Abstract
Background
Patients with bicuspid aortic valves (BAV) are at increased risk of ascending aortic dilatation, dissection, and rupture. We hypothesized that ascending aortic wall stress may be increased in patients with BAV compared to patients with tricuspid aortic valves (TAV).
Methods
Twenty patients with BAV and 20 patients with TAV underwent electrocardiogram-gated computed tomography angiography. Patients were matched for diameter. The thoracic aorta was segmented, reconstructed, and triangulated to create a mesh. Utilizing a uniform pressure load of 120 mmHg, and isotropic, incompressible, and linear elastic shell elements, finite element analysis was performed to predict 99th-percentile wall stress.
Results
For patients with BAV and TAV, aortic root diameter was 4.0 ± 0.6 cm and 4.0 ± 0.6 cm (P=0.724), sinotubular junction diameter was 3.6 ± 0.8 cm and 3.6 ± 0.7 cm (P=.736), and maximum ascending aortic diameter was 4.0 ± 0.8 cm and 4.1 ± 0.9 cm (P=.849), respectively. The mean 99th-percentile wall stress in the BAV group was greater than in the TAV group (0.54 ±0.06 megpascals, MPa, versus 0.50 ± 0.09 MPa), though this did not reach statistical significance (p=0.090). When normalized by radius, the 99th-percentile wall stress was greater in the BAV group (0.31 ± 0.06 MPa/cm versus 0.27 ± 0.03 MPa/cm, P=0.013).
Conclusions
Patients with BAV, regardless of aortic diameter, have increased 99th-percentile wall stress in the ascending aorta. Ascending aortic three-dimensional geometry may account in part for the increased propensity to aortic dilatation, rupture, and dissection in patients with BAV.
Keywords: Aorta/Aortic, Bioengineering, CHD valve
Introduction
Bicuspid aortic valve (BAV) is the most common congenital cardiac abnormality, with a prevalence of approximately 1%.1–3 Patients with BAV are at increased risk of ascending aortic dilatation, dissection, and rupture compared to patients with tricuspid aortic valves (TAV).3–5 As a result, prophylactic ascending aortic replacement is recommended at a smaller size for patients with BAV.3,6–7 However, diameter is a less-than-ideal predictor of acute aortic syndromes.8–9 Given the increased risk of ascending aortic catastrophes associated with BAV, improving our understanding of - and ability to predict - these syndromes represent an important area of investigation, and might result in improved criteria for prophylactic ascending aortic replacement.
Two mechanisms have been proposed to explain the increased prevalence of ascending aortic dilatation, rupture, and dissection in patients with BAV. The first is that higher-velocity and eccentric flow jets in patients with BAV may lead to increased shear stress on the ascending aortic wall, and, thus, increased risk of ascending aortic dilatation, dissection, and rupture.3, 10–11 The second is that a genetic or developmental abnormality in the ascending aorta of BAV patients decreases aortic wall strength, and, therefore, predisposes patients to ascending aortic dilatation, dissection, and rupture.3, 12–13
Prior computational biomechanical studies have utilized finite element analysis (FEA) to investigate the role wall stress plays in aortic pathology. FEA is a numerical method that subdivides complex structures into small elements with defined material properties in order to predict the distribution of wall stress in these structures under physiological loading. FEA has been utilized to examine infrarenal abdominal aortic aneurysm (AAA) rupture risk, and has shown that wall stress is superior to diameter in predicting AAA rupture.14 In addition, our recent study used FEA to show that there are localized maxima of wall stress where type A and type B aortic dissections typically occur.15 Therefore, computational biomechanical analysis may represent a promising lens through which to evaluate – and predict – the aortic complications associated with BAV. In order to identify differences in ascending aortic wall stress in patients with BAV and TAV, FEA was applied to three-dimensional (3-D) meshes of ascending aortas reconstructed from electrocardiogram-gated computed tomography angiography (ECG-gated CTA). To our knowledge, this is the first study of BAV pathophysiology using ECG-gated CTA and FEA.
PATIENTS AND METHODS
Patients
Subjects with tricuspid and bicuspid aortic valves on ECG-gated CTA from June 2008 to January 2010 were retrospectively identified from the Radiology Information System (Centricity RIS-IC, GE Healthcare Waukesha, WI) utilizing PRESTO (Montage Healthcare Solutions, Philadelphia PA) at a single institution. Exclusion criteria included renal dysfunction precluding intravenous contrast; connective tissue disorder; penetrating atherosclerotic ulcer, dissection, or intramural hematoma; and history of cardiac or thoracic aortic surgery, other than coarctation repair in 2 patients with BAV.
One hundred and five subjects met the initial inclusion criteria during the study period, with 36 subjects with bicuspid aortic valves and 69 subjects with tricuspid aortic valves. From this study population, 20 subjects with bicuspid aortic valves and 20 subjects with tricuspid aortic valves were specifically selected for size-matching. There was no difference in medical characteristics or aortic diameters between the subjects who were selected for size-matching and those who were not. Patients were matched for diameter at the aortic root and sinotubular junction, and for maximum diameter in the ascending aorta. Demographic, echocardiographic, and radiographic information was obtained from patient records. Two patients in the BAV group and 5 patients in the TAV group had not undergone echocardiography. Approval was obtained from the Institutional Review Board.
Aortic wall thickness
The thickness of the anterior ascending aortic wall was determined from 2-dimensional (2-D) transthoracic echocardiography (TTE). Standard TTE parasternal long axis digital images of the proximal aorta were recorded and transferred to a TomTec (Tomtec, Inc. Hamden, CT) off-line analysis system. Anterior aortic wall thickness was measured in early systole in the proximal ascending aorta, 2 cm above the sinotubular junction. Aortic wall thickness was measured from leading edge to leading edge. Mean ascending aortic wall thickness was 1.7 mm for both BAV and TAV patients; therefore, a uniform wall thickness of 1.7 mm was used in the FEA modeling.
Image Acquisition and Analysis
Each subject underwent ECG-gated CTA scanning as part of their routine care. Indications for ECG-gated CTA for the patients with TAV included evaluation for aortic aneurysm (n=14), aortic dissection (n=3), or embolic source (n=3). In the BAV group, indications for ECG-gated CTA were evaluation for aortic aneurysm (n=14), aortic dissection (n=2), BAV itself (n=2), and following coarctation repair (n=2). ECG-gated CTA was performed using 64-slice scanners (Siemens Medical Solutions, Malvern, PA) with intravenous injection of nonionic iodinated contrast (Omnipaque 350, GE Amersham, Milwaukee, WI). Thin-section ECG gated-imaging was obtained during the arterial phase of enhancement. The scans were performed with in-plane resolution of 512 × 512 pixels. Scans were interpreted by cardiovascular radiologists.
The digital imaging and communications in medicine (DICOM) data from the ECG-gated CTA scan were imported into an Amira (Visage Imaging™, San Diego, CA) work-station for analysis.15 A semiautomatic segmentation algorithm – utilizing a 2-D region growing method – was used to detect the aortic wall in all images. The algorithm requires user-defined seed points and lower and upper grey-level threshold.15 To obtain an accurate and smooth surface for FEA, each slice was visually inspected, and a Gaussian filter with a 3 × 3 kernel was utilized to smooth the lumen borders. The patient-specific 3-D aortic wall surfaces were constructed from the 2-D contour stack and tessellated so as to avoid skewed elements, which might result in artificial stress concentrations.
Commercial finite element (FE) software (ABAQUS/Explicit 6.3, HKS Inc. Pawtucket, RI) was then used to analyze the deformation and resulting stress distribution in the aortic models.15 The 3-D coordinates of nodes and their interconnection were converted to ABAQUS input file format using customized Matlab (Mathworks™, Natick, MA) functions. Grid convergence studies demonstrated that a total of 9,000 – 10,000 nodes and 18,000 – 20,000 triangular elements were sufficient for a human thoracic aorta. Isotropic, incompressible, and linear elastic triangular finite strain shell elements were utilized.
The 3-D geometric FE model included all essential anatomic components of the ascending aorta (aortic root, coronary sinuses, and sinotubular junction), and aortic arch (supraaortic vessels). Neither aortic valve leaflet geometry nor the effects of valve morphology, such as differences in fluid flow through the bicuspid aortic valve, were included in the FE modeling. Material properties were determined from the literature: Young’s modulus of 3.0 × 106 Pa and a Poisson ratio of 0.46.16–18 All freedom of motion except rotation was fixed for the nodes in the proximal aortic root, at the distal ends of the supraaortic arteries, and in the supraceliac aorta. The aortic lumen was then loaded with a uniform loading pressure of 120 mmHg.
Von Mises stresses in the ascending aorta were calculated in megapascals (MPa). For reporting the highest stress, 99th-percentile wall stress was reported, which has been shown to be more reproducible than peak stress, and protects against detecting nonphysiologic localized peak stresses due to inhomogeneities in the FEA mesh.16 In addition, normalized 99th-percentile wall stress was calculated by dividing the 99th-percentile wall stress by the aortic root, sinotubular junction, or maximum ascending aortic radius. Normalized wall stress was a planned outcome. In examining a system with a uniform pressure load and uniform wall thickness, the local radii of curvature (local surface shape) of the aortic wall is the determinant of load-induced wall stress, as predicted by Laplace’s law. Normalizing by radius “corrects” or accounts for differences in overall aortic size, and thereby isolates the influence of the local wall surface geometry on load-derived stress. Several previous studies have utilized pressure-normalized wall stress to allow comparison between left ventricle responses to loading at different pressures.19–20
Statistical Analysis
Patient demographic and medical characteristics were compared by Pearson’s χ-square test and Student t-test, as appropriate. Maximum diameters and stresses between the two groups were compared with Student t-test. All tests of significance were at the P<0.05 level. Statistical analyses were performed with SPSS software (SPSS Inc, Chicago, Illinois).
RESULTS
Patient Demographic and Echocardiographic Characteristics
Patients in the BAV group were younger than patients in the TAV group (48.9 ± 15.1 versus 57.3 ± 10.3; P=0.050); otherwise there were no differences (Table 1). Echocardiographic findings are reported in Table 2. Patients in the BAV group were more likely to have aortic valve disease (P<0.001).
Table 1.
Patient demographic and medical history characteristics
| BAV (n=20) | TAV (n=20) | P-value | |
|---|---|---|---|
| Age (years) | 48.9 ± 15.1 | 57.3 ± 10.3 | 0.050 |
| Male | 15 (75.0%) | 15 (75.0%) | 1.000 |
| Hypertension | 9 (45.0%) | 13 (65.0%) | 0.431 |
| Diabetes | 1 (5.0%) | 1 (5.0%) | 1.000 |
| Coronary artery disease | 1 (5.0%) | 1 (5.0%) | 1.000 |
| Chronic obstructive pulmonary disease | 1 (5.0%) | 0 (0.0%) | 0.979 |
BAV: bicuspid aortic valve; TAV: tricuspid aortic valve
Table 2.
Patient aortic valve function by echocardiography
| BAV (n=18) | TAV (n=15) | P-value | |
|---|---|---|---|
| No aortic valve disease | 0 | 9 (60.0%) | |
| Trivial, mild, or mild-to-moderate aortic valve disease | 12 (66.7%) | 6 (40.0%) | <0.001 |
| Moderate or worse aortic valve disease | 6 (33.3%) | 0 (0.0%) |
BAV: bicuspid aortic valve; TAV: tricuspid aortic valve
Maximum Diameter of Ascending Aorta
There were no differences between the 2 groups with respect to aortic root, sinotubular junction, or maximum ascending aortic diameter. The aortic root diameter in the BAV and TAV groups was 4.0 ± 0.6 cm and 4.0 ± 0.6 cm, respectively (P=0.724). The sinotubular junction diameter was 3.6 ± 0.8 cm in the BAV group and 3.6 ± 0.7 cm in the TAV group (P=0.736). The maximum ascending aortic diameter was 4.0 ± 0.8 cm in the BAV group and 4.1 ± 0.9 cm in the TAV group (P=0.849).
Wall Stress in the Ascending Aorta
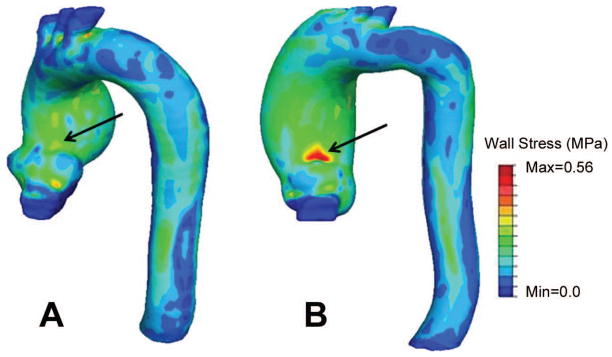
Representative 3-D wall stress distributions in the ascending aorta in a patient with a BAV and a patient with a TAV are shown in Figure 1. The 99th-percentile wall stress was 0.54 ± 0.06 MPa in the BAV group and 0.50 ± 0.09 MPa in the TAV group (P=0.090). The normalized 99th-percentile wall stress was greater in the BAV group than the TAV group for all 3 measured radii (Table 3).
Figure 1.
Wall stress in the ascending aorta of A) a patient with a tricuspid aortic valve, and aortic root, sinotubular, and maximum ascending aortic diameter of 4.0, 3.4 and 5.4 cm, respectively, and B) a patient with a bicuspid aortic valve, and aortic root, sinotubular junction, and maximum ascending aortic diameter of 3.8, 3.5 and 5.1 cm, respectively. Note the increased stress in the latter patient.
Table 3.
Wall stress in the ascending aorta
| BAV (n=20) | TAV (n=20) | P-value | |
|---|---|---|---|
| 99-percentile wall stress (MPa) | 0.54 ± 0.06 | 0.50 ± 0.09 | 0.090 |
| 99-percentile wall stress normalized for aortic root radius (MPa/cm) | 0.28 ± 0.04 | 0.25 ± 0.03 | 0.023 |
| 99-percentile wall stress normalized for sinotubuluar junction radius (MPa/cm) | 0.31 ± 0.06 | 0.27 ± 0.03 | 0.013 |
| 99-percentile wall stress normalized for maximum ascending aortic radius (MPa/cm) | 0.28 ± 0.05 | 0.24 ± 0.03 | 0.029 |
BAV: bicuspid aortic valve; TAV: tricuspid aortic valve
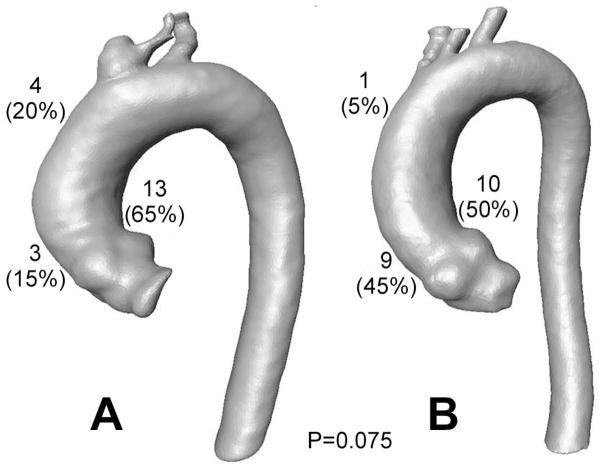
The regional distribution of the location of 99th-percentile wall stess was investigated. In the BAV group, the location of 99th-percentile wall stress was above the left coronary sinus on the concave side of the ascending aorta in 10 (50.0%) patients, above the right coronary sinus on the convex side of the ascending aorta in 9 (40.0%) patients, and in the ascending aorta in 1 (5.0%) patient (Figure 2). In the TAV group, the location of 99th-percentile wall stress was above the left coronary sinus in 13 (65.0%) patients, above the right coronary sinus in 3 (15.0%) patients, and in the ascending aorta in 4 (20.0%) patients. This difference in distribution approached but did not reach statistical significance (P=.075).
Figure 2.
Location of 99-percentile wall stress in the ascending aorta in patients with A) tricuspid and B) bicuspid aortic valves. This differing distribution of peak wall stress approached but did not reach statistical significance (P=0.075).
Wall Stress in the Non-Aneurysmal Ascending Aorta
Ten patients in the BAV group and 10 patients in the TAV group had non-aneurysmal ascending aortas (maximum diameter less than 4 cm). Four (40%) of these 10 BAV patients with non-aneurysmal ascending aortas had moderate or worse aortic valve disease. The 99th-percentile wall stress in the non-aneurysmal BAV group was greater – but did not reach statistical significance – than that in the non-aneurysmal TAV group (0.51 ± 0.07 versus 0.45 ± 0.10 MPa, P=0.151). The normalized 99th-percentile wall stress was greater in the non-aneurysmal BAV group than the non-aneurysmal TAV group for all 3 measured locations (Table 4).
Table 4.
Wall stress in the non-aneurysmal ascending aorta
| BAV (n=10) | TAV (n=10) | P-value | |
|---|---|---|---|
| 99-percentile wall stress (MPa) | 0.51 ± 0.07 | 0.45 ± 0.10 | 0.151 |
| 99-percentile wall stress normalized for aortic root radius (MPa/cm) | 0.29 ± 0.05 | 0.24 ± 0.03 | 0.013 |
| 99-percentile wall stress normalized for sinotubular junction radius (MPa/cm) | 0.36 ± 0.04 | 0.28 ± 0.04 | 0.001 |
| 99-percentile wall stress normalized for maximum ascending aortic radius (MPa/cm) | 0.31 ± 0.04 | 0.26 ± 0.04 | 0.009 |
BAV: bicuspid aortic valve; TAV: tricuspid aortic valve
Wall Stress by Bicuspid Morphology
Seventeen (85%) patients had fusion of the right and left coronary cusps (R/L), and 3 (15%) patients had fusion of the right and non-coronary cusps (R/N). No patient had fusion of the left and non-coronary cusps (L/N). Neither the 99th-percentile wall stress (0.55 ± 0.06 MPa versus 0.51 ± 0.07 MPa, P=0.369) nor the 99th-percentile wall stress normalized for sinotubuluar junction radius (0.31 ± 0.05 MPa versus 0.35 ± 0.07 MPa, P= 0.193) differed between the R/L group and the R/N group. With respect to patients with BAV and non-aneurysmal ascending aortas, 7 patients had R/L cusp fusion, and 3 patients had R/N cusp fusion: between these two groups there was no difference in 99th-percentile wall stress (0.51 ± 0.08 MPa versus 0.51 ± 0.07 MPa, P=00.983) or 99th-percentile wall stress normalized for sinotubular junction radius (0.36 ± 0.03 MPa versus 0.035 ± 0.07 MPa, P=0.865).
COMMENT
This study demonstrates that patients with BAV have a higher normalized 99th-percentile wall stress in the ascending aorta than patients with TAV. Patients with BAV were younger and more likely to have aortic valve disease, which is consistent with BAV epidemiology and pathophysiology.3,6 In addition, patients with non-aneurysmal ascending aortas and BAV had a greater normalized 99th-percentile wall stress than patients with non-aneurysmal ascending aortas and TAV. The current finding that disparate 3-D geometry in the ascending aorta of patients with BAV and TAV results in increased load pressure-induced wall stress in patients with BAV may help explain the increased frequency of ascending aortic dilatation, dissection, and rupture observed in these patients. Future investigations to ascertain the usefulness of wall stress to predict ascending aortic dilatation, dissection, and rupture are warranted, as wall stress might be used to improve risk-stratification, and establish more refined criteria for prophylactic ascending aortic replacement.
Diameter is one of the criteria for recommending prophylactic aortic replacement. However, diameter alone is less than ideal in predicting acute aortic syndromes.8–9, 14 Aortic pathology occurs when wall stress exceeds wall strength, which depends on the 3-D geometry of the aorta, the aortic wall material properties, and the pressure load.14–15, 21–22 Computationally-predicted wall stress is superior to diameter in predicting AAA rupture risk, and is a potential etiology of type A and type B aortic dissection.14–15 Although improvements in biomechanics, medical imaging, and computational techniques have provided a new lens through which to study aortic pathology, there has been little use of these methods. Moreover, there has been little attempt to utilize this technology in designing new rational treatment algorithms.
Several studies have investigated ascending aortic pathophysiology in the setting of BAV. Bauer et al used TTE and duplex ultrasound to investigate patients with BAV or TAV, and found a significantly higher peak systolic velocity on the anterolateral wall of the ascending aorta in patients with BAV, which suggests a hemodynamic etiology of ascending aortic dilation.10 Utilizing four-dimensional flow magnetic resonance angiography, Hope et al showed that patients with R/L cusp fusion tended to have right-handed nested helical flow toward the right-anterior quadrant of the ascending aorta, while patients with R/N cusp fusion had left-handed nested helical flow aimed at the left-posterior quadrant of the ascending aorta.11 Our findings are consistent with these results, and suggest that the ascending aorta in patients with BAV is subject to increased load pressure-induced stress owing to different aortic wall geometry, which may account - in part - for their increased risk for acute aortic syndromes.
It is interesting to note that patients in the BAV group tended to have 99th-percentile wall stress on the convex side of the ascending aorta above the right coronary sinus more often than the patients in the TAV group. In the context of the studies from Bauer et al and Hope et al,10–11 this finding suggests that patients with BAV, the majority of whom have R/L cusp fusion, may have different 3-D aortic geometry due to the eccentric jet flows aimed at the right anterolateral wall of the ascending aorta. Given the increased smooth muscle cell apoptosis that occurs at the convexity of the ascending aorta in patients with bicuspid aortic valves,13 it is possible that differences in the location of 99th-percentile stress may be prove to be more important than the maximal level of 99th-percentile stress. This may be especially true given the observation that most type A dissections occur above the sinotubular junction on the convex side of the ascending aorta, and should be investigated in future studies that correlate location and magnitude of peak wall stress with clinical outcome. Lastly, the finding that patients with BAV and non-aneurysmal ascending aortas had greater normalized 99th-percentile wall stress than patients with TAV and non-aneurysmal ascending aortas suggests that FEA might be useful in the observation and risk-stratification of patients with BAV and non-aneurysmal ascending aortas.
There are several limitations of this study. First, the isotropic and linear elastic material properties utilized in this study do not fully describe biologic ascending aortic tissue. Duprey et al demonstrated that the aneurysmal ascending aorta is anisotropic.23 Okamoto et al showed that the ascending aorta in patients with BAV has hyperelastic material properties.24 Moreover, the latter study found that the equibiaxial stretch ratio at which nonlinear behavior occurs generally decreases with age. Although the assumption of identical material properties for patients with BAV and TAV represents another limitation of the study, different material properties have yet to be elucidated definitively between patients with bicuspid and tricuspid aortic valves, and are not readily available for use in FE modeling. Furthermore, the majority of studies that provided information on the behavior of ascending aortic tissue were ex vivo studies. Material properties have yet to be determined through non-invasive in vivo analysis of the ascending aorta. ECG-gated CTA represents a novel tool in that it may allow for the non-invasive in vivo measurement of mechanical properties in the ascending aorta.25 A third limitation is the utilization of a hydrostatic model, ignoring momentum effects and shear stresses, which may lead to local inhomogeneities in wall stress and momentum transfer; nevertheless, hydrostatic loads are expected to be an order of magnitude greater than either other contributing stress.26 Finally, uniform wall thickness was used for all of the subjects. However, it is important to note that it is not possible to obtain accurate resolution of wall thickness from current ECG-gated CTA data; thus, the use of echocardiography as a substitute for wall thickness is reasonable.
In conclusion, this study employs ECG-gated CTA and FEA to demonstrate that normalized 99th-percentile wall stress in the ascending aorta of patients with BAV is greater than that in patients with TAV. Furthermore, there may be differences in the location of 99th-percentile wall stress in patients with BAV compared to patients with TAV. These findings may help to explain the increased frequency of aortic dilatation, dissection, and rupture observed in patients with BAVs. Moreover, it highlights 3-D geometry and hydrostatic stress as a new mechanism in the pathophysiology of acute aortic syndromes in patients with BAV. Future investigations using ECG-gated CTA and FEA to assess the ability of load pressure-induced wall stress to predict ascending aortic catastrophes are warranted.
Acknowledgments
Grant Support: McCabe Fund, Philadelphia, PA; National Institute of Health F32 HL099172-01
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nistri S, Basso C, Marzari C, Mormino P, Thiene G. Frequency of bicuspid aortic valve in young male conscripts by echocardiogram. Am J Cardiol. 2005;96:718–721. doi: 10.1016/j.amjcard.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 2.Tutar E, Ekici F, Atalay S, Nacar N. The prevalence of bicuspid aortic valve in newborns by echocardiographic screening. Am Heart J. 2005;150:513–515. doi: 10.1016/j.ahj.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 3.Tadros TM, Klein MD, Shapira OM. Ascending Aortic Dilatation Associated With Bicuspid Aortic Valve: Pathophysiology. Molecular Biology, and Clinical Implications Circulation. 2009;119:880–890. doi: 10.1161/CIRCULATIONAHA.108.795401. [DOI] [PubMed] [Google Scholar]
- 4.Beroukhim RS, Kruzick TL, Taylor AL, Gao D, Yetman AT. Progression of aortic dilation in children with a functionally normal bicuspid aortic valve. Am J Cardiol. 2006;98:828–830. doi: 10.1016/j.amjcard.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Edwards WD, Leaf DS, Edwards JE. Dissecting aortic aneurysm associated with congenital bicuspid aortic valve. Circulation. 1978;57:1022–1025. doi: 10.1161/01.cir.57.5.1022. [DOI] [PubMed] [Google Scholar]
- 6.Davies RR, Kaple RK, Mandapati D, et al. Natural history of ascending aortic aneurysms in the setting of an unreplaced bicuspid aortic valve. Ann Thorac Surg. 2007;83:1338–1344. doi: 10.1016/j.athoracsur.2006.10.074. [DOI] [PubMed] [Google Scholar]
- 7.Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002;73:17–28. doi: 10.1016/s0003-4975(01)03236-2. [DOI] [PubMed] [Google Scholar]
- 8.Pape LA, Tsai TT, Isselbacher EM, et al. Aortic diameter > or < 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD) Circulation. 2007;116:1120–1127. doi: 10.1161/CIRCULATIONAHA.107.702720. [DOI] [PubMed] [Google Scholar]
- 9.Parish LM, Gorman JH, 3rd, Kahn S, et al. Aortic size in acute type A dissection: implications for preventive ascending aortic replacement. Eur J Cardiothorac Surg. 2009;35:941–5. doi: 10.1016/j.ejcts.2008.12.047. [DOI] [PubMed] [Google Scholar]
- 10.Bauer M, Siniawski H, Pasic M, Schaumann B, Hetzer R. Different hemodynamic stress of the ascending aorta wall in patients with bicuspid and tricuspid aortic valve. J Card Surg. 2006;21:218–220. doi: 10.1111/j.1540-8191.2006.00219.x. [DOI] [PubMed] [Google Scholar]
- 11.Hope MD, Hope TA, Meadows AK, et al. Bicuspid aortic valve: Four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology. 2010;255:53–61. doi: 10.1148/radiol.09091437. [DOI] [PubMed] [Google Scholar]
- 12.Nataatmadja M, West M, West J, et al. Abnormal extracellular matrix protein transport associated with increased apoptosis of vascular smooth muscle cells in Marfan syndrome and bicuspid aortic valve thoracic aortic aneurysm. Circulation. 2003;108:II329–II334. doi: 10.1161/01.cir.0000087660.82721.15. [DOI] [PubMed] [Google Scholar]
- 13.Della Corte A, Quarto C, Bancone C, et al. Spatiotemporal patterns of smooth muscle cell changes in ascending aortic dilatation with bicuspid and tricuspid aortic valve stenosis: focus on cell-matrix signaling. J Thorac Cardiovasc Surg. 2008;135:8–18. doi: 10.1016/j.jtcvs.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Fillinger MF, Marra SP, Raghavan ML, Kennedy FE. Prediction of rupture risk in abdominal aortic aneurysm during observation: wall stress versus diameter. J Vasc Surg. 2003;37:724–32. doi: 10.1067/mva.2003.213. [DOI] [PubMed] [Google Scholar]
- 15.Nathan DP, Xu C, Gorman JH, et al. Pathogenesis of Acute Aortic Dissection: A Finite Element Stress Analysis. Annals of Thoracic Surgery. 2011;91:458–63. doi: 10.1016/j.athoracsur.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 16.Speelman L, Bosboom EMH, Schurink GWH, et al. Patient-Specific AAA Wall Stress Analysis: 99-Percentile Versus Peak Stress. Eur J Vasc Endovasc Surg. 2008;36:668–76. doi: 10.1016/j.ejvs.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Fukui T, Matsumoto T, Tanaka T, et al. In vivo mechanical properties of thoracic aneurysmal wall estimated from in vitro biaxial tensile test. Bio-Medical Materials and Engineering. 2005;15:295–305. [PubMed] [Google Scholar]
- 18.Beller CJ, Labrosse MR, Thubrikar MJ, Szabo G, Robicsek F, Hagl S. Increased aortic wall stress in aortic insufficiency: clinical data and computer model. Eur J Cardiothorac Surg. 2005;27:270–275. doi: 10.1016/j.ejcts.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Zhong L, Sola S, Tan R, Le T, Ghista DN, Kurra V, Navia JL, Kassab GS. Effects of surgical ventricular restoration on left ventricular contractility assessed by a novel contractility index in patients with ischemic cardiomyopathy. Am J Cardiol. 2009;103:674–679. doi: 10.1016/j.amjcard.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 20.Zhong L, Tan RS, Ghista DN, Ng EYK, Chua LP, Kassab GS. Validation of a novel cardiac index of left ventricular contractility in patients. Am J Physiol Heart Circ Physiol. 2007;292:H2764–H2772. doi: 10.1152/ajpheart.00540.2006. [DOI] [PubMed] [Google Scholar]
- 21.Khanafer K, Berguer R. Fluid-structure interaction analysis of turbulent pulsatile flow within a layered aortic wall as related to aortic dissection. J Biomech. 2009;42:2642–8. doi: 10.1016/j.jbiomech.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Humphrey JD, Taylor CA. Intracranial and abdominal aortic aneurysms: similarities, differences, and need for a new class of computational models. Ann Rev Biomed Eng. 2008;10:221–46. doi: 10.1146/annurev.bioeng.10.061807.160439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duprey A, Khanfer K, SChlicht M, Avril S, Williams D, Berguer R. Eur J Vasc Endovasc Surg. 2010;39:700–707. doi: 10.1016/j.ejvs.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto RJ, Xu H, Kouchoukos NT, Moon MR, Sundt TM., III The influence of mechanical properties on wall stress and distensibility of the dilated ascending aorta. J Thor Cardiovasc Surg. 2003;126:842–50. doi: 10.1016/s0022-5223(03)00728-1. [DOI] [PubMed] [Google Scholar]
- 25.Morrison TM, Choi G, Zarins CK, Taylor CA. Circumferential and longitudinal cyclic strain of the human thoracic aorta: Age-related changes. J Vasc Surg. 2009;49:1029–36. doi: 10.1016/j.jvs.2008.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berne RM, Levy MN. Cardiovascular Physiology. 7. St. Louis, Mo: Mosby-Year Book, Inc; 1997. [Google Scholar]