Abstract
Influenza (H1N1) 2009 occurred in Mexico in April 2009, quickly spread around the world, and was found in Japan in May. Many pediatric patients experienced encephalopathy, acute respiratory distress syndrome, and severe pneumonia. The subjects of this study were 31 pediatric patients who needed mechanical ventilation due to respiratory failure caused by influenza (H1N1) 2009 as reported to the Emergency Medical Information Center of the Japan Pediatric Society in Kanagawa Prefecture in Japan from August 1 to December 31, 2009. The diagnosis of influenza (H1N1) 2009 infection was based on positive results of a real-time polymerase chain reaction. No patient was diagnosed as having a bacterial infection. The average arterial PaO2/FiO2 ratio was significantly decreased to 126. Atelectasis was revealed by chest X-ray in 90.3% of subjects. There was one plastic bronchitis patient. Anti-influenza drugs were used at an average of 14.9 h after onset. Five patients showed abnormal behavior as a complication of encephalopathy. We found that respiratory failure progressed rapidly. The type of respiratory failure was oxygenation failure. It was helpful to attempt to remove more sputum in these cases. Pediatric patients with respiratory failure from influenza (H1N1) 2009 should be carefully monitored for the onset of encephalopathy.
Keywords: Influenza (H1N1) 2009, Children, Respiratory failure, Encephalopathy
Introduction
Influenza (H1N1) 2009 occurred in Mexico in April 2009, quickly spread across the world, and was found in Japan in May 2009 [1] (http://idsc.nih.go.jp/disease/swine_influenza/2009idsc/report_kobe1.html). Later in the epidemic, many pediatric patients experienced encephalopathy, acute respiratory distress syndrome (ARDS), and severe pneumonia [2–6]. The peak of the epidemic occurred in Japan in November 2009, and the first wave subsided in January 2010.
To prepare for the expected second and third waves, we examined the clinical cases of respiratory failure in children caused by influenza (H1N1) 2009 in Kanagawa Prefecture in Japan in 2009.
Materials and methods
Subjects were 31 pediatric patients who needed mechanical ventilation due to respiratory failure caused by influenza (H1N1) 2009 as reported to the Emergency Medical Information Center of the Japan Pediatric Society Regional Meeting in Kanagawa Prefecture in Japan from August 1 to December 31, 2009. The diagnosis of influenza (H1N1) 2009 infection was based on positive results of a real-time polymerase chain reaction (RT-PCR) of the patients’ sputum. We sent questionnaires to hospitals to which the patients were admitted regarding age, gender, past history, clinical course, laboratory data, the kind of anti-influenza drugs, antibiotics and steroids used, as well as complications and outcomes. We retrospectively analyzed the results. (Cooperating facilities included Kanagawa Children Medical Center, Yokohama City University Hospital, Showa University Northern Yokohama Hospital, Kawasaki Municipal Hospital, Eastern Yokohama Hospital, and Odawara Municipal Hospital.)
Results
Patient characteristics
The patients were 22 boys and 9 girls (2.44:1; see Table 1). The age range was from 0.9 to 10 years old (median age was 7 years old). There was a history of bronchial asthma in 12 patients (step 1 in 8 patients, step 2 in 2 patients, and step 3 in 1 patient according to the GINA treatment concept), atopic dermatitis in 2 patients, mental retardation in 3 patients, and isovaleric acidemia and epilepsy in 1 patient each. Forty-seven percent of the patients had a history of allergic disease. Twenty patients had contact with influenza patients in their family or in school. One patient had been vaccinated with influenza (H1N1) 2009 vaccine just 6 days before the onset of influenza (H1N1) 2009. Three patients had been vaccinated with a seasonal influenza vaccine.
Table 1.
Patient characteristics, clinical course and past history
| Case | Gender | Age (years) | Interval of onset to medication (h) | Interval of onset to ventilation (h) | Bronchial asthmaa |
|---|---|---|---|---|---|
| 1 | F | 6.6 | 10 | 6 | Step 2 |
| 2 | F | 7.9 | 18 | 18 | Step 1 |
| 3 | M | 7.3 | 10 | 24 | – |
| 4 | M | 7.8 | 3 | 18 | Step 1 |
| 5 | M | 7.6 | 24 | 24 | Step 1 |
| 6 | F | 10.4 | 8 | 6 | – |
| 7 | M | 7.7 | 12 | 12 | Step 1 |
| 8 | M | 4.3 | 2 | 10 | – |
| 9 | F | 10.7 | 18 | 36 | Step 3 |
| 10 | M | 7.7 | 30 | 30 | – |
| 11 | M | 10.8 | 24 | 24 | Step 1 |
| 12 | M | 8.6 | 12 | 18 | – |
| 13 | M | 5.9 | 3 | 11 | Step 2 |
| 14 | M | 12.5 | 12 | 12 | – |
| 15 | M | 5.8 | 2 | 10 | Step 1 |
| 16 | F | 7.3 | 6 | 12 | – |
| 17 | M | 5 | 2 | 2 | – |
| 18 | M | 6.7 | 12 | 24 | Step 1 |
| 19 | M | 7.5 | 4 | 12 | – |
| 20 | M | 3.8 | 6 | 12 | – |
| 21 | F | 6.4 | 6 | 6 | Step 1 |
| 22 | F | 8.4 | 6 | 6 | – |
| 23 | F | 4.1 | 8 | 8 | – |
| 24 | M | 0.9 | 90 | 96 | – |
| 25 | M | 6.5 | 20 | 24 | – |
| 26 | M | 6.4 | ND | ND | – |
| 27 | M | 7.2 | 6 | 12 | – |
| 28 | M | 5.8 | 24 | 24 | – |
| 29 | M | 7.3 | 7 | 7 | – |
| 30 | F | 6.6 | 10 | 10 | – |
| 31 | M | 5.7 | 16 | 24 | – |
M male, F female, ND no data
aGlobal initiative for asthma (GINA) treatment step
Symptoms and clinical courses
The average body temperatures ranged from 35.5 to 39.7°C (median was 38.0°C) in the emergency room. The average highest body temperature during the hospital course was 38.6°C. Five patients had highest body temperatures of <38.0°C (16.1%) during the hospital course. Fifteen patients had wheezing on auscultation.
An average of 17.9 h (range 6–96 h) elapsed from the beginning of fever or respiratory failure to tracheal intubation (see Table 1). Respiratory failure progressed rapidly. The average time from onset to the administration of the anti-influenza drug was 14.9 h. Sixteen cases were treated with anti-influenza drug after the onset of respiratory failure. The average time from the onset to testing positive with the influenza rapid test was 14 h. All patients had tested positive by 24.0 h from the onset.
Laboratory data and chest X-ray findings
White blood cell (WBC) counts ranged from 7,300 to 28,400/μL (mean 15,000/μL; see Table 2). Neutrophil counts were elevated to 4,800–25,800/μL. C-reactive protein (CRP) levels were elevated from 0.56 to 13.3 mg/dL (mean 4.3 mg/dL). The average hemoglobin was 13.0 g/dL, and the average platelet count was 26 × 104/μL. The average aspartate aminotransferase (AST) level was 33.5 IU/L, the average alanine aminotransferase (ALT) level was 14.9 IU/L, and the average lactate dehydrogenase (LDH) level was 309 IU/L.
Table 2.
Laboratory data, chest X-ray, and culture findings
| Case | PCO2 (mmHg) | P/F ratio | WBC (/μL) | Neu (/μL) | CRP (mg/dL) | Chest X-ray | Blood culture | Sputum culture |
|---|---|---|---|---|---|---|---|---|
| 1 | 39.4 | 100 | 16,000 | 14,900 | 8.7 | A(LU) | − | NF |
| 2 | 27.8 | 250 | 13,000 | 12,300 | 3.7 | A(RL) | − | SA |
| 3 | 54.9 | 179 | 7,300 | 6,500 | 1.6 | A(RL) | − | − |
| 4 | 37.4 | 88 | 15,600 | 14,500 | 4.2 | A(LL) | − | − |
| 5 | 38.3 | 138 | 16,800 | 15,800 | 8.6 | A(LL) | − | − |
| 6 | 45.9 | 80 | 8,400 | 7,700 | 9.8 | A(LL) | − | − |
| 7 | 67.2 | 77 | 14,300 | 13,700 | 6.7 | A(RM) | ND | SP |
| 8 | 39.8 | 63 | 27,200 | 24,800 | 7.1 | A(LU + LL) | − | − |
| 9 | 34.9 | 63 | 15,900 | 14,200 | 8.2 | A(LL) | − | − |
| 10 | 57.9 | 123 | 11,800 | 10,900 | 6.0 | A(LL) | − | ND |
| 11 | 98.5 | 93 | 14,800 | 13,600 | 2.6 | A(RM) | ND | ND |
| 12 | 73.6 | 93 | 13,500 | 12,600 | 10.9 | A(RU + LU) | − | − |
| 13 | 61.2 | 60 | 28,400 | 25,800 | 10.0 | A(RM + LU) | − | − |
| 14 | 36.8 | 109 | 19,600 | 19,000 | 3.7 | A(RL) | − | − |
| 15 | 86.2 | 60 | 9,000 | 7,700 | 1.2 | A(LL) | − | − |
| 16 | 73.0 | 177 | 9,800 | 8,500 | 1.8 | A(LU) | − | − |
| 17 | 46.8 | ND | 17,800 | ND | 7.8 | A(RM) | − | − |
| 18 | 36.8 | 136 | 21,700 | 21,100 | 10.2 | ARDS | − | NF |
| 19 | 37.3 | 208 | 15,300 | 14,700 | 0.6 | A(LU) | − | NF |
| 20 | 38.1 | 332 | 10,500 | 9,600 | 1.7 | A(LU + LL) | − | − |
| 21 | 67.2 | 143 | 16,200 | 15,100 | 10.8 | A(RU + RL + LL) | − | NF |
| 22 | 35.1 | 106 | 12,400 | 11,200 | 8.9 | A(LU) | − | − |
| 23 | 38.4 | 176 | 23,400 | 21,600 | 12.7 | M | ND | NF |
| 24 | 61.3 | 81 | 12,400 | 4,900 | 8.4 | A(RL + LL) | − | NF |
| 25 | 35.0 | 90 | 16,100 | 15,300 | 13.3 | A(LU) | − | − |
| 26 | 59.9 | 59 | 11,500 | 10,700 | 16.5 | ARDS | − | CG |
| 27 | 45.5 | 218 | 15,300 | 14,200 | 2.5 | A(RU + RM + RL) | ND | NF |
| 28 | 44.6 | 155 | 20,200 | 17,900 | 8.9 | A(RL) | − | NF |
| 29 | 49.6 | 180 | 25,800 | 22,000 | 5.3 | A(RU + RL) | ND | NF |
| 30 | 56.7 | 105 | 12,400 | 11,500 | 3.7 | A(RL) | ND | NF |
| 31 | 73.9 | 50 | 14,900 | 14,200 | 3.9 | A(RU) | ND | ND |
P/F ratio PaO2/FiO2 ratio, WBC white blood cell, Neu neutrophil, CRP C-reactive protein, A atelectasis, M minor change, ARDS acute respiratory distress syndrome, ND no data, NF normal flora, − negative, SAStaphylococcus aureus, SPStreptococcus pneumoniae, CGCandida glabrata, RU right upper lobe of lung, RM right middle lobe of lung, RL right lower lobe of lung, LU left upper lobe of lung, LL left lower lobe of lung
The type of respiratory failure was oxygenation failure. The average lowest arterial PaO2/FiO2 ratio was significantly decreased to 126, and the average PCO2 level was 51.1 mmHg.
Blood cultures were negative in all 24 cases tested. There were Staphylococcus aureus in 1 patient, Streptococcus pneumoniae in another, and Candida glabrata in yet another, based on sputum culture. However, phagocytosis was not observed in Gram stains of sputum culture. On chest X-ray, 28 patients had atelectasis (90.3%), 2 patients had ARDS, and 1 patient exhibited a minor change in plastic bronchitis. On chest CT scan, we found mucous plugs in the bronchi of this patient (Figs. 1, 2, 3).
Fig. 1.
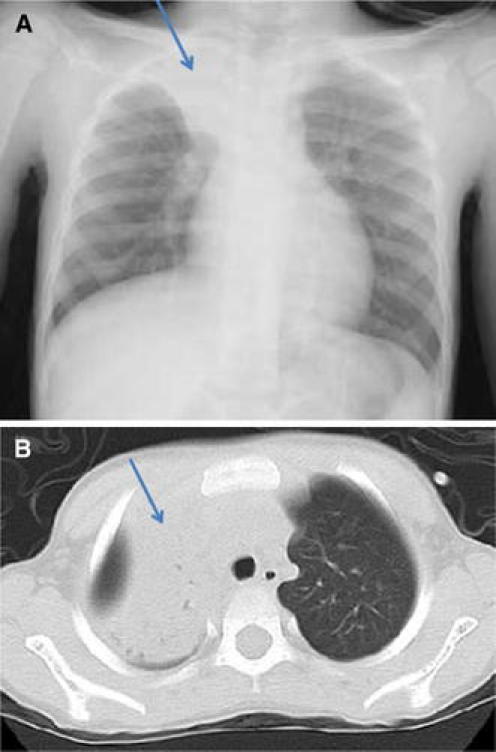
a The right upper lobe showed atelectasis and the right lung field volume was decreased (arrow). b Atelectasis was seen in the right lobe (arrow)
Fig. 2.
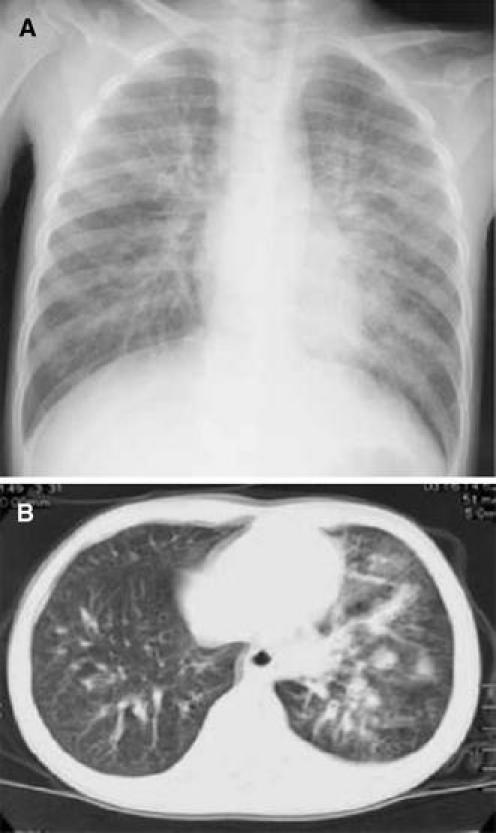
a The lung fields showed interstitial shadow enhancement. b Ground-glass attenuation was seen in the left lobe
Fig. 3.
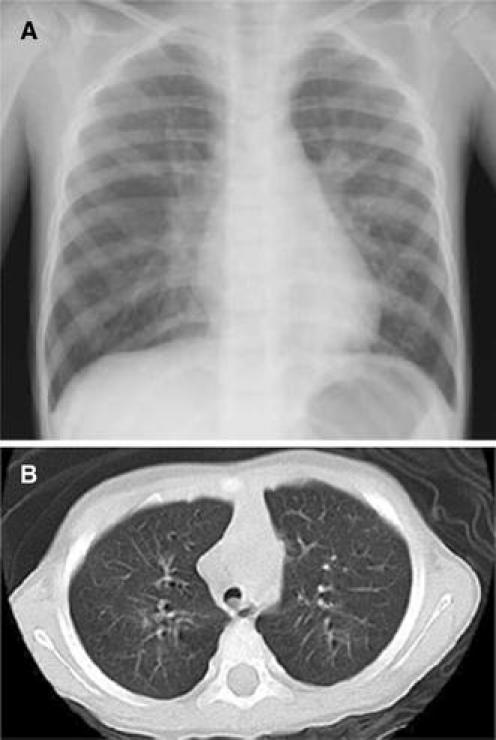
a Chest X-ray within normal limits. b There was a mucous plug in the main bronchus
Treatments
A total of 28 patients (90.3%) were treated with oseltamivir and 6 patients (19.4%) were treated with zanamivir (see Table 3). Two patients were treated with both oseltamivir and zanamivir. Treatment in 2 patients was changed from oseltamivir to zanamivir.
Table 3.
Treatments
| Case | O | Z | Antibiotics | Steroid | Dose of steroid (mg/kg/day)b | Interval of steroid (days)b |
|---|---|---|---|---|---|---|
| 1 | + | + | ABPC | PSL | 2 | 10 |
| 2 | + | + | ABPC | PSL + (mPSL pulse)a | 2a | 3a |
| 3 | + | − | ABPC/SBT | – | – | – |
| 4 | + | − | ABPC/SBT | mPSL | 3 | 7 |
| 5 | + | − | ABPC/SBT | mPSL + (mPSL pulse)a | 4a | 3a |
| 6 | + | − | ABPC/SBT | – | – | – |
| 7 | + | − | ABPC/SBT | – | – | – |
| 8 | + | − | ABPC/SBT | mPSL | 4 | 5 |
| 9 | + | − | ABPC/SBT | mPSL | 4 | 14 |
| 10 | + | − | ABPC/SBT | – | – | – |
| 11 | + | − | ABPC/SBT | mPSL | 2 | 4 |
| 12 | + | − | ABPC/SBT | (mPSL pulse)a | –a | –a |
| 13 | + | − | ABPC/SBT | mPSL | 2 | 3 |
| 14 | + | − | ABPC/SBT | mPSL | 4 | 3 |
| 15 | + | − | ABPC/SBT | mPSL | 4 | 13 |
| 16 | + | − | – | mPSL | 3 | 4 |
| 17 | + | − | ABPC/SBT | – | – | – |
| 18 | + | − | CTRX | PSL | 2 | 5 |
| 19 | + | − | CTRX | PSL | 2 | 5 |
| 20 | + | − | ABPC/SBT | PSL + (mPSL pulse)a | 2a | 4a |
| 21 | + | − | CPR | – | – | – |
| 22 | + | − | CTRX | PSL | 2 | 4 |
| 23 | + | − | CTRX | PSL | 2 | 4 |
| 24 | + | − | CTRX | PSL | 2 | 4 |
| 25 | + | − | CTRX | PSL | 2 | 6 |
| 26 | + | + | CAZ | – | – | – |
| 27 | + | − | – | PSL | 1.7 | 4 |
| 28 | − | + | MEPM | PSL | 1 | 5 |
| 29 | − | + | ABPC/SBT | mPSL | 2 | 4 |
| 30 | + | + | ABPC/SBT | PSL | 2 | 8 |
| 31 | + | − | ABPC/SBT | PSL | 2 | 1 |
O oseltamivir, Z zanamivir, ABPC ampicillin, ABPC/SBT ampicillin/sulbactam, CAZ ceftazidime, CPR cefpirome, CTRX ceftriaxone, MEPM meropenem, mPSL methylprednisolone, PSL prednisolone
aThey received methylprednisolone pulse therapy for encephalopathy after extubation
bAside from the dose of steroid given with mPSL pulse therapy
Twenty-nine patients (93.5%) were given antibiotic therapy, which included ampicillin/sulbactam in 18 patients, ceftriaxone in 6 patients, and ampicillin, meropenem, cefpirome, and ceftazidime in 1 patient each. Two patients were not treated with antibiotic therapy.
Twenty-five patients (80.6%) had been administered steroids, including prednisolone in 12 patients, methylprednisolone in 9 patients and methylprednisolone pulse therapy in 4 patients. Seven patients were not treated with steroids.
Complications
There were 5 cases of encephalopathy (16.1%; see Table 4). Consciousness was clear in 4 patients before tracheal intubation, 1 patient showed abnormal behavior before tracheal intubation, and an 11-month-old male patient did not appear to be doing well. Five patients had been diagnosed with encephalopathy when impaired consciousness and abnormal behavior were observed after extubation. Electroencephalogram (EEG) findings showed diffuse high-voltage-amplitude slow waves in 5 patients. Brain magnetic resonance imaging (MRI) findings were normal in 4 patients. In the other patient, brain MRI findings showed high-intensity areas of the temporal lobe bilaterally.
Table 4.
Complications and outcomes
| Case | Interval of intubation (days) | Plastic bronchitis | Encephalopathy | Outcome |
|---|---|---|---|---|
| 1 | 7 | − | − | Cure |
| 2 | 5 | − | + | Left palsy |
| 3 | 1 | − | − | Cure |
| 4 | 7 | − | − | Cure |
| 5 | 1 | − | + | Cure |
| 6 | 2 | − | − | Cure |
| 7 | 2 | − | − | Cure |
| 8 | 2 | − | − | Cure |
| 9 | 6 | − | − | Cure |
| 10 | 2 | − | − | Cure |
| 11 | 1 | − | − | Cure |
| 12 | 2 | − | − | Cure |
| 13 | 1 | − | − | Cure |
| 14 | 2 | − | − | Cure |
| 15 | 15 | − | − | Cure |
| 16 | 0.5 | − | − | Cure |
| 17 | 1 | − | − | Cure |
| 18 | 2 | − | + | Cure |
| 19 | 2.5 | − | + | Cure |
| 20 | 3 | − | − | Cure |
| 21 | 4 | − | − | Cure |
| 22 | 5 | − | − | Cure |
| 23 | 3 | + | − | Cure |
| 24 | 8 | − | + | Cure |
| 25 | 3 | − | − | Cure |
| 26 | Died | Died | Died | Died |
| 27 | 3.5 | − | − | Cure |
| 28 | 3.5 | − | − | Cure |
| 29 | 5 | − | − | Cure |
| 30 | 3.5 | − | − | Cure |
| 31 | Died | Died | Died | Died |
Outcomes
The mean period of hospitalization was 11.6 days (range 5–40 days), and the mean period of intubation was 3.5 days (range 0.5–8 days; see Table 4).
Twenty-eight patients had no complications. One patient showed hemiplegia and 2 patients died (1 ARDS case and 1 myocarditis case). The consciousness of the hemiplegia patient was clear before tracheal intubation. The patient had been diagnosed with encephalopathy when impaired consciousness and abnormal behavior were observed after extubation.
Discussion
In 31 patients with severe respiratory failure caused by influenza (H1N1) 2009 in Kanagawa Prefecture in Japan, the average WBC counts were 15,000/μL, and CRP levels were elevated, averaging 4.3 mg/dL. No patients were diagnosed with bacterial infections. Thus, influenza (H1N1) 2009 infection alone was thought to be responsible for WBC and CRP elevations in patients with severe respiratory failure.
A few reports have suggested that inflammatory cytokines are elevated in patients with influenza (H1N1) 2009. Ichikawa et al. [7] reported that inflammatory cytokine levels in the blood of patients with respiratory failure with influenza (H1N1) 2009 were elevated. Kawashima et al. reported that cytokine and chemokine levels in the sputa of pediatric patients with influenza (H1N1) 2009 were high [8].
In light of these findings, in pediatric patients with severe respiratory failure, it appears that inflammatory cytokines may be involved in the pathogenesis of influenza (H1N1) 2009 by increasing the number of leukocytes and the levels of CRP.
Chest X-ray findings revealed atelectasis in 93.5% of cases with respiratory failure. These results were similar to previous reports. Plastic bronchitis, which produces mucous plugging of the respiratory tree and atelectasis, has attracted much attention [9–11]. In plastic bronchitis patients, it is necessary to remove the mucous plugs after tracheal intubation. There was one patient with plastic bronchitis in this study. There were 28 patients with atelectasis. Therefore, it seemed to be an important to remove mucous plugs in pediatric patients with severe respiratory failure with influenza (H1N1) 2009.
In this study, anti-influenza drugs such as oseltamivir or zanamivir were administered at an average of 14.9 h after onset of fever. In other words, anti-influenza drugs were administered soon after the onset. In Japan, it has been reported that the administration of anti-influenza drugs early after onset made the hospitalization period shorter and reduced mortality from influenza (H1N1) 2009 compared to other countries [12–14]. In 15 cases, anti-influenza drugs were administered prior to the onset of respiratory failure, but then the respiratory failure became severe enough to require mechanical ventilation. Because there were patients who showed respiratory failure with influenza (H1N1) 2009 in spite of the early administration of anti-influenza drugs, preventive measures are needed before the second wave of influenza (H1N1) 2009 occurs.
Interestingly, 5 out of 31 patients (16.1%) showed abnormal behavior and encephalopathy. Four patients showed no impairment of consciousness before tracheal intubation, and the onset of encephalopathy had not been recognized. According to Japanese guidelines for influenza encephalopathy, 4 patients had been diagnosed with encephalopathy when impaired consciousness and abnormal behavior were observed after extubation. Because sedation is required for respiratory control, it is difficult to confirm the level of consciousness. Therefore, pediatric patients with severe respiratory failure due to influenza (H1N1) 2009 should be carefully monitored for the existence of encephalopathy. The recommended treatment of influenza encephalopathy is steroid pulse therapy (http://www.jpeds.or.jp/influenza/influenza090928.pdf). In patients with respiratory failure and encephalopathy of influenza (H1N1) 2009, steroid pulse therapy should be considered the treatment of choice.
Acknowledgments
The authors thank Dr. Takuya Hayashi of the Kanagawa Children Medical Center, Dr. Yo Umeda of Showa University Northern Yokohama Hospital, Dr. Ayumi Nakao of Kawasaki Municipal Hospital, Dr. Fumihiro Sawa of Eastern Yokohama Hospital, and Dr. Nao Shimizu of Odawara Municipal Hospital.
References
- 1.Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 2.Japanese Ministry of Health, Labour and Welfare. Death due to pandemic (H1N1) 2009 infection. http://www.mhlw.go.jp/kinkyu/kenkou/influenza/houdou/2010/03/dl/infuh0315-01.pdf.
- 3.Japan Pediatric Society. Emergency report of updated surveillance data regarding pandemic (H1N1) 2009 infection in Japanese children. http://www.jpeds.or.jp/influenza/influenza_091119.pdf.
- 4.Yoshida Y, Nishiyama M, Ishikawa T, Imai K, Wakamatsu H, Kawana A, et al. Treatment strategy of pneumonia caused by 2009 H1N1 influenza. J Jpn Pediatr Soc. 2010;114:85–87. [Google Scholar]
- 5.Lister P, Reynolds F, Parslow R, Chan A, Cooper M, Plunkett A, et al. Swine-origin influenza virus H1N1, seasonal influenza virus, and critical illness in children. Lancet. 2009;374:605–607. doi: 10.1016/S0140-6736(09)61512-9. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S, Havens PL, Chusid MJ, Willoughby RE, Jr, Simpson P, Henrickson KJ, et al. Clinical and epidemiologic characteristics of children hospitalized with 2009 pandemic H1N1 influenza A infection. Pediatr Infect Dis J. 2010;29:591–594. doi: 10.1097/INF.0b013e3181d73e32. [DOI] [PubMed] [Google Scholar]
- 7.Ichikawa K, Mori M, Oyama Y, Ogawa M, Shiojima H, Ebina K, et al. Respiratory failure of a 2009 influenza (H1N1) infection in childhood. J Jpn Pediatr Soc. 2010;114:78–81. [Google Scholar]
- 8.Takano T, Tajiri H, Kashiwagi Y, Kimura S, Kawashima H. Cytokine and chemokine response in children with the 2009 pandemic influenza A (H1N1) virus infection. Eur J Clin Microbiol Infect Dis. 2011;30:117–120. doi: 10.1007/s10096-010-1041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mori T, Morii M, Terada K, Wada Y, Kuroiwa Y, Hotsubo T, et al. Clinical characteristics and computed tomography findings in children with 2009 pandemic influenza A (H1N1) viral pneumonia. Scand J Infect Dis. 2011;43:47–54. doi: 10.3109/00365548.2010.515607. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa M, Inamo Y, Fuchigami T, Hashimoto K, Morozumi M, Ubukata K, et al. Bronchial casts and pandemic (H1N1) 2009 virus infection. Emerg Infect Dis. 2010;16:344–346. doi: 10.3201/eid1602.091607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terano C, Miura M, Fukuzawa R, Saito Y, Arai H, Sasaki M, et al. Three children with plastic bronchitis associated with 2009 H1N1 influenza virus infection. Pediatr Infect Dis J. 2011;30(1):80–82. doi: 10.1097/INF.0b013e3181f10fff. [DOI] [PubMed] [Google Scholar]
- 12.Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med. 2009;361:1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 13.Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362:1708–19. [DOI] [PubMed]
- 14.Patients hospitalized with 2009 pandemic influenza A (H1N1)—New York City, May 2009. MMWR Morb Mortal Wkly Rep 2010;58:1436–40. [PubMed]


