressed in ARH neurons and in axon terminals traveling throughout the periventricular zone of the hypothalamus (Bouret et al., 2004a), i.e., where basal autophagy was detected, we next investigated whether autophagy occurs specifically in POMC neurons by using electron microscopy. Ultrastructural analysis of material derived from P24 wild-type mice revealed the presence of double-membraned autophagosomes in both neuronal perikarya and neuronal processes of POMC neurons, including dendrites (Figure 1C).
Together, these data indicate that autophagy occurs constitutively in the hypothalamus. The presence of autophagosomes specifically in POMC neuronal processes suggests a role for hypothalamic autophagy in metabolic regulation.
Specific deletion of Atg7 in POMC neurons causes metabolic disturbances
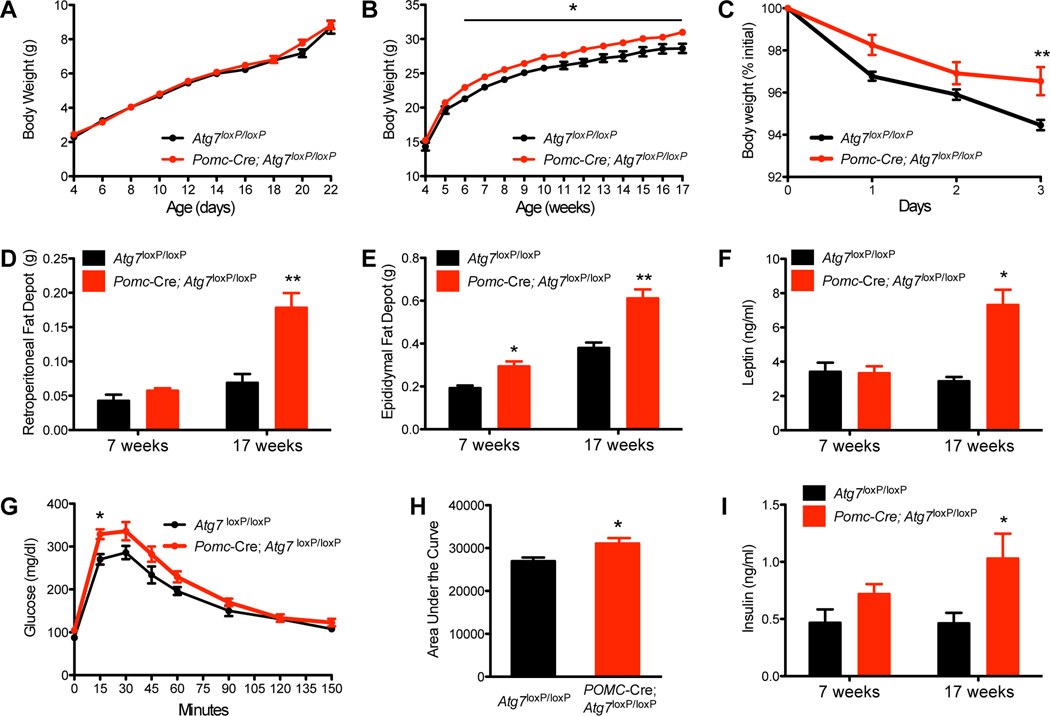
To determine the physiological role of autophagy in POMC neurons, we generated mice that lack Atg7, an essential autophagy gene, specifically in POMC neurons. We crossed mice carrying an Atg7loxP allele (Komatsu et al., 2005) with mice that express Cre recombinase in a POMC-specific manner (Pomc-Cre) (Balthasar et al., 2004). The resulting Pomc-Cre; Atg7loxP/loxP mice were born normally and survived to adulthood. Pomc-Cre; Atg7loxP/loxP mice had body weights undistinguishable from their Atg7loxP/loxP control littermates until 6 weeks of age (group, F(1/322) = 2.08, P = 0.1498; age, F(9/322) = 159.22, P < 0.0001; interaction, F(9/322) = 0.39, P = 0.9413) (Figures 2A–B). However, starting at 6 weeks of age, mutant mice displayed significantly higher body weights compared with control Atg7loxP/loxP mice, and these changes in body weight persisted until 17 weeks of age (group, F(1/311) = 103.80, P < 0.0001; age, F(13/311) = 188.66, P < 0.000; interaction, F(13/311) = 0.35, P = 0.9835) (Figure 2B). In addition, epididymal and retroperitoneal fat pad weights were also significantly higher in adult Pomc-Cre; Atg7loxP/loxP mice compared to Atg7loxP/loxP mice (Figures 2D–E). Moreover, there was a shift in adipocyte size distribution toward larger adipocytes in mutant mice (Figures S2AC). Consistent with these findings, serum leptin levels were also significantly elevated in mutant mice compared to control mice at 17 weeks of age (Figure 2F). Because POMC neurons are major mediators for leptin’s regulatory actions, we performed leptin sensitivity tests and found that the weight loss effect of leptin was also attenuated in Pomc-Cre; Atg7loxP/loxP mice compared to Atg7loxP/loxP mice. This attenuation in leptin sensitivity was observed as early as at 7 weeks of age (data not shown) and persisted at 17 weeks of age (group, F(1/39) = 13.79, P = 0.0006; days of treatment, F(3/39) = 40.71, P < 0.0001; interaction, F(3/39) = 2.44, P = 0.0786) (Figure 2C). Whether these metabolic defects are also associated with changes in locomotor activity and energy expenditure remain to be investigated.
Figure 2.
Altered metabolism in mice lacking autophagy in POMC neurons. (A) Pre- and (B) post-weaning growth curves of Atg7loxP/loxP (n ≥ 9) and Pomc-Cre; Atg7loxP/loxP (n = 15) male mice. (C) Leptin sensitivity of 10-week-old Atg7loxP/loxP (n = 6) and Pomc-Cre; Atg7loxP/loxP (n = 8) male mice. Mass of (D) retroperitoneal and (E) epididymal fat of 7- and 15- to 17-week-old Atg7loxP/loxP (n = 4–5) and Pomc-Cre; Atg7loxP/loxP (n = 5–11) male mice. (F) Serum leptin and (I) insulin levels in Atg7loxP/loxP (n = 8) and Pomc-Cre; Atg7loxP/loxP (n = 6) male mice from 7- and 15–17 weeks of age. (G) Glucose tolerance test (GTT) and (H) area under the GTT curve of 8- to 9-week-old Atg7loxP/loxP (n = 7) and Pomc-Cre; Atg7loxP/loxP (n = 12) male mice. Values are shown as mean ± SEM. *P < 0.05 versus Atg7loxP/loxP.
To examine whether deletion of autophagy in POMC neurons also had consequences on glucose homeostasis, we performed glucose tolerance tests. Fasting glucose levels were significantly elevated in adult Pomc-Cre; Atg7loxP/loxP mice compared to Atg7loxP/loxP mice and when exposed to a glucose challenge, mutant mice displayed impaired glucose tolerance as compared to control mice (Figures 2G–H). Serum insulin levels were also elevated in fed Pomc-Cre; Atg7loxP/loxP mice compared to Atg7loxP/loxP mice at 17 weeks of age (Figure 2I).
Mice deficient in autophagy in POMC neurons also display sexual dimorphism in metabolic regulation. Although female Pomc-Cre; Atg7loxP/loxP mice displayed higher body weights as early as 9 weeks of age, they did not have changes in glucose tolerance, and leptin and insulin levels (Figures S2D–G).
Together, these data indicate that the absence of autophagy in POMC neurons has functional consequences on body weight and glucose regulation and these effects are sexually dimorphic.
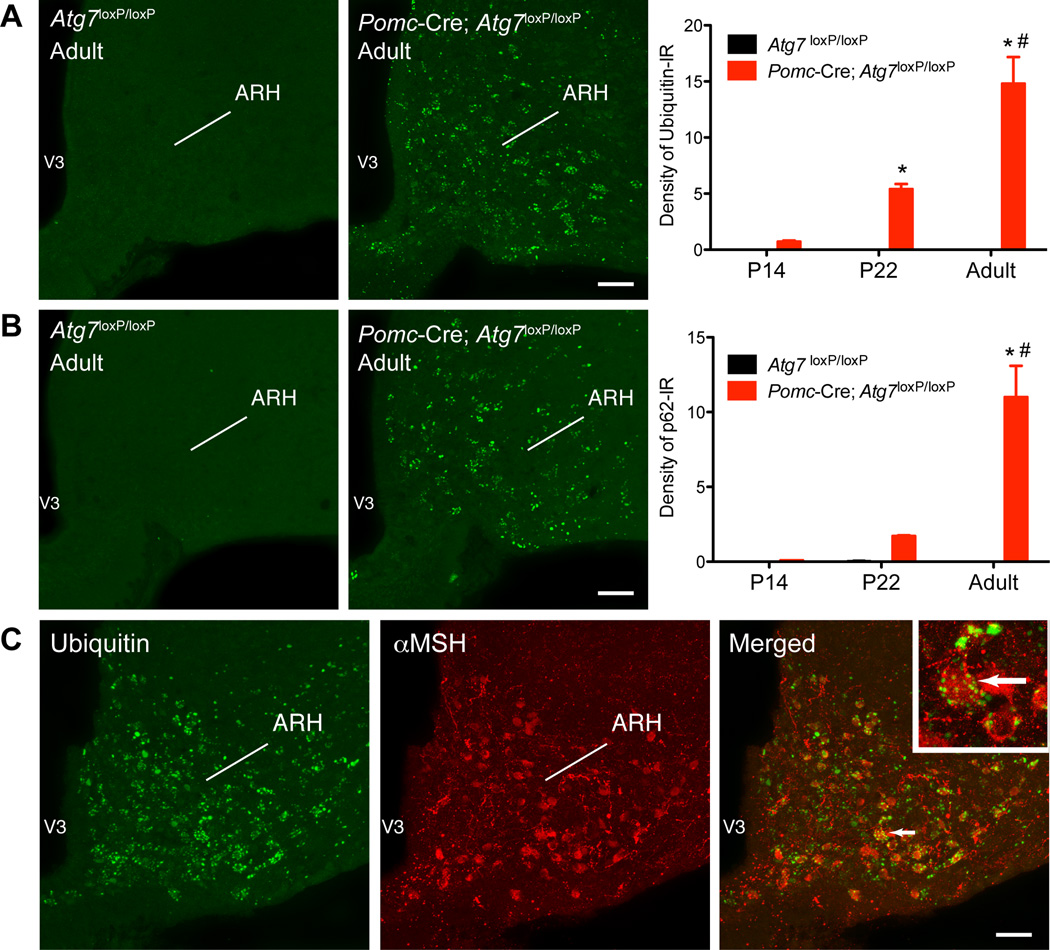
Lack of Atg7 in POMC neurons causes age-dependent accumulation of ubiquitin and p62 aggregates in the arcuate nucleus
Because defects in autophagy have been shown to cause the accumulation of ubiquitincontaining inclusion cell bodies (Komatsu et al., 2006; Komatsu et al., 2005), we next evaluated ubiquitin-immunoreactivity (IR) in Pomc-Cre; Atg7loxP/loxP mice. Hypothalami of control Atg7loxP/loxP mice were devoid of ubiquitin-IR. In contrast, numerous inclusions immunopositive for ubiquitin were detected in the hypothalamus of Pomc-Cre; Atg7loxP/loxP mice (Figure 3A). Notably, the accumulation of ubiquitin was restricted to the ARH (Figure S3A) in an age-dependent manner. A quantitative analysis of the experimental material revealed that although the density of ubiquitin-IR was modest in the ARH of Pomc-Cre; Atg7loxP/loxP mice at P14, levels of ubiquitin-IR in the ARH were increased 8-fold between P14 and P22 and increased 3-fold between P22 and adult mice, indicating a gradual increase in ubiquitin accumulation in the ARH of mutant mice (Figure 3A).
Figure 3.
Lack of autophagy in POMC neurons leads to the gradual accumulation of ubiquitin aggregates in the arcuate nucleus. Quantification of (A) ubiquitin- and (B) p62-immunoreactivity in the arcuate nucleus (ARH) of P14, P22, and adult (15- to 17-week-old) Atg7loxP/loxP (n = 4) and Pomc-Cre; Atg7loxP/loxP (n = 4) male mice. (A, B) Confocal images illustrating (A) ubiquitin- and (B) p62-immunoreactivity in the ARH of adult Atg7loxP/loxP and Pomc-Cre; Atg7loxP/loxP mice. (C) Confocal images showing the presence of ubiquitin-immunoreactivity (green fluorescence) in aMSH-positive cells (red fluorescence) of an adult Pomc-Cre; Atg7loxP/loxP mouse. The arrow points to a doubled labeled cell. V3, third ventricle. Values are shown as mean ± SEM. *P < 0.05 versus P14; #P < 0.05 versus P22. Scale bars, 50 um.
We also investigated the expression of the polyubiquitin-binding protein p62/SQSTM1 that links ubiquitinated proteins to the autophagy apparatus (Komatsu et al., 2007a). In good agreement with our ubiquitin findings, Pomc-Cre; Atg7loxP/loxP mice exhibited substantial p62-IR in the ARH as early as P22 and levels of p62-IR continued to increase until adulthood (Figure 3B). Double-labeling experiments further showed that 85% of ubiquitin-immunopositive cells also express POMC (aMSH-IR) and that 95% of aMSH-IR cells are also immunopositive for ubiquitin (Figures 3C and S3B).
Together, these data indicate that deletion of Atg7 in POMC neurons leads to the age-dependant accumulation of ubiquitin and p62 in arcuate neurons. The finding that ubiquitin is largely restricted to aMSH-IR neurons also confirms the selective reduction of autophagy in POMC neurons and suggests that POMC neurons may have biological alterations.
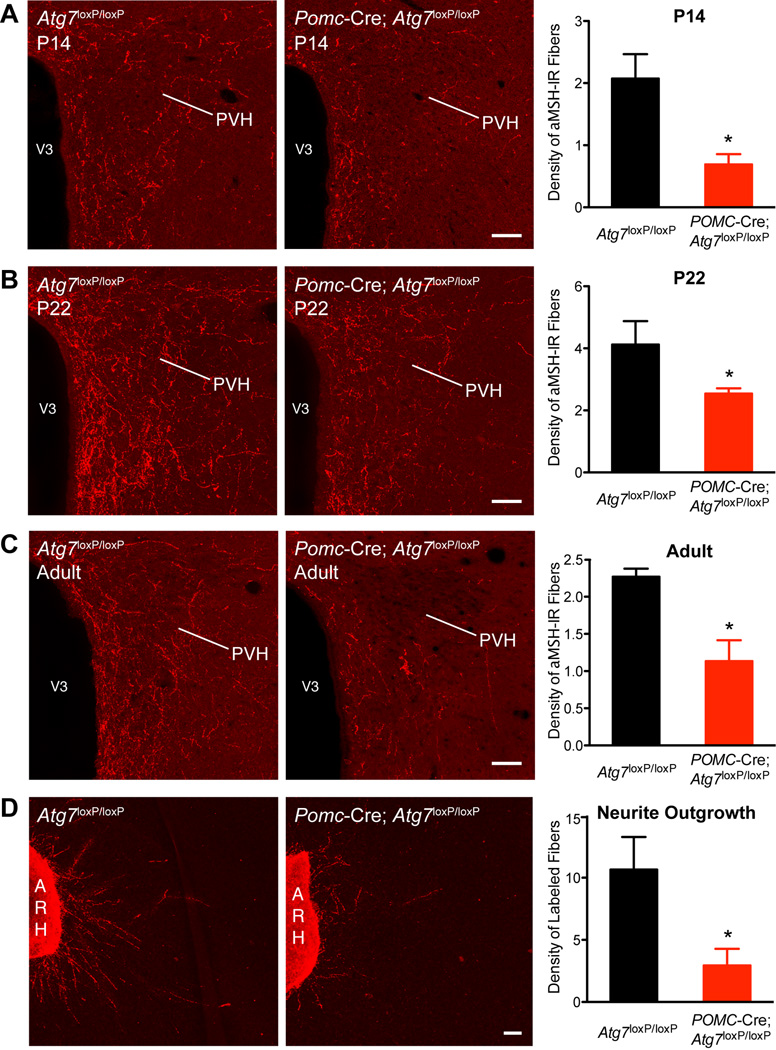
Mice with POMC neuron-specific deletion of Atg7 display abnormal development of POMC neuronal projections
To investigate whether autophagy deficiency in POMC neurons causes structural alterations, we analyzed POMC-containing neural projections in Pomc-Cre; Atg7loxP/loxP and Atg7loxP/loxP mice. We paid particular attention to the POMC-derived aMSH projections to the paraventricular nucleus of the hypothalamus (PVH) because of their well-established importance in the neural control of food intake and energy balance (Ellacott and Cone, 2006; Elmquist et al., 2005; Gao and Horvath, 2007; Sawchenko, 1998). Although the overall distribution of aMSH-IR fibers was quite similar between mutant mice and control animals, clear differences were apparent in the density of the labeled fibers. The density of aMSH-IR fibers in the PVH of Pomc-Cre; Atg7loxP/loxP mice was 1.5–3 fold lower than that observed in the Atg7loxP/loxP mice (Figure 4A–C). The reduction in aMSH fiber density was observed as early at P14 (Figure 4A) and persisted at P22 (Figure 4B). To determine if the defects in aMSH projections observed in neonatal mutant mice were permanent, we also performed immunohistochemical labeling of aMSH in brain sections from adult Pomc-Cre; Atg7loxP/loxP and Atg7loxP/loxP mice. As observed in neonates, the average density of aMSH-IR fibers in the PVH remained diminished in adult Pomc-Cre; Atg7loxP/loxP mice relative to that of Atg7loxP/loxP mice (Figure 4C). In adults, the density of aMSH-IR fibers in the PVH remained 2-fold lower in mutant mice relative to that of control mice. A substantial disruption in the density of labeled fibers was observed in both the parvicellular and magnocellular parts of the PVH. Similar reductions in aMSH fiber density were also observed in the DMH and LHA (Figures S4D–E), indicating that Atg7 deficiency causes extensive disruption of aMSH projections to each of their major terminal fields. To confirm that lack of autophagy in POMC neurons altered the pattern of ARH axonal projections, we implanted a fluorescent axonal tracer DiI into the ARH of Pomc-Cre; Atg7loxP/loxP and Atg7loxP/loxP mice. The density of ARH DiI-labeled fibers was significantly attenuated in the PVH of P14 mutant mice as compared to control mice (Figure S4C).
Figure 4.
Disruption of POMC projections in mice lacking autophagy in POMC neurons. Confocal images and quantification of aMSH-IR fibers in the PVH of (A) P14, (B) P22, and (C) adult (15- to 17-week-old) Atg7loxP/loxP (n = 4) and Pomc-Cre; Atg7loxP/loxP (n = 4) male mice. (D) Images and quantification of b-endorphin (a POMC-derived peptide)-immunopositive fibers derived from isolated organotypic cultures of the ARH from P4 Atg7loxP/loxP and Pomc-Cre; Atg7loxP/loxP mice. ARH, arcuate nucleus of the hypothalamus, PVH, paraventricular nucleus of the hypothalamus, V3, third ventricle. Values are shown as mean ± SEM. *P < 0.05 versus Atg7loxP/loxP. Scale bars, 50 um.
To confirm that the mutation in Pomc-Cre; Atg7loxP/loxP mice does not affect the development of other non-POMC circuits, we examined neuronal projections containing AgRP, another neuropeptide system present in the ARH that sends overlapping projections to the PVH. Another reason for studying this neuronal population specifically is because POMC and AgRP/NPY neurons share a common ontological lineage, and it was therefore possible that some of cre-expressing cells also contain AgRP/NPY (Padilla et al., 2010). However, despite a marked attenuation in the density of aMSH-IR fibers, neural projections containing AgRP appear unaltered in Pomc-Cre; Atg7loxP/loxP mice (Figure S3C). Consistent with these findings, colocalizations between AgRP-and ubiquitin-IR were rare in mutant mice (Figure S3B). Also, Agrp and Npy mRNA levels are similar between mutant and control mice (Figures S3D–E). In addition, in sharp contrast to Agrp-Cre; Atg7loxP/loxP mice that are lean(Kaushik et al., 2011), Pomc-Cre; Atg7loxP/loxP mice have higher overweight and adiposity. Together, these data suggest that the AgRP/NPY system is relatively normal in Pomc-Cre; Atg7loxP/loxP mice and that the mutation present in Pomc-Cre; Atg7loxP/loxP mice selectively affects POMC neurons.
Because autophagy has been suggested to exert neuroprotective actions (Hara et al., 2006; Komatsu et al., 2006; Komatsu et al., 2007b), we also assessed if Atg7 deficiency affects the survival of POMC neurons. The neuroanatomical distribution and number of b-endorphin (a product of POMC neurons)-immunopositive neurons did not differ between Pomc-Cre; Atg7loxP/loxP and Atg7loxP/loxP mice (Figure S4A). Supporting these observations, there were no noticeable differences in the number of TUNEL-positive cells (a marker of apoptosis) or apoptotic blebbed nuclei in the hypothalamus of adult mutant mice as compared to controls (data not shown). In addition, hypothalamic Pomc mRNA levels did not differ between Pomc-Cre; Atg7loxP/loxP and Atg7loxP/loxP mice (Figure S4B). These data suggest that the absence of Atg7 does not alter POMC neuron survival. They also indicate that the low density of POMC-derived fibers observed in mutant mice is due to alterations in axon growth as opposed to a reduction in cell number or peptide content in axons.
To determine whether deficiency of Atg7 might alter the ability of POMC neurons to send axonal projections, we conducted a series of in vitro experiments and evaluated POMC axon growth in both Pomc-Cre; Atg7loxP/loxP and Atg7loxP/loxP mice. After 36 hrs in vitro, organotypic ARH explants derived from Atg7loxP/loxP control mice exhibit marked POMC axon growth, as revealed by the presence of GAP-43 (a marker of axon growth) and b-endorphin (a marker of POMC neurons) double-labeled fibers extending from the edge of ARH explants (Figure 4D). In contrast, this basal POMC axon growth was severely blunted if the explant was derived from Pomc-Cre; Atg7loxP/loxP mice (Figure 4D). The overall density of GAP-43-bendorphin double-labeled neurites extending from explants from Pomc-Cre; Atg7loxP/loxP mice was 2.5-fold lower than that of Atg7loxP/loxP mice (Figure 4D).
Together, these data indicate that Pomc-Cre; Atg7loxP/loxP mice display an abnormal development of POMC neuronal projections that may be the result of the diminished ability of ARH POMC neurons to extend axons during postnatal development.
Discussion
It is well documented that autophagy, a cellular pathway involved in degrading and recycling various intracellular constituents, plays a major role in the peripheral regulation of metabolism by acting on pancreatic, liver, and adipocyte morphology and function (Ebato et al., 2008; Jung et al., 2008; Komatsu et al., 2007a; Singh et al., 2009; Zhang et al., 2009). More recently, a role for autophagy in the central control of energy metabolism has been identified (Kaushik et al., 2011). However the precise contribution of autophagy in various subpopulations of hypothalamic neurons involved in metabolic regulation and the structural consequences of autophagy deficiency on the organization of hypothalamic feeding neural circuits remain unknown. In the present study, we reported that autophagy is constitutively active in key parts of the hypothalamus that play a role in feeding and energy balance, including in POMC neurons. By generating mice with a targeted deletion of a key autophagy gene (Atg7) selectively in POMC neurons, we also showed that the loss of autophagy in POMC neurons resulted in increased postweaning body weight, increased adiposity, and perturbations in glucose homeostasis. Autophagy deficiency in POMC neurons also caused an age-dependent accumulation of ubiquitin and p62 and alterations in the maturation of POMC axonal processes.
Neurons were originally thought to be resistant from autophagy induction. This hypothesis was based on the fact that autophagy is induced in almost all tissues, except the brain, following starvation (Mizushima et al., 2004). Nevertheless, there is now a growing appreciation that constitutive autophagy plays an important role in the CNS. Mice that are deficient in either Atg5 or Atg7 in the CNS develop progressive behavioral defects and motor deficits that are usually associated with neurodegenerative diseases (Hara et al., 2006; Komatsu et al., 2006). Consistent with these behavioral observations, the same mutant mice display early signs of neurodegeneration that include massive neuronal loss and abnormal protein aggregation in the cortex and the cerebellum (Hara et al., 2006; Komatsu et al., 2006). A more recent study has implicated autophagy in the central control of energy balance. Mice lacking autophagy specifically in arcuate AgRP neurons have reduced body weight and adiposity, and display diminished refeeding response to fasting (Kaushik et al., 2011). Our study extends the role of CNS autophagy on metabolic regulation and brain development. It shows the widespread importance of autophagy in the CNS, particularly in POMC neurons, a major neuronal population promoting negative energy balance. The presence of constitutively active autophagy in ARH neurons and processes is supported by the following observations: LC3, a major constituent of the autophagosome, is expressed in the ARH and the periventricular zone of the hypothalamus (the major route for ascending ARH efferent connections) during postnatal and adult life, and the lack of autophagy (as observed in Pomc-Cre; Atg7loxP/loxP mice) leads to accumulation of ubiquitin and p62 in the ARH. Moreover, Pomc-Cre; Atg7loxP/loxP mice, which lack constitutive autophagy, displayed marked structural and physiological alterations. In contrast to mice that lack the autophagy gene in neurons (i.e., Nestin-Cre; Atg5loxP/loxP or Nestin-Cre; Atg7loxP/loxP mice), we did not observe significant neuronal loss in Pomc-Cre; Atg7loxP/loxP mice. Despite a marked reduction in POMC fiber density, POMC cell number was not different between adult control and mutant mice, and there was no evidence cell death induction (as evidenced by the absence of TUNEL-positive cells or apoptotic blebbed nuclei) in the hypothalamus of Pomc-Cre; Atg7loxP/loxP mice. These data suggest that autophagy may exert different functions on different brain regions. They also imply that CNS autophagy is not only important for the protection from neurodegenerative diseases, but it is also an important mechanism involved in normal brain development.
The complex patterns of neuronal wiring in the adult hypothalamus depend on a series of events that establish a framework on which functional circuits can be built. The cellular and molecular processes that are involved in the formation of a functioning hypothalamus remain largely unknown. Previous studies have reported autophagy vacuoles in distal axons and have suggested that autophagy plays an important role in axon remodeling and homeostasis during aging (Yue et al., 2009). The present study suggests that autophagy is an important intracellular process for the normal development of neural projections derived from POMC neurons. The density of aMSH-immunoreactive fibers is markedly attenuated in the hypothalamus of Pomc-Cre; Atg7loxP/loxP mice, and the disruption in POMC neural projections is found in all major terminal fields of POMC axons, such as the PVH, DMH, and LHA. These observations support the idea that the lack of autophagy alters the ability of POMC neurons to send axonal projections to their target nuclei. Consistent with this hypothesis, the reduction in the density of aMSH-immunoreactive fibers is observed as early as P14, i.e., when POMC neurons extend their axonal projections to their target nuclei (Bouret, 2010; Sullivan and Grove, 2010). Importantly, the density of ARH DiI-labeled fibers is also attenuated in the PVH of mutant mice, suggesting that lack of autophagy causes structural changes in axonal projection pattern from ARH POMC neurons. Our in vitro studies further support this hypothesis by showing that the ability of ARH POMC neurons to extend their axons is attenuated in explants derived from Pomc-Cre; Atg7loxP/loxP mice. Notably, the reduction in axon growth specifically affected POMC axons containing GAP-43 (a marker of axons independent of peptide content), supporting the idea that the reduction in aMSH-immunoreactive fibers found in mutant mice is caused by a reduction in axon density as opposed to changes in the peptide content in axons. Consistent with this idea, hypothalamic Pomc mRNA levels did not differ between Pomc-Cre; Atg7loxP/loxP and Atg7loxP/loxP mice. However, we cannot rule out the possibility that the reduction in immunostaining observed in mutant mice is not due to an increase in peptide release. Nevertheless, a reduction in peptide release is more often associated with a lack of autophagy. For example, glucose-stimulated insulin release in isolated islets is reduced in mice lacking Atg7 specifically in pancreatic beta cells (Ebato et al., 2008). We have also checked the density of fibers containing b-endorphin (another peptide derived from POMC) and found that b-endorphin-containing projections were also reduced in Pomc-Cre; Atg7loxP/loxP mice (data not shown), supporting the idea that the lack of autophagy does not alter the pattern of POMC peptide processing and specifically affects axon growth.
Optimal brain wiring, including optimal hypothalamic neuronal circuitry, depends on the capacity to change metabolic states in response to external stimuli that vary from early development to adulthood (Elmquist and Flier, 2004; Horvath and Bruning, 2006). Depending on the availability of nutrients (such as proteins) or trophic stimuli (such as hormones), neurons can metabolically switch from an anabolic state to a state of catabolism. Autophagy has long been characterized as a key cellular mechanism for maintaining energy homeostasis during nutrient-poor conditions. Accordingly, short-term food restriction causes induction of autophagy in various tissues including the hypothalamus (Alirezaei et al., 2010; Kaushik et al., 2011). The present study indicates that under basal conditions, hypothalamic autophagy plays a key role in metabolic regulation. Other cell-intrinsic metabolic-sensing pathways have been implicated in the hypothalamic control of energy balance. These include the kinase mammalian target of rapamycin (mTOR), SIRT1, and AMP-activated protein kinase (Claret et al., 2007; Cota et al., 2006; Minokoshi et al., 2004; Ramadori et al., 2010). The implication of mTOR in the hypothalamic control of appetite regulation is particularly interesting because it also directly regulates autophagy. When nutrients are lacking, mTOR repression shifts cellular metabolism toward autophagy and the recycling of cytosolic constituents. Thus, inadequate mTOR and/or autophagy regulation can lead to overlapping pathological conditions and may predispose an individual to the development of obesity and diabetes. In addition, because leptin directly promotes axon growth from ARH neurons (Bouret et al., 2004b), and because leptin promotes autophagy (Malik et al., 2011), it remains possible that some of the neurotrophic effects of leptin might be mediated through autophagy activation. Similarly, it would be important to determine whether there is an acute role of autophagy regulated by leptin on input organization, membrane properties and firing frequency of POMC neurons, which in turn, could affect feeding behavior. It is also tempting to speculate that autophagy may support a cellular homeostasis permissive of electric changes in these neurons during the course of changing metabolic and hormonal milieu.
Experimental Procedures
Animals
Mice were housed in individual cages under specific pathogen-free conditions, maintained in a temperature-controlled room with a 12 h light/dark cycle, and provided ad libitum access to water and standard laboratory chow (Special Diet Services). Animal usage was in compliance with and approved by the Institutional Animal Care and Use Committee of the Saban Research Institute of the Children’s Hospital of Los Angeles. Mice in which microtubule-associated protein 1 chain light chain (LC3) had been fused to the green fluorescent protein (GFP) were kindly provided by Dr. Mizushima (Mizushima et al., 2004). To generate POMC-specific Atg7 knockout (Pomc-Cre; Atg7loxP/loxP) mice, Pomc-Cre mice (C57BL/6 background) (Balthasar et al., 2004) were mated to mice carrying a loxP-flanked Atg7 allele (Atg7loxP/loxP) (C57BL/6 background) (Komatsu et al., 2005). Breeding colonies were maintained by mating Pomc-Cre; Atg7loxP/+ mice to Atg7loxP/loxP mice. Animals were genotyped by PCR as described previously (Balthasar et al., 2004; Komatsu et al., 2005). Cre-negative Atg7loxP/loxP were used as controls.
Physiological measures
One day after birth, the litter size was adjusted to 7 pups to ensure adequate and standardized nutrition until weaning. Male and female mice (n ≥ 9 per group) were weighed every two days from P4 to P22 (weaning) and weekly from P28 through P119 using an analytical balance. Glucose tolerance was performed at 8–9 weeks of age (n ≥ 7 per group) by an i.p. administration of glucose (1.5 mg/g body weight) after overnight fasting, and then the blood glucose levels were measured 0, 15, 30, 45, 60, 90, 120, and 150 min following glucose challenge, as previously described (Fan et al., 2000; Howard et al., 2004). Leptin sensitivity test was performed in 7- and 10-week-old male mice (n ≥ 6 per group). Briefly, mice were injected i.p. with vehicle (5 mM sodium citrate buffer) or leptin (3 mg/kg body weight, Peprotech) according to the following scheme: vehicle injections for 5 days, followed by leptin injections for 3 days. Body weight was measured during the injection period. Retroperitoneal and epididymal fat depots were collected at 7 weeks of age and between 15 and 17 weeks of age (n ≥ 5 per group) and weighed.
Hormone assays
Serum leptin and insulin levels were assayed at 7 weeks and between 15 and 17 weeks of age (n = 6–8 per group) using leptin and insulin ELISA kits, respectively (Millipore).
Immunohistochemistry and image analysis
Anesthetized male mice were perfused transcardially with 4% paraformaldehyde. The brains were then frozen and sectioned at 30-um thick and processed for immunofluorescence using standard procedures (Bouret et al., 2004b). The primary antibodies used for IHC were as follows: rabbit anti-GFP (1:10,000, Invitrogen), sheep anti-aMSH (1:40,000, Millipore), rabbit anti-AgRP (1:1,000, Phoenix Pharmaceuticals), rabbit anti-b-endorphin (1:10,000, Millipore), rabbit anti-p62/SQSTM1 (1:1,000, Abcam), and rabbit anti-ubiquitin (1,000, Dako). The primary antibodies were visualized with Alexa Fluor 488 goat anti-rabbit IgGs, or Alexa Fluor 568 goat anti-rabbit IgGs, or Alexa Fluor 568 donkey anti-sheep IgGs (1:200, Invitrogen). Sections were counterstained using bis-benzamide (1:10,000, Invitrogen) to visualize cell nuclei, and coverslipped with buffered glycerol (pH 8.5). Image analysis was performed using ImageJ analysis software (NIH) (Bouret et al., 2008). Additional details appear in Supplemental Experimental Procedures.
Isolated ARH explant cultures
Brains were collected from P4 male mice and sectioned 200-um thick with a vibroslicer as previously described (Bouret et al., 2004b). The ARH was then carefully dissected out of each section under a stereomicroscope. Explants (n = 5–6 cases per group) were cultured onto a rat tail collagen matrix (Upstate). Beginning on the first day in vitro, each explant was transferred to fresh Basal Medium Eagle medium (Invitrogen). After 36 hr, the explants were fixed in paraformaldehyde and neurites extending from the explants were stained with GAP-43 (rabbit, 1:5,000, Millipore) and b-endorphin (mouse, 1:5,000, Millipore). Image analysis was performed using ImageJ analysis software (NIH) (Bouret et al., 2008). See Supplemental Experimental Procedures for additional information.
Electron Microscopy
P24 wild-type male mice were perfused with 4% paraformaldehyde, and their brains were processed for immunolabeling for POMC using a rabbit anti-POMC precursor antibody (1:4,000, Phoenix Pharmaceuticals) for electron microscopy examination. Ultrathin sections were then cut on a Leica ultra-microtome, collected on Formvar-coated single-slot grids, and analyzed with a Tecnai 12 Biotwin electron microscope (FEI).
Immunoblots
Frozen microdissected hypothalami derived from 15-week-old C57BL6 wild-type male mice were immunoblotted as described previously (d'Anglemont de Tassigny et al., 2007). Polyclonal antibodies against LC3 (1:300, Cell Signaling) and b-actin (1:1,000, Sigma) were used in this experiment.
Statistical analysis
All values were expressed as means ± SEM. Statistical analyses were conducted using GraphPad PRISM (version 5.0a). Statistical significance was determined using unpaired two-tailed Student’s t-tests and a two-way ANOVA followed by the Bonferroni post-hoc test when appropriate. P-values less than 0.05 were considered to be statistically significant.
Highlights.
Autophagy is constitutively active in hypothalamic feeding regions
The loss of Atg7 in POMC neurons causes metabolic defects
Atg7 is required for the normal maturation of POMC axonal projections
Supplementary Material
Acknowledgements
We thank Pr. Noboru Mizushima for the generous gift of the LC3-GFP mice. We also would like to thank Li Liu and Julien Maillard for the expert technical assistance. This work was supported by the National Institute of Health (Grant DK84142, to SGB and Grant DK080000 to TLH), the “Fondation pour la Recherche Médicale” (to SGB), the EU FP7 integrated project (grant agreement n° 266408, “Full4Health”, to SGB), the “Agence Nationale de la Recherche” (Grant ANR-08-JCJC-0055-01, to SGB), and the American Diabetes Association (Grant ADA 7-08-MN-25, to TLH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alirezaei M, Kemball CC, Flynn CT, Wood MR, Whitton JL, Kiosses WB. Short-term fasting induces profound neuronal autophagy. Autophagy. 2010;6:702–710. doi: 10.4161/auto.6.6.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua JSC. Leptin Receptor Signaling in POMC Neurons Is Required for Normal Body Weight Homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Belgardt BF, Brüning JC. CNS leptin and insulin action in the control of energy homeostasis. Annals of the New York Academy of Sciences. 2010;1212:97–113. doi: 10.1111/j.1749-6632.2010.05799.x. [DOI] [PubMed] [Google Scholar]
- Bouret SG. Role of Early Hormonal and Nutritional Experiences in Shaping Feeding Behavior and Hypothalamic Development. The Journal of Nutrition. 2010;140:653–657. doi: 10.3945/jn.109.112433. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB. Formation of Projection Pathways from the Arcuate Nucleus of the Hypothalamus to Hypothalamic Regions Implicated in the Neural Control of Feeding Behavior in Mice. J. Neurosci. 2004a;24:2797–2805. doi: 10.1523/JNEUROSCI.5369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB. Trophic Action of Leptin on Hypothalamic Neurons That Regulate Feeding. Science. 2004b;304:108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Gorski JN, Patterson CM, Chen S, Levin BE, Simerly RB. Hypothalamic Neural Projections Are Permanently Disrupted in Diet-Induced Obese Rats. Cell Metabolism. 2008;7:179–185. doi: 10.1016/j.cmet.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecconi F, Levine B. The Role of Autophagy in Mammalian Development: Cell Makeover Rather than Cell Death. Developmental Cell. 2008;15:344–357. doi: 10.1016/j.devcel.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LGD, Clements M, Al-Qassab H, Heffron H, Xu AW, Speakman JR, Barsh GS, Viollet B, Vaulont S, Ashford MLJ, Carling D, Withers DJ. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. The Journal of Clinical Investigation. 2007;117:2325–2336. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RD. Studies on the Physiological Functions of the Melanocortin System. Endocrine Reviews. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- Cota D, Proulx K, Smith KAB, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR Signaling Regulates Food Intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny X, Campagne Cl, Dehouck Bnd, Leroy Dl, Holstein GR, Beauvillain J-C, Bu√©e-Scherrer Vr, Prevot V. Coupling of Neuronal Nitric Oxide Synthase to NMDA Receptors via Postsynaptic Density-95 Depends on Estrogen and Contributes to the Central Control of Adult Female Reproduction. The Journal of Neuroscience. 2007;27:6103–6114. doi: 10.1523/JNEUROSCI.5595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagda RK, Zhu J, Kulich SM, Chu CT. Mitochondrially localized ERK2 regulates mitophagy and autophagic cell stress. Autophagy. 2008;4:770–782. doi: 10.4161/auto.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebato C, Uchida T, Arakawa M, Komatsu M, Ueno T, Komiya K, Azuma K, Hirose T, Tanaka K, Kominami E, Kawamori R, Fujitani Y, Watada H. Autophagy Is Important in Islet Homeostasis and Compensatory Increase of Beta Cell Mass in Response to High-Fat Diet. Cell Metabolism. 2008;8:325–332. doi: 10.1016/j.cmet.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Ellacott K, Cone R. The role of the central melanocortin system in the regulation of food intake and energy homeostasis: lessons from mouse models. Philosophical Transactions of the Royal Society B: Biological Sciences. 2006;361:1265–1274. doi: 10.1098/rstb.2006.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. The Journal of Comparative Neurology. 2005;493:63–71. doi: 10.1002/cne.20786. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Flier JS. NEUROSCIENCE: The Fat-Brain Axis Enters a New Dimension. Science. 2004;304:63–64. doi: 10.1126/science.1096746. [DOI] [PubMed] [Google Scholar]
- Fan W, Dinulescu DM, Butler AA, Zhou J, Marks DL, Cone RD. The Central Melanocortin System Can Directly Regulate Serum Insulin Levels. Endocrinology. 2000;141:3072–3079. doi: 10.1210/endo.141.9.7665. [DOI] [PubMed] [Google Scholar]
- Gao Q, Horvath TL. Neurobiology of Feeding and Energy Expenditure. Annual Review of Neuroscience. 2007;30:367–398. doi: 10.1146/annurev.neuro.30.051606.094324. [DOI] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsu iM, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Bruning JC. Developmental programming of the hypothalamus: a matter of fat. Nat Med. 2006;12:52–53. doi: 10.1038/nm0106-52. [DOI] [PubMed] [Google Scholar]
- Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjorbaek C, Flier JS. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med. 2004;10:734–738. doi: 10.1038/nm1072. [DOI] [PubMed] [Google Scholar]
- Jung HS, Chung KW, Won Kim J, Kim J, Komatsu M, Tanaka K, Nguyen YH, Kang TM, Yoon K-H, Kim J-W, Jeong YT, Han MS, Lee M-K, Kim K-W, Shin J, Lee M-S. Loss of Autophagy Diminishes Pancreatic [beta] Cell Mass and Function with Resultant Hyperglycemia. Cell Metabolism. 2008;8:318–324. doi: 10.1016/j.cmet.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Kaushik S, Rodriguez-Navarro JA, Arias E, Kiffin R, Sahu S, Schwartz GJ, Cuervo AM, Singh R. Autophagy in Hypothalamic AgRP Neurons Regulates Food Intake and Energy Balance. Cell Metabolism. 2011;14:173–183. doi: 10.1016/j.cmet.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nature Reviews Molecular Cell Biology. 2007;8:931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Koike M, Sou Y-s, Ueno T, Hara T, Mizushima N, Iwata J-i, Ezaki J, Murata S, Hamazaki J, Nishito Y, Iemura S-i, Natsume T, Yanagawa T, Uwayama J, Warabi E, Yoshida H, Ishii T, Kobayashi A, Yamamoto M, Yue Z, Uchiyama Y, Kominami E, Tanaka K. Homeostatic Levels of p62 Control Cytoplasmic Inclusion Body Formation in Autophagy-Deficient Mice. Cell. 2007a;131:1149–1163. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, Kominami E, Tanaka K, Chiba T. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. The Journal of Cell Biology. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Wang QJ, Holstein GR, Friedrich VLJ, Iwata J, Kominami E, Chait BT, Tanaka K, Yue Z. Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. PNAS. 2007b;104:14489–14494. doi: 10.1073/pnas.0701311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin B. Metabolic imprinting: critical impact of the perinatal environment on the regulation of energy homeostasis. Phil. Trans R. Soc. Lond B. 2006;361:1107–1121. doi: 10.1098/rstb.2006.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nature Reviews Molecular Cell Biology. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- Malik SA, Marino G, BenYounes A, Shen S, Harper F, Maiuri MC, Kroemer G. Neuroendocrine regulation of autophagy by leptin. Cell Cycle. 2011;10:2917–2923. doi: 10.4161/cc.10.17.17067. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferré P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In Vivo Analysis of Autophagy in Response to Nutrient Starvation Using Transgenic Mice Expressing a Fluorescent Autophagosome Marker. Mol. Biol. Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla SL, Carmody JS, Zeltser LM. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nature Medicine. 2010;16:403–405. doi: 10.1038/nm.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G, Fujikawa T, Fukuda M, Anderson J, Morgan DA, Mostoslavsky R, Stuart RC, Perello M, Vianna CR, Nillni EA, Rahmouni K, Coppari R. SIRT1 Deacetylase in POMC Neurons Is Required for Homeostatic Defenses against Diet-Induced Obesity. Cell Metabolism. 2010;12:78–87. doi: 10.1016/j.cmet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko PE. Toward a new neurobiology of energy balance, appetite, and obesity: the anatomists weigh in. The Jounal of Comparative Neurology. 1998;402:435–441. [PubMed] [Google Scholar]
- Singh R, Xiang Y, Wang Y, Baikati K, Cuervo AM, Luu YK, Tang Y, Pessin JE, Schwartz GJ, Czaja MJ. Autophagy regulates adipose mass and differentiation in mice. The Journal of Clinical Investigation. 2009;119:3329–3339. doi: 10.1172/JCI39228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Grove KL. Metabolic imprinting in obesity. Forum Nutr. 2010;63:186–194. doi: 10.1159/000264406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, Friedman L, Komatsu M, Tanaka K. The cellular pathways of neuronal autophagy and their implication in neurodegenerative diseases. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2009;1793:1496–1507. doi: 10.1016/j.bbamcr.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Goldman S, Baerga R, Zhao Y, Komatsu M, Jin S. Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proceedings of the National Academy of Sciences. 2009;106:19860–19865. doi: 10.1073/pnas.0906048106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.