Abstract
Purpose
To examine, in tree shrews, the visual guidance of recovery from negative lens-induced myopia by measuring the effect of wearing low-power negative or positive lenses during recovery. To learn if removing a negative lens for two hours per day, after compensation has occurred, is sufficient to produce recovery.
Methods
Starting 16 days after natural eye opening (days of visual experience), juvenile tree shrews wore a monocular –5 D lens for 11 days to produce compensation (age-appropriate refraction while wearing the lens). Recovery in four groups was started by discontinuing −5 D lens wear, which caused the treated eyes to be refractively myopic, and substituting: no lens (n = 7), a plano lens (n = 8), a −2 D lens (n = 6) or a +2 D lens (n = 10). In a fifth group (n = 6), the −5 D lens was removed for 2 hours each day but worn the remainder of the time. Non-cycloplegic refractive measurements were made daily for the first 10 days and then less frequently. After 31 to 35 days, the lens-guided recovery period was ended for most animals; periodic measures were continued to assess post-lens recovery changes.
Results
All the eyes responded to the −5 D lens and were myopic (-4.8±0.1 D, mean ± SEM) compared to the untreated fellow control eye. In all groups except the −2 D Lens group, some animals exhibited slow or incomplete recovery. During recovery, the treated eye of most animals recovered until its refraction, measured with the recovery-lens in place, was near to that of the control eye. Measured without the lens, the −2 D group was myopic and the +2 D group was hyperopic. With the lens in place, the plano-lens, −2 D lens, and +2 D lens groups remained slightly myopic (−1.0±0.3 D, −0.6±0.2 D and −1.3±0.1 D, respectively). The rate of recovery during the first four days was unrelated to the amount of myopia initially experienced by the recovering eyes. Removal of the −5 D lens for two hours each day produced recovery.
Conclusions
During recovery, the emmetropization mechanism uses the presence of myopia, but perhaps not the magnitude, to guide eyes toward a refractive state similar to the control eye, regardless of whether the optically-recovered eye is longer or shorter than the fellow control eye. Wearing a goggle frame containing a lens of any power limits the recovery. The recovery signal can be intermittent, present for only 2 hours per day, and still mediate recovery in competition with increasing amounts of hyperopia as recovery progresses.
Keywords: emmetropization, myopia, recovery, negative lenses, positive lenses, animal model
Introduction
Studies in animal models have found that emmetropization, the matching of the eye’s axial length to its optical power, is an active process in which visual cues related to the presence and sign of an eye’s refractive error are used to guide the eye’s axial elongation rate in the juvenile period.1-4 The normal progression from hyperopia in (most) young animals toward near-emmetropia in juvenile animals is guided by the eye’s refractive state. This guidance can be demonstrated by using a negative-power lens, held in front of an eye with a goggle frame, to shift the eye optically toward hyperopia. In response, the vitreous chamber of the eye elongates over the course of a few days or weeks until the retina is shifted to the new focal plane, compensating for the lens.5-9 Measured with the lens in place, the eye matches the untreated control eye.10
After negative lens compensation has occurred, if lens wear is discontinued the eye is optically myopic. It also is elongated relative to normal.4,5,11,12 Recovery from the induced myopia typically occurs.8,10,13-17 During recovery from induced myopia, the axial elongation rate of the treated eye is slowed below normal but the optics continue to mature, moving the focal plane away from the cornea back to where it matches the location of the retina.8 When recovery is complete, the eye’s refractive state and axial length match that of the fellow control eye or normal eyes of the same age, typically a small, age-appropriate hyperopia.4,10
An involvement of refractive error signals in the guidance of recovery has been inferred from three observations: 1) monocularly treated eyes typically recover until their refraction matches that of their fellow untreated control eye; 2) recovery can be prevented from occurring if the myopic eyes are optically corrected with a negative lens of the appropriate power, regardless of whether the myopia was induced with a negative lens10 or by form deprivation18,19 In these cases, the eyes are optically corrected and so the visual signal (myopia) that might be used to produce the slowed axial elongation rate needed for recovery is absent, even though the eyes remain elongated. 3) if, after negative-lens compensation has occurred in chick, the negative lens is replaced for one week with a positive lens, making the treated eye highly myopic, the eyes partially compensate for the positive lens and become hyperopic, measured with the lens removed.20
To the extent that recovery is visually guided it should be possible to manipulate the end-point of recovery by having an animal wear a low-power lens during recovery. Following compensation to a −5 D lens, an eye is 5 D myopic when the lens is removed. If the eye begins to wear a −2 D lens, it will be 3 D myopic and one might expect it to recover until it is emmetropic while wearing this lens. With the lens removed, it would be 2 D myopic. If a +2 D lens were substituted at the start of recovery, the myopic refractive error would be increased so the eye would be 7 D myopic. If the eye were to recover until it is emmetropic with the positive lens in place and the lens were then removed, the eye would be 2 D hyperopic. Shifting the refractive target for recovery may help learn the degree to which refractive error signals control recovery.
It has been found that optical/visual signals to slow axial elongation have shorter integration times than do signals to increase axial elongation.21 For instance, two hours of removal per day of a negative lens or form deprivation in chick, monkey and tree shrews is uniformly sufficient to prevent the development of lens-induced or form-deprivation myopia.11,22-24. Nickla et al.25 found that a 2-hr per day relief from form deprivation is sufficient to allow recovery in chicks, suggesting that exposure to myopia may be effective even when this stimulus is present for a relatively short period of time. We thus examined whether a similar result would occur in tree shrews if a -5 D lens were removed for two hours each day in a group of animals that had compensated for a −5 D lens. Portions of this work were published in abstract form.26
Methods
The subjects in this study, juvenile tree shrews (Tupaia glis belangeri), were raised by their mothers in our breeding colony on a 14/10 h light/dark cycle. The procedures in this study were performed according to the ARVO Statement for the Use of Animals in Ophthalmic Research and were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee. Tree shrew pups are born with their eyes closed; they open on about postnatal day 21. The first day that both eyes were open was designated as day 1 of visual experience. On day 16 ± 1 of visual experience, each animal began to wear a goggle frame with a −5 D lens covering one eye and an open frame around the other eye. This was designated as treatment day 1. The −5 D lens was worn for 11 days, until treatment day 12. The treated eye was randomly assigned and the other, fellow eye, served as a within-animal control.
Experimental Groups
Recovery began on treatment day 12, designated as recovery day (RD) 1. The 37 animals were divided into five groups, each containing males and females. To maximize genetic variability, the number of animals from the same parents was minimized within a group. In the no-lens group (n=7), the goggle containing the −5 D lens was permanently removed. Data from five animals in this group were included in a previous report (recovery starting at 27 days of visual experience).10 In the plano-lens group (n=8) the −5 D lens was replaced with a plano (zero-power) lens. In the −2 D-lens group (n=6) the −5 D lens replaced with a −2 D lens and in the +2 D-lens group (n=10) it was replaced with a +2 D lens.
Recovery continued for at least 31 days for the lens-guided recovery groups. End of recovery refractive measures were made with and without the treatment lens, typically after 31, 33 or 35 days of recovery. In the 2-hr lens-off group (n=6), the animals continued to wear the -5 D lens during recovery, but the goggle containing the lens was removed for a two-hour period each morning (at 9:30 – 11:30 a.m., approximately one hour after the colony lights turned on). During the 2 hr off-period the animals remained in their home colony cages and were prevented from entering their dark nest boxes to ensure exposure to light and visual stimuli. In this group, two of the animals recovered quickly and lens wear was discontinued after 25 and 26 days Three other animals continued with daily removal of the −5 D lens for two hours each day until RD 33. The sixth animal showed slow recovery; lens-wear was discontinued on RD 19 while the animal was still myopic and further recovery was monitored.
Post-recovery
After the end-of-recovery measures, lens wear was discontinued and a “post-recovery” period began for many of the animals in the plano-lens, −2 D lens and +2 D lens groups. Additional refractive measures were made on a variable schedule to examine changes in refractive state when recovery-lens wear was discontinued.
Procedures and Measurements
Pedestal Surgery
In order to hold the goggle frame and lens, each tree shrew was anesthetized (ketamine, 90mg/kg; xylazine, 10mg/kg, supplemented with 0.5 – 2.0 % halothane as needed) on day 15 ± 1 of visual experience, and fitted with a dental acrylic pedestal attached to the skull as previously described.27
Refractive Measures
Between approximately 9:00 and 10:00 a.m., awake non-cycloplegic refractive measures were made with a Nidek ARK 700A autorefractor. The refractive measures were made without cycloplegia because atropine has been found to reduce the amount of induced myopia in tree shrews.28 The measures were taken daily during the 11 days of −5 D lens treatment and for the first 10 days of recovery, then less frequently until the end of the lens-guided recovery period. Also, on RD 1 and at the end of recovery, the animals had the refractive measures made with the recovery lens in place. This allowed measurement of the how close the eyes, while wearing the recovery lens, were to full refractive recovery, defined as being within 1 D of the control eye.10 Some animals had more frequent with-the-lens measurements.
Lens related Procedures
Lenses were cleaned twice daily, in the morning at the time of the autorefractor measures and in the afternoon between 4:00 and 5:00 pm. During lens cleaning, the animals were placed in their nest box in a dimly lit room during the brief (1-3 min) procedure. If a lens became severely scratched, it was replaced with a new one. The lens replacement procedure took 10 – 20 min and was necessary approximately every 5-10 days. During lens replacement the animal was kept in its nest box in a dark enclosure to ensure that it received no visual signals while the lens was off.
Ocular Component Dimension Measures
Prior to fitting the pedestal, while the animal was anesthetized, ocular component dimensions (anterior segment, lens thickness vitreous chamber depth and axial length) of both eyes were measured using A-scan ultrasonography.29 This was to ensure that the two eyes were similar at the start of the experiment, as one of the eyes would serve as a control. After recovery from anaesthesia, each animal was returned to an individual cage in the colony.
Because of concerns that the anaesthesia required for measurements might affect the start of the recovery process, no A-scan measures were made at the start of recovery. However, based on numerous studies in tree shrews in which myopia was induced with a negative lens8,12 it is safe to assume that the induced myopia was due to enlargement of the vitreous chamber. The A-scan measures were generally repeated, and cycloplegic refractive measures made, at the end of the study (end of post-recovery period) and verified that the final refractive differences between the treated and control eyes were well correlated with differences in the vitreous chamber depth and axial length. In two animals in the +2 D lens recovery group, A-scan measurements were made at the end of recovery to examine whether the recovered, hyperopic eye was shorter than its fellow control eye. In these animals, ocular component measurements were also made using a Lenstar optical biometer (Lenstar LS 900, Haag-Streit, Basel, Switzerland).
Statistical Analysis
Measures of refractive state and axial component dimensions were entered into Excel spreadsheets. Recovery was measured as the difference between the treated and control eye refractions. The slope of the recovery (dioptres of decreased myopia per day) during the first four days was obtained for each animal by calculating the regression of the five data points from RD 1 – RD5. A similar approach was used to calculate the slope of the regression during the first 10 days (RD1 – RD11). In addition, the recovery data for each animal were fitted with a 3-parameter exponential decay function (SigmaPlot 9, Systat /software Inc., Point Richmond, CA) to obtain a time constant and an asymptote for recovery. A repeated-measures ANOVA (Statistica, StatSoft, Inc., Tulsa, OK) was used to compare the control eye values with a group of normal eyes. One-way ANOVAs were used to learn if there was a significant difference between the measures in the different groups of animals at the start and end of the recovery period.
Results
Control Eyes
Comparison of the refractive measurements from the control eyes of the animals to a previously-reported10 group of age-matched normal eyes showed a normal progression toward emmetropia with no significant effect of −5 D lens wear by the fellow eye on the refractive development of the control eyes throughout the recovery period (repeated-measures ANOVA, p > 0.05). The absence of an effect on these control eyes allowed them to serve as a reference for judging the extent to which treated eyes recover from an induced myopia. All values of the amount of induced myopia and amount of recovery in the following sections are comparisons between the control eye and treated eye refractive measures, either with the lens removed or with it in place.
Lens Compensation
The treated eyes in all animals responded to the −5 D lens by becoming myopic, relative to the control eyes, during the 11-day treatment period. The mean ± SEM induced myopia (treated eye – control eye) at the end of the −5 D lens treatment period (treatment day 12) was -4.8 ± 0.1 D. The amount of induced myopia (Table 1) did not differ across the groups (one-way ANOVA, p > 0.05). Across all groups, the refractive difference (treated eye – control eye) measured with the −5 D lens in place was 0.3 ± 0.2 D indicating that the eyes had fully compensated for the −5 D lens. The with-the-lens refractive differences also did not differ significantly across groups (ANOVA, p > 0.05).
Table1.
Compensation and Recovery Refractive Differences
| Groups | No lens | Plano lens | −2 D lens | +2 D lens | 2 hours off |
|---|---|---|---|---|---|
|
Induced myopia (no lens): Treated eye– control eye (D) (mean ± SEM) |
−4.3 ± 0.4 (n=7) |
−5.0 ± 0.3 (n=8) |
−4.7 ± 0.2 (n=6) |
−4.8 ± 0.2 (n=10 |
−5.3 ± 0.2 (n=6) |
|
Compensation (with −5 D lens): Treated eye – control eye (D) (mean ± SEM) |
0.3 ± 0.7 (n=7) |
0.2 ± 0.3 (n=8) |
0.2 ± 0.2 (n=6) |
0.1 ± 0.4 (n=10 |
0.6 ± 0.4 (n=6) |
|
Myopia with recovery lens: Treated eye – control eye (D) (mean ± SEM) |
−4.3 ± 0.4 (n=7) |
−4.8 ± 0.3 (n=8) |
−3.2 ± 0.3 (n=6) |
−7.0 ± 0.4 (n=10) |
−5.3 ± 0.2 (n=6) |
|
Recovery end (no lens): Treated eye – control eye (D) (mean ± SEM) |
0.0 ± 0.1 § @ RD 34.8 ± 0.2 (n=6) § |
−1.1 ± 0. 1 @ RD 33.5 ± 0.5 (n=6) € |
−2.5 ± 0.2 @ RD 33 (n=6) |
1.0 ± 0.1 @ RD 33.9 ± 0.8 (n=8) € |
−0.4 ± 0.4 @ RD 30 ± 1.8 (n=5)* |
|
Recovery end (with lens): Treated eye – control eye (D) (mean ± SEM) |
------ | −1.0± 0.3 @ RD 33.5 ± 0.5 (n=6) € |
−0.6 ± 0.2 @ RD 33 (n=6) |
−1.3± 0.1 @ RD 33.9 ± 0.8 (n=8) € |
4.6 ± 0.5 @ RD 30 ± 1.8 (n=5)* |
|
Post-recovery (stopped lens wear): Treated eye – control eye (D) (mean ± SEM) |
------ | −0.0 ± 0.1 @RD 46.2 ± 1.4 (n=6) € |
−1.0± 0.5 @ RD 47.3 ± 1.7 (n=6) |
0.3 ± 0.3 @ RD 44.7 ± 0.3 (n=7) ¥ |
------ |
Number after excluding one animal with slow recovery
Number after excluding two animals with slow recovery
Number after excluding one animal, lens wear stopped early
One animal was not measured after lens treatment stopped
Recovery
All groups showed refractive recovery. Overall, the initial recovery slope (decrease in myopia during the first 4 days; Table 2) was similar to that of animals studied previously.10 However, as shown in Fig. 1, the end-point of recovery differed significantly across groups (repeated measures ANOVA, p < 0.05), indicating that the end-point of recovery was affected by the power of the lens worn during recovery.
Table 2.
Recovery Slopes
| Groups | No lens | Plano lens | −2 D lens | +2 D lens | 2 hours off |
|---|---|---|---|---|---|
|
4-day slope (D/day) (mean ± SEM) |
0.86 ± 0.21 (N=6) § | 0.54 ± 0.11 (n=6) € | 0.56 ± 0.12 (n=6) | 0.87 ± 0.16 (n=8) € | 0.37 ± 0.12 (N=6) |
|
10-day slope (D/day) (mean ± SEM) |
0.35 ± 0.03 (N=6) § |
0.33 ± 0.03 (n=6) € |
-----* | 0.46 ± 0.07 (n=8) € |
0.29 ± 0.04 (N=6) |
Number after excluding one animal with slow recovery
Number after excluding two animals with slow recovery
This group had recovered so no 10-day slope was calculated
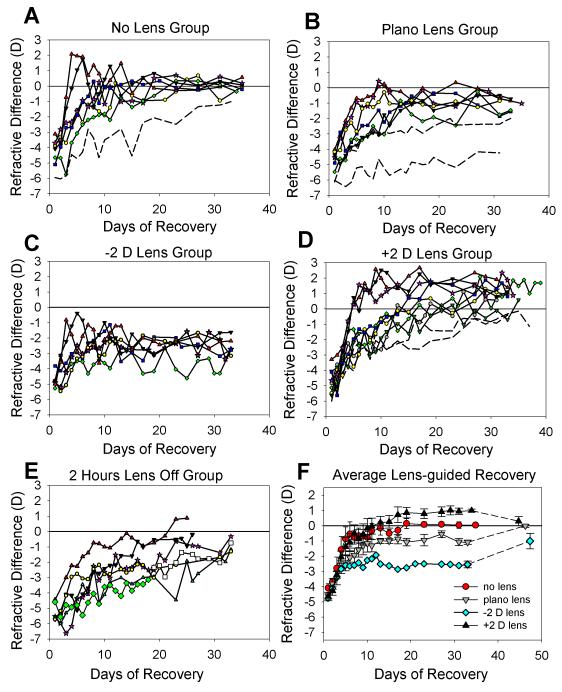
Figure 1.
Recovery from lens-induced myopia. Measurements of the refractive difference (recovering eye – control eye) were made with the recovery lens removed. (a) Animals that wore no lens during recovery. (b) Animals that wore a plano (zero-power) lens. (c) Animals that wore a −2 D lens. (d) Animals that wore a +2 D lens. (e) Animals that continued to wear the −5 D lens, which was removed for 2 hours each day. (f) Mean ± S.E.M. refractive differences for the groups shown in a – d. Post-recovery changes are also shown. Dashed lines in a, b, and d indicate slow-recovering animals not included in the averages shown in f.
No-lens group
The animals in this group were −4.3 ± 0.4 D myopic at the start of recovery (Table 1). Six of the seven treated eyes rapidly recovered until their refractions were very similar to those of their control eyes. The average recovery of these six animals is shown in Fig. 1F. The average refractive difference at the end of the recovery measurement period (measured on RD 34.8 ± 0.2 days), was 0.0 ± 0.1 D, indicating that the recovering eyes had recovered until they matched the refraction of the control eyes.
One animal, (dashed line in Fig. 1A), showed slow recovery that was incomplete (>1.0 D myopic compared to the control eye) on RD 33. Additional measures were made out to RD 49 (data not shown), at which point the recovering eye was still −0.6 D relative to the control eye. The slope of the initial recovery in this slow-recovering animal was 0.40 D/day, much slower than in the other six animals (0.86 ± 0.21 D/ day, Table 2) and the time-constant of the fitted exponential function was more than 2x the standard error of the mean (SEM) of the entire group.
Previous studies in tree shrews have found that some tree shrews do not recover rapidly or fully from negative lens wear or from form deprivation.10,15 Slow, or incomplete, recovery appears to be a characteristic of individual animals and its aetiology is unknown. It also was observed in two animals in the plano-lens group and two in the +2 D lens group. In all cases, the recovery time-constant or the asymptote of exponential decay curves fit to the data differed from the group average by more than 2 times the group SEM. Including such animals in the group refractive averages would potentially mask the effects of visual guidance on recovery. Thus, although the individual data of these animals are shown in Fig. 1, and the final recovered values of the groups still differed significantly if these animals were included (ANOVA, p >0.05), they are not included in the group averages shown in Fig. 1F, Table 1 and Table 2.
Plano-lens group
As shown in Table 1 and Fig. 1B, the animals had developed an induced myopia of −5.0 ± 0.3 D at the end of −5 D lens treatment. Measured with the plano lens in place, the myopia was essentially the same (−4.8 ± 0.3 D), confirming that the plano lens did not produce a shift in refraction, measured on the pupillary axis.
Two animals in this group showed slow, incomplete recovery (dashed lines in Fig. 1B). For one of these, the initial slope of the recovery (first 4 days) was very low, 0.17 D per day, compared with the average slope of 0.54 ± 0.9 D per day for the other seven animals in this group. Extrapolation of the recovery plots beyond the end of the last measurement suggested that it would never have recovered fully. A second animal, also shown with a dashed line in Fig. 1B, was still −2 D myopic when the plano lens wear ended at RD 33. Very slow recovery continued thereafter and the animal was still −0.7 D myopic when measured 30 days later, indicating that the slow recovery was unrelated to plano lens wear. Neither animal was included in the group average.
The other six animals in the group followed a pattern of recovery similar to that of the no-lens group, but stabilized with the recovering eye remaining myopic compared to the control eye (Fig. 1B, Table 1). This relative myopia was the same when measured with the plano lens removed (−1.1 ± 0.1 D) or with the lens in place (−1.0 ± 0.3 D).
At RD 33.5 ± 0.5 days, the goggle frame containing the plano lens was removed. When the eyes were re-measured nearly two weeks later (at RD 46.2 ± 1.4 days) the treated eyes had recovered further so that the refractive difference between the recovering and control eyes was 0.0 ± 0.1 D (Table 1, Fig 1F). Thus, the incomplete recovery appeared to be related to the presence of the goggle containing the plano lens, possibly because the goggle frame affected the visual periphery.30
−2 D Lens group
At the start of recovery, the animals in this group were −4.7 ± 0.2 D myopic, measured without a lens (Table 1 and Fig. 1C). With the −2 D lens in place, the myopia was reduced to −3.2 ± 0.2 D. Thus, if the eyes recovered by 3 D, they would be refractively the same as the control eyes while wearing the lens and would be myopic (−2.0 D) with the lens removed. Fig. 1C plots the recovery of animals in this group, measured with the lens removed. The treated eyes showed a rapid recovery during the first few days, and then stabilized. At the end of the recovery period (RD 33), the recovering eyes were −2.5 ± 0.2 D myopic with the lens removed, a recovery of approximately 2.2 D. As with the plano lens group measured with the lens in place, the eyes stabilized with a small amount of myopia (−0.6 ± 0.2 D) compared with their control eyes.
The −2 D lens wear was discontinued at RD 33, exposing the treated eyes to the without-the-lens myopia. During this post-recovery period, the group showed further recovery, so that at RD 47.3 ± 1.7 days the recovering eyes were −1.0 ± 0.5 D myopic (Fig.1F).
+2 D Lens Recovery
As shown in Table 1 and Fig. 1D, the animals in this group were −4.8 ± 0.2 D myopic at the start of recovery, measured with no lens in place. When the -5 D lens was replaced with a +2 D lens, the myopia experienced by the eyes was increased to −7.0 ± 0.4 D. Full refractive recovery for the treated eyes in this group would mean recovering from the −7 D of myopia so that the recovering eye would match the control eye when measured with the lens in place. However, when measured without the lens, the eye would be +2 D hyperopic.
Three animals recovered very rapidly compared with all other animals in this group. There was no obvious reason for the rapid recovery of this distinct sub-group. Two animals (dashed lines in Fig. 1D) exhibited slow recovery and were still myopic (−0.4 D and −1.1 D), measured with the lens removed, when the lens-recovery period ended and were not included in the averages. The treated eyes of the other eight animals became hyperopic (1.1 ± 0.1 D), measured without the lens, compared with their control eyes by the end of the recovery period (RD 33.9 ± 0.8 days). However, measured with the +2 D lens in place, they, like animals in the plano-lens and −2 D lens groups when measured while wearing their recovery lenses, were slightly myopic (−1.3 ± 0.1 D).
Although A-scan measurements were not generally made at the end of the recovery period, we were interested to learn if the hyperopic eyes were in fact shorter than the control eyes. Thus, at the end of the recovery period two animals in this group were anesthetized and measured with A-scan ultrasound. The average hyperopia was 0.85 D and the vitreous chambers of the recovering eyes were slightly shorter (average, 19 μm) than the control eyes. Additional, awake non-cycloplegic measures of vitreous chamber depth in these two animals were made with a Lenstar optical biometer (Haag-Streit), newly available in the lab. Although this machine has not yet been well calibrated for tree shrews, it was clear that the vitreous chamber of the treated eye was slightly shorter than the control eye in both of the animals (average, approximately 17 μm). The A-scan and optical biometer values are consistent with previous studies in this lab that have found that a 1 D difference in refraction is typically accompanied by a 20 – 25 μm difference in vitreous chamber depth.8
+2 D lens wear was discontinued in the eight animals with hyperopic recovered eyes. After a post-recovery period of 10.4 ± 1.4 days the refractions of the treated eyes “recovered” from the induced hyperopia (Fig. 1F, Table 1) so that they were nearly the same as the control eyes (refractive difference, 0.3 ± 0.3 D).
2-hour Lens-off group
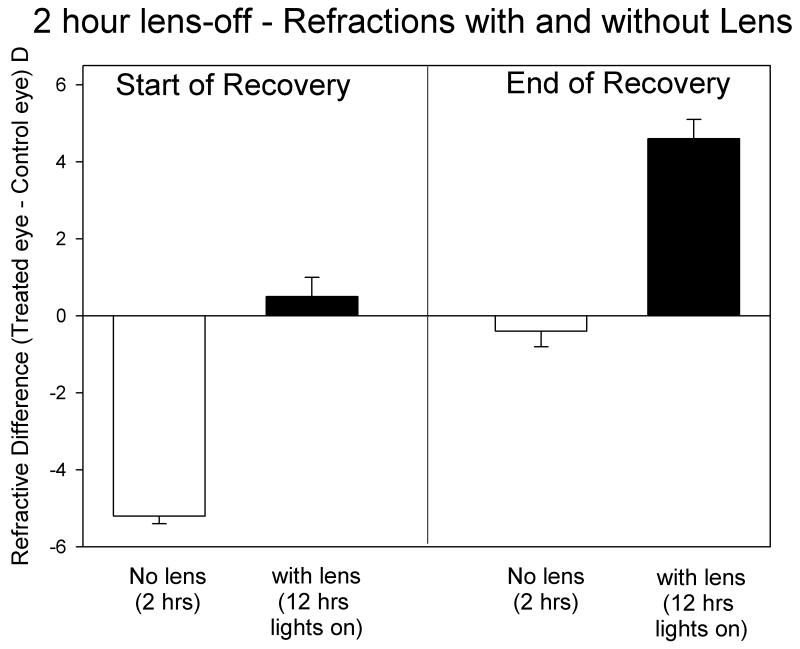
As shown in Table 1 and Fig. 1E, the animals in this group were −5.3 ± 0.2 D myopic at the end of full-time −5 D lens wear, measured with the lens removed. Starting on RD 1, the −5 D lens was removed for 2 hrs every day, but was worn the remainder of the time. As shown in Fig. 2, the animals in this group experienced myopia when the lens was first removed. When the lens was replaced, the recovering eyes initially experienced a small hyperopia (0.5 ± 0.5 D).
Figure 2.
Refractive differences (recovering eye – control eye) for the 2-h lens-off group, measured with, and without, the −5 D lens at the start and end of recovery. Initially, two hours of myopia competed with 12 h of emmetropia. At the end of recovery two hours of emmetropia competed with 12 h of hyperopia.
All animals showed significant recovery during the recovery period. After 26 days, two animals had recovered to the point that the recovering eye was less than 1 D myopic, compared with the control eye, measured with the lens removed and thus recovery measurements were discontinued. Three other animals recovered slightly more slowly and were still myopic (>1 D) when measurements were discontinued at RD 33. One animal (diamond symbols in Fig. 1E) was slower to recover than the rest of the group, and after RD 19 the −5 D lens was permanently removed to learn if the rate of recovery would increase. As shown by the open squares in Fig. 1E, recovery appeared to continue at approximately the same rate, suggesting that this animal’s slower rate of recovery was unrelated to the lens wear.
As recovery progressed, the recovering eyes experienced less myopia during the two hours of lens removal and an increasing level of hyperopia during the rest of the 12-hour period while the colony lights were on. As shown in Fig. 2, on the last day recovery was measured (RD 29.8 ± 0.2 days), the recovering eyes experienced a small amount of myopia (−0.4 ± 0.4 D) during the two hours the lens was removed. During the other 12 hours that the colony lights were on, the eyes experienced 4.6 ± 0.5 D of hyperopia.
Recovery slopes
As shown in Fig. 1F, all groups showed a similar initial pattern of recovery, with relatively rapid initial recovery followed by slower recovery, except for the −2 D lens group in which the initial rapid recovery was sufficient to move the eyes close to the final refractive state. Table 2 shows the initial (first 4-day) recovery slopes and the recovery slopes over the first 10 days of the recovery period. No 10-day slope was calculated for the −2 D lens group because the eyes had already reached a stable refractive level. The slope values were similar to those found previously.10 Neither the initial (4-day slopes) nor the 10-day recovery slopes differed significantly across groups (one-way ANOVA, p > 0.05).
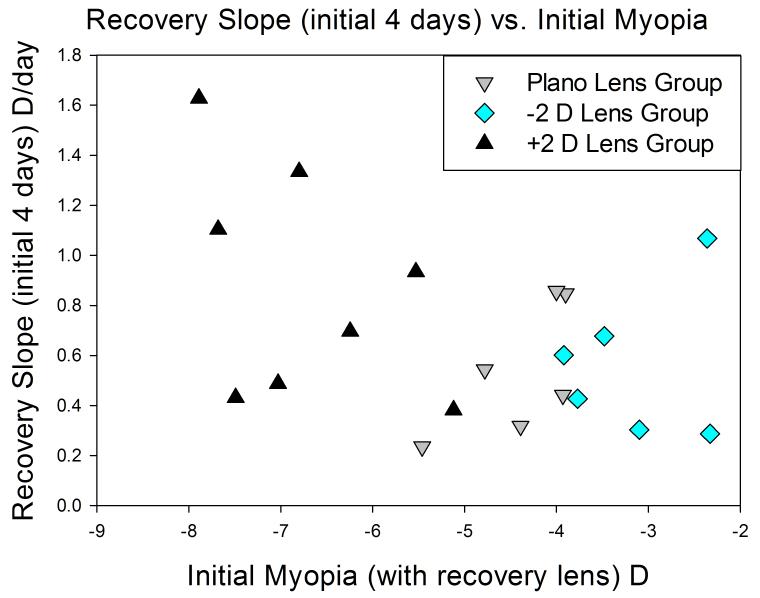
The recovery slopes of the plano, −2 D and 2 D lens-wear groups are of interest because the refractive conditions experienced by the recovering eyes differed considerably, with the +2 D lens group experiencing about 7 D of myopia and the −2 D group experiencing about 3 D. These differences allowed us to ask if the initial rate of recovery was affected by the amount of myopia experienced by the eyes. Fig. 3 is a plot of the initial (4-day) recovery slope vs. the amount of myopia initially experienced by the recovering eyes. Although initial slope of some animals in the +2 D lens group was higher than that of animals in the other groups, there was not a significant correlation (p > 0.05) between the initial amount of myopia and the initial rate of recovery, neither for the initial (4-day) recovery slopes nor the 10-day recovery slopes (Table 2)
Figure 3.
Initial recovery versus the amount of myopia initially experienced by the recovering eye. The regression was not statistically significant (p > 0.05) and the R2 was 0.17.
Discussion
The results of this study clearly confirmed the hypothesis that recovery from lens-induced myopia is visually guided. By the end of the recovery period, the three lens-wearing groups (plano lens, −2 D lens, +2 D lens) were refractively different. Measured with the lens removed, the +2 D group was hyperopic, the plano lens group was slightly myopic and the −2 D group was over 2 D myopic. Further, when lens wear was discontinued (the post-recovery period), the refractions converged toward a point where the treated eye refractions matched those of their fellow control eyes.
That we were able to alter the endpoint of recovery with the use of lenses confirms that visual guidance is utilized when eyes are re-achieving emmetropia from the myopic direction. It has been clear from many previous studies with negative lenses that hyperopia is a powerful stimulus, stimulating axial elongation that continues until the hyperopia dissipates. The present study showed that the emmetropization mechanism also uses the presence of myopia to guide refractive development. For the +2 D lens group, this involved slowing axial elongation to the point that the eyes were shorter than the control eyes in the two animals in which axial measures were made. A similar result was found in chicks by Irving et al.;20 however, the duration of the lens wear in that study was one week which did not provide sufficient time for the eyes to achieve complete compensation for the positive lenses. These results also extend to recovery the results of recent studies in tree shrews using plus lenses31,32 that found that very young tree shrews, made myopic with a plus lens, could slow their axial elongation to become emmetropic while wearing the lens.
Because viewing distance was not controlled or measured in this study, it is not clear whether the recovering eyes used myopic defocus, per se, to guide the recovery. A previous study33 showed that at least some tree shrews can use low amounts of myopic defocus to compete against hyperopic defocus and block negative-lens compensation. However, as is well recognized, a myopic eye can experience clear images when viewing objects at the refractive near point, and hyperopic defocus for objects closer than that. Thus the present study does not speak to the question of whether the eyes could distinguish myopic defocus from hyperopic defocus. However, the recovering eyes in the present study must have experienced more myopic defocus than the untreated fellow control eyes and recovery appeared to cease when that was no longer the case.
As is discussed in the following two sections, the amount of visually-guided recovery found in this study is affected by two factors: 1) some animals appear to have limited capacity to use myopia to slow axial elongation; 2) wearing a goggle frame and a lens, even one with no optical power on the pupillary axis, can limit refractive recovery.
Slow and/or Incomplete recovery
Slow and/or incomplete recovery in some animals is unique neither to this study nor to tree shrews.10,13,17,34 A previous study in tree shrews10 that examined recovery from lens-induced myopia found that some animals, at this and at older ages, did not recover quickly and/or completely in a “no-lens” recovery condition. Incomplete recovery also was found for recovery from form deprivation-induced myopia in tree shrews.15 Although, as shown in Fig. 1A & B, the excluded animals from the no-lens and plano-lens groups had initial myopia levels that were the highest in their groups, the data in Fig. 1 are the refractive differences measured with the lenses removed. While wearing the recovery lenses, all but two of the animals in the +2 D lens recovery group experienced a larger amount of initial myopia than did any of the no-lens or plano-lens recovery animals, yet most recovered quickly and completely. Thus, the slow recovery in the no-lens or plano-lens groups probably did not occur because the eyes experienced a large initial amount of myopia.
The presence of animals with slow recovery in the no-lens, plano-lens and +2 D lens groups suggests that this phenomenon was unrelated to lens wear and appeared to be a characteristic of individual animals. Why some animals are less able than others to use a myopic refractive state, coupled with having an axially-elongated eye, to slow the axial elongation rate and re-achieve a refractive match to the control eyes remains unknown as does the question of whether this occurs because of ineffective retinal signalling or scleral response.
The question for the present study was not the presence of a few slow or incomplete-recovering animals, but rather what to do with their data? The recovery of the refractions across groups was significantly different even if the slow-recovering animals were included. However, to examine the ability of visual guidance to achieve a refractive match to the control eyes, it seemed important to exclude the data from animals that appeared to be impaired in using that visual guidance. Thus, in the group averages reported in Table 1 and Fig. 1F, the data from five animals were excluded: one in the no-lens, 2 in the plano-lens and 2 in the +2 D lens groups.
It is possible that some of the animals included in the −2 D lens group averages may have actually been somewhat impaired in using myopia to produce refractive recovery, but that this was not detected because the −2 D lens reduced the amount the eyes needed to recover to become nearly emmetropic while wearing the lens. However, when lens wear was discontinued in the post-recovery period, the eyes were myopic again. Two animals showed almost no refractive recovery during the following 15 days, unlike the other four animals in the group which became less than 1 D myopic. If their values were not included in the final post-recovery average, the group average would have been near zero. Thus, wearing the −2 D lens may have masked difficulty in these two animals of using myopia as a cue for recovery.
Effects of wearing a goggle containing any lens during recovery
Comparison of the recovery of the animals in the plano-lens group with the animals in the no-lens group suggests that simply wearing a goggle containing a lens has a small (~ 1 D myopic) effect on recovery, even if the lens has no refractive power when measured on the pupillary axis. The myopic “offset” seen in the plano lens group also appeared to be present in the +2 D lens and −2 D lens groups; the final refractions in each group were symmetrically above and below the plano-lens group, but more myopic than expected from the recovery of the no-lens group. Thus, the effects of lens power were superimposed on an apparent shift produced by the presence of a lens during recovery. The reasons for this shift are not known. Two possibilities include, slight degradation of image clarity due to accumulated dust and oils on the lens, despite frequent lens cleaning, and the presence of unmeasured aberrations the periphery of the lenses or even slight peripheral form deprivation caused by the edges of the goggle frame.
Recovery guidance with intermittent lens removal
Intermittent exposure (2 h per day) to myopia each day is sufficient to mediate recovery from lens-induced myopia, confirming a similar result in chicks.25 The eyes recovered toward the point where the recovering eye’s refractive state, measured without the lens, matched the control eye. However, the lens was removed only for 2 hours each day and the −5 D lens was worn for the remaining 12 hours that the colony lights were on. In the first few days of the recovery period, the eyes experienced nearly 5 D of myopia for 2 hours, and were nearly emmetropic for the other 12 hours. Thus, being myopic for a relatively brief portion of the day was sufficient to produce slowed axial elongation so that over time the refractive state, with the lens removed, became less myopic. This extends to recovery the observation that brief exposure to myopic refractive error can produce slowed axial elongation.
By the end of the recovery period, the eyes experienced 2 hours of near emmetropia and 12 hours of hyperopia, a situation that has been found in numerous species to counteract the myopiagenic effects of hyperopia.11,22,35 In this instance, however, the recovering eyes were able to use visual cues to move from a state where myopia competed with emmetropia to the point where emmetropia competed with hyperopia. The ability of the eyes to do this may be related to the elongated state of the sclera after negative lens compensation (an “eye-size” factor).25,31,36,37 In chick, normal eyes exposed to three hours per day of myopia, produced by positive lens wear, developed only a small hyperopic shift.22
Does the emmetropization mechanism respond to the amount of blur?
As was shown in Fig. 3, the initial (4-day) recovery rates did not increase significantly in proportion to the amount of myopia experienced by the eyes. A similar lack of correlation between initial refractive error and the initial rate of recovery was found previously.10 The overall time to full recovery of the −2 D, plano and 2 D lens groups differed, presumably because the group wearing the +2 D lens underwent a larger refractive shift than the plano lens or −2 D lens groups. The absence of an effect of initial refractive error on the initial rate of recovery suggests that, beyond some threshold, the emmetropization mechanism may use the presence of myopia, but not its magnitude, to initiate recovery. When the eye detects that it is myopic, (and, in this study, that it also is elongated axially) the emmetropization mechanism generates a “stop” (or “slow”) signal that reduces the axial elongation rate of the growing juvenile eyes. However, the lack of a strong relationship between the amount of myopia and the rate of recovery suggests that the strength of this signal may not be related to the magnitude of the myopia.
Acknowledgements
We thank Dr. John Siegwart for advice and guidance during this study and Mr. Joel Robertson and Ms. Caroline K. Herman for technical assistance. Supported by National Eye Institute Grants RO1 EY005922 (and ARRA supplement) and P30 EY003909 (CORE).
Footnotes
The authors have no commercial relationships or conflict of interest.
Reference List
- 1.Wildsoet CF. Active emmetropization - evidence for its existence and ramifications for clinical practice. Ophthal Physiol Opt. 1997;17:279–290. [PubMed] [Google Scholar]
- 2.Norton TT. Animal Models of Myopia: Learning How Vision Controls the Size of the Eye. ILAR J. 1999;40:59–77. doi: 10.1093/ilar.40.2.59. [DOI] [PubMed] [Google Scholar]
- 3.Schaeffel F, Howland HC. Mathematical model of emmetropization in the chicken. J Opt Soc Am. 1988;5:2080–2086. doi: 10.1364/josaa.5.002080. [DOI] [PubMed] [Google Scholar]
- 4.Smith EL, III, Hung LF. The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999;39:1415–1435. doi: 10.1016/s0042-6989(98)00229-6. [DOI] [PubMed] [Google Scholar]
- 5.Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988;28:639–657. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- 6.Irving EL, Callender MG, Sivak JG. Inducing myopia, hyperopia, and astigmatism in chicks. Optom Vis Sci. 1991;68:364–368. doi: 10.1097/00006324-199105000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Hung LF, Crawford ML, Smith EL. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nature Med. 1995;1:761–765. doi: 10.1038/nm0895-761. [DOI] [PubMed] [Google Scholar]
- 8.Siegwart JT, Jr., Norton TT. Regulation of the mechanical properties of tree shrew sclera by the visual environment. Vision Res. 1999;39:387–407. doi: 10.1016/s0042-6989(98)00150-3. [DOI] [PubMed] [Google Scholar]
- 9.Shen W, Sivak JG. Eyes of a lower vertebrate are susceptible to the visual environment. Invest Ophthalmol Vis Sci. 2007;48:4829–4837. doi: 10.1167/iovs.06-1273. [DOI] [PubMed] [Google Scholar]
- 10.Norton TT, Amedo AO, Siegwart JT., Jr. The effect of age on compensation for a negative lens and recovery from lens-induced myopia in tree shrews (Tupaia glis belangeri) Vision Res. 2010;50:564–576. doi: 10.1016/j.visres.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaikh AW, Siegwart JT, Norton TT. Effect of interrupted lens wear on compensation for a minus lens in tree shrews. Optom Vis Sci. 1999;76:308–315. doi: 10.1097/00006324-199905000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Moring AG, Baker JR, Norton TT. Modulation of Glycosaminoglycan Levels in Tree Shrew Sclera during Lens-Induced Myopia Development and Recovery. Invest Ophthalmol Vis Sci. 2007;48:2947–2956. doi: 10.1167/iovs.06-0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallman J, Adams JI. Developmental aspects of experimental myopia in chicks: Susceptibility, recovery and relation to emmetropization. Vision Res. 1987;27:1139–1163. doi: 10.1016/0042-6989(87)90027-7. [DOI] [PubMed] [Google Scholar]
- 14.Norton TT. Experimental myopia in tree shrews. In: Bock G, Widdows K, editors. Myopia and the Control of Eye Growth. Wiley; Chichester: 1990. pp. 178–194. 178-94. [PubMed] [Google Scholar]
- 15.Siegwart JT, Jr., Norton TT. The susceptible period for deprivation-induced myopia in tree shrew. Vision Res. 1998;38:3505–3515. doi: 10.1016/s0042-6989(98)00053-4. [DOI] [PubMed] [Google Scholar]
- 16.Troilo D, Nickla DL. The response to visual form deprivation differs with age in marmosets. Invest Ophthalmol Vis Sci. 2005;46:1873–1881. doi: 10.1167/iovs.04-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiao-Grider Y, Hung LF, Kee CS, Ramamirtham R, Smith EL., III. Recovery from form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2004;45:3361–3372. doi: 10.1167/iovs.04-0080. [DOI] [PubMed] [Google Scholar]
- 18.Wildsoet CF, Schmid KL. Optical correction of form deprivation myopia inhibits refractive recovery in chick eyes with intact or sectioned optic nerves. Vision Res. 2000;40:3273–3282. doi: 10.1016/s0042-6989(00)00138-3. [DOI] [PubMed] [Google Scholar]
- 19.McBrien NA, Gentle A, Cottriall C. Optical correction of induced axial myopia in the tree shrew: implications for emmetropization. Optom Vis Sci. 1999;76:419–427. doi: 10.1097/00006324-199906000-00022. [DOI] [PubMed] [Google Scholar]
- 20.Irving EL, Sivak JG, Callender MG. Refractive plasticity of the developing chick eye. Ophthalmic Physiol Opt. 1992;12:448–456. [PubMed] [Google Scholar]
- 21.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Schmid KL, Wildsoet CF. Effects on the compensatory responses to positive and negative lenses of intermittent lens wear and ciliary nerve section in chicks. Vision Res. 1996;36:1023–1036. doi: 10.1016/0042-6989(95)00191-3. [DOI] [PubMed] [Google Scholar]
- 23.Napper GA, Brennan NA, Barrington M, Squires MA, Vessey GA, et al. The duration of normal visual exposure necessary to prevent form deprivation myopia in chicks. Vision Res. 1995;35:1337–1344. doi: 10.1016/0042-6989(94)00226-c. [DOI] [PubMed] [Google Scholar]
- 24.Smith EL, III, Hung LF, Kee CS, Qiao Y. Effects of brief periods of unrestricted vision on the development of form-deprivation myopia in monkeys. Invest Ophthalmol Vis Sci. 2002;43:291–299. [PubMed] [Google Scholar]
- 25.Nickla DL, Sharda V, Troilo D. Temporal integration characteristics of the axial and choroidal responses to myopic defocus induced by prior form deprivation versus positive spectacle lens wear in chickens. Optom Vis Sci. 2005;82:318–327. doi: 10.1097/01.opx.0000159368.31481.de. [DOI] [PubMed] [Google Scholar]
- 26.Amedo AO, Norton TT. Guidance of recovery from induced myopia in tree shrews. Invest Ophthalmol Vis Sci. 2005;46 E-Abstract 1977. [Google Scholar]
- 27.Siegwart JT, Norton TT. Goggles for controlling the visual environment of small animals. Lab Animal Sci. 1994;44:292–294. [PubMed] [Google Scholar]
- 28.McKanna JA, Casagrande VA. Atropine affects lid-suture myopia development. Documenta Ophthalmologica Proc Series. 1981;28:187–192. [Google Scholar]
- 29.Norton TT, McBrien NA. Normal development of refractive state and ocular component dimensions in the tree shrew (Tupaia belangeri) Vision Res. 1992;32:833–842. doi: 10.1016/0042-6989(92)90026-f. [DOI] [PubMed] [Google Scholar]
- 30.Smith EL, III, Kee CS, Ramamirtham R, Qiao-Grider Y, Hung LF. Peripheral vision can influence eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2005;46:3965–3972. doi: 10.1167/iovs.05-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siegwart JT, Jr., Norton TT. Binocular lens treatment in tree shrews: Effect of age and comparison of plus lens wear with recovery from minus lens-induced myopia. Exp Eye Res. 2010;91:660–669. doi: 10.1016/j.exer.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metlapally S, McBrien NA. The effect of positive lens defocus on ocular growth and emmetropization in the tree shrew. J Vis. 2008;8:1–12. doi: 10.1167/8.3.1. [DOI] [PubMed] [Google Scholar]
- 33.Norton TT, Siegwart JT, Jr., Amedo AO. Effectiveness of hyperopic defocus, minimal defocus, or myopic defocus in competition with a myopiagenic stimulus in tree shrew eyes. Invest Ophthalmol Vis Sci. 2006;47:4687–4699. doi: 10.1167/iovs.05-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Troilo D, Nickla D. The response to form deprivation by occluders differs from that by lid suture in marmosets. Invest Ophthalmol Vis Sci. 2000;41 ARVO Abstract 691. [Google Scholar]
- 35.Kee CS, Hung LF, Qiao-Grider Y, Ramamirtham R, Winawer J, et al. Temporal constraints on experimental emmetropization in infant monkeys. Invest Ophthalmol Vis Sci. 2007;48:957–962. doi: 10.1167/iovs.06-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Troilo D, Wallman J. The regulation of eye growth and refractive state: an experimental study of emmetropization. Vision Res. 1991;31:1237–1250. doi: 10.1016/0042-6989(91)90048-a. [DOI] [PubMed] [Google Scholar]
- 37.Schaeffel F, Howland HC. Properties of the feedback loops controlling eye growth and refractive state in the chicken. Vision Res. 1991;31:717–734. doi: 10.1016/0042-6989(91)90011-s. [DOI] [PubMed] [Google Scholar]