Abstract
The risk of hip fracture rises rapidly with age, and is particularly high in women. This increase in fracture risk reflects both the age-related change in the risk of falling and decrements in the strength of the proximal femur. To better understand the extent to which proximal femoral density, structure and strength change with age as a function of gender, we have carried out a longitudinal analysis of proximal femoral volumetric quantitative computed tomographic (vQCT) images in men and women, analyzing changes in trabecular and cortical bone properties, and using subject-specific finite element modeling (FEM) to estimate changes in bone strength. In the AGES-Reykjavik Study vQCT scans of the hip were performed at a baseline visit in 2002–2006 and at a second visit 5.05±0.25 years later. From these, 223 subjects (111 men, 112 women, aged 68–87 years) were randomly selected. The subjects were evaluated for longitudinal changes in three bone variables assessed in a region similar to the total femur region quantified by DXA: areal bone mineral density (aBMD), trabecular volumetric bone mineral density (tBMD) and the ratio of cortical to total tissue volume (cvol/ivol). They were also evaluated for changes in bone strength using FEM models of the left proximal femur. Models were analyzed under single-limb stance loading (FStance), which approximates normal physiologic loading of the hip, as well as a load approximating a fall onto the posterolateral aspect of the greater trochanter (FFall). We computed five-year absolute and percentage changes in aBMD, tBMD, cvol/ivol, FFall and FStance. The Mann-Whitney Test was employed to compare changes in bone variables between genders and the Wilcoxon Signed Rank Test was used to compare changes in bone strength between loading conditions. Multiple (linear) regression was employed to determine the association of changes in FFall and FStance with baseline age and five-year weight loss. Both men and women showed declines in indices of proximal femoral density and structure (aBMD: men −3.9±6.0%, women −6.1±6.2%; tBMD: men −14.8±20.3%, women −23.9±26.8%; cvol/ivol: men −2.6±4.6%, women −4.7±4.8%, gender difference: p<0.001). Both men and women lost bone strength in each loading condition (Fstance: men −4.2±9.9%, women −8.3±8.5%; FFall: men −7.0±15.7%, women −12.8±13.2%; all changes from baseline p<0.0001). The gender difference in bone strength loss was statistically significant in both loading conditions (p<0.001 for FStance and P<0.01 for FFall) and FFall was lost at a higher rate than FStance in men (p<0.01) and women (p<0.0001). The gender difference in strength loss was statistically significant after adjustment for baseline age and weight loss in both loading conditions (p< 0.01). In these multi-linear models, men showed increasing rates of bone loss with increasing age (FFall: p=0.002; FStance: p=0.03), and women showed increasing bone strength loss with higher degrees of weight loss (FStance:p=0.003). The higher loss of FFall compared to FStance supports previous findings in animal and human studies that the sub-volumes of bone stressed under normal physiologic loading are relatively better protected in aging. The gender difference in hip bone strength loss is consistent with the higher incidence of hip fracture among elderly women.
Keywords: Hip fracture, osteoporosis, bone strength, finite element modeling, computed tomography, proximal femur
INTRODUCTION
Age-related bone loss leads to osteoporosis and its associated skeletal fractures. At the proximal femur, the site of the most clinically serious osteoporotic fractures, age-related bone loss occurs through a set of underlying structural changes that collectively result in diminished proximal femoral whole bone strength, increasing the likelihood of structural failure in the case of a fall[1–5]. Clinical evaluation of age-related changes in proximal femoral strength has become possible with the advent of subject-specific finite element modeling (FEM) based on volumetric quantitative computed tomography (vQCT)[6, 7]. The FEM technique employs the vQCT image to specify the three-dimensional bone geometry and distribution of material properties of the proximal femur, allowing estimation of structural strength with respect to clinically relevant loading conditions, such as a fall to the side with an impact on the greater trochanter. This approach has been validated as an important predictor of strength in vitro as well as a predictor of hip fracture in prospective studies[8, 9]. In a recent cross-sectional study, Keaveny et al examined bone strength loss in a cohort of men and women ranging in age from 20–80 years, finding that proximal femoral whole bone strength, evaluated with respect to a lateral fall onto the lateral aspect of the greater trochanter, declined 55% and 39% over the lifetime in women and men respectively[10]. This study was the first to document cross-sectional estimates of lifetime losses of bone strength in both men and women, it was subject to limitations associated with cohort effects and included relatively small number of elderly subjects.
To determine longitudinal patterns of proximal femoral strength loss in elderly subjects, we have carried out a 5-year study, based on vQCT and FEM, of aging men and women enrolled in a population-based epidemiologic cohort, the Age/Gene Environment Susceptibility Study-Reykjavik (AGES-Reykjavik). Our goals were to examine gender differences in rate of bone strength loss, to determine whether the rate of bone strength loss differs between loading conditions simulating normal function and those simulating a fall, and to determine the extent to which the rate of bone strength loss varies with factors such as age and weight loss. By obtaining more accurate and more detailed measures of proximal femoral strength loss, we expect to better understand the pathophysiology of hip fracture, including the three-fold higher rate of hip fracture in elderly women compared to men, and the rapid rise of hip fracture risk at later ages in elderly males.
MATERIALS AND METHODS
Subjects
We studied a group of subjects selected from the Age Gene/Environment Susceptibility (AGES) Reykjavik cohort. The AGES-Reykjavik study is an ongoing population-based study of men and women continuing the Reykjavik Study, which has been described in detail[11]. Baseline CT scans of 5500 subjects from this cohort were obtained between 2002 and 2006. Subjects were rescanned 5.05±0.25 years after the baseline measurements. Informed consent was obtained from all participants in the study, which was approved (VSN 00–063) by the National Bioethics Committee in Iceland as well as the Institutional Review Board of the Intramural Research Program of the National Institute on Aging. Scans of a subset of 223 (111 men, 112 women) subjects were randomly chosen from the cohort, with the only constraint being equal sample sizes per decade of age for each sex and lack of metallic hardware within the field of view of the hip scan. For each subject, in addition to age, height and weight, the history of medications that may induce changes in BMD (e.g. hormone replacement therapy, bisphosphonates, thiazides or glucocorticoids), was recorded. Additionally, the subject reported their state of health on a scale of 1–5 with a score of 1 indicating poor health and a score of 5 indicating excellent health.
Imaging
CT measurements in the hip were performed using a 4-detector CT system (Sensation 4, Siemens Medical Systems, Erlangen, Germany). To calibrate CT Hounsfield units to equivalent bone mineral concentration, all subjects were positioned supine on top of a calibration phantom (Image Analysis, Columbia, KY, USA), which extended from superior to the L1 vertebral body to the mid-femoral shaft. The phantom contained calibration cells of 0, 75 and 150 mg/cm3 equivalent concentrations of calcium hydroxyapatite. A helical study of the hip (120 kVp, 140 mAs, 1-mm slice thickness, pitch = 1, coarsened to 3-mm slice thickness) encompassed the proximal femur from a point 1 cm superior to the acetabulum to a point 3–5 mm inferior to the lesser trochanter.
Finite Element Modeling
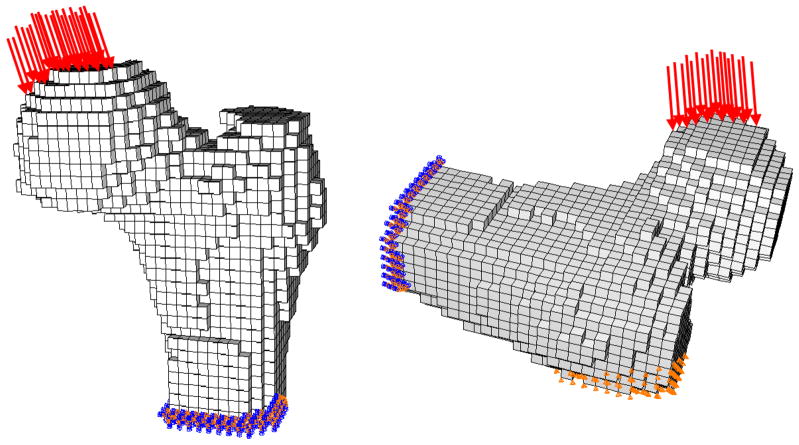
From the baseline (pre-fracture) QCT data for the left hip of each subject at each time point, we computed hip bone strength using our FE modeling method. The FE models incorporated patient-specific geometry and heterogeneous material properties that were computed from the QCT density data. The specific methodology has been described in detail previously and will be briefly outlined here[6, 12, 13]. Two loading conditions were studied (Figure 1). One approximately represented single-limb stance loading, with displacement applied to the femoral head in the coronal plane at 20° to the shaft axis. The second simulated loading from a fall onto the posterolateral aspect of the greater trochanter, with force applied to the femoral head at 60° to the shaft axis and 25° to the coronal plane while the opposing surface of the greater trochanter was constrained in the direction of the force.
Figure 1. Finite Element Models.
Finite element models based on QCT images are used to compute the strength of the proximal femur in two loading configurations: stance (left) and fall (right)
For single-limb stance loading, heterogeneous nonlinear properties were used to describe the nonlinear stress-strain relationship for the 3-mm cube of bone that was represented by each finite element. Use of nonlinear properties allowed modeling of the failure process as displacement was incrementally applied to the femoral head and the equivalent reaction force on the femoral head was calculated by the model. The FE-computed bone strength under stance loading (FStance) was defined as the maximum total reaction force on the femoral head. This nonlinear modeling method was necessary for the stance loading condition because linear models did not provide adequate precision for predicting fracture loads under this type of loading. In contrast, for fall loading, linear models employing heterogeneous linear elastic material properties were used. The fracture load for the fall loading condition (FFall) was defined as the force on the femoral head at the onset of fracture, i.e. the point at which local failure begins within the proximal femur. The fracture loads computed using this FE modeling method and these particular stance and posterolateral fall loading conditions have been validated previously via mechanical testing of cadaveric specimens, with high correlations of finite element prediction fracture load to measured fracture load in both loading conditions (stance, r2=0.93[12]; fall, r2=0.90[13]).
vQCT measures of proximal femoral structure
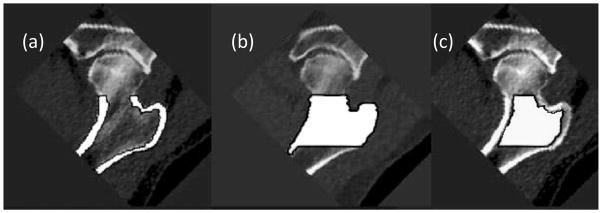
CT images were transferred from the CT scanner to a network of computer workstations equipped with the Linux operating system (Red Hat Version 7.2) and the AVS5 visualization program (AVS, Waltham MA, USA). Proximal femoral vQCT images were processed to extract measures of BMD and cortical structure[14] in the total femur region (Figure 2). This involved calibration of the images and segmentation procedures to determine trabecular, cortical and integral regions of interest in a region of interest similar to the total femur region used in DXA. In this region, we computed several parameters which have been shown to predict hip fracture in cross-sectional and prospective studies[15, 16]. These included trabecular BMD (tBMD, g/cm3), areal BMD (aBMD, g/cm2)(aBMD comparable to the total femur region of DXA) and the ratio of the volume of the cortical region of interest to the total bone volume (cvol/ivol), a fracture-related measure of the integrity of the proximal femoral cortex.
Figure 2. Proximal Femoral Regions for vQCT analysis.
(a) Total femur cortical, (b) integral and (c) trabecular regions of interest overlaid in white on greyscale data. aBMD is derived from region (b) and cvol/ivol is defined as the tissue volume in region (a) divided by the tissue volume of region (b).
Statistical Analysis
We computed the mean baseline values of the study parameters for each gender, and used the Mann-Whitney test to determine the statistical significance of the gender differences. To estimate age-related changes, we determined the five-year change in each parameter by dividing the inter-visit difference by the number of years between the visits, and multiplying by five. We tabulated these results in both percentage and absolute terms. In univariate analyses, we employed the Wilcoxon signed-rank test to determine statistically significant age-related changes within gender, and the Mann-Whitney test to determine the statistical significance of differences in loss rates between genders and loading conditions. To determine if the gender difference in bone strength loss was independent of independent of covariates that are thought to affect BMD and thus bone strength, we constructed linear models with percentage strength losses in stance and fall loading conditions as dependent variables, and baseline age, weight change between the baseline and follow-up visit, use of medications affecting BMD and self-reported health status at baseline. In order to compute the contribution of the predictor variables to percentage changes in bone strength in men and women, these analyses were first carried out separately for each gender. To determine whether differences in the effect of co-variates on percentage change in bone strength were statistically significant by gender, we pooled both genders into a single liner model and computed interaction terms for gender with each of the predictor variables.
RESULTS
Baseline values
Baseline characteristics of the cohort are summarized in Table 1. Men and women had similar ages at baseline, but men were on average taller and heavier. Men had 30–40% higher proximal femoral strength than women, with slightly smaller effect sizes for gender differences in areal BMD and trabecular BMD. Men and women had similar values of cvol/ivol at baseline.
Table 1.
Baseline characteristics of study subjects. Values are given as Mean (SD).
| Men (N = 111) | Women (N = 112) | |
|---|---|---|
| Age (years) | 76.7 (5.6) | 77.0 (5.11) |
| Height (cm) | 176.0 (6.5) | 160.0 (5.6) **** |
| Weight (kg) | 81.3 (13.8) | 69.4 (10.7) **** |
| Health Status | 2.46 (0.11) | 2.55 (0.11) |
| # Subjects on bone-altering medications | 20 | 61 |
| FFall (N) | 1690 (670) | 1200 (463) **** |
| FStance (N) | 10300 (2920) | 7710 (2060) **** |
| aBMD (g/cm2) | 0.822 (0.169) | 0.687 (0.141)**** |
| tBMD (g/cm3) | 0.078 (0.038) | 0.064 (0.033) ** |
| cvol/ivol | 0.342 (0.046) | 0.338 (0.050) |
p-values for gender differences based on Mann-Whitney test
: p<0.05
: p<0.01
p<0.0001
Univariate changes
Univariate changes for each gender are summarized in Table 2. All measures showed a statistically significant change from baseline (p<0.0001). In percentage terms, women had statistically significantly higher rates of percentage loss in aBMD, tBMD, cvol/ivol, FFall and FStance. This was also true of absolute changes, with the exception of FFall, which had a marginally insignificant gender difference (p=0.06).
Table 2.
Longitudinal changes in measures of proximal-femoral whole bone strength and measures of density and structure. Absolute changes (SD) are provided in plain text and percentage changes (SD) are provided in bold italics. Asterisks refer to p-values for gender differences in absolute and percentage change. # symbols refer to p-values for differences in change as a function of loading condition (stance vs fall).
| Men (N = 111) | Women (N = 112) | |
|---|---|---|
| ΔFFall (N) | −122 (281) #### | −164 (217) #### |
| ΔFFall (%) | −7.0 (15.7)## | −12.8 (13.2) **, #### |
|
| ||
| ΔFStance (N) | −451 (980) | −669 (807) * |
| ΔFStance (%) | −4.2 (9.9) | −8.3 (8.5)*** |
|
| ||
| ΔaBMD (g/cm2) | −0.031 (0.046) | −0.043 (0.047) ** |
| ΔaBMD (%) | −3.9 (6.0) | −6.1 (6.2) *** |
|
| ||
| ΔtBMD (g/cm3) | −0.007 (0.010) | −0.012 (0.011) **** |
| ΔtBMD (%) | −14.8 (20.3) | −23.9 (26.8) **** |
|
| ||
| Δcvol/ivol | −0.009 (0.015) | −0.016 (0.017) *** |
| Δcvol/ivol (%) | −2.6 (4.6) | −4.7 (4.8) *** |
In both genders, all absolute and percentage changes from baseline had Wilcoxon signed rank p<0.0001
*: p-values for gender differences in absolute and percentage changes based on Mann-Whitney test
: p<0.05
: p<0.01
p<0.001
p<0.0001
# p-values for significant difference between ΔFFall and ΔFStance based on Wilcoxon Signed Rank Test
p<0.05
p<0.01
p<0.001
p<0.0001
Multiple linear regression models
Table 3 shows results of multiple regression analyses relating five-year percentage changes in FFall and FStance to weight loss, health status, use of bone-active medications, baseline age and gender over the follow-up period. In models which pooled data from both genders, women continued to show greater percentage strength loss than men in both stance (least square means −8.60% vs −3.65%; p=0.0002) and fall (least square means −12.65% vs −7.40%; p=0.005) loading conditions, even after adjustment for co-variates. In models that pooled both genders, the gender interaction term with baseline age was statistically significant (p=0.04) indicating that men show a greater increase in percentage FFall loss with age than women (−0.695%/year-baseline age, p=0.002, in men and 0.04%/year-baseline age in women, p=.9, from gender-specific models). Although five-year percentage Fstance loss was significantly associated with increasing baseline age in men (−0.405%/year-baseline age; p<0.05) but not women (−0.170%/year-baseline age; p=0.2), no significant interaction of gender with baseline age was observed for FStance. Five-year percentage change in FStance was statistically significantly associated with weight loss in women (−0.490%/kg weight loss; p=0.003), but not men and a sex-interaction was significant for Fstance. This relationship was not observed for FFall or in absolute terms No other interactions of gender with predictor variables was observed in any of the linear models.
Table 3.
Multi-linear regression analyses relating five-year percentage changes in FStance and FFall to age, gender, self-reported health score, history of use of bone active medications and change in weight between the baseline and five-year visit.
| GENDER POOLED *# | ||||||
|---|---|---|---|---|---|---|
| FStance | FFall | |||||
| Term | β | Std Error | p | β | Std Error | p |
| Intercept | 14.29 | 8.66 | 0.1 | 16.2 | 12.48 | 0.2 |
| Baseline Age (years) | −0.265 | 0.11 | 0.02 | −0.365 | 0.16 | 0.026 |
| Gender | 2.49 | 0.65 | 0.0002 | 2.64 | 0.935 | 0.005 |
| Health Score | −0.425 | 0.53 | 0.43 | 0.405 | 0.765 | 0.597 |
| Bone Active Meds | 3.315 | 1.34 | 0.01 | 1.89 | 1.93 | 0.329 |
| Weight Change (kg) | 0.19 | 0.12 | 0.11 | −0.03 | 0.17 | 0.859 |
| MEN | ||||||
| FStance | FFall | |||||
| Term | β | Std Error | p | β | Std Error | p |
| Intercept | 24.235 | 12.455 | 0.05 | 44.255 | 16.53 | 0.009 |
| Baseline Age (years) | −0.405 | 0.165 | 0.015 | −0.695 | 0.22 | 0.002 |
| Health Score | 0.625 | 0.805 | 0.44 | 0.475 | 1.07 | 0.658 |
| Bone Active Meds | 6.705 | 2.315 | 0.005 | −0.765 | 3.07 | 0.804 |
| Weight Change (kg) | −0.075 | 0.17 | 0.664 | −0.315 | 0.22 | 0.156 |
| WOMEN | ||||||
| FStance | FFall | |||||
| Term | β | Std Error | p | β | Std Error | p |
| Intercept | 8.75 | 11.555 | 0.451 | −13.71 | 18.715 | 0.465 |
| Baseline Age (years) | −0.17 | 0.145 | 0.245 | −0.02 | 0.24 | 0.932 |
| Health Score | −1.225 | 0.67 | 0.071 | 0.62 | 1.09 | 0.571 |
| Bone Active Meds | 1.165 | 1.52 | 0.446 | 3.485 | 2.465 | 0.16 |
| Weight Change (kg) | 0.49 | 0.165 | 0.003 | 0.27 | 0.265 | 0.311 |
: gender*weight loss interaction was significant (p=0.03) for FStance but not FFall
gender*age interaction was significant (p=0.04) for FFall but not FStance
DISCUSSION
Young men and women appear to have comparable proximal femoral vBMD[5, 17]. However, higher bone loss, in the presence of smaller bone size and a similar periosteal apposition effect to that observed in men[18], appears to result in a higher rate of fracture in elderly women. Cross-sectional data obtained from a QCT-scanned subset of the Rochester Epidemiology Study indicate that the deficits observed in women, in cortical and trabecular vBMD, and in proximal femoral whole bone strength, originate in the period immediately following the menopause[5]. Keaveny et al documented 55% and 39% lifetime bone strength losses for women and men respectively, observing that all but 18% of the gender deficit in bone strength was determined by menopausal bone loss[10]. However, while these QCT-based studies included a broad cohort encompassing a wide age range, the study was not aimed at detailing age-related trends specifically within the elderly subjects. In a cross-sectional analysis carried out in the AGES-Reykjavik cohort, Sigurdsson et al reported that the gender difference in various measures of proximal femoral skeletal integrity, including cortical and trabecular vBMD and estimates of femoral neck axial compressive and bending strength, continued to increase in the elderly subjects, with women showing cross-sectional rates of bone density and estimated strength loss two-four times higher than those of men, depending on the specific measure[18]. However, age-related changes are better represented in longitudinal compared to cross-sectional studies, which are confounded by cohort effects. Differences in longitudinal compared to cross-sectional estimates of bone density loss were examined by Melton et al, who found that cross-sectional measurements systematically overestimated rates of bone loss at the hip, with the error varying with the age- and hormone treatment status of the subjects[19].
Our study represents the first longitudinal QCT data comparing changes in proximal femoral bone density, structure and whole bone strength in men and women. In reasonable agreement with previous longitudinal studies carried out with DXA, our results showed that elderly women lose about 1.2% total femur aBMD per year, compared to roughly 0.8% per year in men[19, 20]. However, the gender differences in aBMD loss tended to underestimate the changes observed for the specifically trabecular and cortical volumetric measures, tBMD and cvol/icol, which were two-fold higher in women compared to men. Similarly, longitudinal changes in proximal femoral whole bone strength as assessed by FEM, a measure that which integrates the effect of differential changes in the cortical and trabecular compartments, were two-fold higher in women then in men. Thus, when averaged across our cohort, women appear to have greater longitudinal rates of loss in bone strength and indices of femoral density and structure, confirming earlier reports that the gender disparity in proximal femoral fragility tends to increase with age[18].
In our multiple regression models relating changes in bone strength to various risk factors, a more complex picture of the gender differences in bone strength loss emerged. We examined the variation of strength change with a range of factors including increasing baseline age and weight loss, which are risk factors for both bone loss[21, 22] and hip fracture[23, 24]. In models relating percentage bone strength loss to age, weight loss, use of BMD active medications and health status, we found that the percentage changes in FFall and FStance continued to vary by gender, even after adjustment for those variables. However, when we carried out gender-specific analyses, we observed that bone strength loss was associated with different factors in men and women. In men, but not women, increasing baseline age was associated with increasing rates of bone strength loss in both loading conditions. In women, but not men, greater weight loss was associated with greater loss of stance (but not fall) strength. Such a finding may support the use gender-specific strategies for preventing or reducing the impact of bone strength loss, reinforcing the importance of maintaining body weight in older women. The accelerating rate of bone strength loss with age in elderly men underscores the need to better understand the etiology of age-related bone loss in men, to continue to investigate pharmaceutical and other interventions for bone health in aging men, as well as to carefully control medications which increase the risks of falls, bone loss and other risk factors for hip fracture.
Among the women of our cohort, although the longitudinal rate of aBMD loss was in keeping with previous studies using DXA, the losses of tBMD and FFall were particularly high. We observed a 25% five year loss rate for total femur tBMD. Our observed five year change is a substantial proportion of the changes reported in cross-sectional comparisons of young and elderly women. Meta et al [4]observed 34% and 62% differences for trochanteric and femoral neck tBMD between groups of women with mean ages of 41 and 74 years. Using a different femoral neck region of interest, Riggs et al reported a 56% decline between 20 and 90 years of age for women in a QCT-scanned subset of the Rochester Study[5]. Similarly, our observation of a 12.8% (160 N) five year loss in fall strength represents nearly a quarter of the 55% loss of strength reported by Keaveny using QCT scan data from the subjects of the Rochester study[10]. By comparison, in a previous study relating low femoral strength to incident hip fracture, we observed that women who experienced incident hip fracture over a seven year follow-up period had at baseline a 337N lower mean value of FFall than age-matched controls[8]. Thus, the five-year FFall loss corresponded to both a significant proportion of previously reported lifetime losses in bone strength and half of the difference in FFall between fractures and controls in our prospective fracture study.
In this study, we also compared longitudinal changes in estimated whole bone strength evaluated in two loading conditions, one representing a single-limb stance, and one representing a fall onto the posterolateral aspect of the greater trochanter. In contrast to the posterolateral fall loading condition, which represents a pathologic load on the hip, the single-limb stance loading condition approximates the activities of walking, jumping and stair-climbing, activities to which the structure of the hip is adapted. We observed that in both genders, the rate of strength loss was 50% higher in the fall loading condition than in stance loading. In stance loading, a substantial portion of the load on the femoral head is transmitted through the trabecular bone to the thick inferomedial cortex of the hip. Thus, our observation of lower bone strength loss in this loading condition is consistent with other studies that have found lower degrees of age-related loss in structures that are associated with routine load bearing[2]. On the other hand, the posterolateral fall loading condition utilizes the load bearing capacity of the inferomedial cortex to a lesser extent (because it lies close to the neutral axis for this loading condition), and calls upon other structures to support the load, i.e. structures such as the superior and anterior femoral neck cortices that normally experience less mechanical stimulation and suffer greater age-related losses[2].
This study has strengths and limitations. The strengths include the inclusion of both genders, the use of finite element modeling to compute whole bone strength, and the longitudinal study design, which eliminates the cohort effects that confound cross-sectional studies. The limitations of our study include the exclusively Northern European, Caucasian study group, which may reduce applicability of our findings to other races and ethnicities. Another limitation is that our categorical variable summarizing use of BMD-active medication did not permit us to discriminate between medications that cause bone loss and those that protect against bone loss or increase BMD. Technical limitations inherent in the FEM approach, such as the assumptions of isotropic material properties and distortion energy failure theory, have been discussed in previous reports[6, 13, 25, 26]. However, despite these limitations, this FEM technique described here provides estimates of whole bone strength that strongly correlate to experimentally measured values in cadaveric specimen studies[6] and that are strongly associated with incident hip fracture in prospective clinical studies, such as the recent study by Keyak et al in this cohort [8].
In conclusion, our longitudinal study has shown that elderly men and women lose proximal femoral whole bone strength at a rate of 4–13% per five years, with women, on average showing twice the loss of strength as men in single-limb stance and posterolateral fall loading conditions. In addition to gender differences in mean bone strength loss, we observed gender differences in the risk factors for bone strength loss, with loss of weight in women (but not men) and increasing age in men (but not women) being significant risk factors for strength loss. Finally, we also observed a clear dependence of age-related bone strength loss on loading condition, with stance strength decreasing at a 50% lower rate than fall strength. Even so, we still observed a substantial loss of stance strength with age, indicating that the adaptive processes (eg periosteal apposition) that have been hypothesized to preserve mechanical homeostasis with age do not fully protect bone strength.
Highlights.
We longitudinally studied age-related changes in hip strength in elderly men and women
Finite element modeling was used to estimate strength changes in stance and fall loading conditions
Women lost twice as much hip strength as men
Women and men had different risk factors for loss of bone strength
Hip strength in fall loading was lost at a 50% greater rate than in stance loading.
Acknowledgments
This study was supported by NIH/NIA R01-AG028832. The Age, Gene/Environment Susceptibility Reykjavik Study is funded by NIH contract N01-AG-12100, the NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). The study was approved by the Icelandic National Bioethics Committee, (VSN: 00–063) and the Data Protection Authority. The researchers are indebted to the participants for their willingness to participate in the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Poole KE, Mayhew PM, Rose CM, Brown JK, Bearcroft PJ, Loveridge N, Reeve J. Changing structure of the femoral neck across the adult female lifespan. J Bone Miner Res. 2010;25:482–91. doi: 10.1359/jbmr.090734. [DOI] [PubMed] [Google Scholar]
- 2.Mayhew PM, Thomas CD, Clement JG, Loveridge N, Beck TJ, Bonfield W, Burgoyne CJ, Reeve J. Relation between age, femoral neck cortical stability, and hip fracture risk. Lancet. 2005;366:129–35. doi: 10.1016/S0140-6736(05)66870-5. [DOI] [PubMed] [Google Scholar]
- 3.Marshall LM, Lang TF, Lambert LC, Zmuda JM, Ensrud KE, Orwoll ES. Dimensions and Volumetric BMD of the Proximal Femur and Their Relation to Age Among Older U.S. Men. J Bone Miner Res. 2006;21:1197–206. doi: 10.1359/jbmr.060506. [DOI] [PubMed] [Google Scholar]
- 4.Meta M, Lu Y, Keyak JH, Lang T. Young-elderly differences in bone density, geometry and strength indices depend on proximal femur sub-region: a cross sectional study in Caucasian-American women. Bone. 2006;39:152–8. doi: 10.1016/j.bone.2005.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riggs BL, Melton Iii LJ, 3rd, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, Rouleau PA, McCollough CH, Bouxsein ML, Khosla S. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19:1945–54. doi: 10.1359/JBMR.040916. [DOI] [PubMed] [Google Scholar]
- 6.Keyak JH, Kaneko TS, Tehranzadeh J, Skinner HB. Predicting proximal femoral strength using structural engineering models. Clin Orthop Relat Res. 2005:219–28. doi: 10.1097/01.blo.0000164400.37905.22. [DOI] [PubMed] [Google Scholar]
- 7.Keaveny TM. Biomechanical computed tomography-noninvasive bone strength analysis using clinical computed tomography scans. Ann N Y Acad Sci. 2010;1192:57–65. doi: 10.1111/j.1749-6632.2009.05348.x. [DOI] [PubMed] [Google Scholar]
- 8.Keyak JH, Sigurdsson S, Karlsdottir G, Oskarsdottir D, Sigmarsdottir A, Zhao S, Kornak J, Harris TB, Sigurdsson G, Jonsson BY, Siggeirsdottir K, Eiriksdottir G, Gudnason V, Lang TF. Male-female differences in the association between incident hip fracture and proximal femoral strength: a finite element analysis study. Bone. 2011;48:1239–45. doi: 10.1016/j.bone.2011.03.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orwoll ES, Marshall LM, Nielson CM, Cummings SR, Lapidus J, Cauley JA, Ensrud K, Lane N, Hoffmann PF, Kopperdahl DL, Keaveny TM. Finite Element Analysis of the Proximal Femur and Hip Fracture Risk in Older Men. J Bone Miner Res. 2008 doi: 10.1359/JBMR.081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keaveny TM, Kopperdahl DL, Melton LJ, 3rd, Hoffmann PF, Amin S, Riggs BL, Khosla S. Age-dependence of femoral strength in white women and men. J Bone Miner Res. 2010;25:994–1001. doi: 10.1359/jbmr.091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, Thorgeirsson G, Aspelund T, Garcia ME, Cotch MF, Hoffman HJ, Gudnason V. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–87. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keyak JH. Improved prediction of proximal femoral fracture load using nonlinear finite element models. Med Eng Phys. 2001;23:165–73. doi: 10.1016/s1350-4533(01)00045-5. [DOI] [PubMed] [Google Scholar]
- 13.Keyak JH, Rossi SA, Jones KA, Skinner HB. Prediction of femoral fracture load using automated finite element modeling. J Biomech. 1998;31:125–33. doi: 10.1016/s0021-9290(97)00123-1. [DOI] [PubMed] [Google Scholar]
- 14.Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19:1006–12. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- 15.Black DM, Bouxsein ML, Marshall LM, Cummings SR, Lang TF, Cauley JA, Ensrud KE, Nielson CM, Orwoll ES. Proximal femoral structure and the prediction of hip fracture in men: a large prospective study using QCT. J Bone Miner Res. 2008;23:1326–33. doi: 10.1359/JBMR.080316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng X, Li J, Lu Y, Keyak J, Lang T. Proximal femoral density and geometry measurements by quantitative computed tomography: association with hip fracture. Bone. 2007;40:169–74. doi: 10.1016/j.bone.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 17.Saeed I, Carpenter RD, Leblanc AD, Li J, Keyak JH, Sibonga JD, Lang TF. Quantitative computed tomography reveals the effects of race and sex on bone size and trabecular and cortical bone density. J Clin Densitom. 2009;12:330–6. doi: 10.1016/j.jocd.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Sigurdsson G, Aspelund T, Chang M, Jonsdottir B, Sigurdsson S, Eiriksdottir G, Gudmundsson A, Harris TB, Gudnason V, Lang TF. Increasing sex difference in bone strength in old age: The Age, Gene/Environment Susceptibility-Reykjavik study (AGES-REYKJAVIK) Bone. 2006 doi: 10.1016/j.bone.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Melton LJ, 3rd, Khosla S, Atkinson EJ, Oconnor MK, Ofallon WM, Riggs BL. Cross-sectional versus longitudinal evaluation of bone loss in men and women. Osteoporos Int. 2000;11:592–9. doi: 10.1007/s001980070080. [DOI] [PubMed] [Google Scholar]
- 20.Kaptoge S, Reid DM, Scheidt-Nave C, Poor G, Pols HA, Khaw KT, Felsenberg D, Benevolenskaya LI, Diaz MN, Stepan JJ, Eastell R, Boonen S, Cannata JB, Glueer CC, Crabtree NJ, Kaufman JM, Reeve J. Geographic and other determinants of BMD change in European men and women at the hip and spine. a population-based study from the Network in Europe for Male Osteoporosis (NEMO) Bone. 2007;40:662–73. doi: 10.1016/j.bone.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 21.Hannan MT, Felson DT, Dawson-Hughes B, Tucker KL, Cupples LA, Wilson PW, Kiel DP. Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15:710–20. doi: 10.1359/jbmr.2000.15.4.710. [DOI] [PubMed] [Google Scholar]
- 22.Ensrud KE, Lewis CE, Lambert LC, Taylor BC, Fink HA, Barrett-Connor E, Cauley JA, Stefanick ML, Orwoll E. Endogenous sex steroids, weight change and rates of hip bone loss in older men: the MrOS study. Osteoporos Int. 2006;17:1329–36. doi: 10.1007/s00198-006-0088-z. [DOI] [PubMed] [Google Scholar]
- 23.Ensrud KE, Ewing SK, Stone KL, Cauley JA, Bowman PJ, Cummings SR. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J Am Geriatr Soc. 2003;51:1740–7. doi: 10.1046/j.1532-5415.2003.51558.x. [DOI] [PubMed] [Google Scholar]
- 24.Langlois JA, Visser M, Davidovic LS, Maggi S, Li G, Harris TB. Hip fracture risk in older white men is associated with change in body weight from age 50 years to old age. Archives of Internal Medicine. 1998;158:990–6. doi: 10.1001/archinte.158.9.990. [DOI] [PubMed] [Google Scholar]
- 25.Keyak JH, Rossi SA, Jones KA, Les CM, Skinner HB. Prediction of fracture location in the proximal femur using finite element models. Med Eng Phys. 2001;23:657–64. doi: 10.1016/s1350-4533(01)00094-7. [DOI] [PubMed] [Google Scholar]
- 26.Keyak JH, Rossi SA. Prediction of femoral fracture load using finite element models: an examination of stress- and strain-based failure theories. J Biomech. 2000;33:209–14. doi: 10.1016/s0021-9290(99)00152-9. [DOI] [PubMed] [Google Scholar]