Abstract
The Smyth line (SL) of chicken is an excellent animal model for human autoimmune vitiligo. In SL vitiligo (SLV) post-natal loss of melanocytes in feathers appears to be due to cell-mediated immunity. In this study, leukocyte-infiltration and associated expression (RNA) of immune function-related cytokines in growing feathers were investigated throughout SLV-development and -progression. Both leukocyte infiltration and cytokine expression levels started to increase near visible SLV onset (early SLV), reached peak levels during active SLV, and decreased to near pre-vitiligo levels after complete loss of melanocytes. Specifically, significant increases were noticed in relative proportions of T cells, B cells, and MHC II-expressing cells during active SLV. Levels of T cell infiltration were higher than those of B cells, with more CD8+ than CD4+ cells throughout SLV. Elevated leukocyte infiltration in early and active SLV was accompanied by increased levels of cytokine expression, especially in interferon-gamma, interleukin (IL)-10 and IL-21. Low expression of IL-4 and IL-17 did not suggest important roles of Th2 and Th17 cells in SLV pathogenesis. Taken together, SLV appears to be a Th1 polarized autoimmune disease, whereby interferon-gamma expression is strongly associated with parallel increases in IL-10 and IL-21, particularly during early and active stages of SLV.
Introduction
The mutant Smyth line (SL) of chicken is an excellent animal model for the study of autoimmune vitiligo (Wick et al, 2006) due to many phenotypic and etiopathological similarities between SL and human vitiligo and the multifactorial nature, high incidence and spontaneous onset of SL vitiligo (SLV) (Erf, 2010; Smyth, 1989). In chickens, melanocytes, the target cells in vitiligo, are located in growing feathers (Figure 1) (Smyth, 1989). Growing feathers can be easily removed and regenerate and, hence, the developing autoimmune lesion can be monitored throughout SLV in the same individual. Moreover, the living portion of the growing feather (feather tip; Figure 1a) provides sufficient tissue sample for various post-collection analyses.
Figure 1.
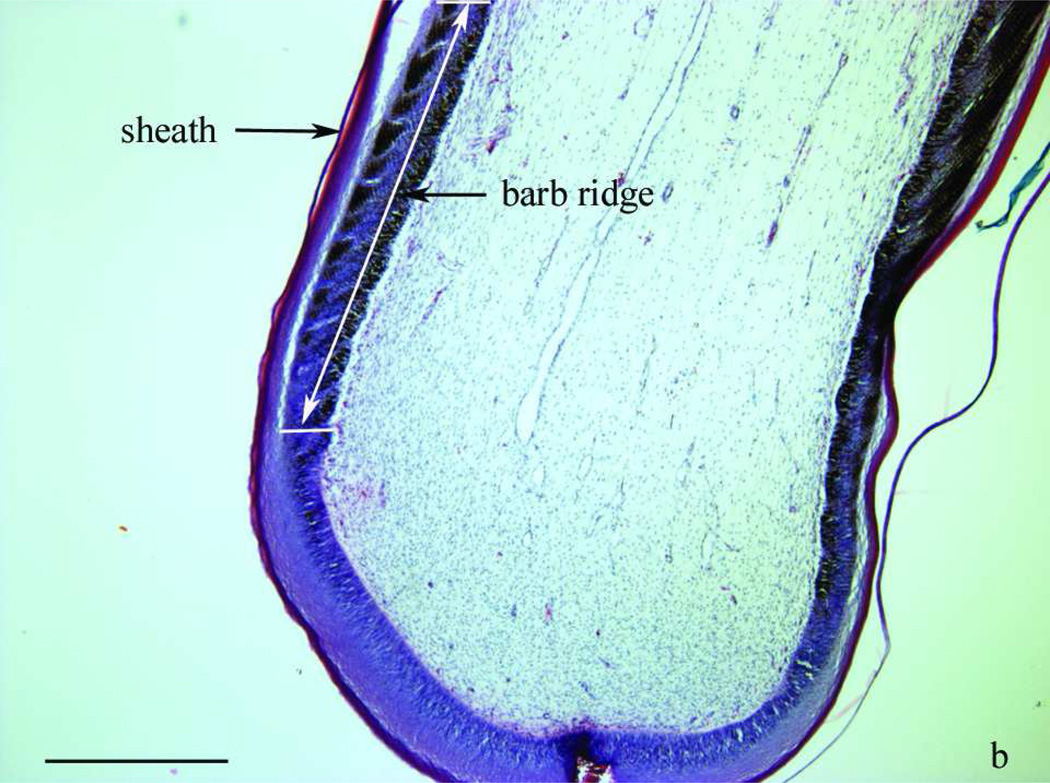
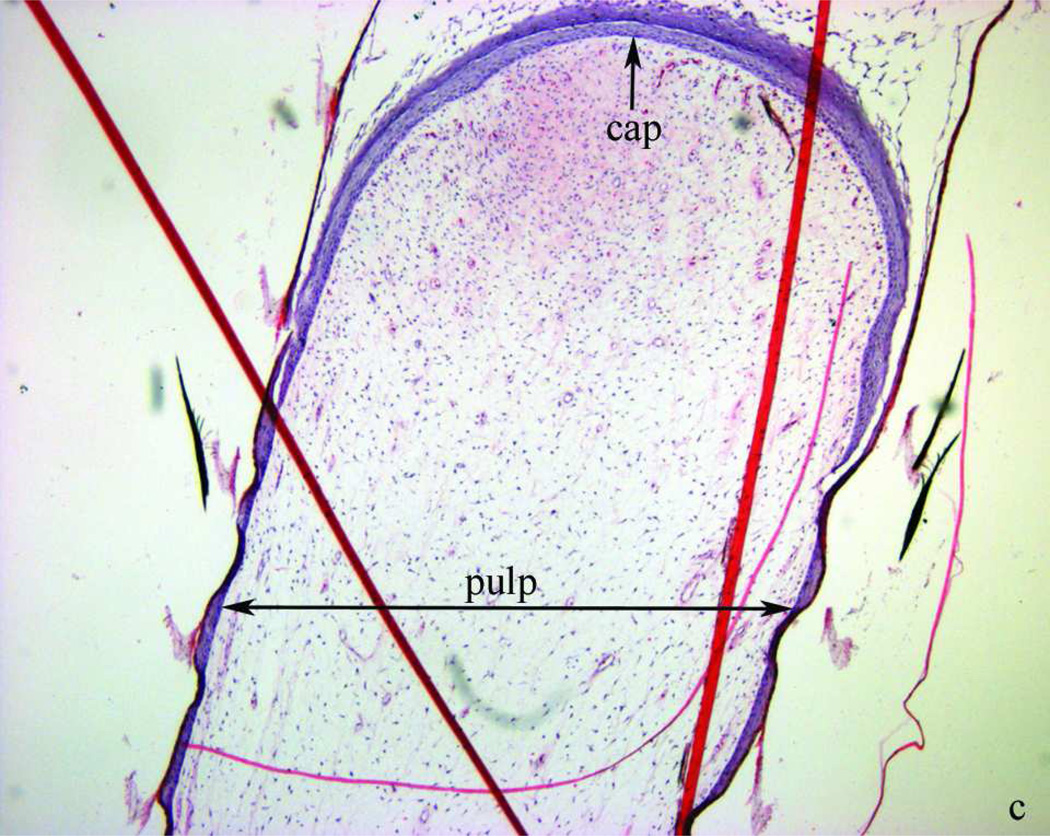
Morphology of two- to three-week-old growing feathers from SL chickens. a) from left to right: normally pigmented, partially depigmented and completely depigmented growing feathers from SL chickens that developed SLV. Growing feathers can be collected from SLV chickens over the whole course of SLV. The living part of growing feathers (newest growth to the epidermal cap) is referred to as “feather tip”. b) microstructure of the newest growth of a feather tip with normal pigmentation; layers shown from the outside to inside are sheath, barb ridge and pulp. c) a cap formed by epidermal layer enclosing the pulp. Longitudinal sections were stained with H&E stain and examined at 40× (b, c) magnification under a bright field microscope. Bar scale = 1 mm.
Similar to human autoimmune vitiligo, both humoral and cellular immunity have been implicated in SLV, with a more prominent role attributed to cellular immunity in melanocyte loss based on phenotypical analysis of infiltrating-leukocytes (Erf et al, 1995; Shresta et al, 1997; Wang and Erf, 2004) and observation of interferon-gamma expression in feather samples collected from SL chickens with active SLV (Shi et al, 2009; Wang, 2001). Moreover, the presence of melanocytes-specific cell-mediated immunity was demonstrated in vivo based on the delayed wattle-swelling response to injection of syngeneic melanocyte lysates in chickens with SLV (Wang and Erf, 2003).
While phenotypic analyses of infiltrating cells in cross-sections of active lesions and in feather pulp cell suspensions support a more prominent role of cell-mediated immunity in melanocyte loss in S LV (Erf et al, 1995; Shresta et al, 1997; Wang and Erf, 2004), immune functional activities associated with SLV development have not been examined. The objective of this study was to monitor cytokine gene expression and determine leukocyte infiltration profiles in feather tips (Figure 1a) collected over the course of SLV development from SLV chickens. Specifically, using two-step quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) gene expression profiles were established for inducible nitric oxide synthase (iNOS) and cytokines of innate immunity (interleukin (IL)-1beta, IL-6, IL-8, IL-10, IL-12alpha, IL-12beta, and IL-15), signature cytokines of Th1, Th2 and Th17 cells (interferon (IFN)-gamma, IL-4 and IL-17F, respectively) and IL-21, a pleiotropic cytokine implicated in organ-specific autoimmune diseases. To relate gene-expression with leukocyte infiltration, the presence of macrophages and T- and B-lymphocytes subsets was also examined in longitudinal sections prepared from feather tips. Knowledge gained from this study will shed more light on the pathogenic mechanisms involved in the autoimmune loss of melanocytes in SLV.
Results
Leukocyte infiltration in growing feathers collected at different states of SLV
For each cell type, the relative amount of staining was expressed as the percentage of the longitudinal sections analyzed (% of area). Leukocyte infiltration data from each individual were grouped with respect to vitiligo state: NV (feather tips from SL chickens that never developed vitiligo), E V, AV and CV (feather tips from SLV chickens just before visible SLV onset, during active and after complete depigmentation, respectively).
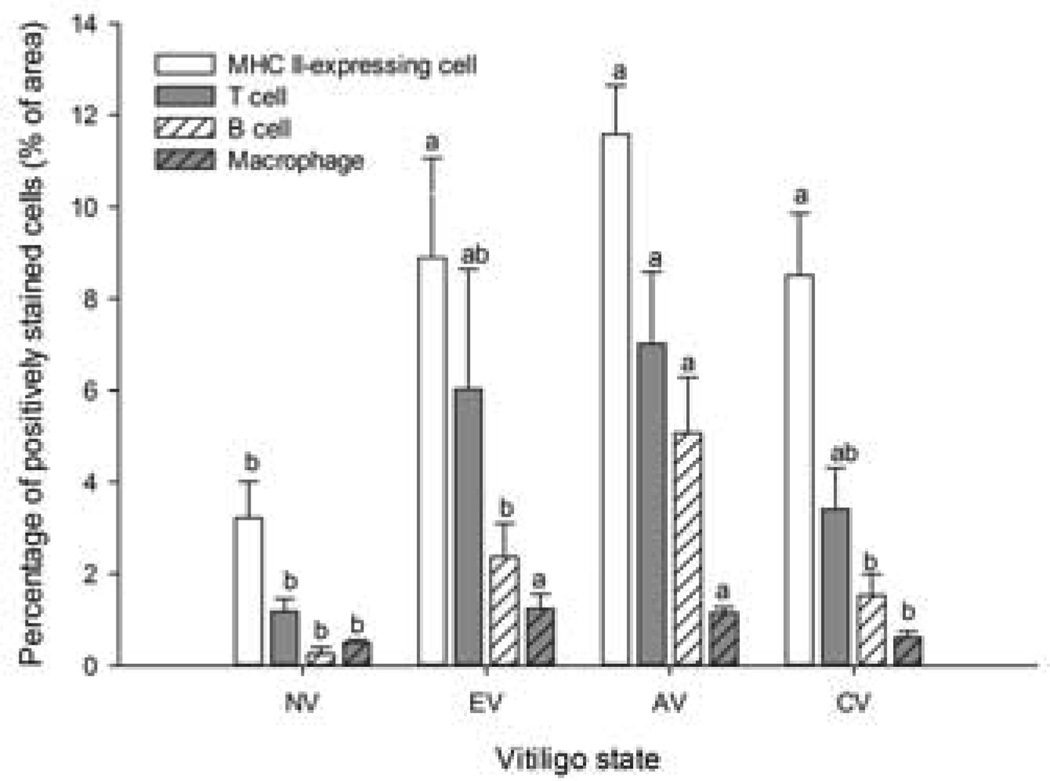
The infiltrating leukocytes consisted primarily of T and B lymphocytes which reached peak levels during AV (P ≤ 0.05). While proportionately fewer macrophages than T and B lymphocytes were present in SLV feathers, macrophage levels were already elevated in EV and remained elevated in AV (P ≤ 0.05) (Figure 2). The relative proportions of MHC II-expressing cells were higher than those of other leukocytes, independent of SLV state. Except for MHC II-expressing cells, the proportions of T cells, B cells, and macrophages returned to near NV levels when melanocyte loss was complete (CV, Figure 2)
Figure 2.
Infiltration profiles of leukocytes in feather tips collected at different states of SLV. MHC II-expressing cells, T cells, B cells and macrophages were identified by indirect immunoperoxidase staining using Ia, TCR gamma-delta and TCR alpha-beta, Bu-1, and KUL01 mouse-anti-chicken monoclonal antibodies, respectively. Stained sections were examined at 40× magnification and the amount of stained cells expressed as the percentage of the whole feather section area analyzed (% of area). NV: feather tips from 3 SL chickens that never developed SLV; E V, AV and CV: feather tips were collected from 7 SLV chickens just before, during and after SLV development, respectively. Each bar represents the mean ± SE. a,b For each cell type, means without common letters are different (P < 0.05).
Both types of TCR-defined T cells (alpha-beta and gamma-delta TCR) followed the same infiltration trend (Table 1). However, while the relative proportions of alpha-beta T cells were greatly elevated (P ≤ 0.05) in AV compared to NV samples, those of gamma-delta T cells did not differ between in NV-, EV-, AV- and CV-samples. CD4+ and CD8+ cells reached peak levels in AV samples compared to NV, EV and CV samples (P ≤ 0.05, Table 1). At all SLV states there were more CD8- than CD4-expressing cells, with CD4:CD8 ratios being lowest in NV samples, intermediate in EV and CV samples, and highest (P ≤ 0.05) in AV samples (Table 1). Based on the proportions of IgM+ cells at the various states of S LV, the majority of infiltrating B cells (Bu-1+) appeared to be IgM+ cells (Table 1). Calculated T to B cell ratios (TCR+ cells versus Bu-1+ cells) were lower (P ≤ 0.05) in AV samples (1.52 ± 0.21) than in NV samples (5.16 ± 2.66) due to a relatively greater increase in B cell- than in T cell-infiltration in AV samples compared to other states of S LV (Table 1).
Table 1.
Relative proportions of leukocytes (% of area)1 in feather tips2 from SL chickens with and without SLV
| Vitiligo state3 | ||||
|---|---|---|---|---|
| NV | EV | AV | CV | |
| T cells (alpha-beta+ TCR) | 0.70 ± 0.17b,4 | 3.95 ± 1.57ab | 4.92 ± 1.10a | 2.15 ± 0.67ab |
| T cells (gamma-delta+ TCR) | 0.47 ± 0.12 | 2.08 ± 1.13 | 2.10 ± 0.57 | 1.25 ± 0.35 |
| CD4+ lymphocytes | 0.61 ± 0.30b, | 2.35 ± 0.67b | 4.81 ± 0.48a | 1.49 ± 0.51b |
| CD8+ lymphocytes | 1.17 ± 0.52b | 3.51 ± 0.88b | 5.71 ± 0.64a | 2.37 ± 0.60b |
| CD4+:CD8+ | 0.48 ± 0.06b | 0.68 ± 0.08ab | 0.87 ± 0.10a | 0.68 ± 0.13ab |
| B cells (Bu-1+) | 0.25 ± 0.15b | 2.37 ± 0.73b | 5.07 ± 1.21a | 1.53 ± 0.46b |
| IgM+ cells | 0.31 ± 0.05b | 1.89 ± 0.62ab | 4.30 ± 1.15a | 1.71 ± 0.48b |
| T:B5 | 5.16 ± 2.66a | 2.69 ± 1.10ab | 1.52 ± 0.21b | 2.94 ± 0.69ab |
leukocyte types were determined by indirect immunoperoxidase staining. Primary unlabeled antibodies were mouse-anti-chicken monoclonal antibodies to alpha-beta T cell receptor (TCR) (cocktail of alpha-beta1 and alpha-beta 2 TCR), gamma-delta TCR, CD4, CD8, Bu-1 (B cell marker) and IgM. Binding of primary antibodies was detected by biotinylated horse-anti-mouse IgG (H+L) with avidin-biotin peroxidase complex reagents and using DAB as the substrate. Image analysis was carried out at 40× magnification under a bright field microscope. The amount of stained cells in feather tip sections was expressed as the percentage of the whole area analyzed (% of area).
three feather tips were collected from three SL chickens without and seven with vitiligo, laid parallel in the same orientation in an aluminum cup containing O.C.T, and snap frozen with liquid nitrogen. Longitudinal sections (6 µm) were cut at −19°C, fixed in acetone and subjected to indirect immunohistochemical staining.
vitiligo state, “NV”, “EV”, “AV” or “CV” means feather tips were from SL chickens that never developed SLV (n = 3), from vitiliginous SL chickens (n = 7) just (<2 weeks) before, during or after SLV development.
data are shown as mean ± SE. a,b Data in the same row without common letters are different at P ≤ 0.05.
T cells include both alpha-beta and gamma-delta TCR+ T cells.
Examination of hematoxylin and eosin (H/E) stained formaldehyde-fixed feather sections confirmed the mononuclear nature of the leukocyte infiltrate and the notable absence of granulocytes, including heterophils (avian equivalent to neutrophils), at all vitiligo states (data not shown). Additionally, NK cells do not appear to make up a significant portion of pulp-infiltrating lymphocytes in SLV based on flow cytometric analysis for CD8+ lymphocytes without surface CD3 (arbitrary definition of avian NK cells; (Gobel et al, 1994)) in pulp cell suspensions from active autoimmune lesions (data not shown).
Profiles of cytokine expression throughout SLV development
Due to limited availability of chicken cytokine specific antibodies, cytokine expression was examined at the transcriptome level using qRT-PCR. Relative expression was calculated by the delta delta Ct method (Wong and Medrano, 2005) and was expressed as fold change with respect to feather tips collected throughout the course of the study from NV SL chickens. As each vitiliginous chicken developed SLV at different weeks of age, to demonstrate the general trend of cytokine expression in SLV development and progression data for each bird were aligned with respect to time of visible SLV onset (set as 0).
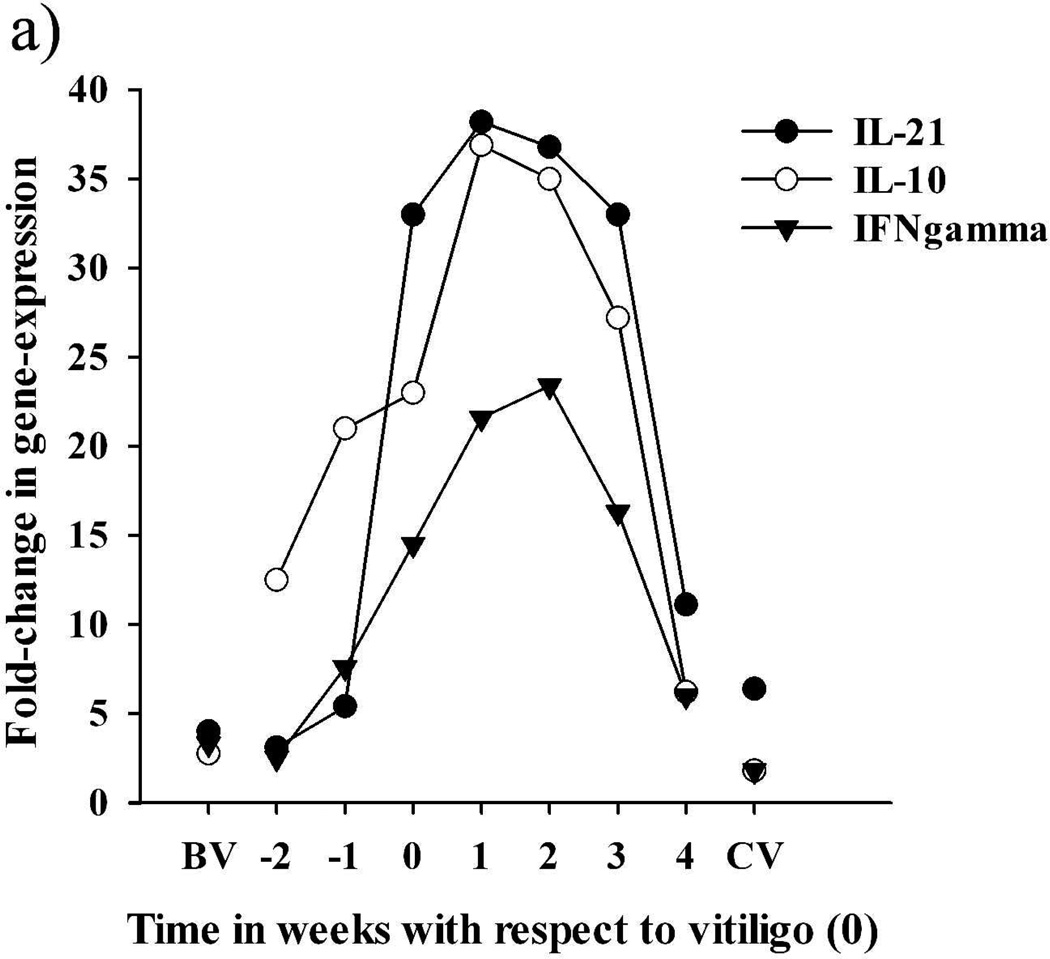
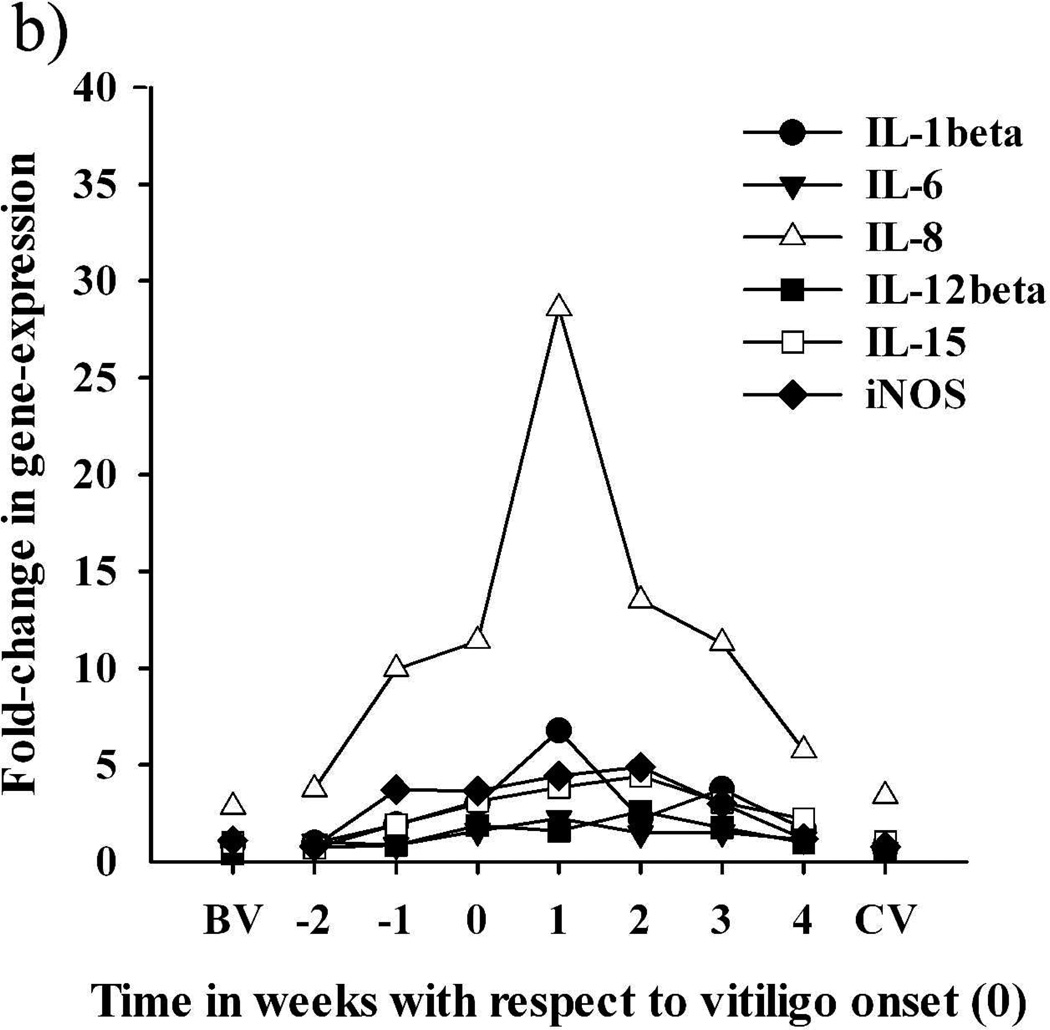
In feather tips collected from SLV chickens several weeks (>2) before SLV onset (BV), expression levels for all targets were similar to those of NV samples, with the exception of IL-8, IL-10 and IL-21, which exhibited ≥ 3 fold higher levels than NV samples (Figure 3). In feather tips collected from SLV chickens closer to (≤2 weeks before; EV) SLV onset, parallel increases in the relative expression of IL-8 and IL-10 could be observed, with IL-10 reaching higher than BV levels in samples collected one week (−1) before SLV onset (Table 2 and Figure 3). With SLV onset, expression of IL-8, IL-10, IL-21 and IFN-gamma increased compared to BV samples, with all 4 cytokines simultaneously reaching peak levels and remaining elevated until 3 weeks post-SLV onset (AV) (P < 0.05) (Table 2 and Figure 3). This active phase of SLV was accompanied with significant, albeit much lower, increases in IL-1beta, IL-6, IL-12beta, IL-15, and iNOS (Table 2 and Figure 3). A notable exception to this expression pattern was IL-4, which did not increase above BV levels and exhibited the highest relative expression at much later time points than other targets (Table 2). With the progression of S LV, levels of most targets returned to BV levels, especially in white growing feathers collected from birds that exhibited complete depigmentation for at least 1 week (CV) (Figure 3). Throughout the course of SLV development, from initiation, progression to complete pigmentation loss, the actual relative expression value of chemokine IL-8 and cytokines IL-10, IL-21 and IFN-gamma, nevertheless, differed greatly between birds (Table 2). However, the combination of these cytokines, especially IL-10, IL-21 and IFN-gamma, emerged as the signature cytokine profile during the early stage and active progression of S LV in all vitiliginous chickens. Expression of IFN-gamma at the protein level was confirmed in a pilot study by indirect immunohistochemistry using mouse-anti-chicken IFN-gamma antibodies developed by Dr. Lillehoj (Animal and Natural Resources Institute, USDA-ARS, Beltsville, MD) (data not shown).
Figure 3.
Time-course of the relative expression of cytokines and iNOS in growing feathers collected from seven SLV chickens throughout vitiligo development. Relative expression was calculated by the delta delta Ct method, using a cDNA pool made from growing feather of 3 SL chickens that never developed vitiligo as the calibrator and chicken 28S as the endogenous control gene. The X-axis represent the time (weeks) of feather tip collection with respect to vitiligo onset (0); BV and CV represent data from feathers collected from the 7 SL chickens >2 weeks before vitiligo and >1 week after complete pigmentation loss, respectively; weeks −2 and −1 represent early vitiligo (EV) and weeks 0–4 correspond to active vitiligo (AV). (a) Expression pattern of IL-21, IL-10, and IFN-gamma. (b) Expression pattern of IL-1beta, IL-6, IL-8, IL-12beta, IL-15 and iNOS. Also see Table 2.
Table 2.
Mean of highest relative expression1, mean time of highest relative expression, and times of significant elevation (P < 0.05) of cytokines and iNOS in growing feathers collected prior to and throughout the development of vitiligo in SL chickens
| Target gene | Highest Expression Mean2 | Time (weeks)3 | Times Elevated4 |
|---|---|---|---|
| IFN-gamma | 31.82 ± 5.53 | 1.43 ± 0.43 | 0, 1, 2, 3 |
| IL-10 | 56.26 ± 12.42 | 1.00 ± 0.53 | −1, 0, 1, 2, 3 |
| IL-21 | 56.49 ± 12.82 | 0.86 ± 0.46 | 0, 1, 2, 3 |
| IL-1beta | 7.47 ± 1.91 | 1.57 ± 0.43 | 0, 1, 3 |
| IL-4 | 2.60 ± 1.01 | 2.72 ± 0.78 | none |
| IL-6 | 4.06 ± 1.59 | 0.43 ± 0.53 | 0, 1, 2, 3 |
| IL-8 | 36.64 ± 9.48 | 1.14 ± 0.58 | 1 |
| IL-12beta | 3.19 ± 0.82 | 1.14 ± 0.77 | 0, 2 |
| IL-15 | 5.70 ± 2.15 | 2.00 ± 0.84 | 2 |
| iNOS | 7.61 ± 2.52 | 1.14 ± 0.55 | 1, 2 |
Relative expression was expressed as fold changes with respect to expression levels in feather samples from three non-vitiliginous SL chickens (calibrator sample).
Mean ± SEM of the 7 SL chickens’ highest expression level of each target gene during SLV development.
Mean time ± SEM (n = 7) in weeks (with respect to SLV onset; week 0) when highest expression occurred.
Time in weeks with respect to SLV onset (week 0) when targets were significantly (P ≤ 0.05) elevated compared to pigmented growing feathers collected from the 7 SLV chickens more than 2 weeks before SLV onset (BV samples). Also see Figure 3.
Relative expression levels for IL-12alpha and IL-17F could not be determined based on the formulae of the delta delta Ct method, because no Ct value was detected in the calibrator sample. The relatively low expression of IL-12alpha and IL-17F in SLV can however be inferred based on calculation of the delta Ct value (Ct of target – Ct of endogenous 28S control), which is a relative measure of target gene expression within a sample.
Discussion
The SL chicken is an excellent animal model for human autoimmune vitiligo as suggested by the many phenotypic and etiologic similarities between the two cases, which are summarized in Table 3. Although SLV is a multifactorial disease in nature, the immune system was shown to play a critical role in SLV expression in susceptible chickens.
Table 3.
Comparison between vitiligo in SL chickens (SLV) and in humans
| SLV | Human vitiligo | References | ||
|---|---|---|---|---|
| Main target tissue | Growing feathers | Skin | ||
| Phenotype | Symptom | Depigmentation in growing feathers | Depigmentation in the skin | |
| Associated autoimmune disorders | Autoimmune thyroiditis, alopecia-like feathering defect | Autoimmune thyroiditis and other disorders of immune origin | (Erf, 2010; Spritz, 2010) | |
| Onset | Early puberty to young adults (6–14 wks of age) | Early puberty to young adults (10–30 y of age) | (Erf, 2010; Smyth, Jr., 1989) | |
| Severity | Erratic to complete | Erratic, occasionally complete | (Erf, 2010; Smyth, Jr., 1989) | |
| Etiology | Multifactorial (genetic, immunologic, metabolic and environmental factors) | Multifactorial (genetic, immunologic, metabolic and environmental factors) | (Boissy and Nordlund, 2011; Erf, 2010) | |
| Genetics | Vitiligo susceptibility genes | Multigenic, identification in progress | Multigenic, candidate genes identified | (Spritz, 2010; Wick et al, 2006) |
| Intrinsic melanocyte defects | Yes | Yes | (Boissy et al, 1986; 1991) | |
| Immunology | Target tissue infiltrating leukocytes | Macrophages, CD4, CD8, and B cells | Macrophages, CD4 and CD8 cells | (Erf et al, 1995; Le Poole et al, 1996) |
| Melanocyte-specific autoantibodies | Yes | Yes | (Austin et al, 1992; Harning et al, 1991) | |
| Cytokines1 and iNOS in the affected tissue | ↑IFN-gamma | ↑IFN-gamma | (Grimes et al, 2004; Wang, 2001) | |
| ↑IL-10 | ↑IL-10 | (Grimes et al, 2004) | ||
| ↑IL-6 | ↑IL-6 | (Moretti et al, 2002; Moretti et al, 2009) | ||
| IL-4 no change | IL-4 no change | (Grimes et al, 2004) | ||
| IL-17 low levels of expression | ↑IL-17 | (Bassiouny and Shaker, 2011) | ||
| ↑IL-21, ↑IL-1beta, ↑IL-8, ↑IL-12beta, ↑IL-15, and ↑iNOS |
Not determined | |||
| Metabolism | Oxidative stress | Yes | Yes | (Erf et al, 2005; Schallreuter et al, 1999) |
| Environmental factor | Viral association | Positive association with live HVT2 vaccination at hatch | Possible association with HCV3 and HIV4 | (Erf et al, 2001; Seyedalinaghi et al, 2009; Tsuboi et al, 2006) |
the expression profile of cytokines and iNOS in the growing feather were determined in the current study.
herpesvirus of turkey
hepatitis C virus
human immunodeficiency virus
Humoral immunity was initially implied by immunosuppressive effects of bursectomy (excision of generative organ for B cells), which resulted in decreased incidence and severity of SLV in SL chickens (Boissy et al, 1984; Lamont and Smyth, Jr., 1981). Findings from the current study also support a potential role of B cells as their infiltration into the target tissue was associated with SLV development (Figure 2 and Table 1). The reason for B cell infiltration and role of B cells in the active SLV lesion is not clear from this study. B cells will follow similar infiltration signals as T cells and can participate in cellular immunity through antigen-uptake and presentation as well as antibody secretion. Considering the late and low expression of IL-4 (Table 2), a cytokine supportive of humoral immunity and isotype switching, together with the predominant IgM+ phenotype of infiltrating B cells (Table 1), it seems that IL-4 is not responsible for B cell activities in this autoimmune response. Evidence for B cell participation in vitiligo is also provided by the presence of melanocyte-specific autoantibodies in sera of vitiliginous SL chickens and vitiligo patients (Austin et al, 1992; Harning et al, 1991). Furthermore, a destructive effect of vitiligo-antibodies on human melanocytes via complement-mediated and antibody-dependent cellular cytotoxicity was demonstrated in vitro and in vivo (Cui et al, 1993; Gilhar et al, 1995). From the current study, it is reasonable to speculate that autoantibodies and feather-infiltrating B cells are more important in amplifying than initiating melanocyte loss in SLV.
Phenotypic characteristics of infiltrating leukocytes and cytokine profiles in the current study confirm a more prominent involvement of cell-mediated, specifically Th1-mediated, than humoral immune activity in melanocyte-loss as previously suggested (Shi et al, 2009; Wang, 2001; Wang and Erf, 2003). Compared to the relative proportions of B (Bu-1+) cells, those of T cells, including alpha-beta and gamma-delta TCR+ cells, were numerically higher at all vitiligo states (Table 1 and Figure 2). Moreover, while both alpha-beta and gamma-delta T cells infiltrated, the infiltration of alpha-beta T cells was proportionately greater during active SLV (Table 1), an observation consistent with previous reports (Erf et al, 1995; Shresta et al, 1997). Also in line with previous reports on S LV (Erf et al, 1995; Wang and Erf, 2004) and in vitiligo patients (Le Gal et al, 2001; Le Poole et al, 1996), the current study showed the predominance of CD8+ compared to CD4+ cells at all vitiligo states (Table 1). Predominance of cytotoxic lymphocytes in active SLV lesions, their close juxtaposition and aggregation around apoptotic melanocytes (Wang and Erf, 2004), together with macrophage infiltration at early and active stages of SLV, further supports a Th1-mediated, melanocyte-specific cellular response in SLV. Macrophages, which are important immune system activators and effector cells of cell-mediated immunity, were also present in perilesional skin from vitiligo patients (Le Poole et al, 1996).
IFN-gamma, which is the signature cytokine of Th1-type cell-mediated immunity and was dramatically increased in samples collected at and after SLV onset (Table 2 and Figure 3), may play a central role in the SLV pathomechanism. IFN-gamma may function in several ways during SLV-expression and -progression. IFN-gamma is important in macrophage activation, phagocytic activity, cytokine and chemokine production, MHC I-and II-expression and nitric oxide production due to transactivation of iNOS (Abbas et al, 2010). In the current study, increased expression of IL-1beta, IL-6, IL-8, IL-12beta and iNOS indicates macrophage activation, which, due to the relatively low levels of macrophage infiltration, does not appear to be a major mechanism by which IFN-gamma exerts its effects in SLV (Table 2, Figure 2&3). IFN-gamma was shown to increase autophagosome formation in human CD4+ T cells (Son et al, 2010) and macrophages (Shi and Kehrl, 2010). Autophagosomes were reported in melanocytes from SL chickens that developed vitiligo (Boissy et al, 1983; Boissy et al, 1986), which may have been promoted by elevated levels of IFN-gamma in the melanocyte’s environment. In addition, IFN-gamma is able to stimulate expression of MHC I and MHC II molecules on various types of cells. In the current study, the heightened and sustained proportions of MHC II-expressing cells in EV, AV and CV samples compared to NV samples (Figure 2), are a strong indication of an immunologically active site, with IFN-gamma supporting antigen-presentation and macrophage-, B cell- and T cell-activation. Lastly, IFN-gamma has been shown to have inhibitory effects on Th17 cell development from CD4+ precursor cells (Kelchtermans et al, 2008), which may explain the relatively low IL-17F expression and absence of heterophils (avian equivalent to neutrophils) in feathers from vitiliginous SL chickens in the current study.
Based on the above discussion, IFN-gamma appears to play an essential role in SLV pathogenesis. Since IFN-gamma expression was not preceded by IL-12 and IL-15 (two cytokines capable of IFN-gamma induction) production, the question regarding the mechanisms driving IFN-gamma expression needs to be addressed. Review of recent literature revealed important positive interrelationships between IFN-gamma and IL-10 and IL-21, the other cytokines with marked expression in EV and AV samples (Table 2 and Figure 3). IL-10 is generally known as a cytokine with anti-inflammatory and immunosuppressive activities (Abbas et al, 2010). IL-10 has, however, recently been shown in human and mice to have immunostimulatory effects by increasing IFN-gamma production and activation of natural killer cells and cytotoxic T lymphocytes (Lauw et al, 2000; Shibata et al, 1998; Tilg et al, 2002). A pre-stimulated immune system and presence of high levels of IL-10 are two important factors that favor immunostimulatory effects of IL-10. Both of these pre-conditions are met in SLV. Marked elevation of IL-10 expression was observed in the current study together with heightened inflammatory immune activities, such as mononuclear cell infiltration, expression of IL-8 chemokine, IFN-gamma and MHC II expression in EV and AV feather tips. The source of immune activation may be the routine vaccination at hatch with live herpesvirus of turkey (HVT) against lymphoma-causing Marek’s disease virus. HVT translocates to and infects the feather follicle epithelium (Cho, 1975) and has been identified as a reliable environmental trigger of S LV expression in susceptible SL chickens (Erf et al, 2001). Recent cytokine gene-expression analysis at the transcriptome level by Abdul-Careem et al (2008) showed that HVT administration in chickens resulted in a concomitant increase in IFN-gamma and IL-10 expression in feathers reaching peak levels at 7 days and returning to levels of control by 10 days post-vaccination.
IL-21, a cytokine with pleiotropic functions, is the target of intensive investigations partially due to its ability to induce differentiation and expansion of Th17 cells in chronic inflammatory diseases (Nurieva et al, 2007). Low expression of IL-17F and absence of heterophils in SLV-lesions does not justify a pathological role of IL-21 through Th17 cells in S LV. IL-21 was also shown to be required for effector function and sustainability of CD8+ T cells, including cytotoxic activities and IFN-gamma production, in chronic viral infection (Elsaesser et al, 2009; Frohlich et al, 2009; Yi et al, 2009); inhibition of Foxp3+ Treg cell development, which indirectly enhanced activity of CD8+ T cells (Li and Yee, 2008); and, induction of IL-10 expression in activated CD4 and CD8 T cells in a mouse model of systemic lupus erythematosus (Spolski et al, 2009). Therefore, overexpression of IL-21 may play an underestimated role in the pathogenesis of SLV directly through its positive effects on cytotoxic T cell function and IFN-gamma expression and indirectly through its support of immunostimulatory effects of IL-10.
Taken together, S LV is an autoimmune disease in which melanocyte loss is associated with mononuclear leukocyte infiltration consisting predominantly of T cells (with more CD8+ than CD4+ cells) followed by B cells and macrophages. Cytokine expression in the target tissue in the same individuals throughout SLV development revealed concomitant increases in the relative expression of IFN-gamma, IL-10 and IL-21 prior to and during active melanocytes loss. The mechanisms underlying this cytokine signature and the interrelationship of immune cells and cytokines in melanocyte loss need to be further examined to gain insight into the etiology and pathology of the spontaneous expression of vitiligo in susceptible SL chickens.
Materials and Methods
Experimental design
Animals
Twenty SL chicks, homozygous for the B101 MHC-haplotype, were randomly selected from 20 families at hatch, vaccinated with live herpesvirus of turkey (Fort Dodge Animal Health, Fort Dodge, Iowa), and assigned to one floor pen at the Arkansas Experiment Station Poultry Farm in Fayetteville, AR, USA. A standard rearing protocol was followed and feed and water were provided ad libitum. Animal use was approved by the University of Arkansas Institutional Animal Care and Use Committee.
Feather collection
Samples (6 feather-tips per bird; Figure 1a) were collected weekly from chickens starting at 5- and ending at 18-weeks of age. Half of the feather tips were snap frozen in Tissue-Tek® O.C.T freezing medium (Sakura Finetek Inc., Torrance, CA) and the other half placed in RNAlater® (Qiagen Inc. Valencia, CA). All samples were kept at −80°C until use. SLV was scored at each feather collection following previous criteria (Wang and Erf, 2003). Basically, SLV was given a score of 1 to 5 if the newest growth of 0%, ≤ 20%, >20% and ≤ 60%, >60% and ≤ 99%, and 100% of the growing feathers demonstrated visible depigmentation. Upon completion of sample collection, feather tips from 7 SL chickens that developed SLV and reached a score of 5 during the 18-week period (SLV chickens) and 3 SL chickens that never developed SLV (NV chickens) were used in this study.
Indirect immunoperoxidase staining of growing feathers
Longitudinal sections (6 µm thick) were cut at −19°C in a cryostat (CM3050-S, Leica Microsystems, Inc., Bannockburn, IL), processed and immunochemically stained as described in Erf et al (Erf et al, 1995). Primary antibodies were mouse monoclonal antibodies (mAbs) specific for chicken T cell receptor gamma-delta (cTCR gamma-delta), cTCR alpha-beta1, cTCR alpha-beta2, cCD4, cCD8, cBu-1 (B cells), cIgM, cKUL01(macrophages) and cMHC II molecules (Southern Biotechnology Associates, Birmingham, AL).
Image analysis
Image analysis was carried out under a bright field microscope (40× magnification) and ImagePro® Plus software (Media Cybernetics, Silver Spring, MD). The amount of positively stained cells was expressed as the percentage of the whole tissue section (epidermis and pulp) analyzed (% area). All evaluations were made by the same person.
RNA isolation, quantification and cDNA synthesis
For each chicken and time point, the three feather tips preserved in RNAlater® were homogenized by Tissue Tearor™ (BioSpec Products, Inc, Bartlesville, OK, Model: 985370-395) in lysis buffer provided by Qiagen RNeasy® Mini kit (Qiagen Inc., Valencia, CA). Total RNA was isolated from homogenates with on-column DNA digestion (Qiagen Inc., Valencia, CA). RNA was eluted in 30 µl RNase-, DNase-free water and stored at −80°C until use. RNA integrity and concentration were determined as previously described (Hamal et al, 2010). RNA (175 ng/sample) was transcribed to cDNA in a 30 µl reaction volume using a High Capacity cDNA reverse transcription kit according to the manufacturer’s protocol (Applied Biosystems, Foster City, CA). Resulting cDNA was aliquoted and stored at −80°C until analysis.
Relative expression of cytokines and iNOS
Primers and probes used in this study are available upon request. Real-time PCR was performed according to Hamal et al (2010). The calibrator sample was a pool of cDNA prepared from feather tips of the three SL chickens without vitiligo (NV). The relative gene expression was determined by the delta delta Ct (∆∆Ct) method (Wong and Medrano, 2005).
Statistical analysis
To determine the effect of SLV state, one-way ANOVA (SYSTAT software Inc., Chicago, IL) was conducted using the general linear model procedure followed by Fisher’s LSD multiple means comparison. For the time course of gene-expression, EV, AV and CV samples were compared to BV samples collected from the same SLV chickens. All data were reported as mean ± SE, and differences were considered to be significant at P ≤ 0.05.
Acknowledgements
This work was supported in part by grants from NIH (R15 AR052670-01, GF Erf, PI) and Arkansas Biosciences Institute (GF Erf, PI). The authors thank Robert Dienglewicz, Lei Dong and Nadezda Stepicheva for their help with sample collection; Bryan Plumlee for advice regarding qRT-PCR; Dr. Edward E. Gbur Jr., Agricultural Statistics Laboratory for statistical advice; the University of Arkansas Molecular Genetics Core Laboratory for use of the Real-Time PCR instrument.
Abbreviations
- SL
Smyth line
- SLV
Smyth line vitiligo
- qRT-PCR
Quantitative reverse transcription-polymerase chain reaction
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- IFN-gamma
interferon-gamma
- Th
T helper
- TCR
T cell receptor
- Ig
immunoglobulin
- MHC II
major histocompatibility complex II
Footnotes
Competing interests
The authors state no conflict of interest.
References
- Abbas A, Lichtman A, Pillai S, editors. Cellular and Molecular Immunology. Philadelphia: SaundersElsevier; 2010. p. 566. [Google Scholar]
- Abdul-Careem MF, Hunter DB, Shanmuganathan S, et al. Cellular and cytokine responses in feathers of chickens vaccinated against Marek's disease. Vet Immunol Immunopathol. 2008;126:362–366. doi: 10.1016/j.vetimm.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Austin LM, Boissy RE, Jacobson BS, et al. The detection of melanocyte autoantibodies in the Smyth chicken model for vitiligo. Clin Immunol Immunopathol. 1992;64:112–120. doi: 10.1016/0090-1229(92)90188-t. [DOI] [PubMed] [Google Scholar]
- Bassiouny DA, Shaker O. Role of interleukin-17 in the pathogenesis of vitiligo. Clin Exp Dermatol. 2011;36:292–297. doi: 10.1111/j.1365-2230.2010.03972.x. [DOI] [PubMed] [Google Scholar]
- Boissy RE, Lamont SJ, Smyth JR., Jr. Persistence of abnormal melanocytes in immunosuppressed chickens of the autoimmune "DAM" line. Cell Tissue Res. 1984;235:663–668. doi: 10.1007/BF00226966. [DOI] [PubMed] [Google Scholar]
- Boissy RE, Liu YY, Medrano EE, et al. Structural aberration of the rough endoplasmic reticulum and melanosome compartmentalization in long-term cultures of melanocytes from vitiligo patients. J Invest Dermatol. 1991;97:395–404. doi: 10.1111/1523-1747.ep12480976. [DOI] [PubMed] [Google Scholar]
- Boissy RE, Moellmann G, Trainer AT, et al. Delayed-amelanotic (DAM or Smyth) chicken: melanocyte dysfunction in vivo and in vitro. J Invest Dermatol. 1986;86:149–156. doi: 10.1111/1523-1747.ep12284190. [DOI] [PubMed] [Google Scholar]
- Boissy RE, Nordlund JJ. Vitiligo: current medical and scientific understanding. G Ital Dermatol Venereol. 2011;146:69–75. [PubMed] [Google Scholar]
- Boissy RE, Smyth JR, Jr., Fite KV. Progressive cytologic changes during the development of delayed feather amelanosis and associated choroidal defects in the DAM chicken line. A vitiligo model. Am J Pathol. 1983;111:197–212. [PMC free article] [PubMed] [Google Scholar]
- Cho BR. Horizontal transmission of turkey herpesvirus to chickens. IV. Viral maturation in the feather follicle epithelium. Avian Dis. 1975;19:136–141. [PubMed] [Google Scholar]
- Cui J, Arita Y, Bystryn JC. Cytolytic antibodies to melanocytes in vitiligo. J Invest Dermatol. 1993;100:812–815. doi: 10.1111/1523-1747.ep12476636. [DOI] [PubMed] [Google Scholar]
- Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erf GF. Animal model. In: Picardo M, Taieb A, editors. Vitiligo. Heidelberg: Springer; 2010. pp. 205–218. [Google Scholar]
- Erf GF, Bersi TK, Wang X, et al. Herpesvirus connection in the expression of autoimmune vitiligo in Smyth line chickens. Pigment Cell Res. 2001;14:40–46. doi: 10.1034/j.1600-0749.2001.140107.x. [DOI] [PubMed] [Google Scholar]
- Erf GF, Trejo-Skalli AV, Smyth JR., Jr. T cells in regenerating feathers of Smyth line chickens with vitiligo. Clin Immunol Immunopathol. 1995;76:120–126. doi: 10.1006/clin.1995.1105. [DOI] [PubMed] [Google Scholar]
- Erf G, Wijesekera H, Lockhart B. Antioxidant capacity and oxidative stress in the local environment of feather-melanocytes in vitiliginous Smyth line chickens. Pigment Cell Res. 2005;18:69. [Google Scholar]
- Frohlich A, Kisielow J, Schmitz I, et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- Gilhar A, Zelickson B, Ulman Y, et al. In vivo destruction of melanocytes by the IgG fraction of serum from patients with vitiligo. J Invest Dermatol. 1995;105:683–686. doi: 10.1111/1523-1747.ep12324456. [DOI] [PubMed] [Google Scholar]
- Gobel TWF, Chen CH, Shrimpf J, et al. Characterization of avian natural killer cells and their intracellular CD3 protein complex. Eur J Immunol. 1994;24:1685–1691. doi: 10.1002/eji.1830240734. [DOI] [PubMed] [Google Scholar]
- Grimes PE, Morris R, vaniss-Aghajani E, et al. Topical tacrolimus therapy for vitiligo: therapeutic responses and skin messenger RNA expression of proinflammatory cytokines. J Am Acad Dermatol. 2004;51:52–61. doi: 10.1016/j.jaad.2003.12.031. [DOI] [PubMed] [Google Scholar]
- Hamal KR, Wideman RF, Anthony NB, et al. Differential gene expression of proinflammatory chemokines and cytokines in lungs of ascites-resistant and -susceptible broiler chickens following intravenous cellulose microparticle injection. Vet Immunol Immunopathol. 2010;133:250–255. doi: 10.1016/j.vetimm.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Harning R, Cui J, Bystryn JC. Relation between the incidence and level of pigment cell antibodies and disease activity in vitiligo. J Invest Dermatol. 1991;97:1078–1080. doi: 10.1111/1523-1747.ep12492607. [DOI] [PubMed] [Google Scholar]
- Kelchtermans H, Billiau A, Matthys P. How interferon-gamma keeps autoimmune diseases in check. Trends Immunol. 2008;29:479–486. doi: 10.1016/j.it.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Lamont SJ, Smyth JR., Jr. Effect of bursectomy on development of a spontaneous postnatal amelanosis. Clin Immunol Immunopathol. 1981;21:407–411. doi: 10.1016/0090-1229(81)90229-4. [DOI] [PubMed] [Google Scholar]
- Lauw FN, Pajkrt D, Hack CE, et al. Proinflammatory effects of IL-10 during human endotoxemia. J Immunol. 2000;165:2783–2789. doi: 10.4049/jimmunol.165.5.2783. [DOI] [PubMed] [Google Scholar]
- Le Gal FA, Avril MF, Bosq J, et al. Direct evidence to support the role of antigen-specific CD8(+) T cells in melanoma-associated vitiligo. J Invest Dermatol. 2001;117:1464–1470. doi: 10.1046/j.0022-202x.2001.01605.x. [DOI] [PubMed] [Google Scholar]
- Le Poole I, Van den Wijngaard RM, Westerhof W, et al. Presence of T cells and macrophages in inflammatory vitiligo skin parallels melanocyte disappearance. Am J Pathol. 1996;148:1219–1228. [PMC free article] [PubMed] [Google Scholar]
- Li Y, Yee C. IL-21 mediated Foxp3 suppression leads to enhanced generation of antigen-specific CD8+ cytotoxic T lymphocytes. Blood. 2008;111:229–235. doi: 10.1182/blood-2007-05-089375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti S, Fabbri P, Baroni G, et al. Keratinocyte dysfunction in vitiligo epidermis: cytokine microenvironment and correlation to keratinocyte apoptosis. Histol Histopathol. 2009;24:849–857. doi: 10.14670/HH-24.849. [DOI] [PubMed] [Google Scholar]
- Moretti S, Spallanzani A, Amato L, et al. New insights into the pathogenesis of vitiligo: imbalance of epidermal cytokines at sites of lesions. Pigment Cell Res. 2002;15:87–92. doi: 10.1034/j.1600-0749.2002.1o049.x. [DOI] [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Martinez G, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- Schallreuter KU, Moore J, Wood JM, et al. In vivo and in vitro evidence for hydrogen peroxide (H2O2) accumulation in the epidermis of patients with vitiligo and its successful removal by a UVB-activated pseudocatalase. J Investig Dermatol Symp Proc. 1999;4:91–96. doi: 10.1038/sj.jidsp.5640189. [DOI] [PubMed] [Google Scholar]
- Seyedalinaghi SA, Karami N, Hajiabdolbaghi M, et al. Vitiligo in a patient associated with human immunodeficiency virus infection and repigmentation under antiretroviral therapy. J Eur Acad Dermatol Venereol. 2009;23:840–841. doi: 10.1111/j.1468-3083.2008.03032.x. [DOI] [PubMed] [Google Scholar]
- Shi CS, Kehrl JH. TRAF6 and A20 regulate lysine 63-linked ubiquitination of Beclin-1 to control TLR4-induced autophagy. Sci Signal. 2010;3:ra42. doi: 10.1126/scisignal.2000751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Plumlee BL, Erf GF. Autoinflammatory/autoimmune vitiligo in Smyth line chickens: Immune system- and melanocyte-related activities in feathers and in feather melanocytes isolated by laser capture microdissection. Pigment Cell Melanoma Res. 2009;22:495–516. (Abstr.) [Google Scholar]
- Shibata Y, Foster LA, Kurimoto M, et al. Immunoregulatory roles of IL-10 in innate immunity: IL-10 inhibits macrophage production of IFN-gamma-inducing factors but enhances NK cell production of IFN-gamma. J Immunol. 1998;161:4283–4288. [PubMed] [Google Scholar]
- Shresta S, Smyth JR, Jr., Erf GF. Profiles of pulp infiltrating lymphocytes at various times throughout feather regeneration in Smyth line chickens with vitiligo. Autoimmunity. 1997;25:193–201. doi: 10.3109/08916939708994728. [DOI] [PubMed] [Google Scholar]
- Smyth JR., Jr. The Smyth chicken: a model for autoimmune amelanosis. Poultry biology. 1989;2:1–19. [Google Scholar]
- Son YM, Kwak CW, Lee YJ, et al. Ginsenoside Re enhances survival of human CD4+ T cells through regulation of autophagy. Int Immunopharmacol. 2010;10:626–631. doi: 10.1016/j.intimp.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Spolski R, Kim HP, Zhu W, et al. IL-21 mediates suppressive effects via its induction of IL-10. J Immunol. 2009;182:2859–2867. doi: 10.4049/jimmunol.0802978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritz R. Shared genetic relationships underlying generalized vitiligo and autoimmune thyroid disease. Thyroid. 2010;20:745–754. doi: 10.1089/thy.2010.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H, van MC, van den EA, et al. Treatment of Crohn's disease with recombinant human interleukin 10 induces the proinflammatory cytokine interferon gamma. Gut. 2002;50:191–195. doi: 10.1136/gut.50.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi H, Yonemoto K, Katsuoka K. Vitiligo with inflammatory raised borders with hepatitis C virus infection. J Dermatol. 2006;33:577–578. doi: 10.1111/j.1346-8138.2006.00135.x. [DOI] [PubMed] [Google Scholar]
- Wang X. The role of cell-mediated immunity in Smyth line autoimmune vitiligo (Ph.D dissertation) Fayetteville, AR: University of Arkansas; 2001. [Google Scholar]
- Wang X, Erf GF. Melanocyte-specific cell mediated immune response in vitiliginous Smyth line chickens. J Autoimmun. 2003;21:149–160. doi: 10.1016/s0896-8411(03)00087-8. [DOI] [PubMed] [Google Scholar]
- Wang X, Erf GF. Apoptosis in feathers of Smyth line chickens with autoimmune vitiligo. J Autoimmun. 2004;22:21–30. doi: 10.1016/j.jaut.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Wick G, Andersson L, Hala K, et al. Avian models with spontaneous autoimmune diseases. Adv Immunol. 2006;92:71–117. doi: 10.1016/S0065-2776(06)92002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ML, Medrano JF. Real-time PCR for mRNA quantitation. Biotechniques. 2005;39:75–85. doi: 10.2144/05391RV01. [DOI] [PubMed] [Google Scholar]
- Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]