Abstract
In the rabbit retina, there are two types of horizontal cell (HC). The axonless A-type HCs form a coupled network via connexin 50 (Cx50) gap junctions in the outer plexiform layer (OPL). The axon-bearing B-type HCs form two independent coupled networks; the dendritic network via gap junctions consisted of unknown Cx and the axon terminal network via Cx57. The present study was conducted to examine the localization and morphological features of Cx50 and Cx57 gap junctions in rabbit HCs at cellular and subcellular levels. The results showed that each gap junction composed of Cx50 or Cx57 showed distinct features. The larger Cx50 gap junctions were located more proximally than the smaller Cx50 gap junctions. Both Cx50 plaques formed symmetrical homotypic gap junctions, but some small ones had an asymmetrical appearance, suggesting the presence of heterotypic gap junctions or hemichannels. In contrast, Cx57 gap junctions were found in the more distal part of the OPL but never on the axon terminal endings entering the rod spherules, and they were exclusively homotypic. Interestingly, about half of Cx57 gap junctions appeared to be invaginated. These distinct features of Cx50 and Cx57 gap junctions show variety of HC gap junctions and may provide insights into the function of different types of HCs.
Keywords: gap junction, Cx50, Cx57, electron microscopy, horizontal cells, rabbit retina
Introduction
Gap junctions composed of connexins (Cxs) form electrical synapses between cells and they are widely distributed throughout the central nervous system. All retinal neurons make gap junctions and four different neuronal Cxs have been found in the mammalian retina [2, 16], where gap junctions are thought to play important roles in visual processing.
Horizontal cells (HCs) provide negative feedback to cones and thus contribute to surround inhibition in bright light [1]. HCs are well coupled via gap junctions and thus form extensively coupled networks [17, 30]. This is the basis for the property that the HC receptive field is considerably larger than the dendritic field and the strength of coupling in the HC network determines the spatial extent of the feedback signal to photoreceptors [3, 5]. In the rabbit retina, there are two morphological types of HC. The A-type HC is an axonless cell with thick dendrites that contacts only cones. In contrast, the B-type HC is axon bearing. The somatic dendrites of B-type HCs contact cones, whereas the axon terminals contact rods exclusively [5, 24]. Together, the two HC types make three different coupled networks: the axonless A-type HC network, the somatodendritic network of B-type HC and the B-type axon terminal network in the outer plexiform layer (OPL) [17, 18, 22].
Our previous work using confocal microscopy has shown that two Cxs, Cx50 and Cx57 contribute to the HC coupled networks in the rabbit retina. The extensively coupled A-type HCs express Cx50 gap junctions whereas B-type HC axon terminals are coupled via Cx57 gap junctions [19, 21]. Neither Cx was reported to be at the tips of dendrites or axon terminals, the presumed sites of feedback to photoreceptors. Rather, Cx50 and Cx57 were located in the HC neuropil. These findings are not consistent with the ephaptic hypothesis of HC feedback [13] based on the electron microscopic findings that hemichannels were located on the dendritic tips of horizontal cells in the goldfish retina.
Recently, in the mouse retina, confocal microscopic observations suggested that Cx57 may be localized at the tips of HC axon terminals where they contact rod spherules [4]. However, in another report, electron microscopic observation did not provide any evidence for the localization of Cx57 at the tips of HC axon terminals [11]. Therefore, in this study, we have examined the location and morphological features of both Cx50 and Cx57 gap junctions in the OPL of the rabbit retina at electron microscopic level.
Materials and methods
Five Adult New Zealand Albino rabbits were anesthetized with urethane (1.5 gm/kg, i.p.). The eyes were removed and hemisected, and the retina was isolated while immersed in bicarbonate-based Ames’ medium (Sigma-Aldrich). The animals were treated according to the regulations of the Catholic Ethics Committee of the Catholic University of Korea, Seoul and Institutional Animal Welfare Committee of the University of Texas Health Science Center at Houston Medical School, which conform to the National Institute of Health (NIH) guidelines for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996. The isolated retina was fixed for 1 hr with 4% formaldehyde in 0.1 M phosphate buffer (PB: pH 7.4) for vertical vibratome sections. Alternatively, for intracellular dye injection, wholemount pieces were submerged with 5 µM 6-diamino-2-pheylindole (DAPI) to prelabel HC nuclei and then fixed for 20 min with 4% formaldehyde in PB.
Pieces of retina prelabeled with DAPI were visualized on an Olympus BX-50WI microscope equipped with epifluorescence. HCs were impaled under visual control by using pipettes whose tips were filled with 4% Neurobiotin (Vector Laboratories,) and 0.5% Lucifer yellow-CH (Molecular Probes) in ddH2O, then back-filled with 3 M LiCl. Electrode resistance was ~100 MΩ. The impaled cells were injected with a biphasic current (±1.0 nA, 3 Hz) for 10 min. After injection, the retinal pieces were fixed in 4% paraformaldehyde.
Rabbit anti-Cx40 antibody that cross-reacts with Cx50 C-terminus was purchased from Chemicon (dilution 1:1000). This antibody specifically stains Cx50 gap junctions in A-type HCs of the rabbit retina [19]. Rabbit anti-Cx57 antibody (dilution 1:200) was used. In our recent work [21], the specificity of antibody was tested and it specifically stained Cx57 gap junctions in B-type HC axon terminal network of the rabbit retina.
Wholemount pieces of retina after intracellular dye injection or vibratome sections were blocked in 5% donkey serum in PB for 2 hr to reduce nonspecific labeling. The tissue was incubated in primary antibody (anti-Cx40/50 or anti-Cx57) in the presence of 1% donkey serum/PB with 0.5% Triton X-100/ 0.1% sodium azide overnight for vertical vibratome sections or for up to 1 week for wholemounts. The vertical vibratome sections were washed with PB and incubated in peroxidase-conjugated donkey anti-rabbit or anti-mouse IgG (Jackson ImmunoResearch) for 2 hr. Washed with PB and 0.1 M Tris buffer (TB: pH 7.6), the sections were preincubated in 0.05% 3,3´-diaminobenzidine tetrahydrochloride (DAB) in TB for 10 min, followed by incubation in the same solution containing 0.05% hydrogen peroxide for an additional 10 min. The wholemounts were washed with PB and incubated in Alexa Fluor 488-conjugated secondary antibodies (Molecular Probes) and Cy3-Streptavidin (Jackson ImmunoResearch). Wholemount preparations were mounted with Vectashield (Vector Laboratories) and viewed with Zeiss LSM 510 Meta confocal microscope equipped with a krypton/argon laser.
For immunoelectron microscopy, retinal vertical sections were prepared and immunostained, as described above for light microscopy but without Triton X-100 and sodium azide. Stained sections were post-fixed in 1% glutaraldehyde in PB for 1 hr and in 1% OsO4 in PB for 1 hr. They were washed and dehydrated in a graded series of alcohol. During the dehydration procedure, they were stained en bloc with 1% uranyl acetate in 70% alcohol for 1 hr, then transferred to propylene oxide, and flat embedded in Epon 812. After curing at 60 °C for 3 days, well-stained areas were cut out and attached to an Epon support for further ultrathin sectioning (Reichert-Jung). Ultrathin sections (90 nm thick) were collected on one-hole grids coated with Formvar, and 180 sections were examined using an electron microscope (Jeol 1200EX).
Results
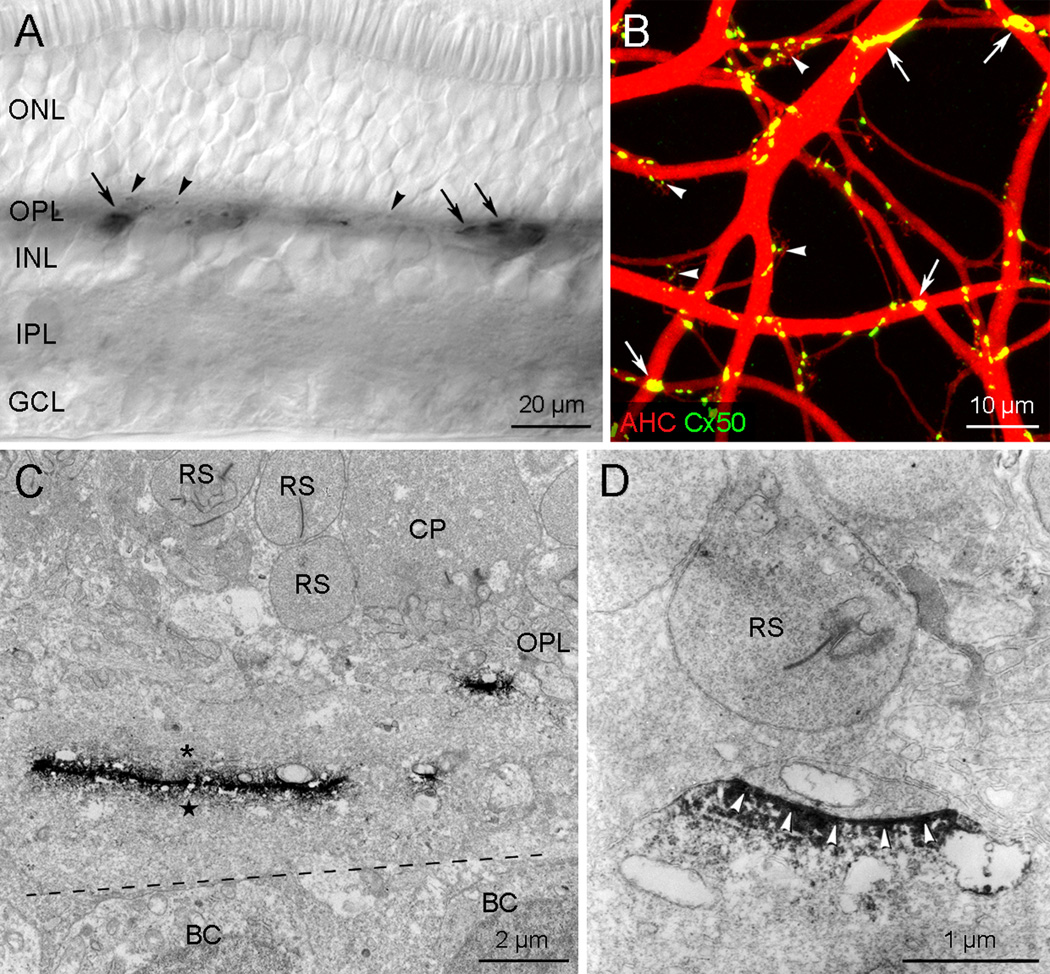
Immunohistochemistry performed on vertical sections of the rabbit retina revealed that there are two types of Cx50-immunoreactive plaques in the outer plexiform layer (OPL) (Fig. 1A). One type was large, often string-like, and localized to the proximal part of the OPL. The second type was small or dot-like and localized to the distal OPL. These two Cx50 plaque types could be distinguished in wholemount preparations (Fig. 1B) where A-type HCs were dye injected with Neurobiotin to visualize the dendritic matrix (Supplementary Fig. 1A). Essentially all the Cx50 plaques were confined to the A-type HC dendritic matrix. Large plaques or strings were found at large A-type HC dendritic crossings whereas small plaques or dots were observed in terminal clusters, each of which is formed by the convergence of fine dendrites from several A-type HCs. These findings suggest that large Cx50 gap junction plaques may be found at the crossings of A-type HC proximal dendrites and the small ones may be localized at distal fine dendrites.
Fig. 1.
Cx50 gap junctions in the rabbit retina. A: The micrograph was taken from a vertical section processed for Cx50 immunoreactivity. Cx50 immunoreactivity is confined to the outer plexiform layer (OPL) and appear as two plaque types: the first type of large or linear plaques (arrows) and the second smaller type (arrowheads). B: This confocal stack image was taken from a wholemount processed for Cx50 immunoreactivity (green) after the injection of Neurobiotin to visualize the A-type HC matrix (red). Cx50 plaques of various sizes are visible on the A-type HC matrix. Large plaques or strings (arrows), corresponding to the first type shown in A, occur at large dendritic crossings in the A-type HC matrix, whereas small plaques or dots (arrowheads), corresponding to the second type shown in A, are localized in terminal clusters. C–D: Electron micrographs showing Cx50 gap junctions in the OPL. C: A large Cx50 gap junction approximately 7 µm in length and a small Cx50 gap junction around 1 µm in length are seen in proximal and distal regions of the OPL, respectively. Cx50 immunoreactivity is found to be associated with both cytoplasmic membranes between two large dendrites (asterisk and star) running horizontally, located in the proximal part of the OPL, and between two small dendrites among many packed beneath a cone pedicle in the distal part of the OPL in the right side. BC, bipolar cell; CP, cone pedicle; RS, rod spherule. D: An example of putative Cx50 hemichannel or heterotypic gap junction formed beneath a RS. Note that only one side of gap junction show Cx50 immunoreactivity (arrowheads).
Using preembedding immunoelectron microscopy, Cx50 gap junctions were easily identified as electron-dense DAB products. Unfortunately, we were unable to observe the typical pentalaminar ultrastructure of gap junctions, probably due to the dense staining. However, the two labeled cell membranes were more closely apposed than other intercellular spaces formed between neighboring unlabeled cell membranes. A total of 166 putative Cx50 gap junctions were observed in the OPL. They could be divided into two types. The first type (n=75) was the large gap junctions (> 2 µm in length) which were exclusively localized in the proximal half of the OPL (Fig. 1C). They were formed between two putative A-type HC proximal dendrites (large one in Fig. 1C) and between somata and passing dendrites of putative A-type HCs (Supplementary Fig. 2). The second type (n=91) was smaller (< 2 µm in length) which were mainly found in the middle of the OPL (small one in Fig. 1C). All large Cx50 gap junctions and most of the small Cx50 gap junctions appeared to be homotypic, i.e. symmetrically labeled on both sides of the gap junction (Fig. 1C and Supplementary Fig. 2). However, in some interesting cases (n=12/91) Cx50 immunoreactivity was restricted to only one dendritic membrane (Fig. 1D and Supplementary Fig. 3), suggesting the presence of heterotypic gap junctions or hemichannels. These putative Cx50 hemichannels or heterotypic gap junctions were found in the distal OPL, beneath rod spherules or cone pedicles, but we never observed contact or penetration of the photoreceptor terminal.
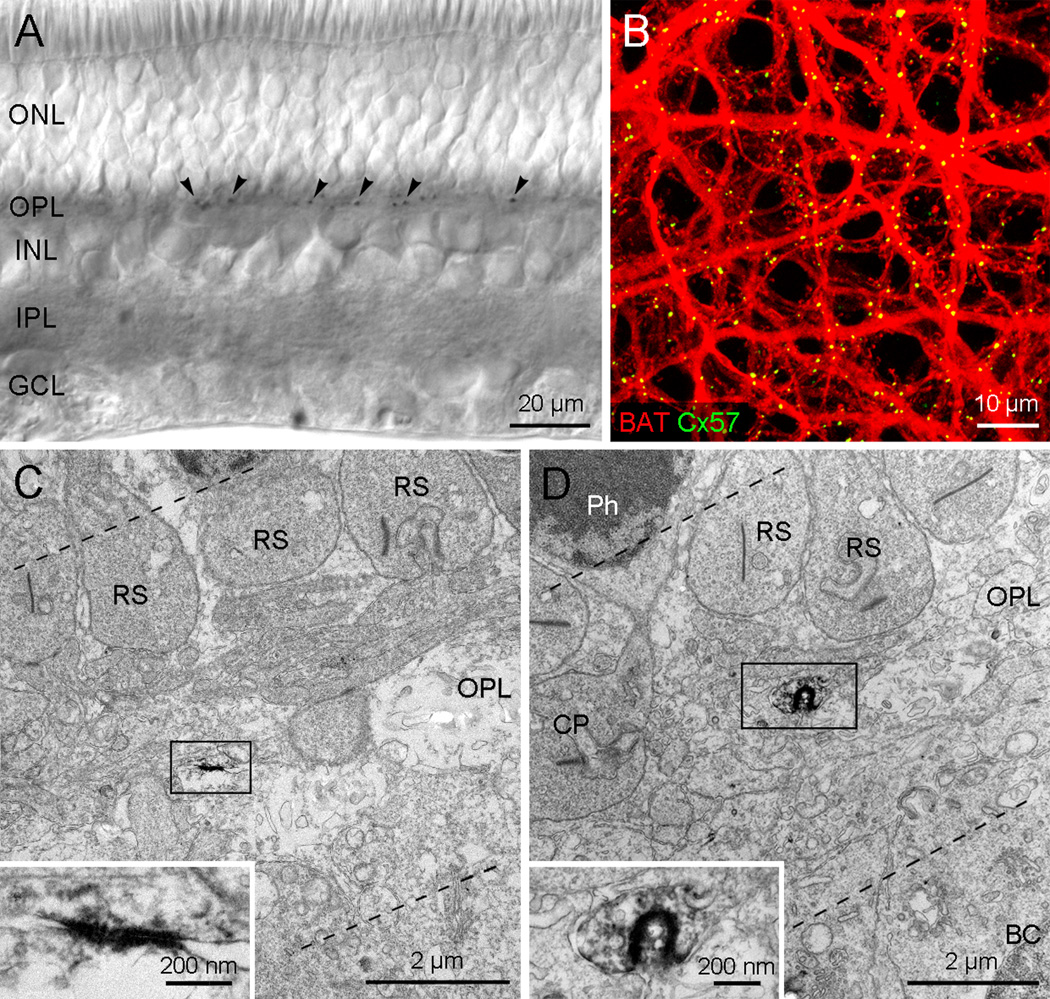
In vertical sections, Cx57 immunoreactivity was found exclusively in the OPL as small plaques. Cx57-immunoreactive plaques were relatively constant in size and localized in the middle to distal part of the OPL (Fig. 2A). In wholemounts, the B-type HC axon terminal matrix was visualized by filling with Neurobiotin (Supplementary Fig. 1B). The resulting network of fine processes could be identified as the axon terminal matrix by the absence of B-type HC somas and the presence of many fine branch tips matching the density of the rod spherules. When this preparation was processed for Cx57 immunoreactivity, the Cx57 plaques were confined to the B-type HC axon terminal matrix (Fig. 2B). Essentially all the Cx57 plaques were colocalized with the axon terminal matrix and therefore appear yellow. Most plaques were distributed along the axon terminal branches but it was difficult to determine whether Cx57 plaques were located at branch crossings, due to the limitation of resolution. Cx57 plaques were not found at the fine branch endings which enter individual rod spherules. These results suggest that Cx57 gap junctions may be localized on axon terminal branches which form a densely coupled matrix beneath the level of rod spherules.
Fig. 2.
Cx57 gap junctions in the rabbit retina. A: The micrograph was taken from a vertical section processed for Cx57 immunoreactivity. Cx57 immunoreactivity is identified as small plaques in the OPL. The plaques appear to be even in size and are located in the middle to distal part of the OPL. B: This confocal stack image was taken from a wholemount processed for Cx57 immunoreactivity (green) after Neurobiotin injection to visualize the B-type HC axon terminal matrix (red). The Cx57 plaques are exclusively colocalized with the axon terminal matrix. C–D: Electron micrographs showing Cx57 gap junctions in the OPL. C: A small linear type of Cx57 gap junction is seen in the middle of the OPL. This gap junction (rectangle) was magnified in inset. This high magnification view shows that the two cell membranes in the gap junction area are more closely apposed than those at other intercellular spaces. D: A small invaginated type of the Cx57 gap junction between both putative B-type horizontal cell axon terminals is seen in the distal OPL below the level of RS. This gap junction (rectangle) is magnified in inset. The membranes of this horseshoe-like gap junction appear to be fused, similar to those shown in inset of C.
As previously for Cx50, preembedding immunoelectron microscopy with specific antibodies against Cx57 was performed to examine the exact localization and the ultrastructural feature of Cx57 gap junctions. In total, 171 putative Cx57 gap junctions were observed in the OPL. Most of Cx57 gap junctions (n=166) were exclusively localized to cell membranes between putative B-type HC fine axons in the distal part of the OPL. Cx57 plaques could be found indiscriminately beneath either rod spherules or cone. We did not observe Cx57 labeling on branch endings which approach the synaptic ribbon in the invaginated region of the rod spherule. This makes it unlikely that Cx57 forms hemichannels mediating feedback to rods. In addition, all Cx57 plaques examined in this study were symmetrical (Figs. 2C and D), that is, both membranes were labeled, and therefore presumed to be homotypic. Cx57 plaques could be classified into two types, according to their shape; we observed 171 of Cx57 plaques and we found 97 were of the linear type which is observed in a flat or a slightly curved surface formed by close apposition of two B-type HC axonal branches (Fig. 2C), and 74 were designated as the invaginated type (Fig. 2D). These were found in the cellular membranes which were formed by the invagination or interlacing of a portion of a putative B-type HC axon terminal into a folded portion of another process. Cx57 gap junctions were small; the length of most flat type Cx57 gap junctions in ultrathin vertical sections of the retina was less than 0.5 µm. For the invaginated type of Cx57 gap junctions, the diameter of the inclusion was always less than 0.5 µm.
Discussion
Extensive coupling among HCs, via gap junctions, is the basis for the property that the HC receptive field is much larger than the dendritic field. The receptive field of A-type HCs was much greater than that of the B-type HC dendritic network which, in turn, was larger than the receptive field of the B-type axon terminal network [3, 5, 18]. These differences were thought to be explained by the electrophysiological and structural features of each gap junction type. Cx50 has a much larger unitary conductance, 220 pS [28], compared to 57 pS [20], for Cx57. In this study, Cx50 gap junctions located close to A-type HC somas were longer than 2 µm in length. In contrast, Cx57 gap junctions were located in the distal OPL, far from the somas, and they were significantly smaller, less than 0.5 µm in length. The area or length of a gap junction has a direct relationship with the number of channels; the larger the plaque, the more channels. Thus, the present results correspond with and corroborate previous studies [3, 17], showing that coupling is strongest in A-type HCs. The combination of gap junction properties and plaque size may determine the coupling parameters for each network.
HCs play a critical role in visual signal processing by providing feedback inhibition to photoreceptors. This plays a key role in the formation of the center-surround receptive field organization of bipolar and ganglion cells [1]. There have been several hypotheses for this feedback inhibition; a GABA-dependent mechanism [29], changes in pH in the synaptic cleft of the photoreceptor terminal [8, 20], and hemichannel-mediated ephaptic mechanism [13]. Among these competing mechanisms, the hemichannel-mediated ephaptic mechanism has drawn our attention because of the requirement for Cxs expression by HCs at photoreceptor terminals. It was proposed, based on the finding that Cx26 labeling was located in HC dendritic tips close to ribbon synapses in the fish retina, that hemichannels modulate voltage dependent calcium channels in the cone pedicle and subsequently the release of glutamate [13]. In this study, however, we could not find either Cx50 or Cx57 labeling on HCs close to ribbon synapses of cone pedicles or rod spherules in the rabbit retina. Thus, our results are aligned with previous reports from the mouse retina which has only one type of axon-bearing HC [23]. Mouse HCs are coupled by Cx57 gap junctions [9, 26] but, in Cx57 knock-out mice, feedback inhibition from HCs to photoreceptors was still present [6, 26]. Furthermore, Cx57 was not localized at the dendritic tips or axon terminal endings of mouse HCs [11]. This suggests, at least in the mammalian retina, that a hemichannel-medicated ephaptic mechanism may not be involved in feedback inhibition from HCs to photoreceptors. On a note of caution, zebrafish HCs express multiple Cxs, Cx55.5 and Cx52.6 [27], and we are unable to rule out the participation of hemichannels constructed from a different or unknown Cx.
In addition, we wish to draw attention to the possibility that Cx50 forms some heterotypic gap junctions. Cx50 can make functional hemichannels with voltage-dependent characteristics modulated by intracellular pH [32]. Cx50 can also participate in heterotypic gap junctions with Cx46 [10, 15]. In this study, in addition to homotypic Cx50 gap junctions, we also observed asymmetric junctions where the Cx50-labeled membrane was closely aligned with the opposing unlabeled membrane. That is, the interval between both Cx50-labeled and unlabeled membranes appears to be narrower than other intercellular spaces. Gap junctions have a narrow (2–3 nm) extracellular space between the membranes of neighboring cells, while hemichannels face wider (~20 nm) extracellular spaces, as reported by Zampighi [31] for Cx50 gap junctions and hemichannels in lens fiber. Thus, there is a possibility that Cx50 may form heterotypic gap junctions with another Cx type.
We observed that almost half of the Cx57 gap junctions had a very distinctive invaginated shape. This finding could perhaps be explained as a biological response to uncoupling, reported in ciliary and iris epithelia consequent to anoxia or low pH due to the prolonged interruption of blood supply during enucleation [7, 25], and may ultimately lead to junction internalization or endocytosis [12, 14]. Thus, our results might reflect the initial stage of gap junction resorption. However, the invaginated shape was peculiar to Cx57 gap junctions and the hypothetical adverse conditions did not produce invaginated Cx50 plaques. This suggests an alternative explanation whereby the purpose of the invaginated shape is to increase the plaque area between cofasciculated processes in a dense overlapping neuropil such as that of the B-type axon terminals. The axon terminal network is certainly dense in the distal OPL, with a coverage of approximately ten [22].
In conclusion, distinct features of Cx50 and Cx57 gap junctions, such as size and shape, readily distinguish between the gap junctions of A- and B-type HCs and show variety of HC gap junctions.
Highlights.
-
➢
Electron microscopy shows variety of horizontal cell gap junctions in the rabbit retina.
-
➢
Heterotypic gap junctions or hemichannels contribute to A-type horizontal cell network.
-
➢
Invaginated forms of Cx57 gap junctions exist in B-type horizontal axon terminal network.
Supplementary Material
Acknowledgements
This work was funded by Catholic Medical Center Research Foundation (Program year of 2007) to I.-B.K. and NIH (EY06515, EY10608) to S.C.M.
Abbreviations
- Cx
connexin
- GCL
ganglion cell layer
- HC
horizontal cell
- INL
inner nuclear layer
- IPL
inner plexiform layer
- ONL
outer nuclear layer
- OPL
outer plexiform layer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baylor DA, Fuortes MG, O'Bryan PM. Receptive fields of cones in the retina of the turtle. J. Physiol. 1971;214:265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloomfield SA, Völgyi B. The diverse functional roles and regulation of neuronal gap junctions in the retina. Nat. Rev. Neurosci. 2009;10:495–506. doi: 10.1038/nrn2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloomfield SA, Xin D, Persky SE. A comparison of receptive field and tracer coupling size of horizontal cells in the rabbit retina. Vis. Neurosci. 1995;12:985–999. doi: 10.1017/s0952523800009524. [DOI] [PubMed] [Google Scholar]
- 4.Ciolofan C, Lynn BD, Wellershaus K, Willecke K, Nagy JI. Spatial relationships of connexin36, connexin57 and zonula occludens-1 in the outer plexiform layer of mouse retina. Neuroscience. 2007;148:473–488. doi: 10.1016/j.neuroscience.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Dacheux RF, Raviola E. Horizontal cells in the retina of the rabbit. J. Neurosci. 1982;2:1486–1493. doi: 10.1523/JNEUROSCI.02-10-01486.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dedek K, Pandarinath C, Alam NM, Wellershaus K, Schubert T, Willecke K, Prusky GT, Weiler R, Nirenberg S. Ganglion cell adaptability: does the coupling of horizontal cells play a role? PLoS One. 2008;3:e1714. doi: 10.1371/journal.pone.0001714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freddo TF. Intercellular junctions of the iris epithelia in Macaca mulatta. Invest. Ophthalmol. Vis. Sci. 1984;25:1094–1104. [PubMed] [Google Scholar]
- 8.Hirasawa H, Kaneko A. pH changes in the invaginating synaptic cleft mediate feedback from horizontal cells to cone photoreceptors by modulating Ca2+ channels. J. Gen. Physiol. 2003;122:657–671. doi: 10.1085/jgp.200308863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hombach S, Janssen-Bienhold U, Sohl G, Schubert T, Bussow H, Ott T, Weiler R, Willecke K. Functional expression of connexin57 in horizontal cells of the mouse retina. Eur. J. Neurosci. 2004;19:2633–2640. doi: 10.1111/j.0953-816X.2004.03360.x. [DOI] [PubMed] [Google Scholar]
- 10.Hopperstad MG, Srinivas M, Spray DC. Properties of gap junction channels formed by Cx46 alone and in combination with Cx50. Biophys J. 2000;79:1954–1966. doi: 10.1016/S0006-3495(00)76444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janssen-Bienhold U, Trümpler J, Hilgen G, Schultz K, Müller LP, Sonntag S, Dedek K, Dirks P, Willecke K, Weiler R. Connexin57 is expressed in dendro-dendritic and axo-axonal gap junctions of mouse horizontal cells and its distribution is modulated by light. J. Comp. Neurol. 2009;513:363–374. doi: 10.1002/cne.21965. [DOI] [PubMed] [Google Scholar]
- 12.Jordan K, Chodock R, Hand AR, Laird DW. The origin of annular junctions: a mechanism of gap junction internalization. J. Cell. Sci. 2001;114:763–773. doi: 10.1242/jcs.114.4.763. [DOI] [PubMed] [Google Scholar]
- 13.Kamermans M, Fahrenfort I, Schultz K, Janssen-Bienhold U, Sjoerdsma T, Weiler R. Hemichannel-mediated inhibition in the outer retina. Science. 2001;292:1178–1180. doi: 10.1126/science.1060101. [DOI] [PubMed] [Google Scholar]
- 14.Leithe E, Brech A, Rivedal E. Endocytic processing of connexin43 gap junctions: a morphological study. Biochem. J. 2006;393:59–67. doi: 10.1042/BJ20050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo WK, Shaw AP, Takemoto LJ, Grossniklaus HE, Tigges M. Gap junction structures and distribution patterns of immunoreactive connexins 46 and 50 in lens regrowths of Rhesus monkeys. Exp. Eye. Res. 1996;62:171–180. doi: 10.1006/exer.1996.0021. [DOI] [PubMed] [Google Scholar]
- 16.Massey SC, O'Brien JJ, Trexler EB, Li W, Keung JW, Mills SL, O'Brien J. Multiple neuronal connexins in the mammalian retina. Cell Commun. Adhes. 2003;10:425–430. doi: 10.1080/cac.10.4-6.425.430. [DOI] [PubMed] [Google Scholar]
- 17.Mills SL, Massey SC. Distribution and coverage of A- and B-type horizontal cells stained with Neurobiotin in the rabbit retina. Vis. Neurosci. 1994;11:549–560. doi: 10.1017/s0952523800002455. [DOI] [PubMed] [Google Scholar]
- 18.Mills SL, Massey SC. A series of biotinylated tracers distinguishes three types of gap junction in retina. J. Neurosci. 2000;20:8629–8636. doi: 10.1523/JNEUROSCI.20-22-08629.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Brien JJ, Li W, Pan F, Keung J, O'Brien J, Massey SC. Coupling between A-type horizontal cells is mediated by connexin 50 gap junctions in the rabbit retina. J. Neurosci. 2006;26:11624–11636. doi: 10.1523/JNEUROSCI.2296-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palacios-Prado N, Sonntag S, Skeberdis VA, Willecke K, Bukauskas FF. Gating, permselectivity and pH-dependent modulation of channels formed by connexin57, a major connexin of horizontal cells in the mouse retina. J. Physiol. 2009;587:3251–3269. doi: 10.1113/jphysiol.2009.171496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan F, Keung J, Snuggs M, Kim IB, Mills SL, O'Brien J, Massey SC. Connexin 57 is expressed by the axon terminal network of B-type horizontal cells in the rabbit retina. J. Comp. Neurol. 2011 doi: 10.1002/cne.23060. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan F, Massey SC. Rod and cone input to horizontal cells in the rabbit retina. J. Comp. Neurol. 2007;500:815–831. doi: 10.1002/cne.21127. [DOI] [PubMed] [Google Scholar]
- 23.Peichl L, Gonzalez-Soriano J. Morphological types of horizontal cell in rodent retinae: a comparison of rat, mouse, gerbil, and guinea pig. Vis. Neurosci. 1994;11:501–517. doi: 10.1017/s095252380000242x. [DOI] [PubMed] [Google Scholar]
- 24.Raviola E, Dacheux RF. Axonless horizontal cells of the rabbit retina: synaptic connections and origin of the rod aftereffect. J. Neurocytol. 1990;19:731–736. doi: 10.1007/BF01188041. [DOI] [PubMed] [Google Scholar]
- 25.Raviola G, Raviola E. Intercellular junctions in the ciliary epithelium. Invest. Ophthalmol. Vis. Sci. 1978;17:958–981. [PubMed] [Google Scholar]
- 26.Shelley J, Dedek K, Schubert T, Feigenspan A, Schultz K, Hombach S, Willecke K, Weiler R. Horizontal cell receptive fields are reduced in connexin57-deficient mice. Eur. J. Neurosci. 2006;23:3176–3186. doi: 10.1111/j.1460-9568.2006.04848.x. [DOI] [PubMed] [Google Scholar]
- 27.Shields CR, Klooster J, Claassen Y, Ul-Hussain M, Zoidl G, Dermietzel R, Kamermans M. Retinal horizontal cell-specific promoter activity and protein expression of zebrafish connexin 52.6 and connexin 55.5. J. Comp. Neurol. 2007;501:765–779. doi: 10.1002/cne.21282. [DOI] [PubMed] [Google Scholar]
- 28.Srinivas M, Costa M, Gao Y, Fort A, Fishman GI, Spray DC. Voltage dependence of macroscopic and unitary currents of gap junction channels formed by mouse connexin50 expressed in rat neuroblastoma cells. J. Physiol. 1999;517(Pt 3):673–689. doi: 10.1111/j.1469-7793.1999.0673s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tachibana M, Kaneko A. gamma-Aminobutyric acid acts at axon terminals of turtle photoreceptors: difference in sensitivity among cell types. Proc. Natl. Acad. Sci. U S A. 1984;81:7961–7964. doi: 10.1073/pnas.81.24.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaney DI. Many diverse types of retinal neurons show tracer coupling when injected with biocytin or Neurobiotin. Neurosci Lett. 1991;125:187–190. doi: 10.1016/0304-3940(91)90024-n. [DOI] [PubMed] [Google Scholar]
- 31.Zampighi GA. Distribution of connexin50 channels and hemichannels in lens fibers: a structural approach. Cell Commun. Adhes. 2003;10:265–270. doi: 10.1080/cac.10.4-6.265.270. [DOI] [PubMed] [Google Scholar]
- 32.Zampighi GA, Loo DD, Kreman M, Eskandari S, Wright EM. Functional and morphological correlates of connexin50 expressed in Xenopus laevis oocytes. J. Gen. Physiol. 1999;113:507–524. doi: 10.1085/jgp.113.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.