Summary
Decapping represents a critical control point in regulating expression of protein coding genes. Here we demonstrate that decapping also modulates expression of long non-coding RNAs (lncRNAs). Specifically, levels of >100 lncRNAs in yeast are controlled by decapping and are degraded by a pathway that occurs independent of decapping regulators. We find many lncRNAs degraded by DCP2 are expressed proximal to inducible genes. Of these, we show several genes required for galactose utilization are associated with lncRNAs that have expression patterns inversely correlated with their mRNA counterpart. Moreover, decapping of these lncRNAs is critical for rapid and robust induction of GAL gene expression. Failure to destabilize a lncRNA known to exert repressive histone modifications results in perpetuation of a repressive chromatin state that contributes to reduced plasticity of gene activation. We propose that decapping and lncRNA degradation serve a vital role in transcriptional regulation specifically at inducible genes.
Introduction
Gene transcription in the nucleus and degradation in the cytoplasm together dictate the level of messenger RNA (mRNA) that is available as a template for protein synthesis. mRNA turnover, therefore, represents a critical control point in regulating gene expression (Franks and Lykke-Andersen, 2008). In eukaryotes, mRNA decay is initiated by removal of the 3′ poly(A) tail (deadenylation) and typically followed by cleavage of the 5′ end 7-methyl-guanosine (m7G) cap and rapid 5′ → 3′ exonucleolytic degradation of the transcript body. Cleavage of the mRNA 5′ cap is catalyzed by a holoenzyme composed of the decapping proteins, DCP1 and DCP2, with DCP2 harboring a conserved NUDIX domain required for catalysis (Dunckley and Parker, 1999). mRNA decapping is regulated by a suite of activators, including DHH1, PAT1 and the LSM1-7 complex (Franks and Lykke-Andersen, 2008). While the role of decapping in controlling mRNA levels is well documented, the contribution of decapping in modulating the levels and function of other RNAs has been largely unexplored.
Eukaryotic genomes express a complex repertoire of RNA molecules that are not protein coding - thousands of which are classified as small non-coding RNAs (i.e. miRNA, siRNA, piRNA) or large non-coding RNAs (i.e. intergenic, antisense, and intronic) (Wilusz et al., 2009; Djuranovic et al., 2011). While some long non-coding RNA (lncRNA) transcripts may represent transcriptional “noise,” several lncRNAs have now been shown to have biological function as bona fide regulators of gene expression both transcriptionally as well as post-transcriptionally (Wilusz et al., 2009; Nagano and Fraser, 2011). Notwithstanding, our understanding of the mechanisms and biological importance of lncRNAs is comparatively scant to that of small non-coding RNAs, which have been the recent focus of intense research (Djuranovic et al., 2011).
lncRNAs have been implicated in regulating a large array of processes in eukaryotic cells include gene imprinting, dosage compensation, cell cycle regulation, innate immunity, pluripotency, retrotransposon silencing, meiotic entry, and telomere length (Wilusz et al., 2009; Nagano and Fraser, 2011). Moreover, altered expression of lncRNAs has been linked to disease states such as cancer and neurological disorders (Qureshi et al., 2010; Tsai et al., 2011). Regulation of gene expression by lncRNAs can be mediated at the level of transcription by interference with mRNA expression, competition at genomic loci for transcription factors, or chromatin remodeling (Berretta and Morillon, 2009; Wilusz et al., 2009). Post-transcriptionally, lncRNAs influence pre-mRNA splicing, nuclear trafficking, and mRNA degradation (Wilusz et al., 2009; Nagano and Fraser, 2011; Gong and Maquat, 2011). Based on the emerging emphasis of lncRNAs on regulating gene expression, the metabolism of the lncRNA itself will likely be a vital aspect of its function.
Similar to most mRNAs transcribed by RNA polymerase II, lncRNAs are both capped and polyadenylated (Berretta and Morillon, 2009; Khalil et al., 2009; Guttman et al., 2009). We, therefore, set out to evaluate if the decapping enzyme, DCP2, and its associated factors play a role in lncRNA metabolism and whether lncRNA turnover impinges on the ability of lncRNAs to regulate gene expression. Using RNA-sequencing to profile transcriptome-wide expression patterns, we determined that over 100 lncRNAs are elevated in cells lacking RNA decapping activity. Importantly, decapping of lncRNA occurs independently of all known regulators of the decapping holoenzyme, and thus represents a unique pathway for RNA turnover. Our study reveals that lncRNAs are often found proximal to inducible genes, and degradation of a lncRNA is required for proper induction of genes involved in galactose metabolism. We propose that lncRNAs are used as a means to tightly maintain repression at inducible genes and that efficient clearance of the lncRNA by DCP2-dependent decapping is vital for robust gene activation.
Results
lncRNAs accumulate when DCP2-dependent decapping is blocked
RNA polymerase II transcribes a large number of lncRNAs that are predicted to receive a 5′ m7G cap structure co-transcriptionally (Berretta and Morillon, 2009; Bentley, 2005). We anticipated that decapping might, therefore, play an important role in modulating abundance and perhaps biological activity of lncRNAs. We monitored the contribution of decapping to global lncRNA levels in budding yeast by high-throughput RNA-sequencing (RNA-Seq). Total RNA was isolated from wild-type (WT) cells and a strain lacking the catalytic subunit of the decapping enzyme (i.e. dcp2Δ), and cDNA libraries were prepared for analysis by the Illumina sequencing platform (see Materials and Methods). Notably, RNA was not subjected to poly(A)+ selection since, without decapping, mRNA (and perhaps lncRNA) accumulate as a deadenylated (i.e. poly(A)−) species. (Dunckley and Parker, 1999). In addition, cDNA libraries were constructed from RNA with strand-specific information retained (see Materials and Methods). RNA-Seq analysis resulted in 84.4 million and 61.2 million mappable reads from WT and dcp2Δ libraries, respectively. Of these, 5.2 million WT and 5.5 million dcp2Δ reads mapped to non-ribosomal loci.
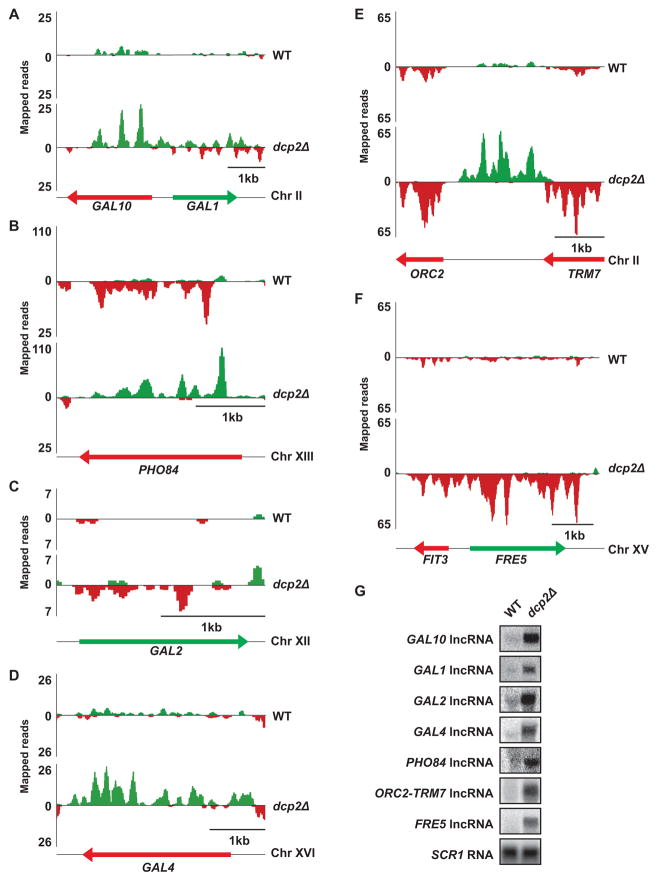
Consistent with our prediction that lncRNAs would be substrates for decapping, we observed a dramatic elevation in the level of several previously characterized lncRNAs in decapping-deficient cells compared to WT (Fig 1A, B, and Table S1). Moreover, our analysis identified approximately 100 putative and previously unannotated lncRNAs based on their accumulation in dcp2Δ cells (Fig 1C–F and Table S2). Notably, several of the putative lncRNAs we identified were predicted in previous studies interrogating the yeast transcriptome, but remain uncharacterized (Nagalakshmi et al., 2008; Xu et al., 2009; Yassour et al., 2010; van Dijk et al., 2011).
Figure 1. lncRNA transcripts accumulate when decapping-dependent decay is blocked.
(A–F) Total RNA from WT and dcp2Δ cells was prepared for strand-specific RNA-Seq. Mapped reads are displayed with diagrams of each locus. Color corresponds to the strand of origin (Watson, green; Crick, red) and arrows indicate direction of orientation 5′ to 3′. (A) GAL10-GAL1, (B) PHO84, (C) GAL2, (D) GAL4, (E) ORC2-TRM7, and (F) FRE5. (G) lncRNAs at the GAL10, GAL1, GAL2, GAL4, PHO84, ORC2-TRM7, and FRE5 loci were confirmed by Northern analysis. SCR1 RNA, a RNA polymerase III transcript, is the loading control (see also Table S1 and S2).
In general, lncRNAs that accumulated in dcp2Δ cells mapped to three types of genomic loci: i) intergenic regions between previously annotated protein-coding genes; ii) locations proximal to telomeres; and, iii) antisense to either the 5′ end or the entire length of known protein-coding genes (Table S2). Unexpectedly, the majority DCP2-sensitive lncRNAs map proximal to genes that could be grouped into specific biological pathways. These pathways include, but are not limited to, iron sensing (i.e. FRE1, FRE5, and FRE7), glucose usage (i.e. HXT5, HXT8, HXT10, and RGS2), maltose metabolism (i.e. MAL11, MAL12, MAL13, and MAL32), flocculation (i.e. FLO5, FLO9, FLO10, and FLO11), inorganic phosphate uptake and utilization (i.e. PHO5 and PHO84), and galactose utilization (i.e. GAL1, GAL10, GAL2, and GAL4). Importantly, most genes within this subset were repressed, and therefore not transcriptionally active, under the conditions assayed in our RNA-seq analysis (Table S3). Their expression is, however, induced by specific environmental cues, which suggests that a large proportion of these lncRNAs map to highly regulated genes.
We analyzed RNA by Northern blot to confirm that the steady-state levels of seven of our identified lncRNAs were indeed elevated in dcp2Δ cells compared to WT. Specifically, lncRNAs that map antisense to GAL10, GAL1, GAL2, GAL4, PHO84, and FRE5 genes were dramatically increased in decapping-deficient cells (Fig 1G, WT vs dcp2Δ). Similarly, a lncRNA mapping intergenic to the OCR2 and TRM7 genes was poorly expressed in WT cells but accumulated in dcp2Δ mutants (Fig 1G).
Our analyses in dcp2Δ cells confirm decapping modulates levels of lncRNAs. Recently, lncRNAs termed XUTs were identified based on their sensitivity to XRN1, a cytoplasmic 5′ → 3′ exonuclease implicated in degrading decapped mRNA (van Dijk et al., 2011). Consistent with the requirement for removal of the 5′ m7Gpp cap before RNA degradation by XRN1 (Stevens and Poole, 1995), 70% of the lncRNAs upregulated in dcp2Δ cells were also classified as XUTs (van Dijk et al., 2011). Interestingly, 30% of lncRNAs we identified were not identified as XUTs (Table S2). It is unclear whether this discrepancy represents differences in annotation of RNA-Seq data or whether some lncRNAs are degraded by an alternative pathway. Indeed, in eukaryotes there are two 5′ → 3′ exonucleases (i.e. XRN1 and RAT1), both of which act downstream of RNA decapping to degrade RNA.
lncRNA levels are regulated by DCP2
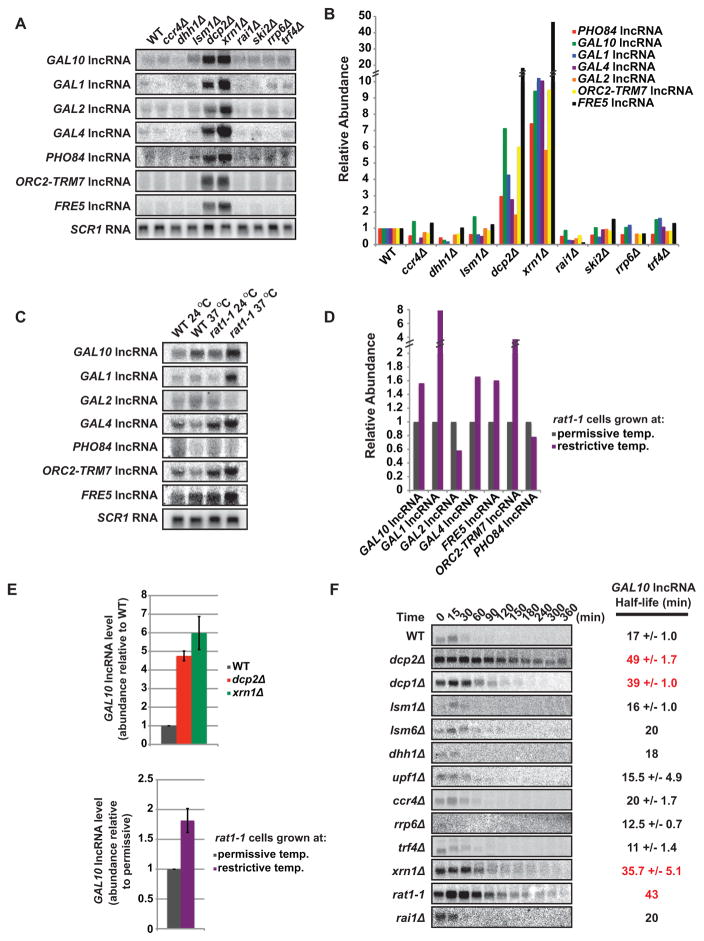
Our observation that lncRNAs are sensitive to decapping, prompted us to evaluate whether they are also modulated by additional proteins implicated in mRNA turnover. Degradation of cytoplasmic mRNA is initiated by removal of the 3′ poly(A) tail by the CCR4-NOT deadenylase, and is followed by decapping catalyzed by the DCP1/DCP2 holoenzyme and 5′ → 3′ exonucleolytic degradation by XRN1. Additional factors, including DHH1, PAT1, and LSM1-7, play an important role in mRNA stability as activators of decapping (Franks and Lykke-Andersen, 2008; Fig S1). We performed Northern blot analysis on RNA isolated from WT or cells lacking an activity important for mRNA turnover. As shown in Fig 2A and B, RNA levels for seven lncRNAs elevated in DCP2-deficient cells (Fig 1G) were also increased in cells lacking XRN1 (5 to 47-fold). Consistent with our Northern analysis, qRT-PCR of the GAL10 lncRNA confirmed its accumulation in dcp2Δ and xrn1Δ cells compared to WT (4.8 and 6-fold respectively, Fig 2E upper panel). Surprisingly, lncRNA levels were unchanged in cells lacking either deadenylase activity (i.e. ccr4Δ) or regulators of mRNA decapping (i.e. dhh1Δ and lsm1Δ; Fig 2A and B). Additionally, inactivation of either nuclear or cytoplasmic 3′ → 5′ exosome activity (i.e. rrp6Δ, trf4Δ, and ski2Δ) failed to result in elevated levels of these lncRNAs (Fig 2A and B). The abundance, therefore, of lncRNAs we identified as decapping substrates, unlike mRNAs, is unaffected by deadenylation, 3′ → 5′ degradation, and, most unexpectedly, proteins required for activating mRNA decapping.
Figure 2. lncRNAs stability is DCP2 dependent.
(A–E) Cells were grown in the presence of glucose and total RNA from WT and RNA decay mutant cells were analyzed by Northern and qRT-PCR. SCR1 RNA (Northerns) and U1 RNA (qRT-PCR) is the loading control. (A–D) Northern analysis probing for GAL10, GAL1, GAL2, GAL4, PHO84, ORC2-TRM7, and FRE5. (B) Quantification of (A) displaying abundance relative to WT with values normalized to SCR1 RNA. (C) WT and rat1-1 cells were grown at permissive temperature (24 °C) then shifted to restrictive temperature (37 °C) for 2 hours. (D) Quantification of (C) displaying the lncRNA increase in rat1-1 cells with values normalized to SCR1 RNA. (E) qRT-PCR analysis of relative GAL10 lncRNA abundance (top panel) WT, dcp2Δ and xrn1Δ cells (bottom panel) rat1-1 cells comparing restrictive and permissive temperature. Error bars represent standard deviation between three experiments. (F) Northern analysis of GAL10 lncRNA half-life analysis in RNA decay defective cells. Time points were taken after transcriptional shutoff (shift from raffinose to galactose growth) and half-lives were determined after normalization to SCR1 RNA (see also Figure S1, S2).
Eukaryotic cells possess two enzymes catalyzing 5′ → 3′ exonucleolytic degradation, XRN1 and RAT1, which are predominantly present in the cytoplasm and nucleus, respectively. To determine whether RAT1 plays a role in modulating lncRNA levels, we used a temperature-sensitive allele of the essential RAT1 gene, rat1-1, that abrogates RAT1 function at restrictive temperature of 37 °C (Amberg et al., 1992). WT and rat1-1 cells were grown at permissive temperature and shifted to 37 °C for two hours before harvesting and isolating RNA. Northern analysis determined several but not all lncRNAs elevated in dcp2Δ cells also accumulated when RAT1 was inactivated (Fig 2C and D). qRT-PCR analysis of the GAL10 lncRNA, confirmed these findings (Fig 2E lower panel). These observations suggest that nuclear 5′ → 3′ exonucleolytic digestion by RAT1 contributes to lncRNA decay.
lncRNA degradation is regulated by a distinct decapping pathway
Steady-state accumulation of lncRNAs in the absence of DCP2, XRN1 or RAT1 strongly implies a role in their degradation. To demonstrate direct involvement of these proteins in mediating lncRNA turnover, kinetic analysis of RNA degradation was performed. We specifically focused on the GAL10 lncRNA because its transcription is regulated by the sugar in the growth media (Houseley et al., 2008). Cells grown in raffinose, where the GAL10 lncRNA is transcriptionally active, were shifted to galactose-containing media, which represses GAL10 lncRNA transcription. RNA isolated from cells at various times after galactose addition was analyzed by Northern blot to evaluate changes in GAL10 lncRNA levels over time and determine lncRNA half-life. As seen in Figure 2F, the half-life of GAL10 lncRNA in WT cells was 17 minutes. In contrast, GAL10 lncRNA stability increased to 49 minutes in dcp2Δ cells, demonstrating that DCP2 is directly involved in the turnover of this lncRNA. Stabilization of GAL10 lncRNA is dependent DCP2’s pyrophosphatase activity (Fig S2B and C). In cells lacking the noncatalytic subunit of the decapping enzyme, DCP1 (dcp1Δ), GAL10 lncRNA were also stabilized with a half-life of 39 minutes. This result is consistent with observations that DCP1 is required for decapping activity both in vivo and in vitro and that the two proteins constitute a holoenzyme (Steiger et al., 2003). Moreover, as expected from steady-state analysis, GAL10 lncRNA was stabilized in cells lacking XRN1 (xrn1Δ; half-life of 36 minutes), but unaffected by inactivation of mRNA decapping activators (lsm1Δ, lsm6Δ, dhh1Δ), the deadenylase complex (ccr4Δ), nonsense-mediated mRNA decay (upf1Δ), the nuclear exosome (rrp6Δ), and the TRAMP complex (trf4Δ) (Fig 2F).
We also evaluated the role of the nuclear 5′ → 3′ exonuclease RAT1 in GAL10 lncRNA stability. Transcriptional shut-off analysis of GAL10 lncRNA in rat1-1 cells at the permissive temperature, where RAT1 function is impaired but not completely abrogated, demonstrated that RAT1 plays an important role in its stability (half-life of 42 min; Fig 2F). In contrast, RAI1, a known cofactor of RAT1 that itself has pyrophosphatase activity (Jiao et al., 2010), did not impact either the steady-state level or stability of GAL10 lncRNA (Fig 2A and F). Taken together our data demonstrate that GAL10 lncRNA turnover is mediated by DCP2, DCP1, XRN1 and RAT1, but not other proteins implicated in decay of mRNA, and therefore its metabolism involves a distinct decay pathway. Given the steady-state data for a number of lncRNAs (Fig 2A), we predict that this decay pathway is used to degrade many, if not most, lncRNAs in the cell. Moreover, these observations support the existence of two separate, yet partially redundant pathways for decay of lncRNAs - a cytoplasmic, XRN1-dependent pathway and a nuclear, RAT1-dependent pathway. Considering that decapping is required to generate a substrate for both 5′ → 3′ exonuclease enzymes (i.e. XRN1 or RAT1), DCP2 constitutes a critical regulator in lncRNA metabolism that likely functions in both the cytoplasm and nucleus based on observations that DCP2 can shuttle (Grousl et al., 2009).
GAL lncRNA expression is regulated by environmental conditions
GAL-inducible gene regulation represents a classic genetic switch regulating sugar metabolism in eukaryotic cells (Lohr et al., 1995). The GAL system has been extensively characterized in yeast and consists of four structural genes, GAL1, GAL10, GAL7 and GAL2, that are coordinately regulated at the level of transcription by GAL4, GAL80, and GAL3. In repressed or non-induced conditions (glucose and raffinose sugar sources, respectively), GAL80 inhibits the ability of GAL4 to recruit the transcription machinery to drive expression of the structural genes. In the presence of galactose, GAL3 sequesters GAL80 in the cytoplasm, thus allowing robust transcriptional activation of GAL1, GAL10, GAL7 and GAL2 by GAL4.
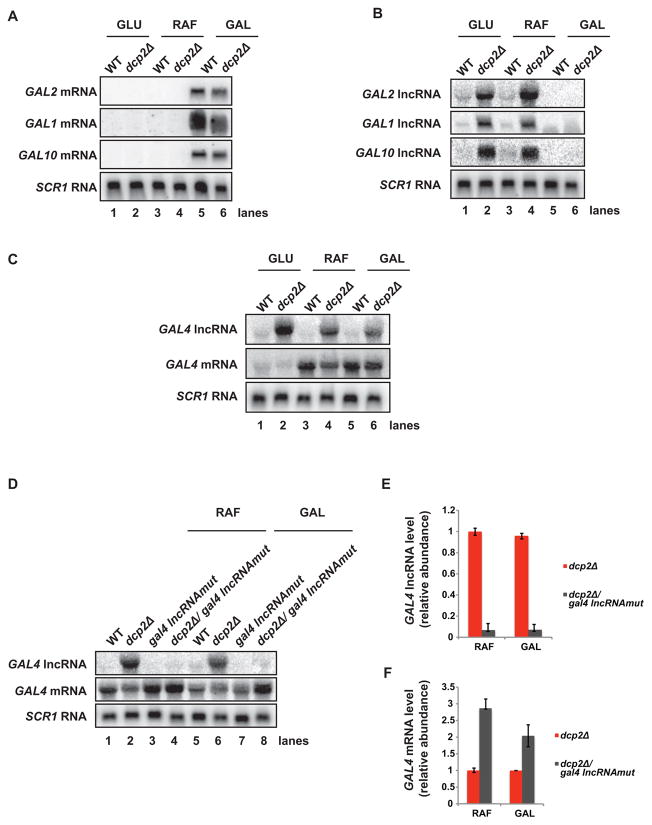
We were interested in determining if GAL lncRNAs, like their mRNA counterparts, exhibit expression patterns in response to sugar availability. To this end, we grew WT and dcp2Δ cells under conditions where GAL gene transcription is either repressed (glucose), non-induced (raffinose), or induced (galactose) with respect to the mRNA at these loci (Fig 3A), and analyzed lncRNA levels by Northern blot. In WT cells grown in glucose, GAL2, GAL1 and GAL10 lncRNAs are present at very low or undetectable levels (Fig 3B, lane 1), consistent with our RNA-Seq data and previous reports characterizing GAL10 lncRNA expression (Fig 1A, 1C, 2A, and 2B; Houseley et al., 2008). Similarly, low levels of these lncRNA were observed for WT cells grown in raffinose or galactose (Fig 3B, lanes 3 and 5). GAL lncRNAs from cells lacking DCP2 were significantly elevated in glucose grown cells (Fig 3B, lane 2), in agreement with their detection by RNA-Seq. These lncRNA levels were also elevated in raffinose-grown cells (Fig 3B, lane 4), but not in cells grown in galactose (Fig 3B, lane 6). Critically, GAL lncRNAs fail to accumulate in cells where GAL gene expression is induced, despite the absence of destabilizing DCP2 activity, indicating a reciprocal pattern of expression and suggesting a role for these lncRNAs in regulating their cognate protein-coding genes (see below).
Figure 3. GAL lncRNA expression is regulated by environmental conditions and lncRNA levels influence mRNA expression.
(A–D) Cells were grown in glucose (GLU), raffinose (RAF), or galactose (GAL) and total RNA was analyzed by Northern analysis probing for (A) mRNAs at GAL-inducible loci (GAL2, GAL1, and GAL10), (B) lncRNAs at GAL-inducible loci (GAL2, GAL1, and GAL10), and (C) lncRNA and mRNA at the GAL4 locus. (D)GAL4 lncRNA expression was attenuated and levels of GAL4 lncRNA and mRNA were determined by Northern analysis in dcp2Δ cells in raffinose (RAF) and galactose (GAL). (E and F) Relative fold changes between dcp2Δ and dcp2Δ/gal4 lncRNAmut cells for (E) GAL4 lncRNA and (F) GAL4 mRNA are displayed with values normalized to SCR1 RNA. Error bars represent standard deviation between three experiments.
GAL4 lncRNA influences expression of GAL4 mRNA
Our RNA-Seq data indicated a lncRNA expressed antisense to the GAL4 locus (Fig 1D), and we were interested in determining if the GAL4 lncRNA also displayed reciprocal expression patterns with regards to its cognate mRNA. Importantly, GAL4, the transcriptional activator of GAL-inducible genes, while subject to glucose repression, is itself not induced by galactose (Lohr et al., 1995). We determined that, similar to GAL2, GAL1 and GAL10 lncRNAs, GAL4 lncRNA levels were low in WT cells grown under all conditions tested (Fig 3C, lanes 1, 3, and 5). Abrogation of decapping activity resulted in robust accumulation of GAL4 lncRNA in glucose-grown cells as expected (Fig 3C, lane 2), but also led to elevated levels in raffinose and galactose-grown cells, where GAL4 mRNA is also expressed (Fig 3C, lanes 4 and 6). GAL4 mRNA and lncRNA do not, therefore, show an inverse expression pattern. GAL4 mRNA levels were, however, reduced in decapping-deficient cells where GAL4 lncRNA accumulated (dcp2Δ; Fig 3C, lanes 4 and 6), suggesting that GAL4 lncRNA may impair GAL4 mRNA expression.
To evaluate whether GAL4 lncRNA impinges upon GAL4 mRNA levels, we attenuated GAL4 lncRNA expression by introducing mutations within the region of its promoter (see Methods and Materials). WT and dcp2Δ cells in which GAL4 lncRNA was either present or absent (gal4 lncRNAmut) were grown under conditions where GAL4 mRNA is expressed (i.e. raffinose or galactose-containing media) to determine the influence of GAL4 lncRNA on GAL4 mRNA expression. Importantly, GAL4 lncRNA levels were reduced greater than 90% in dcp2Δ cells harboring the gal4 lncRNAmut mutation (Fig 3D and 3E). Critically, in the absence of GAL4 lncRNA, GAL4 mRNA levels increased 2–3 fold in dcp2Δ cells grown in either condition (Fig 3D and 3F, lanes 2 vs 4; lanes 6 vs 8). Our data indicate that GAL4 lncRNA levels regulate expression of GAL4 mRNA.
Decapping of a lncRNA at the GAL10-GAL1 locus is required for efficient activation upon galactose addition
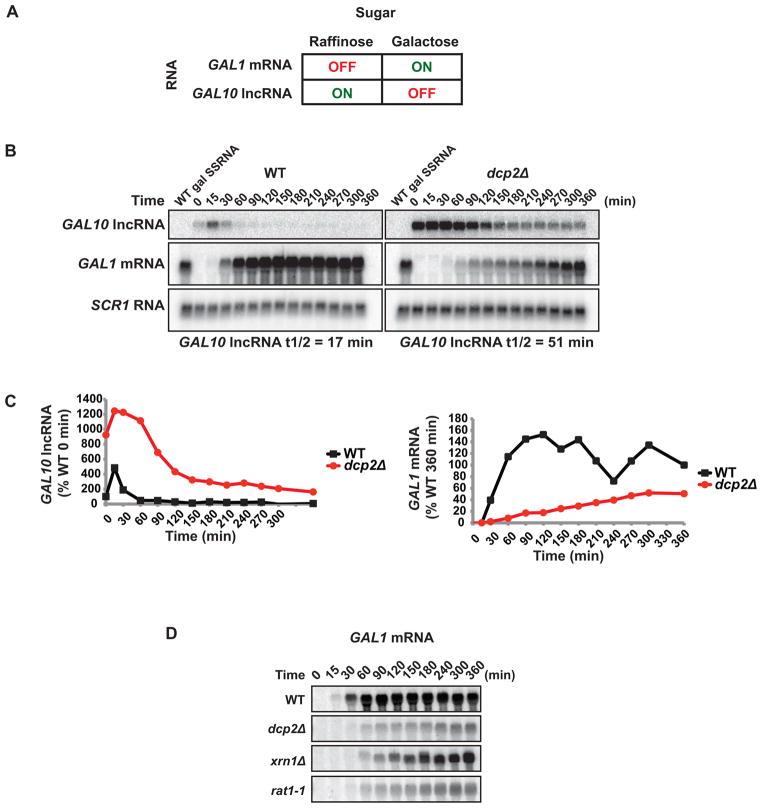
We observed that in dcp2Δ cells several GAL lncRNAs accumulate in cells grown in glucose or raffinose, but fail to accumulate under conditions where GAL mRNAs are expressed (i.e. in the presence of galactose; Fig 3A and B). We hypothesized that GAL lncRNAs are absent in cells grown in galactose because their presence would impinge upon the transcriptional induction of GAL structural genes. GAL lncRNAs would, therefore, need to be rapidly removed from the cell upon an environmental shift from transcriptionally inactive to active conditions. We evaluated whether the GAL10 lncRNA, which spans both GAL10 and GAL1 gene loci, influenced transcriptional activation of GAL1 mRNA upon induction by galactose. Prior to induction, cells were grown in raffinose, non-inducing conditions for GAL1 mRNA, and samples were removed over time following galactose addition. In WT cells, GAL10 lncRNA was present at low levels and decayed quickly upon addition of galactose (half-life = 17 minutes; Fig. 4B). Consistent with reciprocal expression patterns, GAL1 mRNA levels accumulated quickly in these cells and attained maximum levels after 90 min (Fig 4B and C). In cells lacking decapping activity, GAL10 lncRNA persisted for hours following addition of galactose (half-life = 51 minutes), and induction of GAL1 mRNA was significantly delayed (Fig 4B and C). Importantly, however, decay of GAL10 lncRNA strongly correlated with GAL1 mRNA induction in both WT and dcp2Δ cells (Fig 4B and C). This demonstrates that degradation of GAL10 lncRNA through decapping plays an important role in the cells ability to respond to and utilize galactose as a sugar source. Consistent with this, dcp2Δ cells display a pronounced growth defect on galactose containing media (Fig S3).
Figure 4. Decapping of a lncRNA at the GAL10-GAL1 locus is required for efficient activation upon galactose addition.
(A) Schematic of GAL10 lncRNA and GAL1 mRNA expression in raffinose and galactose. (B) Northern analysis of WT and dcp2Δ cells grown in raffinose then shifted to galactose. GAL10 lncRNA decay and GAL1 mRNA accumulation were measured over time with SCR1 RNA as the loading control. (C) Quantification of band intensities of (B) normalized to SCR1 RNA. GAL10 lncRNA levels are represented as a percentage of WT 0 min and GAL1 mRNA levels are represented as a percentage of WT 360 min time points. (D) Northern analysis of WT, dcp2Δ, xrn1Δ and rat1-1 cells grown as in (B) following GAL1 mRNA accumulation (see also Figure S3).
Degradation of GAL10 lncRNA requires RAT1 for efficient induction of GAL1 mRNA
We have demonstrated that GAL10 lncRNA is stabilized in the absence of DCP2-dependent decapping and downstream XRN1 or RAT1-dependent 5′ → 3′ exonucleolytic degradation (Fig 2F). Since RAT1 and XRN1 are localized in distinct cellular compartments, and function in nuclear and cytoplasmic RNA degradation, respectively, we evaluated whether either exonuclease was required for proper induction of GAL1 mRNA expression. GAL1 mRNA induction upon addition of galactose to the media was impaired in cells lacking XRN1, but not to the extent observed in dcp2Δ cells (Fig 4D and E). In contrast, cells only partially active for RAT1 (rat1-1 cells grown at permissive temperature) demonstrated a significant delay in induction of GAL1 mRNA similar to cells lacking decapping activity. These observations suggest a major role for nuclear 5′ → 3′ exonucleolytic degradation in regulating the function of GAL10 lncRNA.
Decapping influences chromatin at the GAL10-GAL1 locus
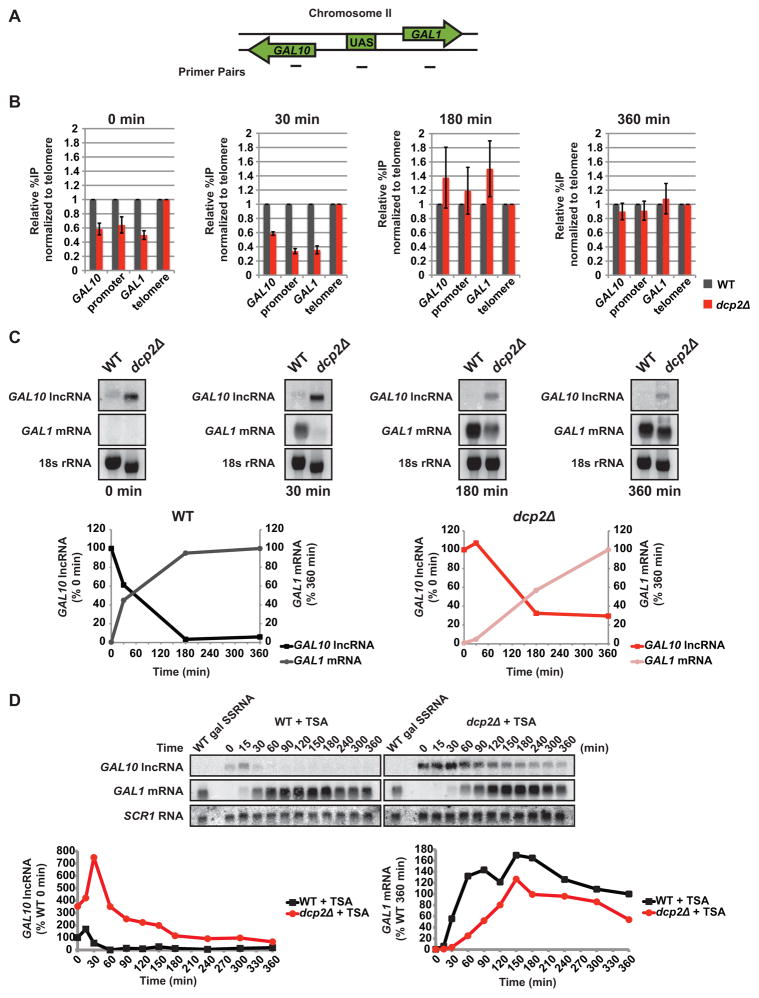
We find that failure to destabilize GAL10 lncRNA by decapping (or nuclear 5′ → 3′ decay) negatively influences induction of GAL1 mRNA in response to galactose. Expression of GAL10 lncRNA has been shown to exert transcriptional repression through methylation of histone H3 at lysine 4 and 36 (H3K4 and H3K36) and, ultimately, deacetylation of the GAL10-GAL1 locus (Houseley et al., 2008; Pinskaya et al., 2009). The influence of GAL10 lncRNA turnover on its function in chromatin remodeling, however, has not been addressed. In cells lacking decapping activity, GAL10 lncRNA is stabilized and we predicted that chromatin surrounding the locus should be hypoacetylated. Moreover, we hypothesized that failure to rapidly degrade GAL10 lncRNA in decapping-deficient cells would correlate with delayed resolution of this hypoacetylated state upon a shift in conditions that transcriptionally activate GAL1 mRNA. We performed chromatin immunoprecipitation (ChIP) followed by quantitative PCR (qPCR) to evaluate the acetylation status at the GAL10-GAL1 locus in WT and dcp2Δ cells. Importantly, acetylation was surveyed over time after cells were shifted from non-inducing to inducing growth conditions (i.e. raffinose to galactose). Acetylation levels were determined based on the percentage of immunoprecipitated DNA normalized against a telomeric region and represented relative to WT.
Acetylation of histone H3 lysine 18 (H3K18) at the GAL10-GAL1 locus (Fig 5A) was reduced in dcp2Δ cells compared to WT cells before galactose addition (Fig 5B; 0 min), indicative of hypoacetylation within this region when GAL10 lncRNA levels are high, as anticipated. We predicted that elimination of GAL10 lncRNA would be required to resolve the repressive chromatin state, and measured H3K18 acetylation at various times after addition of galactose. After 30 minutes post-induction, when GAL10 lncRNA is absent in WT cells but still at high levels in cells lacking DCP2 (Fig 5C), chromatin at the GAL10-GAL1 locus remains hypoacetylated in dcp2Δ cells compared to WT (Fig 5B; 30 min). At 180 minutes when a only paucity of GAL10 lncRNA is present in either WT or dcp2Δ cells (Fig 5C), the locus is no longer hypoacetylated with H3K18 levels similar in both cell types (Fig 5B). Consistent with an attenuation in lncRNA repression, GAL1 mRNA levels in dcp2Δ cells have begun to accumulate (Fig 5C; 180 min). By 360 minutes, H3K18 acetylation between WT and dcp2Δ cells are indistinguishable and GAL1 mRNA levels in dcp2Δ cells are nearing WT (Fig 5B and C). The observed changes in chromatin state at the GAL10-GAL1 locus over time correlate with changing GAL10 lncRNA and GAL1 mRNA levels in the two cell types. As shown in Fig 5C, GAL10 lncRNA levels must fall below a critical threshold (~50%) before productive GAL1 mRNA accumulation occurs. Importantly, in WT this threshold is met approximately 30 minutes after induction, while in dcp2Δ cells this threshold is not achieved until after 2 hours.
Figure 5. Decapping influences chromatin at the GAL10-GAL1 locus.
(A) Diagram of ChIP primer set positions at the GAL10-GAL1 locus. (B) Cells were grown as in Fig 4B and aliquots were crosslinked at 0, 30, 180, and 360 minutes of galactose exposure. Co-immunoprecipitated DNA from ChIP against acetylated H3K18 was amplified by qPCR. Acetylation relative to WT is displayed as a percentage of input normalized to a telomeric location. (C) Cells were grown as in Fig 4B and RNA was analyzed by Northern probing for GAL10 lncRNA and GAL1 mRNA with 18S RNA ethidium stain as the loading control. RNA levels for WT and dcp2Δ were plotted with GAL10 lncRNA levels represented as a percentage of the 0 min and GAL1 mRNA levels as a percentage of the 360 min time points. (D) GAL1 mRNA expression was induced by galactose in cells grown in raffinose and 10 μM trichostatin A (TSA). GAL10 lncRNA decay and GAL1 mRNA accumulation was measured by Northern with SCR1 RNA as the loading control. GAL10 lncRNA levels are represented as a percentage of WT 0 min and GAL1 mRNA levels as a percentage of WT 360 min time points.
To directly test whether the repressive chromatin state induced by GAL10 lncRNA in dcp2Δ cells causes the reduced kinetics of GAL1 mRNA induction, we analyzed GAL1 mRNA induction in cultures containing trichostatin A (TSA), a selective inhibitor of class I and II histone deacetylases (Codd et al., 2009). Importantly, RPD3, the histone deacetylase implicated in GAL10 lncRNA repression is sensitive to TSA (Houseley et al., 2008; Pinskaya et al., 2009; Bernstein et al., 2000). Northern blot analysis indicated that TSA did not significantly influence the decay rate of GAL10 lncRNA in dcp2Δ cells (versus untreated cells; Fig 5D vs Fig 4B and C). Induction of GAL1 mRNA, however, was substantially improved compared to untreated cells (Fig 5D vs Fig 4B and C), and followed kinetics closer to those observed in WT cells (Fig 5D). These results indicate that destabilization of GAL10 lncRNA is important for alleviating its function in histone deacetylation that impinge upon expression of the proximal GAL1 gene.
DCP2 mediates GAL1 mRNA expression through destabilization of lncRNAs
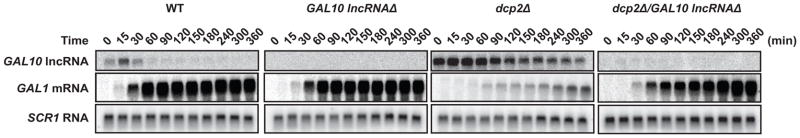
Collectively our data suggest that the decapping enzyme, DCP2, mediates decapping of GAL10 lncRNA and that proper metabolism of the lncRNA has a direct impact on transcriptional activation of GAL1 mRNA. If GAL10 lncRNA stabilization in decapping-deficient cells causes impaired rates of GAL1 mRNA induction, then we would expect that removal of this lncRNA in dcp2Δ cells would result in increased rates of GAL1 mRNA accumulation. To test this hypothesis directly, we introduced mutations in the GAL10 lncRNA promoter that block GAL10 lncRNA expression (Houseley et al., 2008), and determined the impact on induction of GAL1 mRNA. As shown in Fig 6, these mutations completely abrogate expression of GAL10 lncRNA, even in cells where DCP2 is absent. Importantly, loss of GAL10 lncRNA accumulation in dcp2Δ cells resulted in robust and rapid induction of GAL1 mRNA upon addition of galactose to the growth media (Fig 6).
Figure 6. DCP2 mediates GAL1 mRNA expression through stabilization of lncRNAs.
GAL10 lncRNA was deleted by genetically removing the GAL10 gene and complementing GAL10 mRNA expression with a plasmid born copy with silent mutations that disrupt expression of GAL10 lncRNA. Cells were grown in raffinose and GAL1 mRNA expression was induced by shifting to galactose. GAL10 lncRNA decay and GAL1 mRNA accumulation were measured by Northern analysis. SCR1 RNA was the loading control.
Interestingly, while GAL1 mRNA induction was much higher in dcp2Δ/GAL10 lncRNAΔ cells compared dcp2Δ alone, restoration of GAL1 mRNA induction in dcp2Δ/GAL10 lncRNAΔ cells was still delayed compared to that observed in WT cells. We attribute this observation to indicate that repressive factors in addition to GAL10 lncRNA exist that regulate GAL1 mRNA induction. In agreement with this, we annotated a lncRNA expressed antisense to GAL1 based on our RNA-Seq data (GAL1 lncRNA, Fig 1A and G). Moreover, we have shown that expression of GAL4, the transcriptional activator of GAL1, is, in part, controlled by a lncRNA that is itself stabilized in dcp2Δ cells (Fig 3C and D), suggesting that reduced GAL4 expression may also contribute to less robust GAL1 mRNA induction. Together, we suggest that galactose utilization is regulated by a network of partially redundant lncRNAs, and that degradation by DCP2, RAT1, and to a lesser extent XRN1, is required to rapidly and robustly activate expression of GAL mRNAs.
Discussion
Decapping modulates lncRNA levels to regulate inducible genes
In recent years the complexity of the eukaryotic transcriptome has become a subject of intense curiosity as well as debate (Wilusz et al., 2009; Ponting and Belgard, 2010). It is now well established, however, that RNA polymerase II transcribes hundreds of lncRNAs, and that some of these function in such processes as transcriptional regulation, imprinting, and chromosome inactivation (Nagano and Fraser, 2011). The metabolism of these lncRNAs and the impact of turnover on their function has not, until recently, been extensively addressed.
We find that many of the lncRNAs we identified by RNA-Seq are expressed antisense to highly regulated genes whose transcription is responsive to a variety of environmental cues. Specifically, our lncRNAs map to genes involved in regulatory networks for glucose metabolism, maltose utilization, flocculation, iron sensing, meiosis, mating, and sporulation (Table S3). Interestingly, target genes of these pathways are transcriptionally repressed in the absence of stimulation, but are rapidly derepressed upon introduction of the effector (Campbell et al., 2008; Chow et al., 1989; Teunissen and Steensma, 1995; Shakoury-Elizeh et al., 2004).
Galactose utilization in yeast presents a classical paradigm for understanding gene regulation in eukaryotes (Lohr et al., 1995). Our analysis confirms a previously characterized lncRNA antisense to GAL10 (GAL10 lncRNA; Houseley et al., 2008) and reveals lncRNAs mapping antisense to structural genes GAL2 and GAL1, and to the master transcriptional regulator, GAL4. We have, therefore, taken advantage of the wealth of information and robust regulatory circuit underlying the GAL regulon to determine a role for decay in lncRNA function. We demonstrate that decapping of GAL4 lncRNA is required for GAL4 mRNA to reach levels observed in WT (Fig 3C and D). Moreover, transcriptional activation of GAL1 mRNA upon induction by galactose absolutely requires GAL10 lncRNA decapping for rapid and robust expression (Figure 4B).
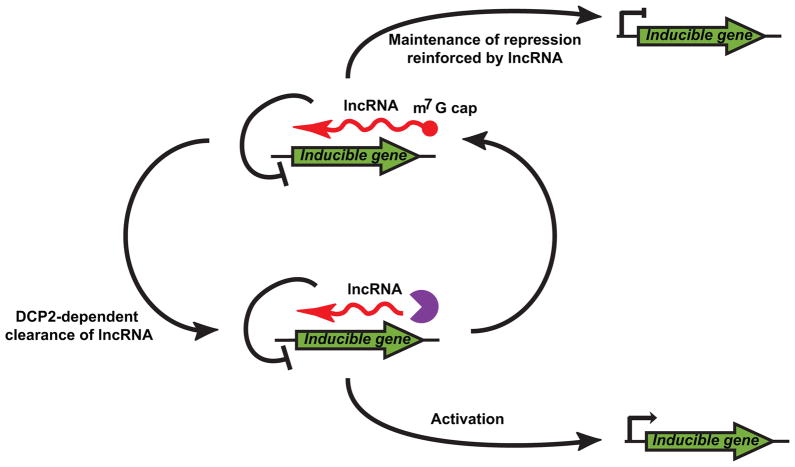
Our data provide the first evidence that GAL lncRNAs are degraded by a DCP2-dependent process and that decapping of lncRNAs is required for robust expression of their cognate protein-coding genes. We propose that, in yeast, regulatory pathways have evolved to include lncRNAs, and that these RNA species play a critical role in maintaining and/or reinforcing gene repression in the absence of activating signals (Fig 7). Moreover, upon stimulation, proper clearance of the lncRNA by decapping-dependent decay is required for rapid and robust gene activation. Our discovery of a network of regulatory GAL antisense lncRNAs adds an additional layer of complexity to this classic genetic switch and suggests that other inducible genes may also require lncRNA degradation for proper transcriptional regulation (Fig 7).
Figure 7. DCP2-dependent clearance of lncRNAs is required for rapid responses to environmental cues.
Inducible genes have evolved lncRNA regulators which are important for maintenance of inactive states. Upon stimulation DCP2 is required to clear these lncRNAs from the cell allowing for activation of inducible genes in response to stimulus (see also Table S3).
Mechanism of lncRNA-dependent transcriptional control and derepression by DCP2
An important and unresolved issue is the precise mechanism by which lncRNA degradation leads to derepression of lncRNA regulated genes. In yeast and metazoans, lncRNAs have been documented to function both in cis and trans to alter methylation and acetylation states of histones through association with chromatin-modifying complexes (Berretta and Morillon, 2009; Nagano and Fraser, 2011). Modulation of lncRNA levels either transcriptionally or by degradation would, therefore, be expected to significantly affect chromatin state and, consequently, expression of lncRNA-regulated genes. In the case of GAL10 lncRNA, transcription of the lncRNA has been suggested to recruit histone modifying enzymes to the C-terminal domain of RNA polymerase II co-transcriptionally which alter chromatin structure and create a chromatin environment that represses spurious transcription (Houseley et al., 2008). In this study, we demonstrate that destabilization of GAL10 lncRNA by DCP2 is essential in order to maintain a chromatin structure that is repressed yet poised for rapid activation.
GAL10 lncRNA regulates the expression of proximal mRNA genes by altering the acetylation status of histones associated with its own locus (Houseley et al., 2008, Fig 5B). We find that in cells deficient for decapping, the GAL10-GAL1 locus is hypoacetylated compared to wildtype, and that pharmacological inhibition of histone deacetylation bypasses GAL10 lncRNA-mediated repression and restores robust GAL1 mRNA induction to near WT levels (Fig 5D). These observations indicate that stabilization of GAL10 lncRNA represses induction of GAL1 mRNA expression through persistent deacetylation of histones.
It remains to be established if impaired induction of GAL1 mRNA expression by stabilized GAL10 lncRNA results from inhibition in cis or in trans. The initial characterization of the GAL10 lncRNA suggested that this lncRNA exerts its effects in cis because transcription of GAL10 lncRNA from one allele in heterozygous diploid cells, failed to downregulate GAL1 mRNA expression from the other allele (Houseley et al., 2008). Although in the absence of changes in transcription, it would be anticipated that elevated lncRNA levels would likely manifest their function in trans, we find no apparent reason to suggest that decapping of GAL10 lncRNA could not modulate its function in cis. Indeed, several lines of evidence place DCP2 as well as GAL10 lncRNA decapping in the right compartment of the cell (i.e. the nucleus) to modulate GAL10 lncRNA function in cis. First, although predominately cytoplasmic, DCP2 has been documented to shuttle and reside in both nuclear and cytoplasmic compartments of the cell (Grousl et al., 2009). Second, a decapping activity involved in turnover of nuclear retained pre-mRNA and mature mRNA has been characterized (Kufel et al., 2004). Third, the elevated level of GAL10 lncRNA in the absence of XRN1 has only a modest effect on GAL1 mRNA induction, while conditional loss of the nuclear exonuclease, RAT1, strongly impairs induction of GAL1 mRNA transcription (Fig 4D). Together, these observations support a model in which DCP2, in concert with RAT1, regulates the function of GAL10 lncRNA by promoting its decapping/destruction in the nucleus and perhaps at its site of transcription.
We envision two possible mechanistic models for how stabilization of GAL10 lncRNA by DCP2 (and RAT1) impairs galactose-induced GAL1 mRNA transcriptional activation. First, stabilization of the lncRNA promotes formation of an R-loop around the transcriptional bubble inhibiting transcriptional elongation, as recently described by Belotserkovskii and Hanawalt (2011). Considering that stabilization of the lncRNA exacerbates GAL10 lncRNA repression, the physical presence of lncRNA might be an important and currently unaccounted for aspect of its mechanism. An R-loop based model reconciles the observed cis-acting nature of GAL10 lncRNA repression (Houseley et al., 2008) with our observations that stabilization and perhaps the physical presence of the lncRNA influences function. Consistent with R-loop structures playing a role in lncRNA physiology, RNA/DNA hybrids have been implicated in the mechanism by which TERRA lncRNA stabilization causes telomere length defects (Luke et al., 2008). It is unclear, however, exactly how an RNA/DNA hybrid model would account for the observed GAL10 lncRNA-mediated histone deacetylation. In the second model, transcription of lncRNA is sufficient to impose a repressive chromatin environment but DCP2 and RAT1 dampen this effect by degrading lncRNA co-transcriptionally. Indeed, RAT1 is vital for termination of RNA polymerase II transcription by the “torpedo” model where mRNA cleavage and polyadenylation generates a downstream monophosphorylated product that is a substrate of RAT1 (Kawauchi et al., 2008). Since lncRNAs have 5′ m7G caps, co-transcriptional decapping by DCP2 would provide RAT1 access to nascent RNA and an opportunity to promote transcriptional termination. Limiting lncRNA transcription by this mechanism would circumvent the repressive alterations in chromatin architecture that result from GAL10 lncRNA transcription. An important next step will, therefore, be to determine precisely how the stability of lncRNAs alter transcriptional events.
Other roles of decapping in lncRNA function
Our observation that RNA decapping and 5′ exonucleolytic decay plays a major role in modulating the levels of lncRNA is consistent with a previous observation that SRG1 lncRNA (controlling expression of the serine biosynthetic gene, SER3) is also a substrate for DCP2 and XRN1 (Thompson and Parker, 2007). Importantly, SRG1 lncRNA turnover was also mediated by the auxiliary decay factors, DHH1 and UPF1. In contrast, none of the lncRNAs analyzed in this study were affected by loss of other factors involved in mRNA metabolism, including deadenylation, activation of decapping, or nuclear and cytoplasmic 3′ exonucleolytic decay (Fig 2A and B). This observation raises an important question regarding how lncRNAs are distinguished from mRNAs and targeted for a distinct decapping pathway. One obvious distinction between mRNA and lncRNAs is their association with the translation apparatus, and considering mRNA decay occurs co-translationally (Hu et al., 2009), a potential determinant in eliciting mRNA decapping rather than lncRNA decapping could be the act of translation itself.
Many of the lncRNAs identified by RNA-Seq that are sensitive to decapping are also substrates of XRN1 (van Dijk et al., 2011). Importantly, however, approximately 30% of lncRNAs that are elevated in cells lacking DCP2 (Table S2) do not appear to be substrates of XRN1 (i.e. XUTs). Although this observation may reflect differences in annotating RNA-Seq data, it also suggests that an alternative pathway exists for the degradation of these RNAs. Indeed, we show evidence that several lncRNAs are sensitive to degradation by RAT1. Differences in lncRNA metabolism likely represent differences in their subcellular distribution. Accordingly, we predict that XUTs and SRG1 lncRNA are predominantly present in the cytoplasm where they are subject to XRN1-mediated decay. In contrast, GAL10 lncRNA is both nuclear and cytoplasmic, since the decapped product is a substrate for both XRN1 and RAT1. Critically, stabilization of only the nuclear pool of GAL10 lncRNA led to an alteration in GAL1 mRNA induction (Fig 4D and 4E) indicating that degradation of the lncRNA in the correct cellular compartment is critical for its proper function. The presence of lncRNAs in the nucleus is consistent with their functional importance in chromatin organization and suggest that decapping can influence gene expression patterns much more broadly than in its characterized role in mRNA stability.
Experimental Procedures
All strains used are the BY4741 genetic background unless indicated, genotypes are listed in Table S4. Cells were grown at 24 °C into mid-log phase (3.0 × 107 cells ml−1) in standard synthetic medium (pH 6.5) with the appropriate amino acids supplemented and either 2% glucose, 2% raffinose, or 2% galactose. Cultures used for RNA-Seq were grown in glucose media. For RNA-Seq Library construction, libraries were prepared according to Illumina’s Directional mRNA-Seq Sample Prep Guide (Part # 15018460 Rev. A). Total RNA was isolated from yeast cells using standard methods. Chromatin immunoprecipitation was carried out using standard methods. All plasmids and oligos used are listed in Tables S5 and S6. See supplemental materials and methods for a detailed description of experimental procedures.
Supplementary Material
Highlights.
lncRNAs are degraded by a DCP2-dependent decapping pathway.
Many lncRNAs targeted to decapping are associated with inducible gene loci.
Decapping of lncRNAs is required for robust activation of GAL gene expression.
lncRNA degradation contributes plasticity to gene regulatory mechanisms.
Acknowledgments
We thank Dr. D. Tollervey for providing reagents and the Coller and Baker labs for discussion. This work was funded by NIH grant GM080465.
Footnotes
Accession Numbers
RNA-seq data are deposited in the NCBI Sequence Read Archive (accession number SRA048128.1)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amberg DC, Goldstein AL, Cole CN. Isolation and characterization of RAT1: an essential gene of Saccharomyces cerevisiae required for the efficient nucleocytoplasmic trafficking of mRNA. Genes Dev. 1992;6:1173–1189. doi: 10.1101/gad.6.7.1173. [DOI] [PubMed] [Google Scholar]
- Belotserkovskii BP, Hanawalt PC. Anchoring Nascent RNA to the DNA Template Could Interfere with Transcription. Biophys J. 2011;100:675–684. doi: 10.1016/j.bpj.2010.12.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley DL. Rules of engagement: co-transcriptional recruitment of pre-mRNA processing factors. Curr Opin Cell Biol. 2005;17:251–256. doi: 10.1016/j.ceb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Tong JK, Schreiber SL. Genome wide studies of histone deacetylase function in yeast. P Natl Acad Sci USA. 2000;97:13708–13713. doi: 10.1073/pnas.250477697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta J, Morillon A. Pervasive transcription constitutes a new level of eukaryotic genome regulation. EMBO Rep. 2009;10:973–982. doi: 10.1038/embor.2009.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RN, Leverentz MK, Ryan LA, Reece RJ. Metabolic control of transcription: paradigms and lessons from Saccharomyces cerevisiae. Biochem J. 2008;414:177–187. doi: 10.1042/BJ20080923. [DOI] [PubMed] [Google Scholar]
- Chow TH, Sollitti P, Marmur J. Structure of the multigene family of MAL loci in Saccharomyces. Mol Gen Genet. 1989;217:60–69. doi: 10.1007/BF00330943. [DOI] [PubMed] [Google Scholar]
- Codd R, Braich N, Liu J, Soe CZ, Pakchung AAH. Zn(II)-dependent histone deacetylase inhibitors: Suberoylanilide hydroxamic acid and trichostatin A. Int J Biochem Cell B. 2009;41:736–739. doi: 10.1016/j.biocel.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Djuranovic S, Nahvi A, Green R. A Parsimonious Model for Gene Regulation by miRNAs. Science. 2011;331:550–553. doi: 10.1126/science.1191138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunckley T, Parker R. The DCP2 protein is required for mRNA decapping in Saccharomyces cerevisiae and contains a functional MutT motif. EMBO J. 1999;18:5411–5422. doi: 10.1093/emboj/18.19.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks TM, Lykke-Andersen J. The control of mRNA decapping and P-body formation. Mol Cell. 2008;32:605–615. doi: 10.1016/j.molcel.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong CG, Maquat LE. lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3′ UTRs via Alu elements. Nature. 2011;470:284–288. doi: 10.1038/nature09701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grousl T, Ivanov P, Frydlova I, Vasicova P, Janda F, Vojtova J, Malinska K, Malcova I, Novakova L, Janoskova D, et al. Robust heat shock induces eIF2 alpha-phosphorylation-independent assembly of stress granules containing eIF3 and 40S ribosomal subunits in budding yeast, Saccharomyces cerevisiae. Journal of Cell Science. 2009;122:2078–2088. doi: 10.1242/jcs.045104. [DOI] [PubMed] [Google Scholar]
- Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houseley J, Rubbi L, Grunstein M, Tollervey D, Vogelauer M. A ncRNA modulates histone modification and mRNA induction in the yeast GAL gene cluster. Mol Cell. 2008;32:685–695. doi: 10.1016/j.molcel.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Sweet T, Chamnongpol S, Baker KE, Coller J. Co-translational mRNA decay in Saccharomyces cerevisiae. Nature. 2009;461:225–229. doi: 10.1038/nature08265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao X, Xiang S, Oh C, Martin CE, Tong L, Kiledjian M. Identification of a quality-control mechanism for mRNA 5′-end capping. Nature. 2010;467:608–611. doi: 10.1038/nature09338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AW. Rat1p and Xrn1p are functionally interchangeable exoribonucleases that are restricted to and required in the nucleus and cytoplasm, respectively. Mol Cell Biol. 1997;17:6122–6130. doi: 10.1128/mcb.17.10.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi J, Mischo H, Braglia P, Rondon A, Proudfoot NJ. Budding yeast RNA polymerases I and II employ parallel mechanisms of transcriptional termination. Gene Dev. 2008;22:1082–1092. doi: 10.1101/gad.463408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, Thomas K, Presser A, Bernstein BE, van Oudenaarden A, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A. 2009;106:11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufel J, Bousquet-Antonelli C, Beggs JD, Tollervey D. Nuclear pre-mRNA decapping and 5′ degradation in yeast require the Lsm2-8p complex. Mol Cell Biol. 2004;24:9646–9657. doi: 10.1128/MCB.24.21.9646-9657.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr D, Venkov P, Zlatanova J. Transcriptional Regulation in the Yeast Gal Gene Family - a Complex Genetic Network. Faseb J. 1995;9:777–787. doi: 10.1096/fasebj.9.9.7601342. [DOI] [PubMed] [Google Scholar]
- Luke B, Panza A, Redon S, Iglesias N, Li Z, Lingner J. The Rat1p 5′ to 3′ exonuclease degrades telomeric repeat-containing RNA and promotes telomere elongation in Saccharomyces cerevisiae. Mol Cell. 2008;32:465–477. doi: 10.1016/j.molcel.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Nagalakshmi U, Wang Z, Waern K, Shou C, Raha D, Gerstein M, Snyder M. The transcriptional landscape of the yeast genome defined by RNA sequencing. Science. 2008;320:1344–1349. doi: 10.1126/science.1158441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano T, Fraser P. No-Nonsense Functions for Long Noncoding RNAs. Cell. 2011;145:178–181. doi: 10.1016/j.cell.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Pinskaya M, Gourvennec S, Morillon A. H3 lysine 4 di- and tri-methylation deposited by cryptic transcription attenuates promoter activation. EMBO J. 2009;28:1697–1707. doi: 10.1038/emboj.2009.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponting CP, Belgard TG. Transcribed dark matter: meaning or myth? Hum Mol Genet. 2010;19:R162–R168. doi: 10.1093/hmg/ddq362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi IA, Mattick JS, Mehler MF. Long non-coding RNAs in nervous system function and disease. Brain Res. 2010;1338:20–35. doi: 10.1016/j.brainres.2010.03.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakoury-Elizeh M, Tiedeman J, Rashford J, Ferea T, Demeter J, Garcia E, Rolfes R, Brown PO, Botstein D, Philpott CC. Transcriptional remodeling in response to iron deprivation in Saccharomyces cerevisiae. Mol Biol Cell. 2004;15:1233–1243. doi: 10.1091/mbc.E03-09-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger M, Carr-Schmid A, Schwartz DC, Kiledjian M, Parker R. Analysis of recombinant yeast decapping enzyme. RNA. 2003;9:231–238. doi: 10.1261/rna.2151403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A, Poole TL. 5′-Exonuclease-2 of Saccharomyces cerevisiae. Purification and features of ribonuclease activity with comparison to 5′-exonuclease-1. J Biol Chem. 1995;270:16063–16069. doi: 10.1074/jbc.270.27.16063. [DOI] [PubMed] [Google Scholar]
- Teunissen AWRH, Steensma HY. Review - the Dominant Flocculation Genes of Saccharomyces-Cerevisiae Constitute a New Subtelomeric Gene Family. Yeast. 1995;11:1001–1013. doi: 10.1002/yea.320111102. [DOI] [PubMed] [Google Scholar]
- Thompson DM, Parker R. Cytoplasmic decay of intergenic transcripts in Saccharomyces cerevisiae. Mol Cell Biol. 2007;27:92–101. doi: 10.1128/MCB.01023-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MC, Spitale RC, Chang HY. Long Intergenic Noncoding RNAs: New Links in Cancer Progression. Cancer Res. 2011;71:3–7. doi: 10.1158/0008-5472.CAN-10-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk EL, Chen CL, Aubenton-Carafa Y, Gourvennec S, Kwapisz M, Roche V, Bertrand C, Silvain M, Legoix-Ne P, Loeillet S, Nicolas A, Thermes C, Morillon A. XUTs are a class of Xrn1-sensitive antisense regulatory non-coding RNA in yeast. Nature. 2011;475:114–117. doi: 10.1038/nature10118. [DOI] [PubMed] [Google Scholar]
- Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs: functional surprises from the RNA world. Gene Dev. 2009;23:1494–1504. doi: 10.1101/gad.1800909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Wei W, Gagneur J, Perocchi F, Clauder-Munster S, Camblong J, Guffanti E, Stutz F, Huber W, Steinmetz LM. Bidirectional promoters generate pervasive transcription in yeast. Nature. 2009;457:1033–1037. doi: 10.1038/nature07728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassour M, Pfiffner J, Levin JZ, Adiconis X, Gnirke A, Nusbaum C, Thompson DA, Friedman N, Regev A. Strand-specific RNA sequencing reveals extensive regulated long antisense transcripts that are conserved across yeast species. Genome Biol. 2010;11:R87. doi: 10.1186/gb-2010-11-8-r87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.