Abstract
Neph proteins are evolutionarily conserved members of the immunoglobulin superfamily of adhesion proteins and regulate morphogenesis and patterning of different tissues. They share a common protein structure consisting of extracellular immunoglobulin-like domains, a transmembrane region, and a carboxyl terminal cytoplasmic tail required for signaling. Neph orthologs have been widely characterized in invertebrates where they mediate such diverse processes as neural development, synaptogenesis, or myoblast fusion. Vertebrate Neph proteins have been described first at the glomerular filtration barrier of the kidney. Recently, there has been accumulating evidence suggesting a function of Neph proteins also outside the kidney. Here we demonstrate that Neph1, Neph2, and Neph3 are expressed differentially in various tissues during ontogenesis in mouse and chicken. Neph1 and Neph2 were found to be amply expressed in the central nervous system while Neph3 expression remained localized to the cerebellum anlage and the spinal cord. Outside the nervous system, Neph mRNAs were also differentially expressed in branchial arches, somites, heart, lung bud, and apical ectodermal ridge. Our findings support the concept that vertebrate Neph proteins, similarly to their Drosophila and C. elegans orthologs, provide guidance cues for cell recognition and tissue patterning in various organs which may open interesting perspectives for future research on Neph1-3 controlled morphogenesis.
Keywords: Neph1, Neph2, Neph3, SYG-1, SYG-2, Kirre-like, IrreC/Rst
Introduction
Neph proteins are members of the immunoglobin superfamily (IgSF), a class of cell–cell-recognition molecules that share a variable number of extracellular immunoglobulin-like domains as a common structure and interact with other IgSF-proteins in a homophilic or heterophilic way. In addition to Neph proteins, the IgSF also comprises neural cell adhesion molecules (NCAMs), integrins, receptor tyrosine kinases, plexins, neuroligins, neurexins, and neuropilins, molecules with a proven role in cell recognition and tissue patterning. These proteins have been shown to be involved in a wealth of ontogenetic mechanisms including synapse formation and axonal pathfinding, cell migration, myoblast fusion, epithelial cell sorting, and cell fate determination [reviewed in (Maness and Schachner 2007)]. All Neph proteins consist of five extracellular Ig-like domains, a transmembrane domain, and a cytoplasmatic tail that contains a podocin-binding motif, a PDZ binding motif, and a SH2-binding site (Sellin et al. 2003). Together with the closely related IgSF protein Nephrin, Neph proteins play a pivotal role for the development and maintenance of the filtration barrier in the kidney (Donoviel et al. 2001; Kestila et al. 1998; Neumann-Haefelin et al. 2010). Neph1 has been shown to interact with Nephrin at the glomerular slit diaphragm, a specialized cell junction of kidney podocytes, the visceral epithelial cells of the kidney (Barletta et al. 2003; Liu et al. 2003). With primary and hundreds of interdigitating secondary processes podocytes enwrap the glomerular capillaries. The extracellular domains of Nephrin and Neph1-3 bind to each other in a heterophilic and homophilic manner constituting a protein complex localized at the slit diaphragm (Gerke et al. 2005; Ihalmo et al. 2003). Mutations in the Nephrin gene cause a congenital nephrotic syndrome in humans (Kestila et al. 1998) and deletion of Neph1 was found to cause severe glomerular disease and perinatal death in mice (Donoviel et al. 2001). In contrast to Neph1, the role of Neph2 and Neph3 in podocyte biology is much less clear. Neph2 and Neph3 were detected at the glomerular slit diaphragm by immunogold and immunofluorescent staining (Gerke et al. 2003; Ihalmo et al. 2003). Basal expression of Neph3 in podocytes is driven by NFkappaB and SP-1, while the transcription factors upstream of Neph1 and Neph2 in the podocyte have remained unclear (Ristola et al. 2009). Soluble fragments of Neph2 were found in urine of healthy individuals due to cleavage of its extracellular domain by metalloproteinases under physiological conditions (Gerke et al. 2005). All mammalian Neph proteins bind the slit diaphragm protein podocin and have been implicated in slit diaphragm centered signaling (Sellin et al. 2003).
Additional functions of Neph proteins have been identified and extensively studied in the model organisms D. melanogaster and C. elegans. Initial studies in D. melanogaster demonstrated a role for the Neph 1 ortholog IrreC/Rst in axonal guidance in the optic chiasms (Ramos et al. 1993; Schneider 1995). These studies revealed restricted homophilic interactions of IrreC/Rst to be required for the correct projection of optical lobe neurons, while overexpression or knock-out leads to erroneous axonal pathfinding. In addition, the two orthologs of Neph1, Kirre and IrreC/Rst (rst) and the two orthologs of Nephrin, Sticks-and-Stones (SNS) and Hibris (Hbs), have been shown to mediate cell–cell recognition in muscle development. In Drosophila embryos, they interact in a trans-heterophilic binding to establish contacts between myoblast founder cells expressing the Neph1 orthologs Kirre/Rst and fusion-competent myoblasts (FCM) expressing the Nephrin orthologs SNS/Hbs. This interaction initiates myoblast fusion to form the final Drosophila muscle units (Chen and Olson 2004; Menon et al. 2005; Ruiz-Gómez et al. 2000; Strunkelnberg et al. 2001). Another process regulated by Neph protein orthologs in the fly that has been investigated very systematically in the past decade is the development of the eye. In the pupal eye disc, rst and hbs are necessary to pattern epithelial cells into the precise ommatidial array. This involves the preferential adhesion between interommatidial precursor cells and primary pigment cells that express Rst or Hbs, respectively (Bao and Cagan 2005). Cell–cell recognition is also the main function of the C. elegans orthologs of Nephrin and Neph1 (SYG-2 and SYG-1). SYG-1 and SYG-2 have been shown to determine the target-specific synaptogenesis of the HSN neuron (hermaphrodite specific motor neuron) onto vulval epithelial cells (Shen et al. 2004). Site-specific cell recognition through the interaction of SYG-2 on guidepost cells and SYG-1 on HSN-type neurons protects on-target synaptic proteins from E3 ubiquitin ligase-mediated degradation and thus restricts synapses to a demarcated region on the HSN neuron. In conclusion, the Neph orthologs in D. melanogaster and C. elegans constitute signaling modules that mediate cell–cell recognition and cell sorting in tissue morphogenesis, axonal pathfinding, and synaptic plasticity (Fischbach et al. 2009; Huber and Benzing 2005).
Recent studies on Neph protein function in extrarenal organ systems revealed that mammalian Neph proteins have similar cell–cell recognition functions. Nephrin and Neph3 expression is driven by the transcription factor Ptf1a in the cerebellum (Mizuhara et al. 2010). Neph3 expressing cells constitute two GABAergic neuronal precursor subpopulations in the rhombic lip, the site of cerebellogenesis, that later differentiate into Purkinje cells of the cerebellar cortex or into neurons of the deep cerebellar nuclei (Minaki et al. 2005; Nishida et al. 2010). A mechanism of how Neph proteins mediate cell–cell-recognition in neuronal tissue has been further clarified in the olfactory system. Here Neph2 and Neph3 are expressed in an antidromic way in certain subsets of olfactory sensory neurons displaying different olfactory receptors. Neph2 and Neph3 bundle axons of similar olfactory sensory neurons by homophilic interaction and cause them to converge in a specific set of olfactory glomeruli (Serizawa et al. 2006). Similar mechanisms might be involved in organizing the topology of the optical chiasms in Drosophila (Clandinin and Zipursky 2002; Schneider 1995; Sugie et al. 2010). Interestingly, patients with a defect in the transcription factor Ptf1a, which acts upstream of Neph3, exhibit cerebellar and pancreatic agenesis and neonatal diabetes mellitus, suggesting a possible role of Neph3 in pancreas and cerebellar development (Hoveyda et al. 1999; Sellick et al. 2004).
Taken together, Neph and Nephrin protein complexes serve as conserved signaling modules that regulate cell–cell recognition and tissue morphogenesis. This module has been shown to be of critical importance for the development and maintenance of several tissues. However, the respective target tissues in vertebrates are still ambiguous. To identify further tissues and organs that require Neph function for morphogenesis and patterning, we performed a comprehensive and systematic analysis of Neph mRNA expression in mice and chicken.
Materials and methods
Animals
Pregnant NMRI mice were killed at appropriate developmental stages [Embryonic day (E) 9.5, E10.5, E11.5, E12.5, E14.5, E16.5] to obtain embryos. Fertilized chicken eggs were staged according to Hamburger-Hamilton (HH) by somite number. All experiments were performed according to the guidelines of animal welfare at our institution.
Histology and tissue preparation
Isolated embryos were fixed in 4% paraformaldehyde solution overnight, dehydrated in phosphate-buffered saline (plus Tween-20; PBT) of increasing methanol concentrations for use as whole-mount embryos, and stored in 100% methanol at −20°C. For histological sections, phosphate-buffered saline (PBS) of increasing ethanol concentrations was used instead, and samples were embedded in paraffin. 8-μm sections were cut using a microtome (Leica).
In situ hybridization
A mouse kidney cDNA library and a chicken cDNA library served to clone fragments of coding sequence and 3′-untranslated region of mouse Neph1, Neph2, Neph3, and chicken homologues of Neph1 and Neph2. The following primers were used: Mouse: Neph1/Neph2: as described previously (Gerke et al. 2006). Neph3: forward primer 5′-CGCCATGGCTCTCTCTCTAAGC-3′, reverse primer 5′-TCACACATGAGTCTGGAGACGCTGGT-3′ Chicken: gga-Neph1: forward primer 5′-ACCCACCGTCACCGACAT-3′, reverse primer 5′-TGCTTCAAGTCCACGTCATC-3′ on template ENSGALT00000010117; gga-Neph2: forward primer 5′-CATTGTGCTGTGGATCAAGG-3′, reverse primer 5′-GTCAGAGCCAAAGCTGTTCC-3′ on template ENSGALT00000041073. PCR fragments were inserted into pBluescript SK+ vector (Invitrogen, Carlsbad, CA) using NotI and MluI restriction sites. pBluescript SK+ vector was linearized and digoxigenin-(DIG)-labeled antisense riboprobes were generated using T7-RNA-polymerase (Ambion).
For whole-mount in situ hybridization (ISH) analysis, embryos were progressively rehydrated, digested with proteinase K (1:1,000, 10–45 min according to sample size), and refixed in 4% PFA/0,2% glutaraldehyde for 20 min. After prehybridization (60 min), hybridization with DIG-UTP probes took place overnight in standard saline citrate (SSC; pH 4.5; containing 50% formamide) at 65–70°C. Specimens were then incubated with alkaline phosphatase-conjugated anti-DIG Fab fragments (Roche, Mannheim, Germany) at a dilution of 1:4,000 overnight at 4°C. Alkaline phosphatase was detected using chromogenic conversion of NBT/BCIP (Roche).
For vibratome sections, whole-mount embryos were stored in 4% PFA after in situ hybridization. Embryos were embedded in heated fluid 3–4% agarose. After cooling for 1 h at room temperature, sectioning was performed with a vibratome at 35–50 μm.
For paraffin section ISH, slides were progressively rehydrated and permeabilized with proteinase K for 3 min. Prehybridization, hybridization, and detection were carried out as described above. To avoid drying up of the slides during hybridization, we placed them in a humidity chamber containing 5×SSC and 40% formamide. Slides were then progressively dehydrated, washed in xylol, and mounted.
Digital images were taken on a stereomicroscope (Leica MZ8) equipped with a digital camera. For figure preparation, brightness and contrast were adjusted using Photoshop.
Results
Expression analysis of Neph1 during mouse development
To analyze Neph mRNA expression, we performed in situ hybridization studies using murine embryos of different developmental stages. Whole-mount in situ hybridization of E9.5–E11.5 embryos revealed persistent expression of Neph1 transcripts in the olfactory placode, branchial arches, and somites (Fig. 1a–c). In the developing mouse brain, intense Neph1 expression was visible in the telocele.
Fig. 1.
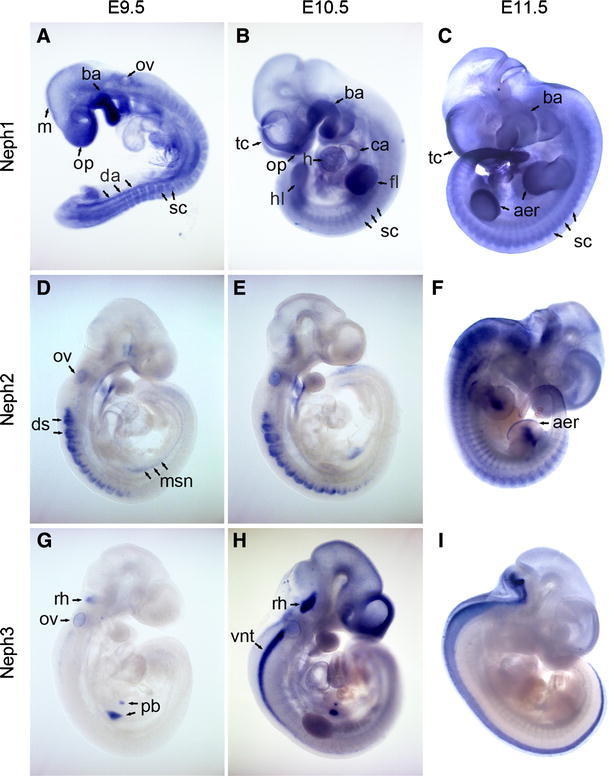
Whole-mount in situ hybridization analysis of Neph1 (a–c), Neph2 (d–f), and Neph3 (g–i) expression in mouse embryos at E9.5 (a, d, g), E10.5 (b, e, h), and E11.5 (c, f, i). Blue NBT/BCIP precipitates are indicative of mRNA expression. aer apical ectodermal ridge, ba branchial arch, ca common atrium, da dorsal aorta, ds dorsal somite, fl fore limb, h heart, hl hind limb, m mesencephalon, msn mesonephros, vnt ventral neural tube, op olfactory placode, ov otic vesicle, pb pancreatic bud, rh rhombencephalon, sc sclerotom, tc telocele
Specifically at E9.5 (Fig. 1a), two long streaks of Neph1-positive cells aligned with the somites at their ventral edge. This expression pattern correlates best with the dorsal aortae, which extend to the tip of the tail, and are in the process of uniting to become the unpaired abdominal aorta at this stage of development. In addition, Neph1 mRNA was detected around the otic vesicle. However, this did not persist at later stages. In the course of embryonic development, expression intensity increased in the left ventricle and the common atrium of the heart. The limb buds were also positive for Neph1 from E9.5 to E11.5 without discriminating particular zones (Fig. 1b). At E11.5, an increased level of Neph1 was found at the apical ectodermal ridge at the extremity of the buds (Fig. 1c). Interestingly, the initially broad expression in the central nervous system became restricted at E11.5 to the now paired teloceles leaving mesencephalon and rhombencephalon almost void of expression.
Hybridization on paraffin sections of mouse embryos allowed for a more detailed analysis of Neph1 expression domains. At stage E11.5, we detected almost ubiquitous expression with locally increased intensity in the areas of the nasal pit and the branchial arches (Fig. 2a). With proceeding embryonic development, Neph1 expression in the neocortex and the superior and inferior colliculi of the mesencephalon (Fig. 2b) became layer-specific, and two tracts of faintly Neph1-positive cells spanned the spinal cord in a cranio-caudal direction (Fig. 2b; E14.5). Also, small domains of the thalamus and the tegmentum stained positive. Higher magnifications of Neph1-positive cell bands in the developing neocortex (Fig. 2e), the hippocampus (Fig. 2f), and the ganglionic eminence (Fig. 2g) showed Neph1 to be layer- or, as in case of the ganglionic eminence, population-specific since Neph1-negative cells seemed to be interspersed here. However, further investigation is needed to determine affiliation of Neph1-positive cells with particular layers or cell types, the more so as the brain at this point is remodeling rapidly, and neuronal cell populations migrate toward the marginal zone of the neocortex by passing through preexistent cell layers (Goffinet 1979).
Fig. 2.
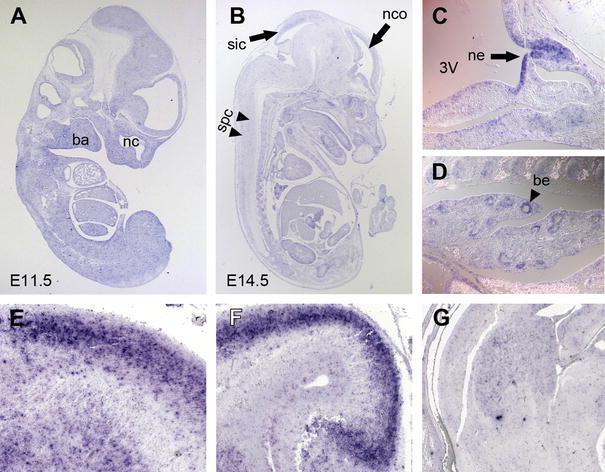
In situ hybridization analysis of Neph1 expression. Paramedian sagittal section of E11.5 (a) and E14.5 (b) mouse embryos. Ubiquitous expression of Neph1 is detected in tissues derived from all three germ layers. Higher magnification of Rathke’s pouch (c) and of the pseudoglandular lung anlage (d) at E12.5. Neph1 expression is found in different regions of the developing brain at E16.5: neocortex (E sagittal); hippocampus (F sagittal); ganglionic eminence (G frontal). 3V third ventricle, ba branchial arches, be bronchial epithelia, nc nasal cavity, nco neocortex, ne neuroepithelium, spc spinal cord, sic superior and inferior colliculi of the mesencephalon
Additional Neph1 transcripts were detected at Rathke’s pouch giving rise to the anterior pituitary lobe as well as at the base of the third ventricle in the neuroepithelium of the developing posterior lobe (Fig. 2c, ne—black arrow), and, intriguingly, in the epithelia of the developing bronchial system (Fig. 2d, black arrow head).
Expression pattern of Neph2 in murine embryonic stages
Whole-mount in situ hybridization revealed an initially precisely circumscribed expression of Neph2 transcripts, which became broader at later stages. At E9.5 (Fig. 1d) and E10.5 (Fig. 1e), the dorsal somites, the otic vesicle, and the second and third branchial arches exhibited Neph2 expression. Expression in the somatic mesoderm at these stages was limited to cranial somites and extended to the caudal somites in the course of embryonic development (Fig. 1f). The diencephalic neuroepithelium or the ectodermal epithelium of Rathke’s pouch stained positive for Neph2, arguably suggesting a role for Neph2 (in concert with Neph1) in the development of the pituitary gland. However, at this magnification expression cannot be easily ascribed to either anterior or posterior lobe. The mesonephros, a linear structure expressing Neph2, is visible ventral of the caudal somites at E9.5 (Fig. 1d), while at stage E10.5 the identity of this domain is unclear (Fig. 1e). With the transition to stage E11.5, major parts of the prosencephalon and the brain stem including pons, medulla oblongata, and the cranial segments of the spinal cord transition zone now stained positive for Neph2 (Fig. 1f). Labeled cells were also found at the site of the primitive intervertebral discs. The anatomically most defined Neph2 expression was localized to the apical ectodermal ridge of fore and hind limb buds and the limb mesenchyme as can be seen in more detail in Fig. 3a. Expression at the apical ectodermal ridge was also present at earlier stages while expression in the mesenchymal domain started later (Fig. 3b; E10.5 insert). Note that the kidney anlage is presumably covered by the hind limb.
Fig. 3.
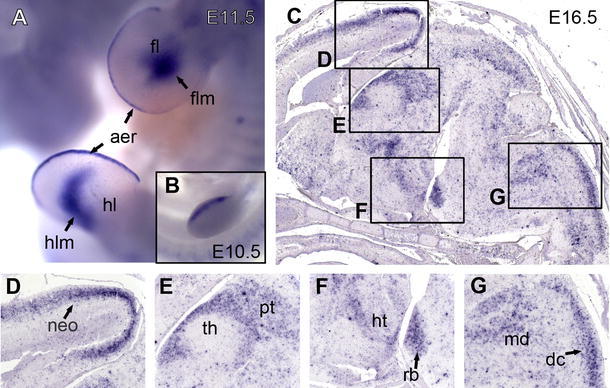
Neph2 expression as revealed by in situ hybridization analysis. Magnification of fore- and hindlimb buds of an E11.5 mouse embryo (a). Embryo is shown from the left, the back directed to the right. The insert shows earlier stage (E10.5) of fore limb development (b). Sagittal sections of mouse brain at stage E16.5 (c). Higher magnification of neocortex (d), diencephalon (e), pons (f), and oblong medulla (g) as indicated by boxes. aer apical ectodermal ridge, dc dorsal column tract, fl fore limb; flm fore limb mesenchyme, hl hind limb, hlm hind limb mesenchyme, ht hypothalamus, md medulla, neo neocortex, pt pretectum, rb rhombomere basal plate
We next sectioned mouse brains at stage E16.5. The overview (Fig. 3c) revealed a distinct expression pattern within the neocortex (Fig. 3d), the diencephalon (Fig. 3e, f), and the rhombencephalon (Fig. 3g). Neph2 transcripts in the neocortex (Fig. 3d) were localized in superficial layers. In the diencephalon at a paramedian section, Neph2 mRNA was detected in the pretectal region, the adjacent intermediate tier of the thalamus, and a small band of cells bordering the rostral part of the dorsal tier of the thalamus (Fig. 3e). Basal plates of rhombomeres at the rostral part of the ponto-medullary transition zone showed intense expression (Fig. 3f rb). Juxtaposed to this area, we also found staining in the peduncular hypothalamus (Fig. 3f ht).
At the spino-medullary transition, an oblong area of Neph2 expression extended caudally into the spinal cord (Fig. 3g). This correlates best with the trigeminal column or the dorsal column tract. Marginally rostral of the cranial end of the column-shaped domain, a cell cluster with strong Neph2 expression stood out. This may represent the alar plates of caudal rhombomeres.
Expression of Neph3 during mouse development
In comparison with the previously described expression domains of Neph1 and Neph2, Neph3 showed a more limited and highly specific expression in the whole-mount in situ hybridizations. While at stage E9.5 (Fig. 1g), Neph3 was exclusively transcribed in cells of the otic vesicle, the pancreas bud, the metanephros, and a small area at the bottom of the fourth ventricle, expression at stage E10.5 (Fig. 1h) also included parts of the rhombencephalon, differentiating cells of the neural crest and the teloceles. Interestingly, expression in all structures except for the neuronal crest and the cerebellum anlage vanished completely at stage E11.5 (Fig. 1i) suggesting a rather specific role for Neph3 in development.
Detailed analysis of Neph3 expression domains in the CNS on paraffin sections identified a group of cells in the cranial roof and the floor of the fourth ventricle to be expressing Neph3 at high levels (Fig. 4a). This expression was consistent throughout all of the screened stages (i.e. E11.5, E12.5, E14.5, Fig. 4a–c) and only widened slightly in the rostro-occipital axis. A dorsal view of a whole-mount specimen revealed the rhombomere-specific pattern in the neural crest (Fig. 4d; E10.5). Note the position of the otic vesicle next to rhombomere 5 as a point of orientation (Young et al. 2009). Rhombomeres r2, r3, and r7 stained for Neph3 as did a thin layer of cells delineating the border of the single ependymal cell layer comprising the roof plate and the alar plate. A sagittal section of an E12.5 embryo (Fig. 4e) revealed Neph3 expression domains in the entire thalamus, the cerebellum anlage, and the pontal flexure. On detailed analysis, Neph3 positive cells at the pontal flexure extend processes towards the floor plates of the pertaining rhombomeres, while their cell bodies appeared to be ventricularly localized (Fig. 4f). This domain did not span the entire rhombencephalon but initiated cranially at the level of r2 in the pontine hindbrain and reached far into the medullary hindbrain. In case of the expression domain in the cerebellum anlage, areas with highly elevated expression levels were distinguished from areas with almost no Neph3 transcripts present. At higher magnification, these areas could be correlated with the external (Neph3-negative) and the internal germ layer (Neph3-positive) (Fig. 4h). Additionally, Neph3 was also expressed at the roof of the nasal cavity, from where the olfactory epithelium extends axons towards the prosencephalon at this point of embryonic development and induces the olfactory bulb in the rostral part of the prosencephalon (Fig. 4g).
Fig. 4.
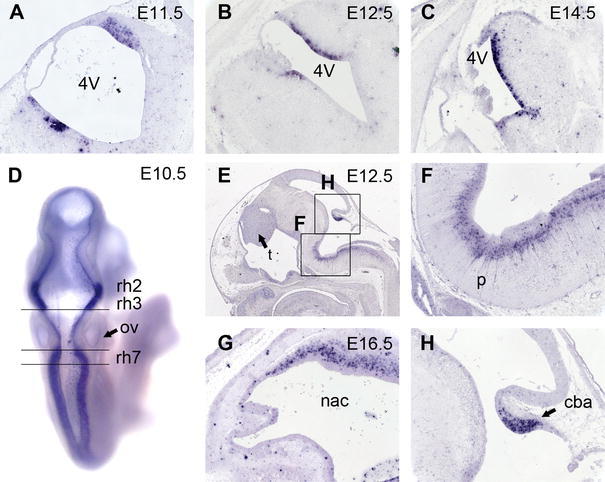
In situ hybridization analysis of Neph3 expression. Continued expression at the bottom and the roof of the fourth ventricle in E11.5 (a), E12.5 (b), and E14.5 (c) mouse embryos. Dorsal view of an E10.5 embryo (d) reveals rhombomere-specific expression. Note the position of the otic vesicle (ov). Sagittal section of an E12.5 embryo (e, f, h) with demarcated expression in the thalamus (t), the cerebellum anlage (cba), and cell populations in the pons (p). At a later time point (i.e. E16.5, g), cells populating the roof of the nasal cavity express Neph3. 4V fourth ventricle, cba cerebellum anlage, nac nasal cavity, ov otic vesicle, p pons, rh rhombomere, t thalamus
Expression of Neph homologues in chicken development
To check for an evolutionary preserved role of Neph proteins, we searched genome databases of Gallus gallus for homologues of Neph like genes. While Neph1, Neph2, and slit-diaphragm-associated Podocin were present, no homologues for Nephrin or Neph3 could be detected (Fig. 5). Next, we conducted in situ hybridization analysis on chicken embryos at stages 20 and 23 according to Hamburger and Hamilton (HH). Strikingly, expression patterns did not vary greatly from those observed in mice. Expression of Neph1 and Neph2 homologues (consequently named gga-Neph1 and gga-Neph2) included the central nervous system, kidney precursors, limb buds, otic vesicle, branchial arches, and, in case of Neph2, the heart and its outflow tract (Fig. 6).
Fig. 5.
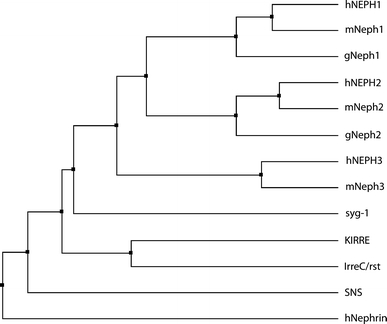
Phylogenetic tree showing homology between Nephrin- and Neph-like genes in vertebrates and invertebrates as discussed in the text. Accession numbers used for analysis: hNEPH1 AAI09194, mNeph1 AAH23765, gNeph1 ENSGALT00000010117, hNEPH2 AAI01776, mNeph2 AAH63072, gNeph2 ENSGALT00000041073, hNEPH3 AAP72166, mNeph3 AAI58022, syg-1 AAC47074, KIRRE AAF86308, IrreC/rst AAA16632, SNS AAF77184, hNephrin AAG17141
Fig. 6.
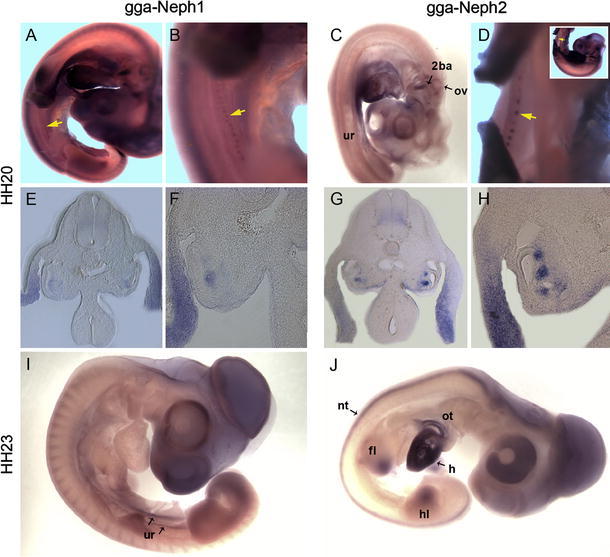
Expression of Neph1- and Neph2-homologues during chicken embryo development. At stage HH20, Neph1 and Neph2 transcripts are detectable in the the mesonephros (yellow arrows in a, b, and d/Insert for orientation) and urogenital ride (c). Additional expression domains are found in the otic vesicle (a, c, i), the branchial arches (a, c), and the limb mesenchyme (a, i, j). On vibratome sections, the expression of Neph1 and Neph2 in the peri-Wolffian ductal mesonephric mesoderm and in the lateral plate mesoderm is evident (e, f, g, h). At HH23, Neph1 is expressed in the urogenital ridge and the limbs (i), whereas Neph2 exhibits a broader expression pattern in the limbs, the heart and its outflow tract (j), and the neural tube (g, j). 2ba second branchial arch, fl fore limb, h heart, hl hind limb, nt neural tube, ov otic vesicle, ot outflow tract, ur urogenital ridge
Gga-Neph1 was strongly expressed not only in the central nervous system, particularly in the teloceles and the mesencephalon, but also in the optic cup, the dorsal parts of the somites, and the limb buds. Expression of gga-Neph1 in the mesonephric kidney starts at HH19 as a faint diffuse expression domain in the peri-Wolffian duct region (data not shown). From stage HH20 onwards, the staining intensity increased, and the tissue condensed into the maturing early pre-nephron vesicles and tubules appearing as beads-like structures along the mesonephric ridge. An additional expression domain was found in the lateral plate mesoderm. Gga-Neph2 expression pattern at HH20 were seen in the developing nephrons adjacent to the Wolffian duct. This expression domain was broader and more prominent in the nephrogenic mesoderm in comparison to gga-Neph1. Gga-Neph2 was expressed in the urogenital ridge at earlier stages (HH20) but expression was attenuated at later stages (HH23). Conversely, CNS expression had broadened by HH23 and extended into the neural crest, the mesencephalon, and the optic cup while none of these areas stained positive at HH20. In the second branchial arch, a dash-like cell cluster stains for gga-Neph2 (Fig. 6c). Hind and fore limb buds showed gga-Neph transcription in the apical and central parts of the bud but not at the base, while very strong expression in the developing heart remained consistent over time.
Discussion
In the present study, we showed that Neph1-3 mRNAs are expressed in a wide range of vertebrate organ systems. Although some previous studies addressed Neph protein expression in development (Gerke et al. 2006; Hornberg et al. 2009; Komori et al. 2008; Morikawa et al. 2007; Serizawa et al. 2006), the present study for the first time provides a comprehensive analysis of Neph gene expression in mouse and chick development. Our study identified Neph2 and Neph3 mRNA expression in sensory organs like the olfactory epithelium and the otic vesicle consistent with previously reported results (Serizawa et al. 2006). Recent evidence suggested that Nephrin-positive nerve terminals in the cochlea project onto Neph2-positive inner and outer hair cells, thus presenting a process that possibly requires Neph proteins for precise axonal projections onto target cells (Morikawa et al. 2007). Based on these observation and on data from C. elegans and D. melanogaster our results suggest that cell populations in the neocortex and the neuroepithelium, which give rise to the future neurohypophysis, might express Neph proteins as guidance cues for projecting axons. Neph2 and Neph3 are mutually exclusively expressed in neurons of the olfactory bulb together with axonal repellent Ephrin A5 and its tyrosine receptor kinase EphA5 (Serizawa et al. 2006). Moreover, the Neph3 expression pattern in the spinal cord resembles that of EphA5 and EphrinA5 as previously reported (Fig. 1h, i) (Cooper et al. 2009; Yue et al. 1999). Here, EphA5 and EphrinA5 help to establish dorso-ventral patterning, and Neph3 could constitute a potential new player in this morphogenetic event. The ligand/receptor pair EphrinA5 and EphA5 also exerts influence on basal insulin secretion by pancreatic islet cells to regulate glucose homeostasis (Konstantinova et al. 2007). Since Nephrin and Neph3 have been shown to be expressed in the brain under the control of pancreatic transcription factor 1a (Ptf1a), and transcripts of both mRNAs have been detected in the pancreas, it is intriguing to surmise that Neph3 represents one part of another receptor/ligand pair that participates in fine-tuning insulin release (Nishida et al. 2010). This is further supported by the recent finding that Nephrin localizes to the plasma membrane of insulin vesicles of pancreatic beta cells (Fornoni et al. 2010).
Cell migration is another essential mechanism in ontogenesis and very similar to axonal pathfinding as it requires cell-bound or soluble factors for directional outgrowth and site-specific settling (Dickson 2002). Data from the fruit fly have already suggested a role for Neph proteins in cell migration and fusion (Ruiz-Gómez et al. 2000; Strunkelnberg et al. 2001). In fact, many organs that depend on cells of high migratory activity during development stain positive for either Neph1/2 or Neph3 (i.e. neural crest, heart, neocortex, neurohypophysis, branchial arches). A strong Neph2 signal was found in avian embryos beginning at stage HH20 in the heart and its outflow tract, which to some part derives from migrating neural crest cells. Therefore, it is conceivable that Neph proteins either contribute to directing neural crest cells to the heart anlage or help sorting cells to form the intricate structure of the outflow tract. In addition, branchial arches show Neph1 and Neph2 expression. From these structures, migrating neural crest cells later form a great variety of tissues ranging from bone to neurons and thymus. In the neurohypophysis, the migrating diencephalic neuroepithelium invaginates to form the infundibular stalk.
Neuron migration in the neocortex is essential to organize neurons in layers. Abrogation of the mechanism—e.g. in the reeler mouse mutant—leads to the disorganization of the layer structure (Frotscher et al. 2009; Goffinet 1979). A similar phenotype was observed when simultaneously knocking out the kinases src and fyn which are known to phosphorylate Nephrin-Neph1 complexes to induce intracellular signaling (Kuo et al. 2005; Manto 2008). In combination with the expression patterns we observe in this study, this strongly suggests a role for Neph proteins in neo- and allocortical neuron migration. However, no severe neuronal phenotype has been observed in Nephrin or Neph1 knock-out animals (Donoviel et al. 2001; Putaala et al. 2001). The murine Nephrin gene is specifically expressed in kidney, brain, and pancreas. Inactivation of the gene leads to massive proteinuria and neonatal death. In humans, however, Nephrin-deficient patients display cerebellar ataxia in about 10% of all cases (Putaala et al. 2001), and a recent publication links Neph2 to cases of intellectual disability (Bhalla et al. 2008).
Further, mutations in CASK, a downstream effector of Neph1 and Neph2, cause a brain malformation phenotype with microcephaly and hypoplasia of the brainstem and cerebellum (Gerke et al. 2006; Najm et al. 2008; Piluso et al. 2009; Tarpey et al. 2009). A possible explanation for the lack of an apparent neuronal phenotype in Neph mutants could be that immunoglobin-superfamily molecules act in a redundant manner (Neumann-Haefelin et al. 2010). As a perspective, it would be of great interest to create double- or triple-knock-out animals for either Neph1/2/3, possibly in combination with Nephrin, to screen for CNS defects.
Other functions of IgSF-proteins inferred from experiments in Drosophila include cell sorting and cell fate determination in morphogenesis. In the cerebellum, Ptf1a expression triggers Neph3 expression and determines neuronal precursor cell fate by causing differentiation into GABAergic subpopulations (Hoshino et al. 2005; Mizuhara et al. 2010; Pascual et al. 2007). Similar processes might take place in some of the organs we report here to be positive for Neph mRNA expression. Neph1 in the lung potentially promotes cell sorting during embryonic induction of epithelial ducts or contributes to the continuing interaction between the endodermal epithelium and the lung mesenchyme during organogenesis. We detected Neph1 and Neph2 transcripts in overlapping areas of the limb buds at synchronous time points (E11.5). Expression seems to specifically take place in the apical ectodermal ridge, which serves as a signaling center controlling limb mesenchyme proliferation and keeps mesenchymal cells in an undifferentiated state (Duboc and Logan 2009). Important effectors of limb development are fibroblast growth factors (FGFs), Hox-transcription factors, sonic hedgehog (Shh), and Wnt-signaling (Newman and Bhat 2007). They cooperate to establish dorso-ventral polarity, promote proliferation and growth, and trigger mesenchymal condensations, which in turn differentiate into muscles, bones, tendons, and cartilage. Concomitant Neph expression, thus, suggests a role in forming mesenchymal condensations similar to myoblast aggregation and fusion in Drosophila. It is also conceivable that by controlling cell affinity, they regulate the position and sorting of newly spawned cell populations during the outgrowth of the limb bud as proximal limb structures appear first and pertaining cells need to cluster accordingly. A comparable function has already been described for a structurally related protein N-cadherin giving positional identity to future cartilagineous elements of the limb (Tuan 2003; Yajima et al. 2002). Beyond that, Nephs might provide survival signals to certain cell populations and prevent apoptosis, which is known to be an important morphogenetic factor. In our eyes, it would be worthwhile to see whether some of the well-characterized transcription factors involved in limb development differentially control Neph expression in the apical ectodermal ridge as well as other expression domains, as Neph genes seem to be controlled by different sets of transcription factors (Guo et al. 2004; Nishida et al. 2010; Ristola et al. 2009).
In addition to the data on rodent development, we present an expression analysis in chicken embryos. Homologues of Neph proteins have been described in a number of species ranging from fruit fly to nematodes, zebra fish, rodents, and humans (Fig. 5). So far, expression in Gallus gallus has not been reported. With the presented work, we add another species to the list of potential model organism in research on Neph gene function. In contrast to the mouse, the avian embryo is much better accessible in vivo and can be subjected to many well-described methods in developmental biology such as in vivo electroporation, microinjection, or in vivo time-lapse microscopy (Funahashi and Nakamura 2008; Wilson 1996). These options would greatly expand the methods available to basic molecular research on adhesion molecules of the Neph family. Our analyses show that the expression patterns in Gallus gallus closely mimic those in the mouse and suggest a common theme in Neph function.
In conclusion, our data suggest that Neph-dependent cell recognition may play a role in a variety of different tissues and organs in vertebrates. Future studies will help to address the role of these proteins in tissue morphogenesis by creating tissue- and organ-specific knock-out animals.
Acknowledgments
We thank Stefanie Keller, Christina Engel, Charlotte Meyer and Petra Dämisch for excellent technical assistance, and members of the laboratories for helpful discussions. T.B. received funding through the Deutsche Forschungsgemeinschaft (BE2212, SFB572 and SFB635), B.S. received funding through the Deutsche Forschungsgemeinschaft (SCHE1562, SFB 832).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Martin Höhne, Phone: +49-221-47889033, FAX: +49-221-47889041, Email: martin.hoehne@uk-koeln.de.
Andreas Kispert, Phone: +49-511-5324017, FAX: +49-511-532-4283, Email: kispert.andreas@mh-hannover.de.
References
- Bao S, Cagan R. Preferential adhesion mediated by hibris and roughest regulates morphogenesis and patterning in the drosophila eye. Dev Cell. 2005;8:925–935. doi: 10.1016/j.devcel.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Barletta G-M, Kovari IA, Verma RK, Kerjaschki D, Holzman LB. Nephrin and neph1 co-localize at the podocyte foot process intercellular junction and form cis hetero-oligomers. J Biol Chem. 2003;278:19266–19271. doi: 10.1074/jbc.M301279200. [DOI] [PubMed] [Google Scholar]
- Bhalla K, Luo Y, Buchan T, Beachem MA, Guzauskas GF, Ladd S, et al. Alterations in CDH15 and KIRREL3 in patients with mild to severe intellectual disability. Am J Hum Genet. 2008;83:703–713. doi: 10.1016/j.ajhg.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EH, Olson EN. Towards a molecular pathway for myoblast fusion in Drosophila. Trends Cell Biol. 2004;14:452–460. doi: 10.1016/j.tcb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Clandinin TR, Zipursky SL. Making connections in the fly visual system. Neuron. 2002;35:827–841. doi: 10.1016/S0896-6273(02)00876-0. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Crockett DP, Nowakowski RS, Gale NW, Zhou R. Distribution of EphA5 receptor protein in the developing and adult mouse nervous system. J Comp Neurol. 2009;514:310–328. doi: 10.1002/cne.22030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- Donoviel DB, Freed DD, Vogel H, Potter DG, Hawkins E, Barrish JP, et al. Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol. 2001;21:4829–4836. doi: 10.1128/MCB.21.14.4829-4836.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duboc V, Logan MP. Building limb morphology through integration of signalling modules. Curr Opin Genet Dev. 2009;19:497–503. doi: 10.1016/j.gde.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Fischbach K-F, Linneweber GA, Andlauer TF, Hertenstein A, Bonengel B, Chaudhary K. The irre cell recognition module (IRM) proteins. J Neurogenet. 2009;23:48–67. doi: 10.1080/01677060802471668. [DOI] [PubMed] [Google Scholar]
- Fornoni A, Jeon J, Varona Santos J, Cobianchi L, Jauregui A, Inverardi L, et al. Nephrin is expressed on the surface of insulin vesicles and facilitates glucose-stimulated insulin release. Diabetes. 2010;59:190–199. doi: 10.2337/db09-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frotscher M, Chai X, Bock HH, Haas CA, Förster E, Zhao S. Role of Reelin in the development and maintenance of cortical lamination. J Neural Transm. 2009;116:1451–1455. doi: 10.1007/s00702-009-0228-7. [DOI] [PubMed] [Google Scholar]
- Funahashi J, Nakamura H. Electroporation in avian embryos. Methods Mol Biol. 2008;461:377–382. doi: 10.1007/978-1-60327-483-8_27. [DOI] [PubMed] [Google Scholar]
- Gerke P, Huber TB, Sellin L, Benzing T, Walz G. Homodimerization and heterodimerization of the glomerular podocyte proteins nephrin and NEPH1. J Am Soc Nephrol. 2003;14:918–926. doi: 10.1097/01.ASN.0000057853.05686.89. [DOI] [PubMed] [Google Scholar]
- Gerke P, Sellin L, Kretz O, Petraschka D, Zentgraf H, Benzing T, et al. NEPH2 is located at the glomerular slit diaphragm, interacts with nephrin and is cleaved from podocytes by metalloproteinases. J Am Soc Nephrol. 2005;16:1693–1702. doi: 10.1681/ASN.2004060439. [DOI] [PubMed] [Google Scholar]
- Gerke P, Benzing T, Hohne M, Kispert A, Frotscher M, Walz G, et al. Neuronal expression and interaction with the synaptic protein CASK suggest a role for Neph1 and Neph2 in synaptogenesis. J Comp Neurol. 2006;498:466–475. doi: 10.1002/cne.21064. [DOI] [PubMed] [Google Scholar]
- Goffinet AM. An early development defect in the cerebral cortex of the reeler mouse. Anat Embryol. 1979;157:205–216. doi: 10.1007/BF00305160. [DOI] [PubMed] [Google Scholar]
- Guo G, Morrison DJ, Licht JD, Quaggin SE. WT1 activates a glomerular-specific enhancer identified from the human nephrin gene. J Am Soc Nephrol. 2004;15:2851–2856. doi: 10.1097/01.ASN.0000143474.91362.C4. [DOI] [PubMed] [Google Scholar]
- Hornberg M, Gussing F, Berghard A, Bohm S. Retinoic acid selectively inhibits death of basal vomeronasal neurons during late stage of neural circuit formation. J Neurochem. 2009;110:1263–1275. doi: 10.1111/j.1471-4159.2009.06216.x. [DOI] [PubMed] [Google Scholar]
- Hoshino M, Nakamura S, Mori K, Kawauchi T, Terao M, Nishimura YV, et al. Ptf1a, a bHLH transcriptional gene, defines GABAergic neuronal fates in cerebellum. Neuron. 2005;47:201–213. doi: 10.1016/j.neuron.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Hoveyda N, Shield JP, Garrett C, Chong WK, Beardsall K, Bentsi-Enchill E, et al. Neonatal diabetes mellitus and cerebellar hypoplasia/agenesis: report of a new recessive syndrome. J Med Genet. 1999;36:700–704. [PMC free article] [PubMed] [Google Scholar]
- Huber TB, Benzing T. The slit diaphragm: a signaling platform to regulate podocyte function. Curr Opin Nephrol Hypertens. 2005;14:211–216. doi: 10.1097/01.mnh.0000165885.85803.a8. [DOI] [PubMed] [Google Scholar]
- Ihalmo P, Palmén T, Ahola H, Valtonen E, Holthöfer H. Filtrin is a novel member of nephrin-like proteins. Biochem Biophys Res Commun. 2003;300:364–370. doi: 10.1016/S0006-291X(02)02854-1. [DOI] [PubMed] [Google Scholar]
- Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, et al. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/S1097-2765(00)80057-X. [DOI] [PubMed] [Google Scholar]
- Komori T, Gyobu H, Ueno H, Kitamura T, Senba E, Morikawa Y. Expression of kin of irregular chiasm-like 3/mKirre in proprioceptive neurons of the dorsal root ganglia and its interaction with nephrin in muscle spindles. J Comp Neurol. 2008;511:92–108. doi: 10.1002/cne.21838. [DOI] [PubMed] [Google Scholar]
- Konstantinova I, Nikolova G, Ohara-Imaizumi M, Meda P, Kucera T, Zarbalis K, et al. EphA-ephrin-A-mediated beta cell communication regulates insulin secretion from pancreatic islets. Cell. 2007;129:359–370. doi: 10.1016/j.cell.2007.02.044. [DOI] [PubMed] [Google Scholar]
- Kuo G, Arnaud L, Kronstad-O’Brien P, Cooper JA. Absence of Fyn and Src causes a reeler-like phenotype. J Neurosci. 2005;25:8578–8586. doi: 10.1523/JNEUROSCI.1656-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Kaw B, Kurfis J, Rahmanuddin S, Kanwar YS, Chugh SS. Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability. J Clin Invest. 2003;112:209–221. doi: 10.1172/JCI18242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maness PF, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;10:19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- Manto M. The cerebellum, cerebellar disorders, and cerebellar research—two centuries of discoveries. Cerebellum. 2008;7:505–516. doi: 10.1007/s12311-008-0063-7. [DOI] [PubMed] [Google Scholar]
- Menon SD, Osman Z, Chenchill K, Chia W. A positive feedback loop between Dumbfounded and Rolling pebbles leads to myotube enlargement in Drosophila. J Cell Biol. 2005;169:909–920. doi: 10.1083/jcb.200501126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minaki Y, Mizuhara E, Morimoto K, Nakatani T, Sakamoto Y, Inoue Y, et al. Migrating postmitotic neural precursor cells in the ventricular zone extend apical processes and form adherens junctions near the ventricle in the developing spinal cord. Neurosci Res. 2005;52:250–262. doi: 10.1016/j.neures.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Mizuhara E, Minaki Y, Nakatani T, Kumai M, Inoue T, Muguruma K, et al. Purkinje cells originate from cerebellar ventricular zone progenitors positive for Neph3 and E-cadherin. Dev Biol. 2010;338:202–214. doi: 10.1016/j.ydbio.2009.11.032. [DOI] [PubMed] [Google Scholar]
- Morikawa Y, Komori T, Hisaoka T, Ueno H, Kitamura T, Senba E. Expression of mKirre in the developing sensory pathways: its close apposition to nephrin-expressing cells. Neuroscience. 2007;150:880–886. doi: 10.1016/j.neuroscience.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Najm J, Horn D, Wimplinger I, Golden JA, Chizhikov VV, Sudi J, et al. Mutations of CASK cause an X-linked brain malformation phenotype with microcephaly and hypoplasia of the brainstem and cerebellum. Nat Genet. 2008 doi: 10.1038/ng.194. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin E, Kramer-Zucker A, Slanchev K, Hartleben B, Noutsou F, Martin K, et al. A model organism approach: defining the role of Neph proteins as regulators of neuron and kidney morphogenesis. Hum Mol Genet. 2010;19:2347–2359. doi: 10.1093/hmg/ddq108. [DOI] [PubMed] [Google Scholar]
- Newman SA, Bhat R. Activator-inhibitor dynamics of vertebrate limb pattern formation. Birth Defects Res C Embryo Today. 2007;81:305–319. doi: 10.1002/bdrc.20112. [DOI] [PubMed] [Google Scholar]
- Nishida K, Hoshino M, Kawaguchi Y, Murakami F. Ptf1a directly controls expression of immunoglobulin superfamily molecules Nephrin and Neph3 in the developing central nervous system. J Biol Chem. 2010;285:373–380. doi: 10.1074/jbc.M109.060657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Abasolo I, Mingorance-Le Meur A, Martínez A, Del Rio JA, Wright CVE, et al. Cerebellar GABAergic progenitors adopt an external granule cell-like phenotype in the absence of Ptf1a transcription factor expression. Proc Natl Acad Sci USA. 2007;104:5193–5198. doi: 10.1073/pnas.0605699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piluso G, D’Amico F, Saccone V, Bismuto E, Rotundo IL, Di Domenico M, et al. A missense mutation in CASK causes FG syndrome in an Italian family. Am J Hum Genet. 2009;84:162–177. doi: 10.1016/j.ajhg.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putaala H, Soininen R, Kilpelainen P, Wartiovaara J, Tryggvason K. The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet. 2001;10:1–8. doi: 10.1093/hmg/10.1.1. [DOI] [PubMed] [Google Scholar]
- Ramos RG, Igloi GL, Lichte B, Baumann U, Maier D, Schneider T, et al. The irregular chiasm C-roughest locus of Drosophila, which affects axonal projections and programmed cell death, encodes a novel immunoglobulin-like protein. Genes Dev. 1993;7:2533–2547. doi: 10.1101/gad.7.12b.2533. [DOI] [PubMed] [Google Scholar]
- Ristola M, Arpiainen S, Saleem MA, Mathieson PW, Welsh GI, Lehtonen S, et al. Regulation of Neph3 gene in podocytes–key roles of transcription factors NF-kappaB and Sp1. BMC Mol Biol. 2009;10:83. doi: 10.1186/1471-2199-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Gómez M, Coutts N, Price A, Taylor MV, Bate M. Drosophila dumbfounded: a myoblast attractant essential for fusion. Cell. 2000;102:189–198. doi: 10.1016/S0092-8674(00)00024-6. [DOI] [PubMed] [Google Scholar]
- Schneider T. Restricted expression of the irreC-rst protein is required for normal axonal projections of columnar visual neurons. Neuron. 1995;15:259–271. doi: 10.1016/0896-6273(95)90032-2. [DOI] [PubMed] [Google Scholar]
- Sellick GS, Barker KT, Stolte-Dijkstra I, Fleischmann C, Coleman RJ, Garrett C, et al. Mutations in PTF1A cause pancreatic and cerebellar agenesis. Nat Genet. 2004;36:1301–1305. doi: 10.1038/ng1475. [DOI] [PubMed] [Google Scholar]
- Sellin L, Huber TB, Gerke P, Quack I, Pavenstädt H, Walz G. NEPH1 defines a novel family of podocin interacting proteins. FASEB J. 2003;17:115–117. doi: 10.1096/fj.02-0242fje. [DOI] [PubMed] [Google Scholar]
- Serizawa S, Miyamichi K, Takeuchi H, Yamagishi Y, Suzuki M, Sakano H. A neuronal identity code for the odorant receptor-specific and activity-dependent axon sorting. Cell. 2006;127:1057–1069. doi: 10.1016/j.cell.2006.10.031. [DOI] [PubMed] [Google Scholar]
- Shen K, Fetter RD, Bargmann CI. Synaptic specificity is generated by the synaptic guidepost protein SYG-2 and its receptor, SYG-1. Cell. 2004;116:869–881. doi: 10.1016/S0092-8674(04)00251-X. [DOI] [PubMed] [Google Scholar]
- Strunkelnberg M, Bonengel B, Moda LM, Hertenstein A, de Couet HG, Ramos RG, et al. rst and its paralogue kirre act redundantly during embryonic muscle development in Drosophila. Development. 2001;128:4229–4239. doi: 10.1242/dev.128.21.4229. [DOI] [PubMed] [Google Scholar]
- Sugie A, Umetsu D, Yasugi T, Fischbach KF, Tabata T. Recognition of pre- and postsynaptic neurons via nephrin/NEPH1 homologs is a basis for the formation of the Drosophila retinotopic map. Development. 2010;137:3303–3313. doi: 10.1242/dev.047332. [DOI] [PubMed] [Google Scholar]
- Tarpey PS, Smith R, Pleasance E, Whibley A, Edkins S, Hardy C, et al. A systematic, large-scale resequencing screen of X-chromosome coding exons in mental retardation. Nat Genet. 2009;41:535–543. doi: 10.1038/ng.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan RS. Cellular signaling in developmental chondrogenesis: N-cadherin, Wnts, and BMP-2. J Bone Joint Surg Am. 2003;85(A Suppl 2):137–141. doi: 10.2106/00004623-200300002-00019. [DOI] [PubMed] [Google Scholar]
- Wilson LM. Methods in Cell Biology. London: Academic Press; 1996. [Google Scholar]
- Yajima H, Hara K, Ide H, Tamura K. Cell adhesiveness and affinity for limb pattern formation. Int J Dev Biol. 2002;46:897–904. [PubMed] [Google Scholar]
- Young HM, Anderson RB, Newgreen DF. Neural Crest. In: Lemke G, editor. Developmental Neurobiology. 1. London: Academic Press; 2009. pp. 271–281. [Google Scholar]
- Yue Y, Su J, Cerretti DP, Fox GM, Jing S, Zhou R. Selective inhibition of spinal cord neurite outgrowth and cell survival by the Eph family ligand ephrin-A5. J Neurosci. 1999;19:10026–10035. doi: 10.1523/JNEUROSCI.19-22-10026.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


