Abstract
A predominant symptom of nicotine withdrawal is cognitive deficits, yet understanding of the neural basis for these deficits is limited. Withdrawal from chronic nicotine disrupts contextual learning in mice and this deficit is mediated by direct effects of nicotine in the hippocampus. Chronic nicotine treatment upregulates nicotinic acetylcholine receptors (nAChR); however, it is unknown whether upregulation is related to the observed withdawal-induced cognitive deficits. If a relationship between altered learning and nAChR levels exists, changes in nAChR levels after cessation of nicotine treatment should match the duration of learning deficits. To test this hypothesis, mice were chronically administered 6.3 mg/kg/day (freebase) nicotine for 12 days and trained in contextual fear conditioning on day 11 or between 1 to 16 days after withdrawal of treatment. Changes in [125I]-epibatidine binding at cytisine-sensitive and cytisine-resistant nAChRs and chronic nicotine-related changes in α4, α7, and β2 nAChR subunit mRNA expression were assessed. Chronic nicotine had no behavioral effect but withdrawal produced deficits in contextual fear conditioning that lasted 4 days. Nicotine withdrawal did not disrupt cued fear conditioning. Chronic nicotine upregulated hippocampal cytisine-sensitive nAChR binding; upregulation continued after cessation of nicotine administration and the duration of upregulation during withdrawal paralleled the duration of behavioral changes. Changes in binding in cortex and cerebellum did not match behavioral changes. No changes in α4, α7, and β2 subunit mRNA expression were seen with chronic nicotine. Thus, nicotine withdrawal-related deficits in contextual learning are time-limited changes that are associated with temporal changes in upregulation of high-affinity nAChR binding.
Keywords: Nicotine, Addiction, Acetylcholine, Learning, Withdrawal, Receptor Binding
1. Introduction
Statistics attest to the problematic nature of nicotine addiction; over 440,000 deaths per year in the US alone are attributed to smoking (CDC, 2002; Mokdad et al., 2004). Perhaps in recognition of this fact, 70% of smokers indicate they want to quit, but unfortunately only 3-5% of the 42% that attempt to quit are successful (Nides, 2008). What remains unknown is why nicotine addiction is so difficult to treat. Interactions between many environmental and genetic factors contribute to the development and maintenance of nicotine addiction. The multiplicity and variability of these interactions most likely give rise to the difficulty in treating nicotine addiction. Thus understanding these factors should advance the development of treatments for nicotine addiction.
Avoidance of withdrawal symptoms is one factor that contributes to the maintenance of smoking and relapse during quit attempts as the severity and the duration of withdrawal symptoms predict relapse (Piasecki et al., 1998; Piasecki et al., 2000). However, nicotine withdrawal syndrome is complex. While multiple withdrawal symptoms exist, over 65% of smokers cite changes in cognition as a serious withdrawal symptom (Ward et al., 2001). Cognitive changes observed in abstinent smokers include deficits in working memory, verbal memory, digit recall, and associative learning (Jacobsen et al., 2007; Jacobsen et al., 2005; Kleinman et al., 1973; Mendrek et al., 2006; Snyder and Henningfield, 1989).
We have focused on understanding the neurobiology of nicotine withdrawal-associated changes in learning in the mouse. Mice withdrawn from chronic treatment with a dose of nicotine that produces plasma nicotine levels in the range seen in smokers showed deficits specifically in hippocampus-dependent learning such as contextual fear conditioning, trace conditioning, and spatial object recognition (Davis et al., 2005; Kenney et al., 2011; Raybuck and Gould, 2009). This work is complemented by independent investigations demonstrating that changes in cognition during periods of abstinence predicted relapse for smokers (Patterson et al., 2010; Rukstalis et al., 2005). In order to understand and treat nicotine withdrawal-related changes in cognition, it is important to identify the underlying neural substrates. These withdrawal deficits in learning are mediated by nicotinic acetylcholinergic receptors (nAChRs) containing the β2 subunits (β2* nAChR) but not by those containing the α7 subunit (α7 nAChR) (Davis and Gould, 2009; Portugal et al., 2008). Direct drug infusion studies have demonstrated that nicotine specifically acts in the hippocampus to produce withdrawal-induced changes in learning (Davis and Gould, 2009). While these studies have helped define the cognitive effects of nicotine withdrawal and identified an underlying nAChR subtype and brain region, the duration of these deficits and the critical neural changes responsible for the deficits remain unknown.
With chronic nicotine treatment, nAChRs upregulate (Marks et al., 1983; Schwartz and Kellar, 1983) and this upregulation lasts beyond cessation of nicotine treatment. Abstinent smokers compared to nonsmokers had higher levels of β2* nAChRs, which returned to baseline between 3-12 weeks, and elevated nAChR levels correlated with urge to smoke (Cosgrove et al., 2009; Mamede et al., 2007; Staley et al., 2006). Similarly, a study in mice indicated that α4β2* nAChRs binding sites increased during chronic nicotine treatment and returned to control levels following cessation of treatment; these changes were related to the development and loss of tolerance (Marks et al., 1985). It is not clear if similar changes contribute to cognitive withdrawal deficits.
If nAChR upregulation contributes to withdrawal symptoms, one would predict that the duration of receptor upregulation would parallel the duration of withdrawal symptoms. While this prediction is straightforward, testing it has been confounded by the observation that different brain regions and nAChR subtypes have different upregulation parameters (Collins et al., 1989; Mao et al., 2008; Perry et al., 2007) and a lack of understanding of which brain regions are critically involved in specific withdrawal symptoms. However, withdrawal from chronic nicotine infusion into the hippocampus, but not cortex or thalamus, produced deficits in contextual fear conditioning (Davis and Gould, 2009). Furthermore, infusion of DHβE (an α4β2 nAChR antagonist) into the hippocampus of mice treated systemically with chronic nicotine precipitated withdrawal deficits in contextual fear conditioning (Davis and Gould, 2009). These experiments demonstrate that the hippocampus is necessary and sufficient for nicotine withdrawal-related disruption of contextual fear conditioning. Thus, the prediction emerges that if nAChR upregulation contributes to learning-related withdrawal deficits, then the duration of nAChR upregulation in the hippocampus should parallel the duration of withdrawal deficits in contextual fear conditioning. The present experiments examined the duration of nicotine withdrawal deficits in contextual fear conditioning, the duration of changes in cytisine-sensitive (i.e., α4β2* (Marks et al., 2006); * indicates potential additional nAChRs subunits) and resistant (i.e., α3β2*, α3β4*, and α6β2* (Marks et al., 2006)) nAChR binding sites measured with radiolabeled epibatidine in cortex, hippocampus, and cerebellum after nicotine withdrawal, and chronic nicotine-related changes in hippocampal nAChR subunit mRNA expression.
2. Material and Methods
2.1 Subjects
Male C57BL/6J mice (8 and 12 weeks of age; n= 5-13 per group, depending on experiment) (Jackson Laboratory, Bar Harbor, ME) weighing between 20-28 g were group housed four to a cage with ad libitum food and water. This strain was chosen because it performs well in fear conditioning (Owen et al., 1997) and is sensitive to the effects of nicotine on learning (Kenney and Gould, 2008). The light-dark cycle was 12:12 h with lights on at 07.00; all testing occurred between 08.00 and 17.00. The Temple University Institutional Animal Care and Use Committee approved all behavioral procedures.
2.2 Apparatus
Training and context testing took place in four identical conditioning chambers (18 × 19 × 38 cm; Plexiglas walls in the front and back, and stainless steel on the sides) housed in sound-attenuating boxes with a tan interior (MED Associates, St. Albans, VT, USA). Ventilation provided air exchange and background noise (69 dB). A speaker, mounted on the right wall of each chamber, produced the 85-dB white noise conditioned stimulus (CS). The shock unconditioned stimulus (US) was a 0.57 mA foot shock for 2 s delivered via grid floors. A computer running MED-PC software controlled stimuli administration. The CS test, which occurred one hour after contextual testing on test day, took place in a separate room with altered conditioning chambers that differed in size (20 × 23 × 19 cm), construction, and environmental cues; specifically, the chamber walls were constructed from Plexiglas on all sides, the floors were white plastic, the speakers were mounted on the left wall of each chamber, a vanilla extract olfactory cue was added, and the sound attenuating boxes that housed the chambers had black interior walls. All chambers were cleaned with 70% ethanol before each subject was tested.
2.3 Drug and Administration
Mini-osmotic pumps (model 1002; Alzet, Cupertino, CA) containing 100 μl of saline or nicotine bitartrate (Sigma Co., St Louis, MO, USA) dissolved in saline were implanted subcutaneously under aseptic conditions with 5% isoflurane as the anesthetic. Solutions were administered at a rate of 0.25 l/hour to deliver 6.3 mg/kg/day of nicotine freebase. Pumps were removed 12 days after surgery under aseptic conditions with 5% isoflurane as an anesthetic. The dose of nicotine was chosen based on our prior research demonstrating that it produced withdrawal deficits in contextual fear conditioning (André et al., 2008; Davis and Gould, 2007b) with plasma nicotine levels of approximately 13.00 ng/ml (Davis et al., 2005), which is within the range seen in smokers (Henningfield and Keenan, 1993).
2.4 Fear conditioning
Training lasted 5 minutes and 30 seconds. Baseline freezing behavior was recorded during the first 120 seconds of the training session followed by two co-terminating CS (30 second, 85 dB white noise)-US (2 second, 0.57 mA foot shock) presentations at 120 and 270 seconds. Immediate freezing behavior was recorded during the 120 second intertrial interval. Mice remained in the chamber 30 seconds after the second CS-US presentation. During training and testing, each mouse was judged as either freezing or active once every 10 seconds (Gould and Higgins, 2003). Freezing was defined as an absence of visible movement except for respiration (Blanchard and Blanchard, 1969).
Twenty-four hours after training, mice were placed in the training chamber and contextual freezing behavior was recorded for 5 minutes. An hour after testing for contextual freezing, freezing to the CS was evaluated. Mice were placed in the altered context chamber for 6 minutes. During the first 180 seconds (preCS period), freezing in the absence of the CS was assessed to measure generalized freezing. During the final 180 seconds, freezing to the CS was assessed (cued freezing).
Two sets of mice were tested. The first set established the duration of withdrawal-induced deficits in contextual fear conditioning examining days 1-8, and day 16 after nicotine withdrawal. Previous work demonstrated that nicotine withdrawal disrupts the acquisition but not the recall of contextual fear conditioning (Portugal and Gould, 2009) and therefore withdrawal time points are discussed in relationship to training. The second set of mice was used for the binding studies. Mice were conditioned on the 11th day of chronic treatment or days 1-6 days after nicotine withdrawal. Half of the mice were used for the binding data and the other half were tested to replicate the first set; for the mice behaviorally tested only baseline freezing, immediate freezing, and contextual conditioning were measured because no effects were seen for cued fear conditioning in the first set. The first set of mice and the second set were tested by independent investigators that were blind to experimental conditions. Prior to the study, inter-rater reliability was established at >95%. Behavior was scored live.
2.5 Nicotinic acetylcholine receptor binding
Mice were euthanized and the cortex, cerebellum, and hippocampus were dissected and homogenized in ice-cold hypotonic buffer (NaCl, 14.4 mM; KCl, 0.2 mM; CaCl2, 0.2 mM; MgSO4, 0.1 mM, HEPES, 2.0 mM; pH = 7.5) using a glass-Teflon tissue grinder. Particulate fraction was obtained by centrifugation at 20,000g for 10 min in a Sorvall RC-2B centrifuge. Pellets were resuspended in fresh homogenization buffer, incubated at 37°C for 10 min, and collected after centrifugation. Samples washed three more times by resuspension and centrifugation were stored in homogenization buffer at −70°C until use.
[125I]-epibatidine binding was measured as described previously (Whiteaker et al., 2000). Frozen, washed pellets were resuspended in the overlying buffer and centrifuged at 20,000g for 10 min. The supernatant was discarded and the pellet was resuspended in ice-cold solution. Resuspension volume varied among brain regions and was adjusted such that less than 10% of the [125I]-epibatidine was bound to the protein at the highest ligand concentration. α-bungarotoxin binding, which would assess α7 nAChR binding, was not examined because α-bungarotoxin binding returns to baseline almost immediately after chronic treatment (Marks et al., 1985) and because prior work has shown that α7nAChRs are not involved in the effects of nicotine on contextual fear conditioning or nicotine withdrawal-associated deficits in hippocampus-dependent learning (André et al., 2011; Davis and Gould, 2006; Davis and Gould, 2007a; Davis and Gould, 2009; Portugal et al., 2008; Raybuck and Gould, 2009).
Samples were incubated in 96-well polystyrene plates for 2 hours at room temperature in a final incubation volume of 30 l with either buffer (total) or buffer containing cytisine (50nM) or cytisine (100 μM), along with 200 pM [125I]-epibatidine. Buffer composition was (NaCl 144 mM; KCl, 1.5 mM; CaCl2, 2.0 mM; MgSO4, 1.0 mM, HEPES, 25 mM; pH = 7.5). The 100 μM cytisine defines non-specific binding whilst the total and 50 nM cytisine enables determination of cytisine-sensitive and cytisine-resistant binding sites (of note cytisine-resistant sites are a relatively small fraction of total epibatidine binding in cortex and hippocampus) (Marks et al., 1998). After incubation, samples were diluted with 200 l of ice-cold wash buffer and filtered under vacuum (0.2 atm) onto glass fiber filters treated with 0.5% polyethelenimine (top filter, MFS Type B; bottom filter, TypeA/E, Pall Bioscience). An Inotech Cell Harvester (Inotech Biosystems International, Rockville, MD) collected the samples, which were subsequently washed six times with ice-cold buffer. Filters were transferred to a 96-well scintillation plate and counted using a Wallac TriLux 1450 MicroBeta scintillation counter (PerkinElmer Life and Analytical Sciences, Waltham, MA) at 30% efficiency after the addition of 150μl of Optiphase Supramix scintillation mixture (PerkinElmer Life and Analytical Sciences, Waltham, MA). Protein was measured using the Lowry method (Lowry et al., 1951) with bovine serum albumin standard.
2.6 RNA extraction and RT-qPCR
The effects of chronic nicotine administration (6.3 mg/kg/day for 12 days, sc) and withdrawal from chronic nicotine administration on hippocampal α4, α7, and β2 nAChR subunit mRNA were measured. On the 12th day of chronic nicotine or saline administration, or 24 hours following cessation of chronic treatment, mice were euthanized and whole hippocampi were rapidly dissected, placed in RNA later (Sigma-Aldrich, St. Louis, MO), and stored at −80° C. Total RNA was isolated from tissue using the RNAqueous Kit (Ambion, Austin, TX) and Turbo DNA Free (Ambion, Austin, TX) was used to remove possible genomic DNA contamination. RNA quantity and purity were assessed using the NanoDrop 2000 (Thermo Scientific,Wilmington, DE). RNA was reverse transcribed and RT-qPCRs were performed using the Power SYBR Green RNA-to-CT 1-step kit (Applied Biosystems, Austin, TX).
RT-qPCRs were performed in triplicate in a 20 μL volume in 96-well plates using 20 μg RNA, 200 nM concentration of primers, 10 uL Power SYBR Green RT-PCR master mix, and 0.16 uL of RT Enzyme mix per well. Reactions were carried out in the 7500 Fast Real-time PCR system (Applied Biosystems, Austin, TX) using the following conditions: reverse transcription for 30 minutes at 48°C, and polymerase activation for 10 minutes at 95°C followed by 40 cycles of 15 seconds at 95°C and 60 seconds at 60°C. The mRNA expression levels for each gene of interest were normalized by the geometric mean of two housekeeping genes, GAPDH and HPRT (Vandesompele et al., 2002), and relative expression was calculated with qbasePLUS software via the Ct method (Hellemans et al., 2007). All primers (see Table 1) were tested for efficiency (100 ± 10%) prior to use and specificity was examined using melt curves and running RT-qPCR products on an ethidium bromide stained agarose gel visualized via UV fluorescence.
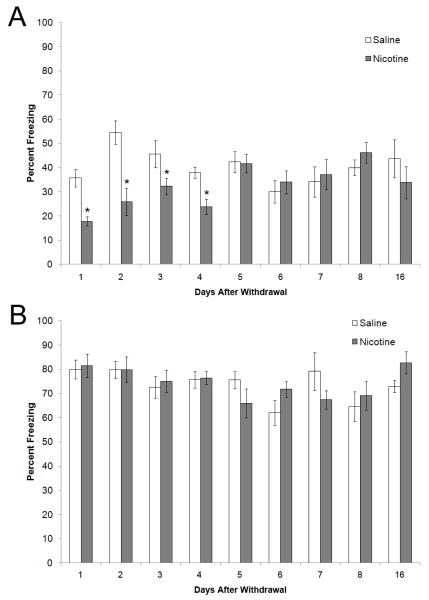
Figure 1.
The time course of contextual and cued fear conditioning after nicotine withdrawal. A) Contextual fear conditioning was examined 1-8, and 16 days after cessation of nicotine treatment; deficits in learning were seen on days 1-4. B) Cued fear conditioning was examined 1-8, and 16 days after cessation of nicotine treatment; no deficits were seen. All time points are separate groups of mice. * = significant difference from saline (n=7-13). Data are presented as mean and standard error of the mean.
2.7 Statistical Analysis
Data was analyzed with two-way ANOVAs (SPSS Version 16.0) and when an interaction or main effect of drug treatment was detected, planned independent samples t-tests with Bonferroni corrections were conducted comparing treatment conditions at different time points. Expression data are represented as fold change compared to control group. Results were considered significant at p≤0.05. All data are expressed as means ± SEM.
3. Results
3.1 Behavioral results
For the experiment in which mice were conditioned 1 day, 2 days, 3 days, 4 days, 5 days, 6 days, 7 days, 8 days, or 16 days after nicotine withdrawal, a 2 × 9 (treatment × time) ANOVA revealed a significant main effect for treatment [F(1, 150) = 13.56, p < 0.05], time [F(8, 150) = 2.79, p < 0.05], and a significant interaction between treatment and time [F(17, 150) = 3.30, p < 0.05]. Planned Bonferroni comparisons revealed that nicotine withdrawal disrupted contextual fear conditioning 1, 2, 3, and 4 days post withdrawal (p < 0.05) but no significant effect of nicotine withdrawal was seen at days 5, 6, 7, 8 or 16 (Figure 1a). There was no significant effect of nicotine withdrawal on baseline freezing, generalized freezing, or freezing to the cue (data for cued shown in Figure 1b).
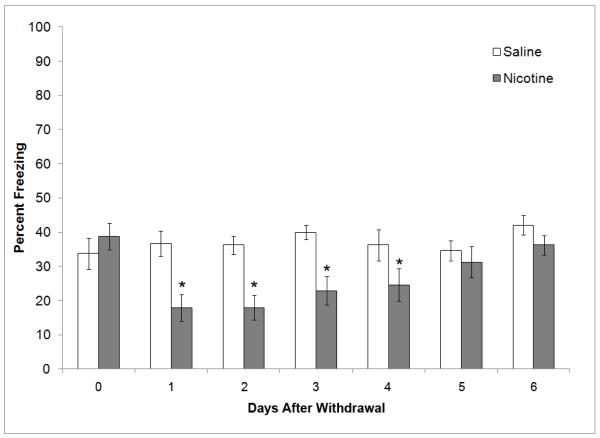
For the mice that were behaviorally tested as part of the binding study, a 2 × 7 (treatment × time) ANOVA revealed a significant main effect for treatment [F(1, 98) = 28.53, p < 0.05], time [F(6, 98) = 3.24, p < 0.05], and a significant interaction between treatment and time [F(13, 98) = 3.30, p < 0.05]. Bonferroni corrected post hoc comparisons revealed that withdrawal deficits were again seen at 1, 2, 3, and 4 days after withdrawal (p < 0.05), whereas no effects were observed in mice treated with chronic nicotine and mice withdrawn from chronic nicotine for 5 or 6 days (Figure 2). Furthermore, no effects of chronic nicotine or nicotine withdrawal were observed in baseline freezing or immediate freezing for all groups.
Figure 2.
The effects of chronic nicotine and withdrawal from chronic nicotine on contextual fear conditioning. A second set of animals were tested by an independent investigator to examine behavior on the last day of chronic treatment and to replicate withdrawal findings. Chronic nicotine had no effect on contextual fear conditioning but withdrawal deficits in learning were seen on the first 4 days of withdrawal. All time points are separate groups of mice. * = significant difference from saline (n=8). Data are presented as mean and standard error of the mean.
3.2 Nicotinic acetylcholine receptor binding
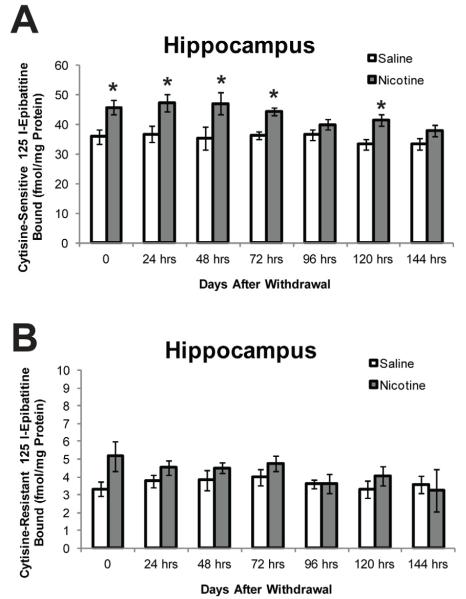
Cytisine-sensitive and resistant nAChR binding was measured in the cortex, hippocampus, and cerebellum during chronic nicotine treatment and at 1 – 6 days after mini-osmotic pump removal. For cytisine-sensitive nAChR binding in the hippocampus, a 2 × 7 (treatment × time) ANOVA revealed a significant main effect for treatment [F(1, 98) = 43.67, p < 0.05] and time [F(6, 98) = 3.05, p < 0.05], whereas the interaction between treatment and time was not significant. Post hoc tests revealed that cytisine-sensitive nAChR binding in the hippocampus was significantly greater in mice treated with chronic nicotine and in mice withdrawn from chronic nicotine for 1, 2, 3, or 5 days (p < 0.05; Figure 3). This upregulation of nAChRs in the hippocampus was consistent with the time course of nicotine withdrawal-related deficits in contextual learning (Figures 1 & 2). No significant differences in cytisine-resistant binding in the hippocampus were observed at any of the time points tested.
Figure 3.
The time course of cytisine-sensitive and cytisine-resistant epibatidine binding in the hippocampus. Chronic nicotine was associated with significantly increased binding in (cytisine-sensitive), and withdrawal was associated with significantly increased binding in the hippocampus (days 1-3 and 5 cytisine-sensitive). All time points are separate groups of mice. * = significant difference from saline (n=8). Data are presented as mean and standard error of the mean.
Cytisine-sensitive nAChR binding in the cortex was examined with a 2 × 7 (treatment × time) ANOVA (see Table 2 for group means and standard error). A significant main effect for treatment [F(1, 98) = 10.18, p < 0.05] was observed, whereas the main effect of time and the interaction between treatment and time were not significant. For cytisine-resistant nAChR binding in the cortex, a 2 × 7 (treatment × time) ANOVA revealed a significant main effect for treatment [F(1, 98) = 21.68, p < 0.05], but the main effect of time and the interaction between treatment and time were not significant. Planned Bonferroni comparisons revealed that cytisine-sensitive nAChR binding in the cortex was significantly increased only 3 days after withdrawal from chronic nicotine, and cytisine-resistant nAChR binding was elevated in mice treated with chronic nicotine and in mice withdrawn from chronic nicotine for 3 days (p < 0.05).
Table 2.
Cerebellar and cortical cytisine-sensitive and cytisine-resistant nAChR binding data
| Cerebellum | Hours After Withdrawa1 | ||||||
|---|---|---|---|---|---|---|---|
| Cytisine Sensitive | 0 | 24 hrs | 48hrs | 72hrs | 96hrs | 120 hrs | 144 hrs |
| Saline | 13.80 | 15.59 | 15.17 | 12.95 | 10.49 | 10.84 | 13.48 |
| Standard Error | 1.24 | 1.29 | 1.27 | 0.73 | 1.73 | 1.09 | 0.92 |
| Nicotine | 21.71 * | 18.29 | 18. 16 | 13.47 | 16.65 * | 14.19 | 15.30 |
| Standard Error | 0.94 * | 1.10 | 1.24 | 0.60 | 1.49 * | 1.56 | 0.89 |
|
| |||||||
| Cytisine Resistant | 0 | 24hrs | 48hrs | 72hrs | 96hrs | 120 hrs | 144 hrs |
|
| |||||||
| Saline | 4.09 | 4.89 | 4.72 | 4.14 | 5.24 | 5.50 | 5.50 |
| Standard Error | 0.18 | 0.21 | 0.20 | 0.23 | 0.26 | 0.29 | 0.28 |
|
| |||||||
| Nicotine | 4.88 | 4.62 | 5.47 | 4.11 | 5.86 | 5.93 | 5.45 |
| Standard Error | 0.33 | 0.47 | 0.31 | 0.13 | 0.53 | 0.31 | 0.26 |
| Cortex | Hours After Withdrawa1 | ||||||
|---|---|---|---|---|---|---|---|
| Cytisine Sensitive | 0 | 24hrs | 48hrs | 72hrs | 96hrs | 120 hrs | 144 hrs |
| Saline | 36.26 | 34.81 | 37.03 | 35.35 | 26. 87 | 39.61 | 36.90 |
| Standard Error | 3.90 | 4.13 | 6.16 | 4.86 | 3.59 | 4.19 | 2.74 |
| Nicotine | 49.17 | 40.78 | 46.34 | 51.85* | 38.82 | 40.93 | 32.45 |
| Standard Error | 4.84 | 1.34 | 4.49 | 3,79* | 4.46 | 6.06 | 5.95 |
|
| |||||||
| Cytisine Resistant | 0 | 24hrs | 48hrs | 72hrs | 96 hrs | 120 hrs | 144 hrs |
|
| |||||||
| Saline | 4.15 | 4.43 | 3.87 | 4.16 | 3.86 | 4.15 | 3.77 |
| Standard Error | 0.27 | 0.32 | 0.21 | 0.19 | 0.23 | 0.38 | 0.16 |
| Nicotine | 5.76 * | 5.13 | 4.99 | 5.65 * | 4.99 | 4.25 | 4.17 |
| Standard Error | 0.43 * | 0.47 | 0.50 | 0.73 * | 0.24 | 0.45 | 0.29 |
Indicates significant difference between nicotine and saline. Data presented as mean and standard error of the mean.
In the cerebellum, cytisine-sensitive nAChR binding was with a 2 × 7 (treatment × time) ANOVA (see Table 2 for group means and standard error). A significant main effect for treatment [F(1, 98) = 32.59, p < 0.05], time [F(6, 98) = 6.12, p < 0.05], and a significant interaction between treatment and time [F(13, 98) = 2.31, p < 0.05] were observed. Subsequent Bonferroni post-hoc tests revealed that cytisine-sensitive nAChR binding was significantly increased in mice treated with chronic nicotine and in mice withdrawn from chronic nicotine for 4 days relative to saline treated mice (p < 0.05). Cytisine-resistant binding in the cerebellum was not significantly different between groups. Changes in nAChR binding in the cortex and cerebellum were not consistent with the effects of nicotine withdrawal on contextual fear conditioning.
3.3 Quantification of nAChR mRNA expression on the 12th day of chronic nicotine administration and following one day of withdrawal from chronic nicotine administration
The effect of chronic nicotine exposure on hippocampal nAChR mRNA expression was examined with RT-qPCR. Changes in CHRNA4, CHRNA7, and CHRNB2 mRNA levels were assessed on the 12th day of chronic nicotine (6.3 mg/kg/day, sc) or saline administration. No significant change in mRNA expression was seen for any of the receptor subtypes examined. The effect of 24 hours of withdrawal from chronic nicotine administration on hippocampal CHRNA4, CHRNA7, and CHRNB2 mRNA expression were also assessed. No change in mRNA level expression was seen for any of the nAChR subtypes examined (p’s > 0.05).
4. Discussion
The results from this study aid in understanding the impact of nicotine withdrawal on learning and the underlying neural changes. It was demonstrated that withdrawal from chronic nicotine produces a deficit in the learning of contextual information that remained for four days after cessation of nicotine administration. Chronic nicotine treatment upregulated nAChRs in the cerebellum, cortex, and hippocampus but only the duration of nAChR upregulation in the hippocampus paralleled the duration of the withdrawal-associated deficits in contextual learning. Finally, chronic nicotine did not produce any significant changes in α4, α7, or β2 nAChR subunit mRNA expression in the hippocampus.
In smokers, cessation of smoking is associated with cognitive deficits that include altered associative learning and working memory (Hughes, 2007; Jacobsen et al., 2007; Jacobsen et al., 2005; Mendrek et al., 2006). While these deficits are a major feature of the nicotine withdrawal syndrome, they are not global deficits affecting all forms of cognition but instead affect selected cognitive tasks. This is in agreement with the present study that found that withdrawal from chronic treatment with a dose of nicotine that produces plasma nicotine levels in mice similar to those seen in smokers (Davis et al., 2005) resulted in a time-limited disruption of contextual fear conditioning with no effect on cued fear conditioning. The withdrawal deficit in contextual learning was no longer present by the 5th day after cessation of nicotine treatment. The fact that the withdrawal deficits were time-limited demonstrates that they are not due to long-lasting neurotoxic effects but instead resemble withdrawal symptoms in humans that dissipate with time (Hughes et al., 1994). In addition, the finding that withdrawal disrupted contextual but not cued fear conditioning adds to a growing literature that, at least in the mouse model, nicotine withdrawal disrupts hippocampus-dependent learning but not hippocampus-independent learning as contextual fear conditioning requires the hippocampus whereas cued fear conditioning does not (Fanselow et al., 1994; Logue et al., 1997; Phillips and Ledoux, 1992). This hippocampal specificity for learning-related withdrawal deficits has been shown for other learning tasks such as trace conditioning and spatial object recognition (Kenney et al., 2011; Raybuck and Gould, 2009), and direct drug infusion studies have confirmed that chronic nicotine is acting directly in the hippocampus to produce the withdrawal symptoms when treatment ceases (Davis and Gould, 2009). Because nicotine effects in the hippocampus are necessary and sufficient to produce the withdrawal-related learning deficits, neurobiological changes that underlie these deficits must occur in the hippocampus.
As discussed earlier, chronic nicotine treatment is associated with upregulation of nAChRs (Marks et al., 1983; Schwartz and Kellar, 1983), and one interesting possibility is that this upregulation contributes to withdrawal-related deficits in learning. Central nervous system nAChRs are broadly divided into two groups, α7 nAChRs and non-α7 nAChRs. Non-α7 nAChRs show a longer lasting upregulation than α7 nAChRs, which return to baseline almost immediately after cessation of nicotine treatment (Marks et al., 1985). The non-α7 nAChRs, which are measured by high affinity epibatidine binding, can be further subdivided by sensitivity to cytisine binding. Cytisine-sensitive binding is associated with α4β2* nAChRs whereas cytisine-resistant binding is associated with α3β2*, α3β4*, and α6β2* nAChRs (Marks et al., 2006). In the present study, chronic treatment with nicotine increased cytisine-sensitive binding in the cerebellum and hippocampus and cytisine-resistant binding in the cortex, suggesting that different nAChR subtypes are upregulated in the cortex compared to cerebellum and hippocampus. The reversal of upregulation after cessation of chronic nicotine treatment varied among brain regions. In cerebellum, cytisine-sensitive binding returned to baseline immediately with only significantly more binding seen on the 4th day after withdrawal. In cortex, cytisine-resistant binding returned to baseline immediately, with only significantly more binding seen on the 4th day after withdrawal; a significant difference in cytisine-sensitive binding in cortex was seen only on the 3rd day after withdrawal.
Of the three areas examined, the binding in the hippocampus may be the most interesting. Cytisine-sensitive binding in the hippocampus remained elevated after withdrawal with significantly increased levels of binding seen in the nicotine withdrawn mice on the 1st, 2nd, 3rd, and 5th days after cessation of nicotine treatment. No change in cytisine-resistant binding, which was at a lower level than cytisine-sensitive binding, was seen in the hippocampus. The duration of increased cytisine-sensitive binding in the hippocampus was highly similar to the duration of nicotine withdrawal-associated deficits in contextual learning. This finding fits well with our prior data demonstrating that direct nicotine effects in the hippocampus mediate withdrawal-related deficits in contextual learning (Davis and Gould, 2009), that direct infusion of acute nicotine into the hippocampus enhances learning (Davis et al., 2007; Gulick and Gould, 2009; Raybuck and Gould, 2010), and acute nicotine ameliorates withdrawal-related deficits in hippocampus-dependent learning (Davis et al., 2005). Together, current and prior research suggests that changes in hippocampal nAChR function, potentially α4β2 nAChRs, may be an important contributing factor to these deficits. However, it should be noted that learning deficits dissipated by day 5 but hippocampus binding was elevated at day 5, suggesting that either a level of upregulation that must occur before deficits are seen or other factors contribute to the deficits.
No changes in α4, α7, and β2 nAChR subunit mRNA expression in the hippocampus on the 12th day of chronic nicotine treatment were found. The α4, α7, and β2 nAChR subunits were targeted because they show high expression in the hippocampus (Baddick and Marks, 2011) and hippocampal β2* nAChRs are involved in nicotine withdrawal learning deficits (Davis and Gould, 2009; Portugal et al., 2008; Raybuck and Gould, 2009). Our finding replicates prior in situ hybridization work that found no chronic nicotine-associated change in hippocampal (and other areas) α4 or β2 mRNA levels (Marks et al., 1992; Pauly et al., 1996); though a study using northern blot analysis of PC12 cells found an increase in β2 mRNA and a decrease in α3 mRNA with chronic nicotine treatment (Madhok et al., 1995). Thus, upregulation of α4β2 nAChRs may involve an increase in receptor protein through post-translational events that may include changes in nAChR maturation and trafficking (Darsow et al., 2005; Kane et al., 2004; Lomazzo et al., 2011; Rezvani et al., 2007).
Based on current results and prior studies, a model of a potential underlying mechanism of nicotine withdrawal-related deficits in hippocampus-dependent learning can be put forth. This model is extrapolated from an earlier model proposed by Dani and Heinemann (1996) in which during periods of low nicotine levels, upregulated nAChRs would be excessively responsive to ACh. Chronic nicotine treatment results in an upregulation of β2* nAChRs (Marks et al., 1983; Schwartz and Kellar, 1983) but also a desensitization of nAChRs (Gentry and Lukas, 2002). This combined upregulation and desensitization that occurs while nicotine is present may help to maintain a functional homeostatic state for the nACh system and thus support proper cognitive functioning. When nicotine is no longer present for an extended period of time (i.e, nicotine withdrawal), the desensitized nAChRs may return to a functional state while upregulation persists. This could result in a hyper-sensitive nACh system, which could produce cognitive deficits. In support, nicotine withdrawal was associated with a persistent increase in hippocampal CA1pyramidal cell activity (Penton et al., 2011). Furthermore, in vitro studies have shown that nAChRs return from a desensitized state in minutes to hours (see Gentry and Lukas (2002) for review). In vivo, Pietilä et al. (1998) found that a 7 week chronic nicotine treatment produced an increase in cortical nAChR binding that lasted over 3 days after cessation of chronic treatment but tolerance to the acute locomotor stimulatory effects of nicotine only lasted 1 day. As it has been proposed that tolerance is related to nAChR desensitization (Robinson et al., 2007), these findings thus suggest that upregulation lasts longer than desensitization.
If nicotine withdrawal deficits in learning and cognition are due to a hyper-sensitive nAChR system that results from functional upregulation, then drugs that ameliorate withdrawal symptoms should desensitize or dampen nAChR signaling. It has been shown that nicotine, varenicline, and bupropion ameliorate nicotine withdrawal-associated deficits in hippocampus-dependent learning (Davis et al., 2005; Portugal and Gould, 2007; Raybuck et al., 2008). Nicotine could ameliorate deficits through desensitizing nAChRs, bringing the system back towards baseline. Similarly, varenicline is a partial agonist and thus could ameliorate the withdrawal deficits by occupying nAChRs. Papke and colleagues (2011) provide support for this position by demonstrating that while varenicline provided weak tonic activation of the nACh system, it also decreased the effects of full agonists. Finally, bupropion is a noradrenergic reuptake inhibitor but also a noncompetitive nAChR antagonist (Slemmer et al., 2000). The nAChR antagonist property of bupropion could dampen nAChR response during withdrawal alleviating withdrawal symptoms, if the symptoms are due to a hyper-sensitive nACh system. These results would suggest that a nAChR antagonist could work as a smoking cessation aid. In support, Rose and colleagues (1994) compared the effectiveness of the nicotine patch versus the nicotine patch paired with administration of the nAChR antagonist mecamylamine on maintenance of abstinence and found that patients receiving the paired treatment had a 9 times higher rate of abstinence at 12 months after quitting. The current data and these studies support a model of nicotine withdrawal where withdrawal deficits in cognition are related to increased sensitivity of the nACh system; however, this model is not without limitations.
In summary, the present results suggest that changes in nAChR upregulation may be an important factor in nicotine withdrawal-related deficits in hippocampus-dependent learning. Upregulation of hippocampal nAChRs and withdrawal deficits in hippocampus-dependent learning shared a common time course. The present results and prior work (Davis and Gould, 2009; Portugal et al., 2008; Raybuck and Gould, 2009) suggest that these effects are mediated by β2* nAChRs. This is in agreement with results from studies of abstinent smokers showing a relationship between β2* nAChR concentration and cravings to smoke to reduce withdrawal symptoms (Staley et al., 2006). However, it should be noted that not all withdrawal symptoms are mediated by β2* nAChRs; for example, β4*, α5*, and α7 nAChRs, but not β2* nAChRs, contribute to somatic withdrawal symptoms (Jackson et al., 2008; Salas et al., 2007; Salas et al., 2004). Because different withdrawal symptoms involve different nAChRs and brain regions and because upregulation varies across brain regions and nAChR types, a future question is whether nAChR upregulation is universally associated with all withdrawal systems. Finally, a caveat is that the current results show a correlational relationship but have not demonstrated causation. Further in vitro and in vivo studies on the role of receptor-related changes in withdrawal are needed.
Highlights.
The duration of nicotine withdrawal-related deficits in learning is 4-5 days
Changes in hippocampal cytisine-sensitive binding parallel changes in behavior
Chronic nicotine does not increase α4, β2, or α7 subunit mRNA expression
Table 1.
Primers used for RT-qPCR.
| Gene | Forward (5′ – 3′) | Reverse (5′ – 3′) |
|---|---|---|
| CHRNA4 | GAAGCAGGAGTGGCATGACT | GAACAGGTGGGCTTTGGTTA |
| CHRNA7 | CCGTGTACTTCTCCCTGAGC | TCAAAGCGTTCATCTGCACT |
| CHRNB2 | GAGTGTGAGGGAGGATTGGA | GGGAGCTGAGTGGTCAGAGT |
| Hprt | TTGCTGACCTGCTGGATTACA | CCCCGTTGACTGATCATTACA |
| GAPDH | TGCACCACCAACTGCTTAGC | GGCATGGACTGTGGTCATGA |
Acknowledgments
The authors would like to acknowledge grant support from the National Institute of Drug Abuse (DA01749, DA024787, TJG; CA143187, PI Caryn Lerman; DA003194, MJM). JWK was supported by a NIDA training grant (DA07237). All behavioral procedures were performed in accordance with NIH guidelines for the use of laboratory animals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- André JM, Gulick D, Portugal GS, Gould TJ. Nicotine Withdrawal Disrupts Both Foreground and Background Contextual Fear Conditioning but not Pre-Pulse Inhibition of the Acoustic Startle Response in C57BL/6 Mice. Behavioural Brain Research. 2008;190:174–181. doi: 10.1016/j.bbr.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André JM, Leach PT, Gould TJ. Nicotine ameliorates NMDA receptor antagonist-induced deficits in contextual fear conditioning through high-affinity nicotinic acetylcholine receptors in the hippocampus. Neuropharmacology. 2011;60:617–625. doi: 10.1016/j.neuropharm.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddick CG, Marks MJ. An autoradiographic survey of mouse brain nicotinic acetylcholine receptors defined by null mutants. Biochemical Pharmacology. 2011;82:828–841. doi: 10.1016/j.bcp.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Passive and active reactions to fear-eliciting stimuli. J. Comp. Physiol. Psychol. 1969;68:129–135. doi: 10.1037/h0027676. [DOI] [PubMed] [Google Scholar]
- CDC Cigarette smoking among adults - United States. MMWR. 2002;51:642–645. [PubMed] [Google Scholar]
- Collins AC, Marks MJ, Pauly JR. Differential effect of chronic nicotine treatment on nicotinic receptor numbers in various brain regions of mice. J. Subst. Abuse. 1989;1:273–286. [PubMed] [Google Scholar]
- Cosgrove KP, Batis J, Bois F, Maciejewski PK, Esterlis I, Kloczynski T, Stiklus S, Krishnan-Sarin S, O’Malley S, Perry E, Tamagnan G, Seibyl JP, Staley JK. β2-Nicotinic Acetylcholine Receptor Availability During Acute and Prolonged Abstinence From Tobacco Smoking. Arch Gen Psychiatry. 2009;66:666–676. doi: 10.1001/archgenpsychiatry.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Heinemann S. Molecular and Cellular Aspects of Nicotine Abuse. Neuron. 1996;16:905–908. doi: 10.1016/s0896-6273(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Darsow T, Booker TK, Piña-Crespo JC, Heinemann SF. Exocytic Trafficking Is Required for Nicotine-induced Up-regulation of α4β2 Nicotinic Acetylcholine Receptors. Journal of Biological Chemistry. 2005;280:18311–18320. doi: 10.1074/jbc.M501157200. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. The effects of DHBE and MLA on nicotine-induced enhancement of contextual fear conditioning in C57BL/6 mice. Psychopharmacology. 2006;184:345–52. doi: 10.1007/s00213-005-0047-y. [DOI] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. Journal of Neuroscience. 2005;25:8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Kenney JW, Gould TJ. Hippocampal alpha4beta2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. J Neurosci. 2007;27:10870–10877. doi: 10.1523/JNEUROSCI.3242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J, Gould T. Beta2 subunit-containing nicotinic receptors mediate the enhancing effect of nicotine on trace cued fear conditioning in C57BL/6 mice. Psychopharmacology. 2007a;190:343–352. doi: 10.1007/s00213-006-0624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. Atomoxetine Reverses Nicotine Withdrawal-Associated Deficits in Contextual Fear Conditioning. Neuropsychopharmacology. 2007b doi: 10.1038/sj.npp.1301315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. Hippocampal nAChRs mediate nicotine withdrawal-related learning deficits. European Neuropsychopharmacology. 2009;19:551–561. doi: 10.1016/j.euroneuro.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Kim JJ, Yipp J, De Oca B. Differential effects of the N-methyl-D-aspartate antagonist DL-2-amino-5-phosphonovalerate on acquisition of fear of auditory and contextual cues. Behav Neurosci. 1994;108:235–240. doi: 10.1037//0735-7044.108.2.235. [DOI] [PubMed] [Google Scholar]
- Gentry CL, Lukas RJ. Regulation of nicotinic acetylcholine receptor numbers and function by chronic nicotine exposure. Curr. Drug Targets. CNS. Neurol. Disord. 2002;1:359–385. doi: 10.2174/1568007023339184. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Higgins JS. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiology of Learning and Memory. 2003;80:147–157. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. The hippocampus and cingulate cortex differentially mediate the effects of nicotine on learning versus on ethanol-induced learning deficits through different effects at nicotinic receptors. Neuropsychopharmacology. 2009;34:2167–2179. doi: 10.1038/npp.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biology. 2007;8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. J Consult Clin. Psychol. 1993;61:743–750. doi: 10.1037//0022-006x.61.5.743. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine. Tob. Res. 2007;9:315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Bickel WK. Nicotine withdrawal versus other drug withdrawal syndromes: similarities and dissimilarities. Addiction. 1994;89:1461–1470. doi: 10.1111/j.1360-0443.1994.tb03744.x. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther. 2008 doi: 10.1124/jpet.107.132977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Constable RT, Westerveld M, Pugh KR. Impact of smoking abstinence on working memory neurocircuitry in adolescent daily tobacco smokers. Psychopharmacology (Berl) 2007;193:557–566. doi: 10.1007/s00213-007-0797-9. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biological Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Kane JK, Konu O, Ma JZ, Li MD. Nicotine coregulates multiple pathways involved in protein modification/degradation in rat brain. Molecular Brain Research. 2004;132:181–191. doi: 10.1016/j.molbrainres.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Kenney JW, Adoff MD, Wilkinson DS, Gould TJ. The effects of acute, chronic, and withdrawal from chronic nicotine on novel and spatial object recognition in male C57BL/6J mice. Psychopharmacology. 2011;217:353–365. doi: 10.1007/s00213-011-2283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Gould TJ. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol. Neurobiol. 2008;38:101–121. doi: 10.1007/s12035-008-8037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman KM, Vaughn RL, Christ TS. Effects of cigarette smoking and smoking deprivation on paired-associate learning of high and low meaningful nonsense syllables. Psychol. Rep. 1973;32:963–966. doi: 10.2466/pr0.1973.32.3.963. [DOI] [PubMed] [Google Scholar]
- Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behavioral Neuroscience. 1997;111:104–113. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- Lomazzo E, Hussmann GP, Wolfe BB, Yasuda RP, Perry DC, Kellar KJ. Effects of chronic nicotine on heteromeric neuronal nicotinic receptors in rat primary cultured neurons. Journal of Neurochemistry. 2011;119:153–164. doi: 10.1111/j.1471-4159.2011.07408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- Madhok TC, Matta SG, Sharp BM. Nicotine regulates nicotinic cholinergic receptors and subunit mRNAs in PC 12 cells through protein kinase A. Brain Res. Mol. Brain Res. 1995;32:143–150. doi: 10.1016/0169-328x(95)00073-2. [DOI] [PubMed] [Google Scholar]
- Mamede M, Ishizu K, Ueda M, Mukai T, Iida Y, Kawashima H, Fukuyama H, Togashi K, Saji H. Temporal Change in Human Nicotinic Acetylcholine Receptor After Smoking Cessation: 5IA SPECT Study. Journal of Nuclear Medicine. 2007;48:1829–1835. doi: 10.2967/jnumed.107.043471. [DOI] [PubMed] [Google Scholar]
- Mao D, Perry DC, Yasuda RP, Wolfe BB, Kellar KJ. The α4β2α5 nicotinic cholinergic receptor in rat brain is resistant to up-regulation by nicotine in vivo. J Neurochem. 2008;104:446–456. doi: 10.1111/j.1471-4159.2007.05011.x. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. Journal Of Pharmacology And Experimental Therapeutics. 1983;226:817–825. [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, Collins AC. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci. 1992;12:2765–2784. doi: 10.1523/JNEUROSCI.12-07-02765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Smith KW, Collins AC. Differential agonist inhibition identifies multiple epibatidine binding sites in mouse brain. J. Pharmacol. Exp. Ther. 1998;285:377–386. [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Collins AC. Time course study of the effects of chronic nicotine infusion on drug response and brain receptors. J Pharmacol Exp Ther. 1985;235:619–628. [PubMed] [Google Scholar]
- Marks MJ, Whiteaker P, Collins AC. Deletion of the α7, β2, or β4 Nicotinic Receptor Subunit Genes Identifies Highly Expressed Subtypes with Relatively Low Affinity for [3H]Epibatidine. Mol Pharmacol. 2006;70:947–959. doi: 10.1124/mol.106.025338. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Monterosso J, Simon SL, Jarvik M, Brody A, Olmstead R, Domier CP, Cohen MS, Ernst M, London ED. Working memory in cigarette smokers: comparison to non-smokers and effects of abstinence. Addict. Behav. 2006;31:833–844. doi: 10.1016/j.addbeh.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual Causes of Death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Nides M. Update on pharmacologic options for smoking cessation treatment. Am. J. Med. 2008;121:S20–S31. doi: 10.1016/j.amjmed.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Owen EH, Logue SF, Rasmussen DL, Wehner JM. Assessment of learning by the Morris water task and fear conditioning in inbred mouse strains and F1 hybrids: implications of genetic background for single gene mutations and quantitative trait analyses. Neuroscience. 1997;80:1087–1099. doi: 10.1016/s0306-4522(97)00165-6. [DOI] [PubMed] [Google Scholar]
- Papke RL, Trocmé-Thibierge C, Guendisch D, Al Rubaiy SAA, Bloom SA. Electrophysiological Perspectives on the Therapeutic Use of Nicotinic Acetylcholine Receptor Partial Agonists. Journal Of Pharmacology And Experimental Therapeutics. 2011;337:367–379. doi: 10.1124/jpet.110.177485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, Frey J, Gur R, Lerman C. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug And Alcohol Dependence. 2010;106:61–64. doi: 10.1016/j.drugalcdep.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly JR, Marks MJ, Robinson SF, van de Kamp JL, Collins AC. Chronic nicotine and mecamylamine treatment increase brain nicotinic receptor binding without changing alpha 4 or beta 2 mRNA levels. J Pharmacol Exp Ther. 1996;278:361–369. [PubMed] [Google Scholar]
- Penton RE, Quick MW, Lester RAJ. Short- and Long-Lasting Consequences of In Vivo Nicotine Treatment on Hippocampal Excitability. The Journal of Neuroscience. 2011;31:2584–2594. doi: 10.1523/JNEUROSCI.4362-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry DC, Mao D, Gold AB, McIntosh JM, Pezzullo JC, Kellar KJ. Chronic Nicotine Differentially Regulates α6- and α3-Containing Nicotinic Cholinergic Receptors in Rat Brain. Journal Of Pharmacology And Experimental Therapeutics. 2007;322:306–315. doi: 10.1124/jpet.107.121228. [DOI] [PubMed] [Google Scholar]
- Phillips RG, Ledoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Fiore MC, Baker TB. Profiles in discouragement: two studies of variability in the time course of smoking withdrawal symptoms. J. Abnorm. Psychol. 1998;107:238–251. doi: 10.1037//0021-843x.107.2.238. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Niaura R, Shadel WG, Abrams D, Goldstein M, Fiore MC, Baker TB. Smoking withdrawal dynamics in unaided quitters. J. Abnorm. Psychol. 2000;109:74–86. doi: 10.1037//0021-843x.109.1.74. [DOI] [PubMed] [Google Scholar]
- Pietilä K, lähde T, Attila M, Ahtee L, Nordberg A. Regulation of nicotinic receptors in the brain of mice withdrawn from chronic oral nicotine treatment. Naunyn-Schmiedeberg’s Archives of Pharmacology. 1998;357:176–182. doi: 10.1007/pl00005152. [DOI] [PubMed] [Google Scholar]
- Portugal GS, Gould TJ. Bupropion dose-dependently reverses nicotine withdrawal deficits in contextual fear conditioning. Pharmacol Biochem. Behav. 2007;88:179–187. doi: 10.1016/j.pbb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Kenney JW, Gould TJ. β2 Containing Acetylcholine Receptors Mediate Nicotine Withdrawal Deficits in Learning. Neurobiology of Learning and Memory. 2008;89:106–113. doi: 10.1016/j.nlm.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Gould TJ. Nicotine withdrawal disrupts new contextual learning. Pharmacology Biochemistry And Behavior. 2009;92:117–123. doi: 10.1016/j.pbb.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. Nicotine withdrawal-induced deficits in trace fear conditioning in C57BL/6 mice--a role for high-affinity β2 subunit-containing nicotinic acetylcholine receptors. Eur. J Neurosci. 2009;29:377–387. doi: 10.1111/j.1460-9568.2008.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. The role of nicotinic acetylcholine receptors in the medial prefrontal cortex and hippocampus in trace fear conditioning. Neurobiol. Learn. Mem. 2010;94:353–363. doi: 10.1016/j.nlm.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Portugal GS, Lerman C, Gould TJ. Varenicline ameliorates nicotine withdrawal-induced learning deficits in C57BL/6 mice. Behav. Neurosci. 2008;122:1166–1171. doi: 10.1037/a0012601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani K, Teng Y, Shim D, De Biasi M. Nicotine Regulates Multiple Synaptic Proteins by Inhibiting Proteasomal Activity. The Journal of Neuroscience. 2007;27:10508–10519. doi: 10.1523/JNEUROSCI.3353-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Vann R, Britton A, O’Connell M, James J, Rosecrans J. Cellular nicotinic receptor desensitization correlates with nicotine-induced acute behavioral tolerance in rats. Psychopharmacology. 2007;192:71–78. doi: 10.1007/s00213-006-0687-6. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Levin ED, Stein RM, Ripka GV. Mecamylamine combined with nicotine skin patch facilitates smoking cessation beyond nicotine patch treatment alone. Clin. Pharmacol. Ther. 1994;56:86–99. doi: 10.1038/clpt.1994.105. [DOI] [PubMed] [Google Scholar]
- Rukstalis M, Jepson C, Patterson F, Lerman C. Increases in hyperactive-impulsive symptoms predict relapse among smokers in nicotine replacement therapy. J Subst. Abuse Treat. 2005;28:297–304. doi: 10.1016/j.jsat.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Salas R, Main A, Gangitano D, De Biasi M. Decreased withdrawal symptoms but normal tolerance to nicotine in mice null for the α7 nicotinic acetylcholine receptor subunit. Neuropharmacology. 2007;53:863–869. doi: 10.1016/j.neuropharm.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M. Decreased Signs of Nicotine Withdrawal in Mice Null for the β4 Nicotinic Acetylcholine Receptor Subunit. Journal of Neuroscience. 2004;24:10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ. Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science. 1983;220:214–216. doi: 10.1126/science.6828889. [DOI] [PubMed] [Google Scholar]
- Slemmer JE, Martin BR, Damaj MI. Bupropion is a nicotinic antagonist. J. Pharmacol. Exp. Ther. 2000;295:321–327. [PubMed] [Google Scholar]
- Snyder FR, Henningfield JE. Effects of nicotine administration following 12 h of tobacco deprivation: assessment on computerized performance tasks. Psychopharmacology (Berl) 1989;97:17–22. doi: 10.1007/BF00443406. [DOI] [PubMed] [Google Scholar]
- Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E, Dubin JA, Estok K, Brenner E, Baldwin RM, Tamagnan GD, Seibyl JP, Jatlow P, Picciotto MR, London ED, O’Malley S, van Dyck CH. Human tobacco smokers in early abstinence have higher levels of β2* nicotinic acetylcholine receptors than nonsmokers. J. Neurosci. 2006;26:8707–8714. doi: 10.1523/JNEUROSCI.0546-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MM, Swan GE, Jack LM. Self-reported abstinence effects in the first month after smoking cessation. Addictive Behaviors. 2001;26:311–327. doi: 10.1016/s0306-4603(00)00107-6. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Jimenez M, McIntosh JM, Collins AC, Marks MJ. Identification of a novel nicotinic binding site in mouse brain using [125I]-epibatidine. British Journal of Pharmacology. 2000;131:729–739. doi: 10.1038/sj.bjp.0703616. [DOI] [PMC free article] [PubMed] [Google Scholar]