Abstract
Most patients with Hodgkin lymphoma (HL) are cured with first and second-line treatment; however for those who fail high dose chemoradiotherapy with autologous stem cell transplant (HDT-ASCT), outcome is unknown. This report is an analysis of patients with relapsed and primary refractory HL who were treated with HDT-ASCT and failed due to progression of disease (POD). Two hundred and two patients received HDT-ASCT at Memorial Sloan Kettering Cancer Center for relapsed or refractory HL between December 1994 and December 2005 and 71 failed due to POD. The median survival following HDT-ASCT failure was 25 months. Only 16 (23%) of the 71 patients are currently alive, 9 of whom are in remission. Multivariate analysis revealed two factors associated with poor outcome: relapse within 6 months of HDT-ASCT and primary refractory disease. The only factor associated with improved survival was the ability to receive a second transplant, in particular, reduced intensity allogeneic transplant (RIT). Novel therapies are needed for patients who fail HDT-ASCT, particularly those with primary refractory disease and those who relapse within 6 months of HDT-ASCT. Future studies should focus on prospectively evaluating RIT following HDT-ASCT failure in patients with remission duration from HDT-ASCT of greater than 6 months.
Introduction
While the majority of patients diagnosed with Hodgkin lymphoma (HL) are cured of their disease with primary treatment, up to 20% of patients have primary refractory disease and an additional 20% will relapse following frontline treatment(Duggan, et al 2003). The standard treatment for relapsed and primary refractory HL is salvage chemotherapy (ST) followed by high dose chemoradiotherapy and autologous stem cell transplant (HDT-ASCT)(Linch, et al 1993, Schmitz, et al 2002); this treatment achieves 5 year PFS rates of approximately 50% (Moskowitz, et al 2001, Sureda, et al 2001). The most common cause of HDT-ASCT failure is progression of disease (POD) (Kewalramani, et al 2003, Lavoie, et al 2005, Paltiel, et al 2003, Vose, et al 1992).
The reported median survival following HDT-ASCT failure ranges from 7.3 to 25 months (Lavoie, et al 2005, Paltiel, et al 2003, Vose, et al 1992). Treatment options for these patients include additional chemotherapy (such as MOPP or gemcitabine based treatment), radiotherapy, a second autologous transplant, or reduced-intensity allogeneic transplant (RIT). It is unknown whether particular therapies or patient characteristics influence survival following HDT-ASCT failure. We conducted a retrospective review of patients who underwent HDT-ASCT for relapsed or primary refractory HL at our institution from 1994-2005 and failed HDT-ASCT because of disease progression. We aimed to determine the overall survival and the factors influencing outcome for this patient population.
Methods
Patients
Consecutive patients with HL who received high dose chemotherapy with HDT-ASCT for relapsed or primary refractory disease from December 1994 to December 2005 and failed due to progression of disease were identified. Relapsed or primary refractory disease was confirmed by biopsy. Patient records were reviewed to determine time to HDT-ASCT failure, treatments following failure, and disease status. A waiver of authorization to carry out this retrospective analysis was approved by the IRB at Memorial Sloan Kettering Cancer Center (MSKCC).
Treatment
Patients were treated on one of four consecutive IRB approved protocols at MSKCC or as per protocol. Patients received ICE (ifosfamide, carboplatin, and etoposide) salvage therapy (Moskowitz, et al 1999) followed by 1 of 4 possible conditioning regimens based upon previous radiation exposure and protocol availability. In addition, one study allowed patients with high risk disease, as defined as having active B symptoms, remission duration < 1 year and extranodal involvement, to receive tandem transplants; this treatment regimen consisted of high dose ICE and autologous stem cell rescue followed by a second HDT-ASCT or allogeneic stem cell transplant (if an HLA-identical sibling was available) (Moskowitz, et al 2009). Chemosensitive disease, defined as attaining at least a minor response (less than 50% reduction in tumor size) to treatment, was a requirement prior to proceeding to HDT-ASCT, except for patients receiving tandem transplants, whose cytoreductive therapy required ASCT.
Statistical analysis
Time to progression (TTP) was defined as time from stem cell infusion to documentation of disease progression. Primary refractory disease was defined as failure to achieve a complete remission following first-line treatment, documented by biopsy. Survival time was calculated from the time of HDT-ASCT failure using the method of Kaplan and Meier. Hazard ratios (HR) were estimated from Cox regression models. Wald statistics were used to generate the p-values. Both univariate analysis and multivariate analysis were performed. For the multivariate analysis, only significant factors were retained in the final model, which was determined by the stepwise backward selection algorithm. Propensity scores were used to adjust for selection bias when evaluating the effect of second transplantation. The propensity score took into account the number of pre-HDT-ASCT risk factors, time to transplant failure, and number of chemotherapy regimens administered after transplant failure.
Results
Between December 1994 and December 2005, 196 patients with relapsed or primary refractory HL were treated with HDT-ASCT on one of four consecutive IRB approved protocols at MSKCC. Six additional patients who were treated off study but according to the available protocol at the time were included in the analysis.
Of the 202 patients who underwent HDT-ASCT between December 1994 and December 2005, there were 81 transplant failures, which included 10 patients with treatment related complications (4 deaths within 100 days from transplant, 5 deaths due to secondary malignancies, and 1 death 10 years after transplant due to cardiomyopathy) and 71 patients with POD.
Characteristics of the 71 HL-related failures are presented in Table I and included 43 males (60%). The median age at the time of transplant was 29 (range 17-65). Thirty-one (44%) patients had primary refractory disease. The majority (62%) of patients had a partial response to ICE salvage therapy while 20% had a complete response. Twenty-six (37%) patients received a TLI-based conditioning regimen prior to HDT-ASCT. Tandem transplants were intended for 12 patients; however 5 patients progressed following the first transplant and therefore did not proceed to the second transplant. Of the 7 completed tandem transplants, 2 included allogeneic transplantation from an HLA-identical sibling. Treatments administered following HDT-ASCT failure included gemcitabine-based treatment (63%), MOPP (39%), radiation (13%), and second transplantation (34%).
Table I.
Patient Characteristics
| Characteristics | Patients (n=71) |
|---|---|
| Median age at transplant, years (range) | 29 (17-65) |
| Males (%) | 43 (60%) |
| Response to first-line therapy | |
| Relapse | 40 (56%) |
| Primary refractory | 31 (44%) |
| Risk factors prior to salvage treatment | |
| 0 | 6 (9%) |
| 1 | 15 (21%) |
| 2 | 31 (44%) |
| 3 | 19 (27%) |
| Response to ICE | |
| CR | 14 (20%) |
| PR | 44 (62%) |
| MR | 6 (9%) |
| POD | 7 (10%) |
| Conditioning regimen | |
| TLI based | 26 (37%) |
| Chemotherapy only | 45 (64%) |
| Tandem transplants | |
| Intended | 12 |
| Achieved | 7 |
| Auto-Auto | 5 |
| Auto-Allo | 2 |
| Time to HDT-ASCT failure (median) | 5.7 months |
| Within 6 months (%) | 36 (51%) |
| Within 12 months (%) | 56 (79%) |
| Within 24 months (%) | 68 (96%) |
| Regimens following HDT-ASCT failure | |
| Gemcitabine-based | 45 |
| MOPP | 28 |
| Radiation | 9 |
| Second transplant | 24 (5 Autologous, 19 Allogeneic)* |
| DLI | 5** |
| Disease status | |
| Alive in remission | 9 (13%) |
| Alive with disease | 7 (10%) |
| Deceased | 55 (77%) |
This does not include the seven patients who received second transplants as part of tandem transplants
This includes two patients who received DLI following Auto-Allo tandem transplants CR, complete response; PR, partial response; MR, minor response
The majority of patients (79%) relapsed within 1 year following HDT-ASCT and all but three patients relapsed within 2 years. The median time to progression (TTP) was 5.7 months (range 1.4 to 83.5 months). The median survival from HDT-ASCT failure was 25 months (Fig 1). Sixteen (23%) of the 71 patients are currently alive, with a median follow-up for surviving patients of 46 months. Among the 16 surviving patients, there are 5 females and 11 males, and the median age at the time of HDT-ASCT was 32 (range 22-45). Only nine (13%) patients are in remission from HL, of whom there are 2 females and 7 males (median follow-up 26 months, range 18 to 124 months). Limited data is available regarding gonadal function for the patients currently in remission. One woman underwent laparoscopic oophorectomy (with removal of 1 ¼ ovaries) for fertility preservation prior to HDT-ASCT and subsequently developed amenorrhea. The second woman had normal menstruation at the time of HDT-ASCT failure; however data regarding her fertility following her subsequent RIT (performed at another institution) is not available. For the eight patients who are currently in remission and continue to be followed at our institution, there have been no documented births.
Fig 1.
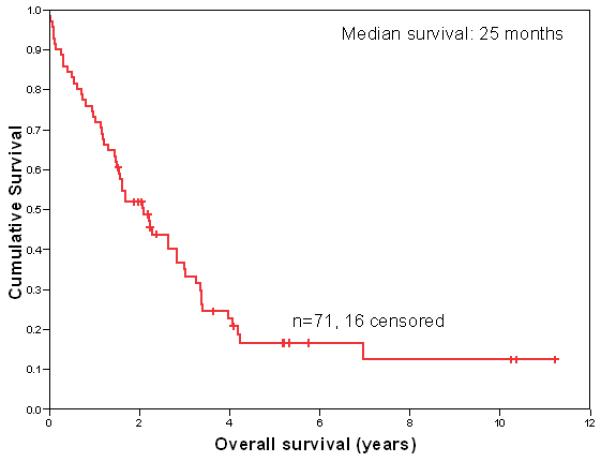
Overall Survival Following HDT-ASCT Failure
In univariate analysis for survival, three factors were found to be statistically significant: response to first line therapy, relapse within 6 months of transplant, and treatment with a second transplant (Table II). Response to first line therapy and treatment with a second transplant remained significant in multivariate analysis (p=0.007 and p=0.004 respectively). Relapse within 6 months of HDT-ASCT was marginally significant (p=0.06) after multivariate analysis. After using a propensity score adjustment to account for the selection bias associated with undergoing a second transplant, second transplant remained significant for impacting survival (p=0.002).
Table II.
Univariate and multivariate analysis of survival following HL-related HDT-ASCT failure
| Variable | Hazard ratio | p† | p‡ |
|---|---|---|---|
| Response to first line therapy (refractory vs relapsed) |
0.57 | 0.04 | 0.007 |
| Risk factors prior to salvage therapy (3 vs 0) |
1.97 | 0.23 | |
| Response to ICE (CR vs PR, MR) |
1.27 | 0.52 | |
| Conditioning regimen (TLI vs no TLI) |
0.92 | 0.78 | |
| Time to HDT-ASCT failure | |||
| After 6 months | 0.49 | 0.01 | 0.06 |
| After 12 months | 0.48 | 0.06 | |
| Regimens following HDT- ASCT failure |
|||
| Gemcitabine-based | 1.31 | 0.34 | |
| MOPP | 0.98 | 0.96 | |
| Radiation | 0.67 | 0.27 | |
| Second transplant | 2.26 | 0.009 | 0.004 |
Univariate analysis
Multivariate analysis
The median survival for patients who relapsed within 6 months of HDT-ASCT (n=36) was 15 months compared to 36 months for patients who relapsed after 6 months (n= 35)(Fig 2). Patients with a history of primary refractory disease (n=31) survived a median of 19 months compared to 27 months for patients who achieved a complete response to their primary treatment (n=40). Eight of the 31 patients with primary refractory disease were treated with RIT following HDT-ASCT and three are currently in remission.
Fig 2.
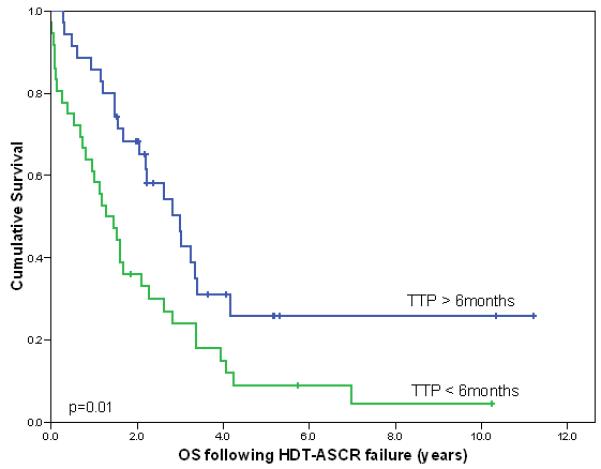
Survival following relapse within 6 months and after 6 months from HDT-ASCT
Outcomes following second transplant appear in Table III. Among the second transplants, there were 19 RIT and 5 second autologous transplants. The median survival for patients who underwent a second transplant was 40 months compared to 19.3 months for patients who were unable to proceed to a second transplant (p=0.004). Three autologous transplant patients (1 in remission) and 7 allogeneic transplant patients (6 in remission) are currently alive. Deaths in the allogeneic transplant group were secondary to GVHD (2 patients), transplant related mortality (2 patients), and relapsed disease (8 patients). Direct comparison of second autologous transplants and RIT following HDT-ASCT failure reveals that second autologous transplants appear to be associated with improved survival (p=0.04), however this is likely due to the marked difference in follow-up time for patients who underwent autologous transplants versus RIT (mean follow-up time for survivors: 8.6 years compared to 2.5 years respectively).
Table III.
Outcomes following second transplant
| Second transplant | n |
|---|---|
| Allogeneic transplant (n=19) | |
| Remission | 6 |
| Alive with disease | 1 |
| HL-related deaths | 8 |
| Transplant related deaths | 4 |
|
| |
| Autologous transplant (n=5) | |
| Remission | 1 |
| Alive with disease | 2 |
| HL-related deaths | 2 |
Five patients received donor leukocyte infusions (DLI) for relapse following allogeneic transplant (median time to DLI after transplant: 8 months; range: 6-22 months). Four of the 5 patients developed GVHD following DLI, including the patient who had a partial response.
Table IV displays the treatment course for the 9 patients in this series who are currently in remission. Interestingly, 2 of these patients achieved prolonged remission without receiving a second transplant. One patient received only chemotherapy and another received only radiation therapy. The patient who received radiotherapy alone had relapsed disease isolated to the mediastinum, an area which had been radiated with his primary treatment. He was treated with intensity-modified radiation therapy (IMRT) in order to reduce radiation exposure to previously irradiated tissue. He remains disease-free over 5 years out from HDT-ASCT failure. The patient who was salvaged with chemotherapy alone relapsed over 6 years following HDT-ASCT. This patient remains disease-free over 2 years after HDT-ASCT failure.
Table IV.
Characteristics of patients who are in remission
| Patient | TTP (months) |
Post-HDT-ASCT treatment | OS (months) |
|---|---|---|---|
| 1 | 7.7 | MOPP, vinblastine, RIT | 26.2 |
| 2 | 8.5 | GND, RIT | 24.4 |
| 3 | 9.8 | MOPP, RIT | 23.7 |
| 4 | 11.2 | IFRT (with IMRT) | 62.0 |
| 5 | 14.3 | GND, RIT | 43.6 |
| 6 | 15.5 | GND, RIT | 18.2 |
| 7 | 19.0 | Methotrexate/bleomycin/vinblastine, HDT-ASCT |
124.3 |
| 8 | 29.3 | GND, MOPP, RIT | 28.4 |
| 9 | 83.5 | GND | 26.7 |
TTP: time to progression following HDT-ASCT; IFRT: involved field radiation therapy; IMRT: intensity-modified radiation therapy; GND: gemcitabine, vinorelbine, and liposomal doxorubicin; HDT-ASCT: high dose chemotherapy and autologous stem cell rescue; RIT: reduced intensity allogeneic stem cell transplant
Discussion
Limited data is available regarding the outcome for patients with relapsed or refractory HL who fail HDT-ASCT. The median survival in our series was 25 months. We found two factors to be associated with poor outcome following HDT-ASCT failure: relapse within 6 months of HDT-ASCT and a history of primary refractory disease. There were 36 patients who relapsed within 6 months and only 3 are alive, all with active disease. Among the 5 of these patients who proceeded to RIT, 4 died due to POD and 1 died from GVHD. In contrast, of the 14 patients who relapsed after 6 months and proceeded to RIT, 6 (43%) are currently in remission. Therefore RIT was unsuccessful for patients who relapsed within 6 months of HDT-ASCT. Overall, the prognosis for these patients is dismal and novel treatment strategies are needed.
It was surprising that a history of primary refractory disease was associated with poor outcome following HDT-ASCT failure since the majority of patients were required to respond to ICE-based salvage treatment prior to proceeding to HDT-ASCT. The German Hodgkin Lymphoma Study Group previously reported outcomes and prognostic factors for patients with primary progressive disease, defined as disease progression during induction treatment or within 90 days after the end of treatment (Josting, et al 2000). While the outcome for this group was poor overall, three factors associated with inferior OS and PFS were Karnofsky performance scores less than 90, age over 50, and never achieving a temporary remission with induction therapy. Patients who never attained a temporary remission with first-line chemotherapy had an 18% lower survival probability than patients who relapsed within 90 days of treatment, indicating that this group may have biologically more aggressive disease. In our series, all of the patients defined as “primary refractory” never achieved a temporary remission with induction therapy and thus would be considered poor risk. Fewer patients with primary refractory disease underwent a second transplant following HDT-ASCT failure (26% compared to 40%) which suggests a lower response rate to salvage therapy following HDT-ASCT failure. Nevertheless, 3 of the 31 patients with primary refractory disease are currently in remission following treatment with RIT, indicating that these patients can respond to allogeneic transplant. Thus, in our series, patients who never achieved a temporary remission to initial therapy do appear to have biologically more aggressive disease, which is reflective in a lower response rate to salvage therapy following HDT-ASCT failure; however if they respond to salvage therapy then referral for a RIT is reasonable.
In multivariate analysis, only the ability to receive a second transplant improved outcome following HDT-ASCT failure. Twenty-four patients (34%) underwent second transplants, the majority of whom (80%) underwent RIT. Undoubtedly there is significant bias associated with which patients received a second transplant. In particular, only patients who demonstrated chemosensitivity to salvage therapy were referred to RIT in our series. Despite this, RIT was associated with only 32% PFS at 2 years. This result is consistent with data recently reported by the EBMT which included 285 patients, the largest published series of HL patients treated with RIT thus far (Robinson, et al 2008). In their series, the OS and PFS at 3 years were 29% and 25% respectively. In addition, Thomson, et al recently reported the outcomes for 38 patients who underwent RIT compared to 34 historical controls(Thomson, et al 2008). The historical controls presumably would have been eligible for RIT because they demonstrated chemosensitivity to salvage treatment and survived at least 12 months following HDT-ASCT failure. The overall survival for the RIT group was 48% at 10 years compared to 15% for the historical control group. Progression free survival (PFS) for the RIT group was 34% at 5 years. While about one-third of patients achieved prolonged PFS in each of the series described above, a considerably high relapse rate associated with RIT was reported from the Fred Hutchinson Cancer Research Center. In their series, the 3 year PFS and OS for 35 patients with relapsed or refractory HL treated with RIT following failed HDT-ASCT were 8% and 35% respectively (Baron, et al 2006). The inferior results observed in this series may have been due to the inclusion of patients with chemoresistant disease (29% of the HL patients). Clearly, the role for RIT following HDT-ASCT failure is not certain and prospective studies investigating this are needed.
Although RIT had a positive impact on survival in our series, it is not essential for long term survival. Two patients in our series achieved prolonged remission with radiotherapy or chemotherapy alone. It is possible that prolonged time to relapse is indicative of disease that is amenable to treatment with chemotherapy alone. In addition, low volume relapse within a radiation field appears amenable to radiotherapy alone.
Despite the apparent advantage associated with RIT, only 1 of the patients who received DLI in our series responded. The response rate to DLI in HL has been reported as 30-50% (Schmitz, et al 2007) and in the EBMT series it was 32%(Robinson, et al 2008). Four of the 5 patients in our series who received DLI developed GVHD, which has been reported to be associated with response (Thomson, et al 2008). The low response rate in our series may simply be due to the small number of patients who received DLI.
In conclusion, relapse within 6 months of HDT-ASCT and a history of primary refractory disease are associated with poor outcome following HL-related HDT-ASCT failure. Treatment with RIT following HDT-ASCT failure can lead to remission and may prolong survival; however this was not the case for patients transplanted following a less than 6 month remission duration after HDT-ASCT. Based on this data, we believe that patients who relapse greater than 6 months after HDT-ASCT should be offered curative treatment which may include RIT and other investigative therapies. Furthermore, patients referred for RIT must have chemosensitive disease at the time of transplant. For patients who relapse within 6 months of HDT-ASCT, palliative treatment is most appropriate. Future studies should focus on prospectively evaluating RIT following HDT-ASCT failure and developing new therapies for patients who fail HDT-ASCT, particularly those with primary refractory disease and those who relapse within 6 months of HDT-ASCT.
References
- Baron F, Storb R, Storer BE, Maris MB, Niederwieser D, Shizuru JA, Chauncey TR, Bruno B, Forman SJ, McSweeney PA, Maziarz RT, Pulsipher MA, Agura ED, Wade J, Sorror M, Maloney DG, Sandmaier BM. Factors associated with outcomes in allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning after failed myeloablative hematopoietic cell transplantation. Journal of clinical oncology. 2006;24:4150–4157. doi: 10.1200/JCO.2006.06.9914. [DOI] [PubMed] [Google Scholar]
- Duggan DB, Petroni GR, Johnson JL, Glick JH, Fisher RI, Connors JM, Canellos GP, Peterson BA. Randomized Comparison of ABVD and MOPP/ABV Hybrid for the Treatment of Advanced Hodgkin’s Disease: Report of an Intergroup Trial. Journal of clinical oncology. 2003;21:607–614. doi: 10.1200/JCO.2003.12.086. [DOI] [PubMed] [Google Scholar]
- Josting A, Rueffer U, Franklin J, Sieber M, Diehl V, Engert A. Prognostic factors and treatment outcome in primary progressive Hodgkin lymphoma: a report from the German Hodgkin Lymphoma Study Group. Blood. 2000;96:1280–1286. [PubMed] [Google Scholar]
- Kewalramani T, Nimer SD, Zelenetz AD, Malhotra S, Qin J, Yahalom J, Moskowitz CH. Progressive disease following autologous transplantation in patients with chemosensitive relapsed or primary refractory Hodgkin’s disease or aggressive non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2003;32:673–679. doi: 10.1038/sj.bmt.1704214. [DOI] [PubMed] [Google Scholar]
- Lavoie JC, Connors JM, Phillips GL, Reece DE, Barnett MJ, Forrest DL, Gascoyne RD, Hogge DE, Nantel SH, Shepherd JD, Smith CA, Song KW, Sutherland HJ, Toze CL, Voss NJS, Nevill TJ. High-dose chemotherapy and autologous stem cell transplantation for primary refractory or relapsed Hodgkin lymphoma: long-term outcome in the first 100 patients treated in Vancouver. Blood. 2005;106:1473–1478. doi: 10.1182/blood-2004-12-4689. [DOI] [PubMed] [Google Scholar]
- Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A, Chopra R, Milligan D, Hudson GV. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet. 1993;341:1051–1054. doi: 10.1016/0140-6736(93)92411-l. [DOI] [PubMed] [Google Scholar]
- Moskowitz C, Yahalom J, Zelenetz AD, Hamlin PA, Horwitz SM, Noy A, Portlock CS, Straus DJ, Kewalramani T, Gerecitano J, Vanak J, Nimer SD. Risk-adapted high dose chemoradiotherapy and ASCT for patients with relapsed or refractory Hodgkin’s disease: An intent to treat analysis. 2009. Submitted.
- Moskowitz CH, Bertino JR, Glassman JR, Hedrick EE, Hunte S, Coady-Lyons N, Agus DB, Goy A, Jurcic J, Noy A, O’Brien J, Portlock CS, Straus DS, Childs B, Frank R, Yahalom J, Filippa D, Louie D, Nimer SD, Zelenetz AD. Ifosfamide, Carboplatin, and Etoposide: A Highly Effective Cytoreduction and Peripheral-Blood Progenitor-Cell Mobilization Regimen for Transplant-Eligible Patients With Non-Hodgkin’s Lymphoma. J Clin Oncol. 1999;17:3776–3785. doi: 10.1200/JCO.1999.17.12.3776. [DOI] [PubMed] [Google Scholar]
- Moskowitz CH, Nimer SD, Zelenetz AD, Trippett T, Hedrick EE, Filippa DA, Louie D, Gonzales M, Walits J, Coady-Lyons N, Qin J, Frank R, Bertino JR, Goy A, Noy A, O’Brien JP, Straus D, Portlock CS, Yahalom J. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 2001;97:616–623. doi: 10.1182/blood.v97.3.616. [DOI] [PubMed] [Google Scholar]
- Paltiel O, Rubinstein C, Or R, Nagler A, Gordon L, Deutsch L, Polliack A, Naparstek E. Factors associated with survival in patients with progressive disease following autologous transplant for lymphoma. Bone marrow transplantation. 2003;31:565–569. doi: 10.1038/sj.bmt.1703888. [DOI] [PubMed] [Google Scholar]
- Robinson SP, Sureda A, Canals C, Russell N, Caballero D, Bacigalupo A, Iriondo A, Cook G, Pettitt A, Socie G, Bonifazi F, Bosi A, Michallet M, Liakopoulou E, Maertens J, Passweg J, Clarke F, Martino R, Schmitz N, on behalf of the Lymphoma Working Party of the EBMT Reduced intensity conditioning allogeneic stem cell transplantation for Hodgkin’s lymphoma: identification of prognostic factors predicting outcome. Haematologica. 2008;94:230–238. doi: 10.3324/haematol.13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz N, Dreger P, Glass B, Sureda A. Allogeneic transplantation in lymphoma: current status. Haematologica. 2007;92:1533–1548. doi: 10.3324/haematol.11185. [DOI] [PubMed] [Google Scholar]
- Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, Boissevain F, Zschaber R, Muller P, Kirchner H, Lohri A, Decker S, Koch B, Hasenclever D, Goldstone AH, Diehl V. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359:2065–2071. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- Sureda A, Arranz R, Iriondo A, Carreras E, Lahuerta J, García-Conde J, Jarque I, Caballero M, Ferrà C, López A, García-Laraña J, Cabrera R, Carrera D, Ruiz-Romero M, León A, Rifón J, Díaz-Mediavilla J, Mataix R, Morey M, Moraleda J, Altés A, López-Guillermo A, de la Serna J, Fernández-Rañada J, Sierra J, Conde E. Autologous stem-cell transplantation for Hodgkin’s disease: results and prognostic factors in 494 patients from the Grupo Español de Linfomas/Transplante Autólogo de Médula Osea Spanish Cooperative Group. Journal of clinical oncology. 2001;19:1395–1404. doi: 10.1200/JCO.2001.19.5.1395. [DOI] [PubMed] [Google Scholar]
- Thomson KJ, Peggs KS, Smith P, Cavet J, Hunter A, Parker A, Pettengell R, Milligan D, Morris EC, Goldstone AH, Linch DC, Mackinnon S. Superiority of reduced-intensity allogeneic transplantation over conventional treatment for relapse of Hodgkin’s lymphoma following autologous stem cell transplantation. Bone Marrow Transplant. 2008;41:765–770. doi: 10.1038/sj.bmt.1705977. [DOI] [PubMed] [Google Scholar]
- Vose JM, Bierman PJ, Anderson JR, Kessinger A, Pierson J, Nelson J, Frappier B, Schmit-Pokorny K, Weisenburger D, Armitage JO. Progressive disease after high-dose therapy and autologous transplantation for lymphoid malignancy: clinical course and patient follow-up. Blood. 1992;80:2142–2148. [PubMed] [Google Scholar]


