Abstract
Transforming growth factor beta 1 (TGF-β1) has been implicated in the pathogenesis of prostate cancer (PCa) bone metastasis. In this study, we tested the antitumor efficacy of a selective TGF-β receptor I kinase inhibitor, LY2109761, in preclinical models. The effect of LY2109761 on the growth of MDA PCa 2b and PC-3 human PCa cells and primary mouse osteoblasts (PMOs) was assessed in vitro by measuring radiolabeled thymidine incorporation into DNA. In vivo, the right femurs of male SCID mice were injected with PCa cells. We monitored the tumor burden in control- and LY2109761-treated mice with MRI analysis and the PCa-induced bone response with x-ray and micro-CT analyses. Histologic changes in bone were studied by performing bone histomorphometric evaluations. PCa cells and PMOs expressed TGF-β receptor I. TGF-β1 induced pathway activation (as assessed by induced expression of p-Smad2) and inhibited cell growth in PC-3 cells and PMOs but not in MDA PCa 2b cells. LY2109761 had no effect on PCa cells but induced PMO proliferation in vitro. As expected, LY2109761 reversed the TGF-β1–induced pathway activation and growth inhibition in PC-3 cells and PMOs. In vivo, LY2109761 treatment for 6 weeks resulted in increased volume in normal bone and increased osteoblast and osteoclast parameters. In addition, LY2109761 treatment significantly inhibited the growth of MDA PCa 2b and PC-3 in the bone of SCID mice (p < 0.05); moreover, it resulted in significantly less bone loss and change in osteoclast-associated parameters in the PC-3 tumor–bearing bones than in the untreated mice. In summary, we report for the first time that targeting TGF-β receptors with LY2109761 can control PCa bone growth while increasing the mass of normal bone. This increased bone mass in nontumorous bone may be a desirable side effect of LY2109761 treatment for men with osteopenia or osteoporosis secondary to androgen-ablation therapy, reinforcing the benefit of effectively controlling PCa growth in bone. Thus, targeting TGF-β receptor I is a valuable intervention in men with advanced PCa.
Keywords: Prostate cancer, Bone metastases, TGF-β, TGF-β receptor type I kinase inhibitor
Introduction
Prostate cancer (PCa), the second-leading cause of cancer-related death among men in the United States [1] can be cured when it is confined to the gland, but when metastatic dissemination occurs, the prospect for cure decreases. Androgen ablation is the most effective way to halt the growth of advanced PCa. However, responses are short lived, the disease then becomes castrate resistant, and only a modest survival advantage is achieved by administering chemotherapies. Bone is the primary site of castrate-resistant progression, and PCa is the only malignancy that consistently produces bone-forming metastases, although osteolysis is also an important component of the pathogenesis of the disease in bone [1]. The unique tropism of PCa cells for bone suggests that specific biologic interactions occur between those cells and the bone environment and that these interactions contribute to the lethal progression of the disease. To date, there is no effective treatment for bone metastases. One added burden for these patients is that androgen-ablation therapy is one of the causes of cancer treatment–induced bone loss, which increases the incidence of bone complications [2]. Thus, to reduce the suffering and prolong the lives of PCa patients, the development of effective therapies for the treatment and prevention of bone metastasis is urgently needed.
Previous studies identified the plasma concentration of transforming growth factor beta 1 (TGF-β1) as a predictor of PCa progression and metastasis development [3–6]. TGF-β1 is a pleiotropic growth factor that regulates cellular proliferation, chemotaxis, differentiation, immune response, and angiogenesis [7, 8]. Production of TGF-β by PCa-associated stroma has been shown to increase the growth and invasiveness of prostate epithelial cells [9]. Further, TGF-β was recently shown to favor osteoblastic bone metastases in experimental systems [10]. Bone is one of the most abundant reservoirs of TGF-β1, which can be released from the bone matrix during bone remodeling after PCa cells migrate to and grow there [11]. Thus, TGF-β is a candidate target for therapy of advanced PCa.
In humans, three isoforms of TGF-β have been described: TGF-β1, TGF-β2, and TGF-β3. Active TGF-β signals through a transmembrane receptor serine–threonine complex that comprises types I and II receptor kinases [12]. Binding of TGF-β1 to the type II receptor leads to the formation of a heterodimeric complex with the type I receptor, which is then phosphorylated. The receptor-associated Smads, Smad2 and Smad3, are subsequently recruited to the activated receptor I complex and are phosphorylated at the carboxyl terminus by the type I receptor. Phosphorylated Smad2/3 interacts with the co-Smad, Smad4, translocates to the nucleus, binds to specific DNA sequences, and recruits co-activators or co-repressors to regulate the transcription of TGF-β target genes [13]. Efforts in targeted drug discovery have thus led to the development of TGF-β receptor type I (TGF-β RI) kinase inhibitors [14].
In this study, we tested the antitumor efficacy of LY2109761, a new selective inhibitor of TGF-β1 RI kinases, on the growth of PCa cells in bone. We assessed its effects in two PCa cell lines that represent the osteoblastic and osteolytic components that are always present in bone metastases. Our findings support the development of therapies targeting TGF-β1 for advanced PCa.
Materials and methods
Cell lines and cultures
The human cell line MDA PCa 2b, a well-established osteoblastic PCa model developed in our laboratory [15], was propagated in BRFF-HPC1 medium (Athena Enzyme Systems, Baltimore, MD) with 20% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO). The other human cell line we used, PC-3, an osteolytic PCa model, was purchased from the American Type Culture Collection (Manassas, VA) and maintained in RPMI 1640 medium (Invitrogen, Carlsbad, CA) with 10% FBS. Primary mouse osteoblasts (PMOs) were isolated from the calvaria of CD1 mouse pups as previously described [16]. All cells were incubated at 37°C in 95% air and 5% CO2.
TGF-β1 protein levels in conditioned medium
MDA PCa 2b and PC-3 cells and the PMOs were grown with complete growth medium in six-well plates. When the cells reached 85%–95% confluence, the medium was changed to serum free. Twenty-four-hour conditioned medium was collected, and the TGF-β1 concentration was measured by using a TGF-β1 ELISA kit (Enzo Life Sciences, Inc., Farmingdale, NY) and following the manufacturer’s instructions. Measurements were performed in three biological replicates.
TGF-β RI kinase inhibitor
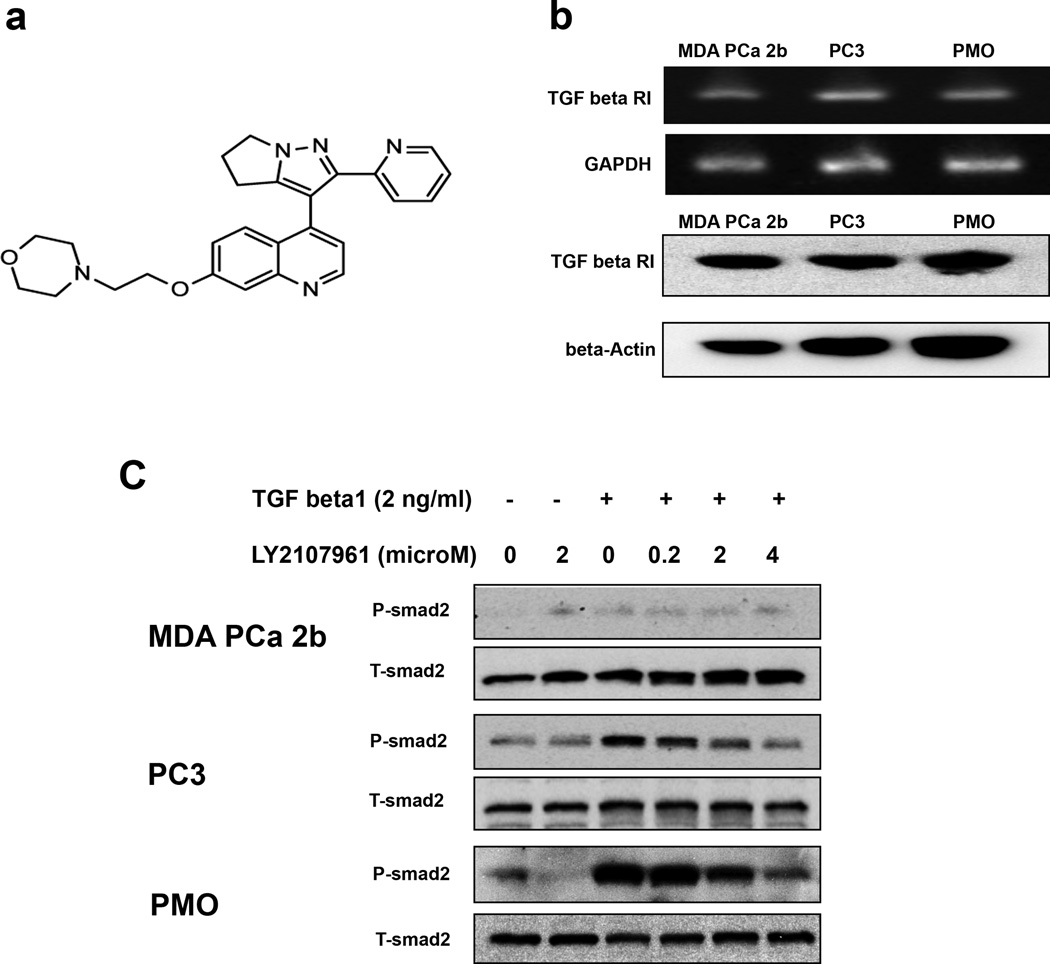
The TGF-β RI kinase inhibitor LY2109761 was synthesized and generously provided by Lilly Research Laboratories (Eli Lilly and Company, Indianapolis, IN). Its structure is shown in Fig. 1a. A stock solution of 5 mM LY2109761 was prepared in 100% DMSO and kept at – 20°C
Fig. 1.
TGF-β receptor I (TGF-β RI) expression and the effect of LY2109761 on TGF-β1 pathway activation in prostate cancer cells and primary mouse osteoblasts (PMOs). a) Structure of LY2109761. b) Expression of TGF-β RI in MDA PCa 2b and PC-3 human prostate cancer cells and in PMOs. (Upper panel) Semiquantitative RT-PCR results; GAPDH was used as the loading control. (Lower panel) Results of Western blot analysis using antibody to human TGF-β RI; beta actin was used as the loading control. c) Results of Western blot analysis of total Smad2 (T-Smad2) and phosphorylated Smad2 (P-Smad2) expression. MDA PCa 2b and PC-3 cells and PMOs were treated with recombinant human TGF-β1 and various concentrations of LY2109761 (as indicated) for 24 h.
Mitogenic cell-proliferation assay
The human PCa cell lines MDA PCa 2b and PC-3 and the PMOs were seeded in six-well plates at densities of 4 × 105, 1 × 105, and 5 × 104 cells per well, respectively, so that they reached 60%–70% confluence after 72 h. At that time, fresh medium containing the indicated amounts of recombinant human TGF-β1 (rhTGF-β1; R&D Systems, Inc., Minneapolis, MN), LY2109761, or rhTGF-β1 + LY 2109761 was added. After 24 h of treatment, cell proliferation was assessed by incorporating [3H]thymidine (NEN Life Science Products, Inc., Boston, MA) into the cells’ DNA; the labeled thymidine was added for the final 3 h of culturing, and its degree of incorporation was measured as previously described [17].
Co-culturing of PMOs and human PCa cells
The PMOs were co-cultured with the PCa cells in a bicompartmental system in which two cell types share medium but are not in physical contact [16]. For controls, we used untreated PMOs and PCa cells, each growing alone in alpha-MEM with 2% FBS (Sigma-Aldrich). Culturing and co-culturing were performed with both the control cells and the cells treated as indicated. After 24 h of co-culturing, the numbers of PMOs and PCa cells were estimated by using the mitogenic assay described above.
Semiquantitative reverse transcription–polymerase chain reaction (RT-PCR)
RNA extracted from the cultured cells was treated with DNase I (Invitrogen), and RT was performed by using Superscript II reverse transcriptase (Invitrogen) according to the manufacturer’s protocol. cDNA was then amplified by PCR with gene-specific primers in standard reaction conditions, resulting in a 273-bp product. The primers for TGF-β RI were purchased from R&D Systems (cat. no. RDP-131). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal control. The PCR products were resolved on 2% agarose gels.
Western blot analysis
Proteins extracted from MDA PCa 2b, PC-3, and PMO cell lysates were loaded into 4%–20% Tris-glycine polyacrylamide gels and transferred to nitrocellulose membranes (Novex, San Diego, CA). TGF-β RI was detected by enhanced chemiluminescence (Amersham, Arlington Heights, IL) after we incubated the membranes with anti–TGF-β RI antibody (R&D Systems) and then with the corresponding secondary antibodies. For detection of total and phosphorylated Smad2 (T-Smad2 and p-Smad2), cells were first grown to 70% confluence and then serum starved for 3 h. Next, we added rhTGF-β1 (2 ng/mL) with and without LY2109761 for an additional 24 h of incubation. T-Smad2 and p-Smad2 were detected by using mouse anti–T-Smad2 (BD Biosciences, Sparks, MD) and rabbit anti–p-Smad2 (Lilly Research Laboratories) primary antibodies, followed by the corresponding secondary antibodies.
In vivo PCa intrabone mouse models treated with LY2109761
Male SCID mice were obtained from Charles River Laboratories (Wilmington, MA) and housed in a certified specific pathogen–free facility. All animal experiments were conducted in accordance with accepted standards of humane animal care and were approved by the Institutional Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center.
To generate the intrabone MDA PCa 2b PCa tumors, we injected 3 µL of medium containing 3 × 105 of the cells into the right femurs of 25 male SCID mice, as previously reported [16]. Four weeks after the cell injections, we determined tumor volumes in the femurs by using magnetic resonance imaging (MRI) analysis according to established procedures [19, 20]. At that point, the mice bearing tumors (19 of the 25 injected developed tumors) were randomly distributed into three groups to receive oral treatment with vehicle alone (control; n = 6) or with 100 (n = 7) or 200 mg/kg/day (n = 6) of LY2109761.
We repeated the tumor volume calculations on MRI at weeks 8 and 10 after the tumor-cell injections. At week 10, the mice were euthanized, and both their injected and contralateral control femurs were dissected out and fixed in 4% paraformaldehyde. Both femurs of each mouse were then subjected to microscopic computed tomographic (micro-CT) imaging analysis and subsequently processed for bone histomorphometric assessment of undecalcified sections, following previously established protocols [20, 21].
Similarly, to generate the intrabone PC-3 tumors, we injected 5 µL of medium containing 3 × 105 of the cells into the right femurs of 30 male SCID mice. One week after the cell injections, the mice were randomly separated into two groups (n = 15 each) to receive vehicle alone (control) or 200 mg/kg/day of LY2109761 orally. (In preliminary studies with LY2109761, we had found a dosage-dependent effect in PC-3 cells, with the maximal effect achieved by using 200 mg/kg/day; thus, we treated PC-3 tumor–bearing mice daily with vehicle alone or the 200 mg/kg dosage of LY2109761.) Tumor volume was monitored on x-ray analysis (see below) and MRI at week 3. Mice were then euthanized, and both their injected and contralateral control femurs were dissected out and fixed in 4% paraformaldehyde. The femurs were then subjected to micro-CT analysis and subsequent bone histomorphometric assessment of undecalcified sections, following previously established protocols [18].
Because some comparisons would be done between tumor-bearing femurs and the contrlateral (left) femurs, we performed a pilot study in which we injected growth medium intrafemorally into 4 mice to assess whether the inoculation procedure induced any obvious histologic change due to bone remodeling. Four weeks after the injection in the distal end of the femur, we did not find any obvious histologic alteration (data not shown). This could be the result of our having used a very small needle (28 gauge, 1/2 inch) to drill a hole in the bone and the small volume (5 µL growth medium) we injected; this is the same procedure we use to inject PCa cells.
X-ray examination
For x-ray analysis of tumor-bearing bones, animals were anesthetized and placed in prone and then lateral positions on a transparent board. The board was placed against an x-ray film (Kodak X-OMAT AR; Eastman Kodak Company, Rochester, NY), and the animals were exposed to x-rays at 20 kV for 15 s in a Faxitron radiographic inspection unit (model 43855A; Faxitron X-Ray Corp., Wheeling, IL). Exposed films were developed in an automatic film processor (RP X-OMAT; Kodak), and the radiographs were evaluated for the presence of bone lesions.
Micro-CT analysis
Micro-CT analysis was performed in the Small Animal Imaging Facility at MD Anderson with an Enhanced Vision Systems hybrid specimen scanner (GE Medical Systems, London, ON, Canada) at a resolution of 20 µm. The images were reconstructed by using GE Healthcare–provided software and a back-projection method, and the volumes were constructed of 20-µm isotropic voxels. Images were calibrated in Hounsfield units with the use of a separately scanned water–air–bone phantom provided by GE. Once reconstructions were carried out, the volumes were analyzed by using software provided by GE (MicroView build 2.0.29). A 3-mm midshaft region of cortical bone, identified as the center of each femur relative to the proximal and distal ends, was evaluated for each bone.
Histomorphometric analysis of bone
Mice were euthanized at the end of the study period. Disarticulated right and left femurs were fixed by immersion in 10% buffered formalin and subsequently processed for assessment of undecalcified sections in the Bone Histomorphometry Core facility at MD Anderson (M.W.S.) according to previously established protocols [18]. The femurs were positioned so that sagittal 5-µm-thick sections could be obtained through the entire width of each bone.
Slides were stained with toluidine blue for assessing osteoblast numbers and surfaces and with TRAP, an enzyme specifically expressed by osteoclasts in the bone marrow, for assessing osteoclast parameters. Both osteoblasts and osteoclasts were quantified on 25–30 adjacent high-magnification fields obtained from one representative 5-µm tissue section, by using the OsteoMeasure software system (OsteoMetrics, Inc., Decatur, GA).
Statistical analysis
Two-sample t testing for equal variance was used to identify the statistical significance of differences between the means of the different treatment groups; p < 0.05 was considered statistically significant.
Results
PCa cells and PMOs express TGF-β RI
Because LY2109761 is a TGF-β RI–selective kinase inhibitor, we assessed the expression level of TGF-β RI in MDA PCa 2b and PC-3 cells and in PMOs. As shown in Fig. 1b, all three cell types express the receptor at both the RNA and protein levels.
PC-3 PCa cells and PMOs express TGF-β1
We subsequently assessed whether the PC-3 cells and PMOs secrete TGF-β1 into the medium: the PMOs released 258 ± 13 pg/mL/24 h and the PC-3 cells, 603 ± 40 pg/mL/24 h. TGF-β1 was undetectable in the growth medium from MDA PCa 2b cells.
LY2109761 inhibits TGF-β1–induced Smad2 activation in PC-3 cells and PMOs
A crucial step in the transduction of TGF-β1 signals is the phosphorylation of receptor-activated Smad2 and Smad3 [19]. We thus assessed the phosphorylation of Smad2 in lysates of MDA PCa 2b cells, PC-3 cells, and PMOs treated with rhTGF-β1. We found that TGF-β1 induces phosphorylation of Smad2 in PC-3 cells and PMOs but not in MDA PCa 2b cells (Fig. 1c). Further, treatment with LY2109761 reverses the Smad2 phosphorylation induced by rhTGF-β1 (Fig. 1c).
LY2109761 effectively blocks the effects of TGF-β1 on cell proliferation in vitro
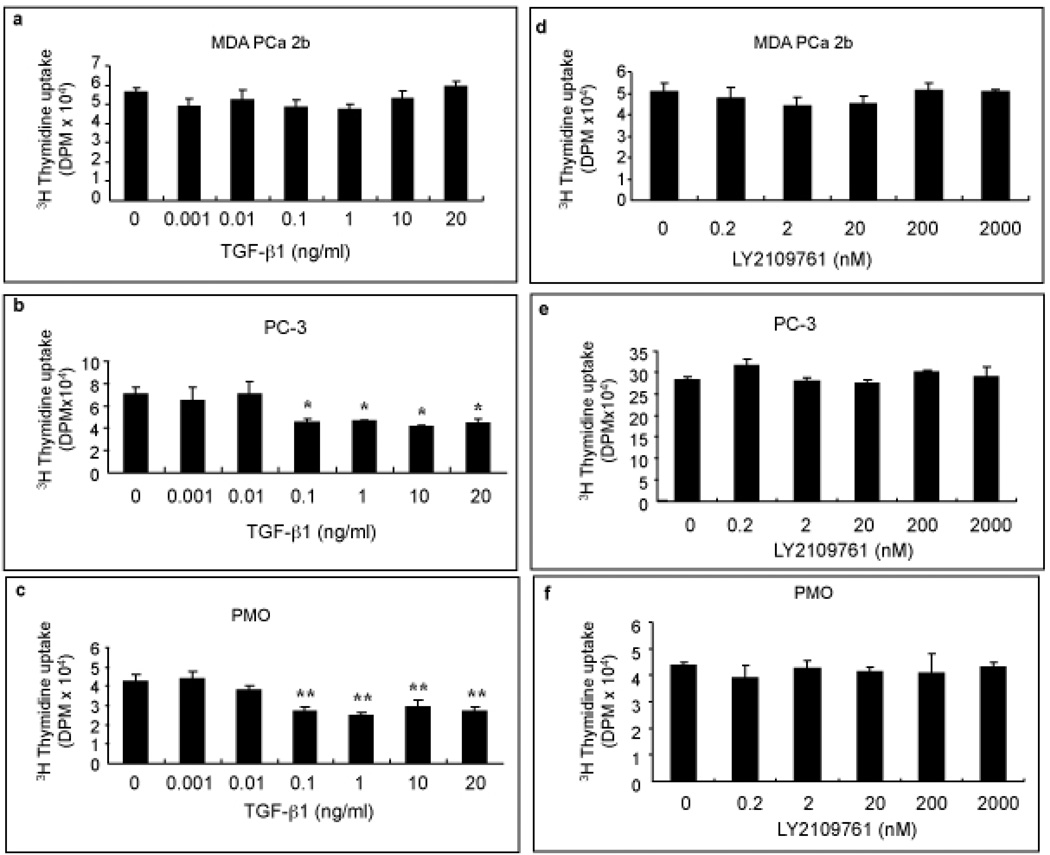
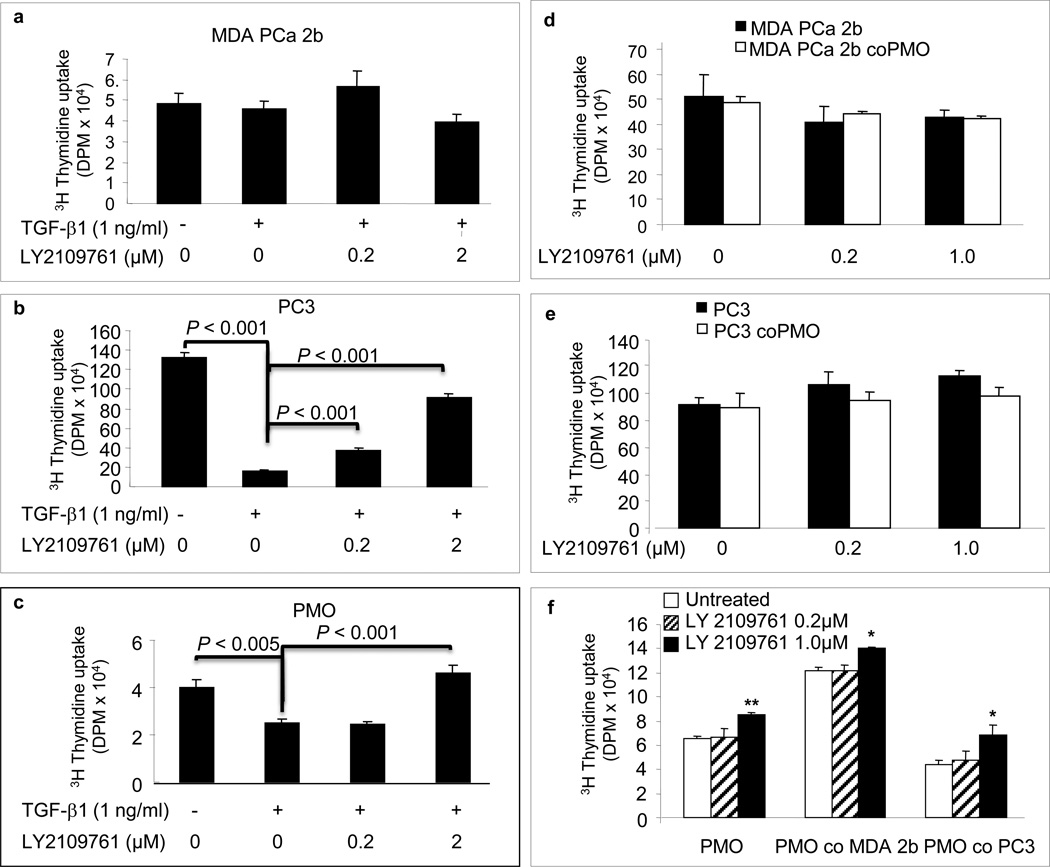
TGF-β1 is known to produce various effects, including regulation of cell proliferation, in different cell types [11]. Thus, we first studied its effect on cell proliferation. We found that TGF-β1 inhibits cell proliferation in PC-3 cells and PMOs but not in MDA PCa 2b cells (Fig. 2). We subsequently found that LY2109761 had no direct effect on cell proliferation at any of the concentrations we tested (Fig. 2) but effectively blocked the inhibition of cell proliferation produced by TGF-β1 in PC-3 cells and PMOs (Fig. 3a–c).
Fig. 2.
The effects of TGF-β1 and LY2109761 on the proliferation of human prostate cancer cells and primary mouse osteoblasts (PMOs). Cell proliferation was assessed by [3H]-thymidine incorporation into the DNA of MDA PCa 2b cells (a and d), PC-3 cells (b and e), and PMOs (c and f). Cells were treated for 24 h with the indicated concentrations of recombinant human TGF-β1 or LY2109761. *p < 0.05 and **p < 0.01 vs. control. Error bars, SEM.
Fig. 3.
The effects of TGF-β1 and of LY2109761 on proliferation of human prostate cancer cells and primary mouse osteoblasts (PMOs) growing alone or in co-culture. Cell proliferation was assessed by [3H]-thymidine incorporation into the DNA of MDA PCa 2b cells (a and d), PC-3 cells (b and e), and PMOs (c and f) grown alone and in co-culture (respectively). Cells were treated for 24 h with recombinant human TGF-β1 alone or in combination with the indicated concentrations of LY2109761. co, co-cultured. *p < 0.05 and **p < 0.01 vs. untreated; other p values are as indicated for individual comparisons. Error bars, SEM.
LY2109761 induces osteoblast proliferation in vitro
Because the main goal of this work was to assess the effect of the TGF-β RI kinase inhibitor on the growth of PCa cells in bone, we studied whether LY2109761 affects the interaction between PCa cells and osteoblasts. For that purpose, we co-cultured the PCa cells and PMOs and found that LY2109761 had no effect on the growth of PCa cells in the presence of PMOs (Fig. 3d, e). However, we consistently found an increased number of PMOs (whether they were grown alone or in the presence of PCa cells) when they were grown in the presence of LY2109761 (with 2% FBS supplementation) at the highest concentration tested (Fig. 3f).
Taken together, these results suggest that TGF-β1 does not participate in proliferation signaling between PCa cells and osteoblasts. Instead, we found that 1 µM LY2109761 increased PMO growth in vitro, suggesting that TGF-β1 is involved in autocrine proliferation signaling in osteoblasts (Fig. 3f).
LY2109761 induces increases in various parameters of normal bone
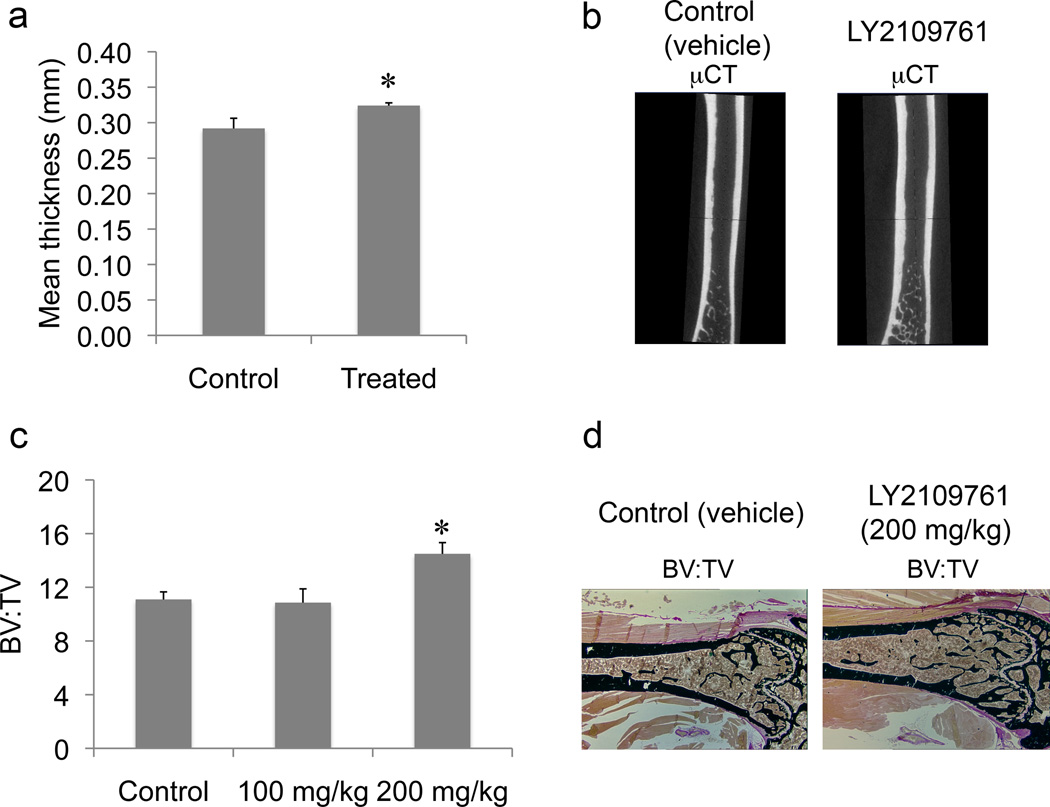
Because we had observed that the 1 µM LY2109761 increased PMO growth in vitro, we assessed whether the inhibitor had any effects on the parameters of normal bone in vivo using, for this analysis, the contralateral (uninjected) femur of the tumor-bearing mice. On micro-CT, we found a statistically significant increase in the mean thickness of the nontumorous control femurs of mice treated with LY2109761 (p = 0.0138) relative to the thickness in the untreated mice (Fig. 4a, b). Furthermore, on bone histomorphometric analysis, we found an increase in the ratio of bone volume (BV) to tissue volume in the nontumorous femurs of mice treated with 200 mg/kg/day of LY2109761 (p = 0.00263) (Fig. 4c, d). These findings suggest that in normal bone, the inhibitor increases mineralized (cortical and trabecular) bone.
Fig. 4.
The effects of 6 weeks of treatment with LY2109761 on bone volume (BV) of normal (uninjected left) femurs in mice whose right femurs were injected with MDA PCa 2b human prostate cancer cells. a) Mean thickness (mm) of of normal femurs in untreated control and treated mice (the two LY2109761-treated groups were considered together), as assessed on micro-CT scanning. p = 0.014 vs. control mice. b) Representative micro-CT images of femurs in mice injected with vehicle only (control) in the left femur and with MDA PCa 2b human prostate cancer cells in the right femur. c) Results of bone histomorphometric analysis of normal femurs in untreated control and treated mice; ratio of bone volume to tissue volume (BV:TV). Group sizes: control, n = 6; 100 mg/kg, n = 6, and 200 mg/kg, n = 4 mice. *p = 0.003 vs. control. d) Representative longitudinal histologic sections of undecalcified normal femurs from control and high-dosage (200 mg/kg/day) LY2109761-treated mice. von Kossa stain; original magnification ×200. Error bars, SEM.
On bone histomorphometric analysis, we also found increases in both osteoblast and osteoclast parameters in the nontumorous femurs in treated mice relative to those in the untreated mice. The increases in the osteoblast parameters did not reach the level of statistical significance (Table 1), whereas those in the osteoclast parameters were significant (Table 2).
Table 1.
The effect of LY2109761 on osteoblast parametersa (assessed by bone histomorphometry) in trabecular bone of the left femur in mice whose right femurs were injected with MDA PCa 2b cells, followed by daily treatment for 6 weeks with vehicle or LY2109761 at the indicated dosages
| Osteoblast parameter | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment group |
Mean Ob.S/BS |
p vs. Control |
Mean N.Ob/T. Ar |
p vs. Control |
Mean N. Ob/B.Pm |
p vs. Control |
Mean N.Ob |
p vs. Control |
Mean N. Ob/Ob. Pm |
p vs. Control |
| Vehicle control | 13.462 | 94.51 | 12.992 | 54.6 | 103.584 | |||||
| 100 mg/kg LY2109761 |
16.0775 | 0.6002 | 122.19 | 0.4819 | 16.67 | 0.4507 | 68.25 | 0.5341 | 100.8125 | 0.7963 |
| 200 mg/kg LY2109761 |
14.1675 | 0.9023 | 138.875 | 0.3886 | 14.045 | 0.8432 | 84 | 0.2758 | 98.935 | 0.6423 |
Abbreviations: Ob.S/BS: osteoblast surface as a percentage of bone surface; N.Ob/T. Ar: number of osteoblasts per area of tissue; N. Ob/B.Pm: number of osteoblasts per bone perimeter; N.Ob: number of osteoblasts ; N. Ob/Ob. Pm: number of osteoblasts per osteoblast perimeter.
Osteoblast parameters were measured by using the semiautomatic mode in the OsteoMeasure system (TAS) in undecalcified mouse femur embedded in methyl methacrylate and stained with von Kossa stain to identify areas of mineralized bone.
Table 2.
The effect of LY2109761 on osteoclast parametersa (assessed by bone histomorphometry) in trabecular bone of the left femur of mice whose right femurs were injected with MDA PCa 2b cells, followed by daily treatment for 6 weeks with vehicle or LY2109761 at the indicated dosages
| Osteoclast parameter | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment group | Mean Oc./BS |
p vs. Control |
Mean N. Oc/T. Ar |
p vs. Control |
Mean N. Oc/B. Pm |
p vs. Control |
Mean N. Oc/Oc Pm |
p vs. Control |
| Vehicle control | 7.5983 | 29.225 | 3.655 | 47.055 | ||||
| 100 mg/kg LY2109761 | 10.29 | 0.1366 | 35.91 | 0.2618 | 5.07 | 0.2085 | 48.338 | 0.7689 |
| 200 mg/kg LY2109761 | 12.57 | 0.00504 | 60.855 | 0.00680 | 6.24 | 0.0101 | 49.880 | 0.526 |
Abbreviations: Oc./BS: osteoclast surface as a percentage of bone surface; N. Oc/T. Ar: number of osteoclasts per area of tissue; N. Oc/B. Pm: number of osteoclasts per bone perimeter; N. Oc/Oc Pm: number of osteoclasts per osteoclast perimeter.
Osteoclast parameters were measured by using the semiautomatic mode in the OsteoMeasure system (TAS) in undecalcified mouse femur embedded in methyl methacrylate and stained with von Kossa stain to identify areas of mineralized bone.
Together, these results suggest that the increased BV observed after treatment with LY2109761 does not result from osteoclast inhibition but rather, from increased bone formation.
LY2109761 inhibits the growth of osteoblastic MDA PCa 2b PCa cells in mouse bone
In vivo, both doses of LY2109761 significantly reduced the growth rate of MDA PCa 2b cells relative to that in untreated control mice (p < 0.05) (Fig. 5). However, we found no differences in the parameters on micro-CT (BV, bone mineral content [BMC], or bone mineral density [BMD]) or on bone histomorphometry (BV vs. tissue volume and osteoblast and osteoclast parameters) of the tumor-bearing bones between LY2109761-treated and untreated mice (data not shown).
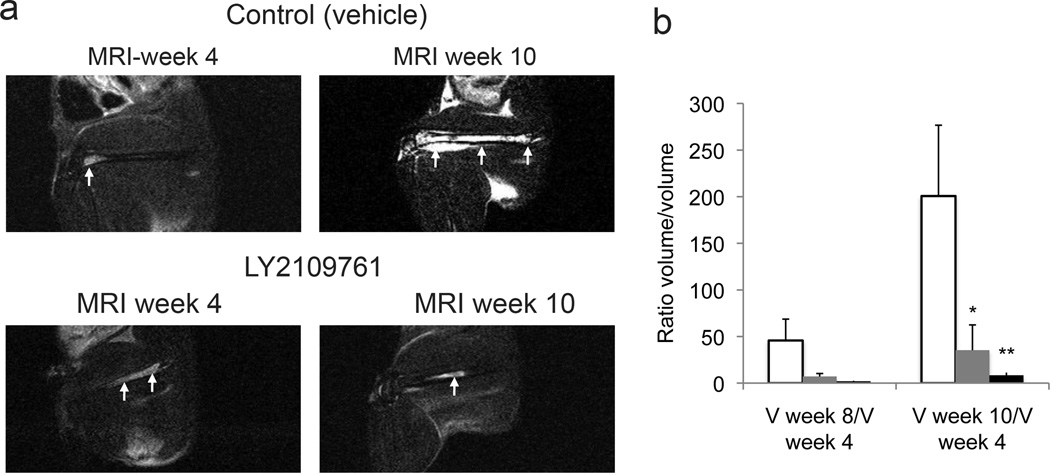
Fig. 5.
The effects of LY2109761 in MDA PCa 2b human prostate cancer cells growing in the bone of mice. The right femurs of male SCID mice were injected with MDA PCa 2b cells, and MRI was performed at 4, 8, and 10 weeks after the injection. At the 4-week point, mice were randomly separated into three groups to receive vehicle alone (control) or LY2109761 at 100 mg/kg or 200 mg/kg daily. a) MDA PCa 2b tumors were visualized and quantified on MRI by obtaining T2-weighted, fat-suppressed images; arrows indicate the tumor in the femur on representative sagittal MR images. b) Degree of change in tumor size (assessed on MRI) was measured as the ratio between tumor volume (V) at 8 and 10 weeks and that at 4 weeks. If the tumor volume at 4 weeks was zero, we used the value at 8 or 10 weeks. Clear bars, control; gray bars, 100 mg/kg of LY2109761; black bars, 200 mg/kg of LY2109761. Group sizes: control, n = 5; 100 mg/kg, n = 7; and 200 mg/kg, n = 5 mice. *p = 0.042 and **p = 0.035 vs. control. Error bars, SEM.
LY2109761 inhibits the growth of osteolytic PCa cells in mouse bone and restores bone parameters to normal
Finally, to confirm that the growth-inhibitory effect of LY2109761 is not restricted to the MDA PCa 2b osteoblastic PCa cell line, we assessed its effect on the PC-3 osteolytic PCa cell line.
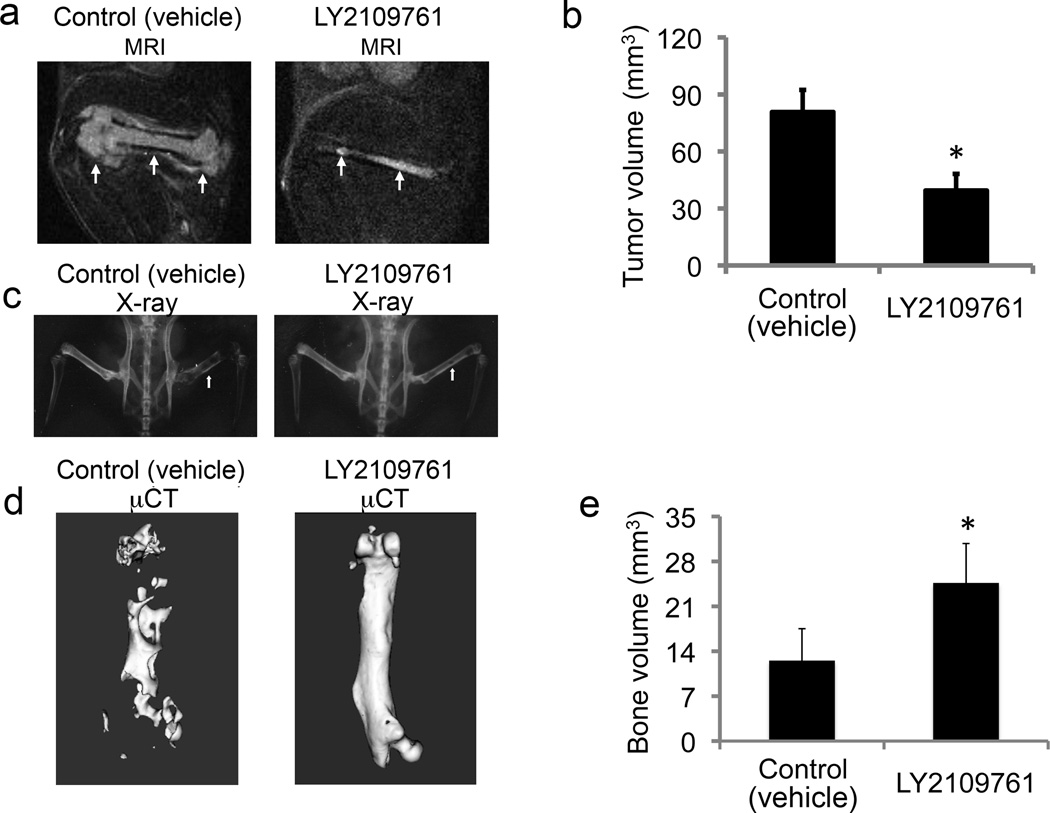
After 3 weeks of treatment, x-ray analysis of the vehicle control group revealed two broken bones and loss of 30%–70% of the radiopaque areas in the tumor-bearing bones (Fig. 6c). In contrast, no broken bones were detected in the treated mice (Fig. 6c), and radiolucent areas in the tumor-bearing bones were localized, constituting less than 20% of the total femur area. MRI analysis showed a significantly smaller tumor volume in the treated group than in the controls (p = 0.012) (Fig. 6a, b). Micro-CT analysis of the tumor-bearing bones of the controls and treated mice demonstrated significantly lower BV (p = 0.00043), BMC (p = 0.000132), and BMD (p = 0.000085) in the control mice (Fig. 6e, f and Table 3). Furthermore, BV, BMC, and BMD in the treated group were restored to values found in the normal (uninjected) femurs (Table 3), which supports the efficacy of treatment. Finally, bone histomorphometric analysis demonstrated that LY2109761 inhibited PC-3–induced activation of osteoclasts (Table 4).
Fig. 6.
The effects of treatment with 200 mg/kg/day of LY2109761 on PC-3 human prostate cancer cells growing in the bone of mice. a) Representative sagittal MRI scans of femurs in mice injected intrafemorally with PC-3 cells. PC-3 tumors were visualized on T2-weighted, fat-suppressed MRI scans obtained after 3 weeks of treatment with LY2109761 or vehicle only (Control). Arrows indicate tumor. b) Mean tumor volumes in regions of increased signal in the PC-3–injected femurs as quantified on T2-weighted, fat-suppressed MRI scans after 3 weeks of treatment (p < 0.012 vs. control). c) Plain radiographs show representative mouse pelves and hind limbs 4 weeks after intrafemoral injection of PC-3 cells in the control and treatment groups. Arrow indicate injected femur. Note extensive areas of bone loss (radiolucent) in the femur of the control mice while the femur in treated mice is less damaged d) micro-CT images of tumor-bearing femurs treated with vehicle only (left image; note the widespread lysis areas) and LY2109761 (right image). e) LY2109761-treated mice had greater bone volume than control mice had (p < 0.00044). Error bars, SEM.
Table 3.
The effect of LY2109761 on bone parameters assessed by micro-CT analysis in PC-3 tumor–bearing (right femur) and normal (uninjected femur) bones of mice treated with vehicle or LY2109761 at the indicated dosage
| Bone parameter | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment group |
Femur | Mean BV (mm3) |
p vs. Vehicle control |
p vs. Normal (uninjected) femur |
Mean BMC (mg) |
p vs. Vehicle control |
p vs. Normal (uninjected) femur |
Mean BMD (mg/cm3) |
p vs. Vehicle control |
p vs. Normal (uninjected) femur |
| Vehicle control |
Tumor- bearing |
12.5 | 7.91 × 10−5 | 8.0 | 2.012 × 10−5 | 613.8 | 3.93 × 10−7 | |||
| Normal (uninjected) |
25.8 | 18.4 | 682.1 | |||||||
| LY2109761 (200 mg/kg) |
Tumor- bearing |
24.6 | 0.00043 | 0.194 | 18.6 | 0.00013 | 0.278 | 718.2 | 0.00027 | 0.488 |
| Normal (uninjected) |
28.0 | 0.170 | 20.7 | 0.070 | 709.4 | 0.017 | ||||
Abbreviations: BV: bone volume; BMC: bone mineral content; BMD: bone mineral density.
Table 4.
The effect of LY2109761 on osteoclast parametersa (assessed by bone histomorphometry) in trabecular bone of PC-3 tumor–bearing bones of mice treated with vehicle or LY2109761 at the indicated dosages
| Osteoclast parameter | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment group | Mean Oc./BS |
p vs. Conrol |
Mean N. Oc/T. Ar |
p vs. Conrol |
Mean N. Oc/B. Pm |
p vs. Conrol |
Mean N. Oc/Oc Pm |
p vs. Conrol |
| Vehicle control | 13.19 | 37.6 | 5.39 | 42.19 | ||||
| 100 mg/kg LY2109761 | 7.41 | 0.2868 | 41.00 | 0.9115 | 3.38 | 0.3478 | 41.54 | 0.9751 |
| 200 mg/kg LY2109761 | 4.07 | 0.0076 | 32.18 | 0.7822 | 1.76 | 0.0031 | 35.98 | 0.6446 |
Abbreviations: Oc./BS: osteoclast surface as a percentage of bone surface; N. Oc/T. Ar: number of osteoclasts per area of tissue; N. Oc/B. Pm: number of osteoclasts per bone perimeter; N. Oc/Oc Pm: number of osteoclasts per osteoclast perimeter.
Osteoclast parameters were measured by using the semiautomatic mode in the OsteoMeasure system (TAS) in undecalcified mouse femur embedded in methyl methacrylate and stained with von Kossa stain to identify areas of mineralized bone.
Discussion
Our results showed for the first time, to our knowledge, that LY2109761, a selective TGF-β RI kinase inhibitor, has antitumor effects against PCa cells growing in the bone of mice. The role of TGF-β in cancer progression is complex, and reports of both tumor-suppressing and -promoting roles have been published [20]. In normal tissues, the suppressor activities are predominant, but during tumorigenesis, changes in TGF-β expression and cellular responses favor its oncogenic activities in certain cancer cells.
Our in vitro studies explored the effect of TGF-β1 in the growth or PCa cells in isolation, and the results demonstrate that TGF-β1 retains its growth suppressor activities in PC-3 cells. Conversely, when growing in vivo, PCa cells interact with the microenvironment, which ultimately influences their growth rate. TGF-β1, one of the most abundantly stored cytokines in bone matrix, is known to stimulate tumor-mediated bone resorption, possibly by promoting PTHrP production by the tumor cell, which in turn stimulates bone resorption [21, 22]. Accordingly, the growth-inhibitory effect of the TGF-β RI kinase inhibitor LY2109761 in vivo is associated with a reduction in osteoclast-associated parameters. These results thus suggest that the blockade of osteoclast activation or function has a profound effect on the growth of PC-3 cells in bone, which counteracts the consequences of a direct blockade of the growth-promoting effects of TGF-β1 on PC-3 cells.
TGF-β1 plays a major role in bone metabolism (e.g. coupling bone formation and resorption) physiologically [23, 24]. However, the specific effects of TGF-β1 signaling on bone formation are complex, and in vitro results have been inconsistent and often not recapitulated in vivo. The best documented model of the effects of TGF-β1 in osteoblasts is that TGF-β1 inhibits osteoblast diferentiation, possibly by repressing the transcriptional activity of Runx2 through Smad3. Because RUNX2 activates transcription from its own promoter, this mechanism likely results in decreased cbfa1 expression [25]. Further, endogenous TGF-β1 was found to induce the expression of inhibitory Smads during the maturation phase of osteoblastic differentiation induced by BMP-4 [26]. In agreement with that model, our studies showed that TGF-β1 inhibits osteoblast proliferation, which is rescued by LY2109761. Further, LY2109761 induces osteoblasts proliferation at 1 µM concentration in 2% FBS. Accordingly, LY2109761 treatment of tumor-bearing mice resulted in increased BV of the nontumorous bone and in a dosage-related increase in osteoblast-related parameters, suggesting that osteoblast function was increased. In agreement with our findings, pharmacologic blockade of TGF-β1 signaling with another TGF-β type I receptor inhibitor resulted in an increase of bone mass [27]. Thus, inhibition of TGF-β signaling by LY2109761 likely results in [release of Runx2 inhibition combined with suppressed induction of inhibitory Smads, which would lead to acceleration of BMP signaling.
The effects of TGF-β1 in osteoclastogenesis are equally complex and must be evaluated considering the presence of other cytokines and hormones that modulate or are modulated by TGF-β1 signaling in different ways. On one hand, active osteoclasts are capable of activating TGF-β, and this in turn may attenuate bone resorption by impairing osteoclastogenesis [24]. Also, TGF-β increases osteoprotegerin secretion from osteoblastic and bone marrow stromal cells and decreases osteoblastic production of RANKL (receptor activator of NF-κB ligand), which may lead to decreased osteoclast differentiation [13]. However, in vivo data in genetically modified mice as well as some treated with TGF-β inhibitors, showed that TGF-β promotes osteoclastogenesis and bone resorption [13]. Our studies, on the other hand, showed that LY2109761 treatment resulted in increased osteoclast parameters in normal bone. This could be due to a compensatory mechanism to the increased bone mass. Together, these results reinforce the concept of the complex role of TGF-β signaling in normal bone biology. Because our studies were performed in the normal bone of tumor-bearing mice, it is possible that the presence of cytokines in the bloodstream of those mice could also be a contributing factor for the effects of TGF-β RI inhibition in normal bone. In any event, this secondary effect of increasing bone mass would be beneficial for men undergoing androgen-ablation therapy because it could alleviate the skeletal complications (i.e. osteopenia, osteoporosis) frequently found in these patients. It is important, though, to identify the status of osteoclast activation, because the benefits of TGF-β RI kinase blockade could synergize with, for example, inhibition of osteoclast activation through the use of a RANKL inhibitor.
The effect of LY2109761 in bones bearing PC-3 tumors was different than that observed in nontumorous bones and resulted in a reduction of tumor-associated osteoclast-related parameters. Accordingly, the antitumor efficacy of LY2109761 was greater in the PC-3 cell line, an osteolytic PCa model, than it was in the MDA PCa 2b cell line, an osteoblastic PCa model. These results concur with the in vivo data in genetically modified mice that have consistently shown that TGF-β promotes osteoclastogenesis and bone resorption [23, 27, 28].
Of note is that in our study, LY2109761 inhibited PC-3–induced osteoclast activation after 3 weeks of treatment but increased the numbers of osteoclasts in normal bone after 6 weeks of treatment. These differences in the effect of LY2109761 could be due to the difference in treatment duration, but a plausible alternative explanation is that the mechanism underlying PC-3–induced osteoclast activation is different from what takes place in the normal bone.
In conclusion, the results of these studies support the promise of TGF-β1 inhibitors for use in the treatment of men with advanced PCa. Morover, the increase in bone mass we observed in nontumorous bone may be a desirable side effect of LY2109761 treatment for men with osteopenia or osteoporosis secondary to androgen-ablation therapy, further reinforcing the benefit of effectively controlling PCa growth in bone.
Highlights.
Treatment with the transforming growth factor beta receptor I inhibitor LY2109761 increased the volume of normal bone in tumor-bearing mice.
The transforming growth factor beta receptor I inhibitor LY2109761 significantly inhibited bone growth of prostate cancer cells in mice.
The LY2109761-induced increased bone mass may be a desirable effect in men with osteopenia or osteoporosis secondary to androgen-ablation therapy.
Acknowledgements
We are grateful to the staffs of the Bone Histomorphometry Core Laboratory and the Small Animal Imaging Facility at MD Anderson for their participation in these studies.
Funding: This research was supported in part by the National Institutes of Health through MD Anderson's Cancer Center Support Grant, CA016672, and by Lilly Research Laboratories, the Prostate Cancer Foundation, and the Rolanette and Berdon Lawrence Bone Disease Program of Texas.
Abbreviations used
- PCa
prostate cancer
- TGF-β1
transforming growth factor beta 1
- TGF-β RI
TGF-β receptor type I
- PMOs
primary mouse osteoblasts
- rhTGF-β1
recombinant human TGF-β1
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- BV
bone volume
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Charhon SA, Chaput MC, Delvin EE, Valentin-Opran A, Edouard CM, Meunier PG. Histomorphometric analysis of sclerotic bone metastases from prostatic carcinoma with special reference to osteomalacia. Cancer. 1983;51:918–924. doi: 10.1002/1097-0142(19830301)51:5<918::aid-cncr2820510526>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 2.Saad F, Adachi JD, Brown JP, Canning LA, Gelmon KA, Josse RG, Pritchard KI. Cancer treatment-induced bone loss in breast and prostate cancer. J Clin Oncol. 2008;26:5465–5476. doi: 10.1200/JCO.2008.18.4184. [DOI] [PubMed] [Google Scholar]
- 3.Shariat SF, Shalev M, Menesses-Diaz A, Kim IY, Kattan MW, Wheeler TM, Slawin KM. Preoperative Plasma Levels of Transforming Growth Factor Beta1 (TGF-{beta}1) Strongly Predict Progression in Patients Undergoing Radical Prostatectomy. J Clin Oncol. 2001;19:2856–2864. doi: 10.1200/JCO.2001.19.11.2856. [DOI] [PubMed] [Google Scholar]
- 4.Shariat SF, Kattan MW, Traxel E, Andrews B, Zhu K, Wheeler TM, Slawin KM. Association of Pre- and Postoperative Plasma Levels of Transforming Growth Factor {beta}1 and Interleukin 6 and Its Soluble Receptor with Prostate Cancer Progression. Clin Cancer Res. 2004;10:1992–1999. doi: 10.1158/1078-0432.ccr-0768-03. [DOI] [PubMed] [Google Scholar]
- 5.Shariat SF, Walz J, Roehrborn CG, Montorsi F, Jeldres C, Saad F, Karakiewicz PI. Early postoperative plasma transforming growth factor-beta1 is a strong predictor of biochemical progression after radical prostatectomy. J Urol. 2008;179:1593–1597. doi: 10.1016/j.juro.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 6.O'Brien PJ, Ramanathan R, Yingling JM, Baselga J, Rothenberg ML, Carducci M, Daly T, Adcock D, Lahn M. Analysis and variability of TGFbeta measurements in cancer patients with skeletal metastases. Biologics. 2008;2:563–569. [PMC free article] [PubMed] [Google Scholar]
- 7.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 8.Wakefield LM, Roberts AB. TGF-beta signaling: positive and negative effects on tumorigenesis. Curr Opin Genet Dev. 2002;12:22–29. doi: 10.1016/s0959-437x(01)00259-3. [DOI] [PubMed] [Google Scholar]
- 9.Ao M, Franco OE, Park D, Raman D, Williams K, Hayward SW. Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 2007;67:4244–4253. doi: 10.1158/0008-5472.CAN-06-3946. [DOI] [PubMed] [Google Scholar]
- 10.Mishra S, Tang Y, Wang L, Degraffenried L, Yeh IT, Werner S, Troyer D, Copland JA, Sun LZ. Blockade of transforming growth factor-beta (TGFbeta) signaling inhibits osteoblastic tumorigenesis by a novel human prostate cancer cell line. Prostate. doi: 10.1002/pros.21361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alliston T, Piek E, Derynck R. TGF-beta family in skeletal development and disease. In: Derynck R, Miyazono K, editors. The TGF-β Family. Woodbury, NY: Cold Spring Harbor Press; 2008. pp. 667–723. [Google Scholar]
- 12.Yingling JM, Blanchard KL, Sawyer JS. Development of TGF-[beta] Signaling Inhibitors for Cancer Therapy. Nature Reviews Drug Discovery. 2004;3:1011–1022. doi: 10.1038/nrd1580. [DOI] [PubMed] [Google Scholar]
- 13.Juarez P, Guise TA. TGF-beta in cancer and bone: implications for treatment of bone metastases. Bone. 48:23–29. doi: 10.1016/j.bone.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Li HY, McMillen WT, Heap CR, McCann DJ, Yan L, Campbell RM, Mundla SR, King CH, Dierks EA, Anderson BD, Britt KS, Huss KL, Voss MD, Wang Y, Clawson DK, Yingling JM, Sawyer JS. Optimization of a dihydropyrrolopyrazole series of transforming growth factor-beta type I receptor kinase domain inhibitors: discovery of an orally bioavailable transforming growth factor-beta receptor type I inhibitor as antitumor agent. J Med Chem. 2008;51:2302–2306. doi: 10.1021/jm701199p. [DOI] [PubMed] [Google Scholar]
- 15.Navone NM, Olive M, Ozen M, Davis R, Troncoso P, Tu SM, Johnston D, Pollack A, Pathak S, von Eschenbach AC, Logothetis CJ. Establishment of two human prostate cancer cell lines derived from a single bone metastasis. Clin Cancer Res. 1997;3:2493–2500. [PubMed] [Google Scholar]
- 16.Yang J, Fizazi K, Peleg S, Sikes CR, Raymond AK, Jamal N, Hu M, Olive M, Martinez LA, Wood CG, Logothetis CJ, Karsenty G, Navone NM. Prostate cancer cells induce osteoblast differentiation through a Cbfa1-dependent pathway. Cancer Res. 2001;61:5652–5659. [PubMed] [Google Scholar]
- 17.Freshney RI. Culture of animal cells. A manual of basic techniques. 3rd ed. New York: Wiley-Liss, Inc; 1994. [Google Scholar]
- 18.Baron R, Vignery A, Neff L, Silverglate A, Santa Maria A. Processing of undecalcified bone specimens for bone histomorphometry. In: Recker RR, editor. Bone Histomorphometry: Techniques and Interpretation. Boca Raton: CRC Press; 1983. pp. 13–35. [Google Scholar]
- 19.Feng X-H, Derynck R. Specificity and versatility in TGF-β signaling through Smads. Annual Review of Cell and Developmental Biology. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 20.Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 10:415–424. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 21.Kakonen SM, Selander KS, Chirgwin JM, Yin JJ, Burns S, Rankin WA, Grubbs BG, Dallas M, Cui Y, Guise TA. Transforming growth factor-beta stimulates parathyroid hormone-related protein and osteolytic metastases via Smad and mitogen-activated protein kinase signaling pathways. J Biol Chem. 2002;277:24571–24578. doi: 10.1074/jbc.M202561200. [DOI] [PubMed] [Google Scholar]
- 22.Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, Massague J, Mundy GR, Guise TA. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, Zhao L, Nagy TR, Peng X, Hu J, Feng X, Van Hul W, Wan M, Cao X. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009;15:757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janssens K, ten Dijke P, Janssens S, Van Hul W. Transforming growth factor-beta1 to the bone. Endocr Rev. 2005;26:743–774. doi: 10.1210/er.2004-0001. [DOI] [PubMed] [Google Scholar]
- 25.Alliston T, Choy L, Ducy P, Karsenty G, Derynck R. TGF-beta-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. EMBO J. 2001;20:2254–2272. doi: 10.1093/emboj/20.9.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maeda S, Hayashi M, Komiya S, Imamura T, Miyazono K. Endogenous TGF-beta signaling suppresses maturation of osteoblastic mesenchymal cells. EMBO J. 2004;23:552–563. doi: 10.1038/sj.emboj.7600067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohammad KS, Chen CG, Balooch G, Stebbins E, McKenna CR, Davis H, Niewolna M, Peng XH, Nguyen DH, Ionova-Martin SS, Bracey JW, Hogue WR, Wong DH, Ritchie RO, Suva LJ, Derynck R, Guise TA, Alliston T. Pharmacologic inhibition of the TGF-beta type I receptor kinase has anabolic and anti-catabolic effects on bone. PLoS One. 2009;4:e5275. doi: 10.1371/journal.pone.0005275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balooch G, Balooch M, Nalla RK, Schilling S, Filvaroff EH, Marshall GW, Marshall SJ, Ritchie RO, Derynck R, Alliston T. TGF-beta regulates the mechanical properties and composition of bone matrix. Proc Natl Acad Sci U S A. 2005;102:18813–18818. doi: 10.1073/pnas.0507417102. [DOI] [PMC free article] [PubMed] [Google Scholar]