Abstract
The role of diet temperature in ingestive behavior is poorly understood. We examined the importance of stimulus temperature and water-restriction state on the preference for and intake of water and sucrose. Using custom-designed equipment that allows us to monitor and maintain solution temperatures during testing (±0.1 °C), we conducted a series of 2-bottle preference tests (10 °C water vs. sucrose 10–40 °C) and brief access tests (10–40 °C water and sucrose). Water-restricted rats preferred cold water over any sucrose concentration (0.0–1.0 M) if the sucrose was 30 or 40 °C, whereas the same rats preferred sucrose at all concentrations and temperatures when unrestricted suggesting that the water-restriction state interacts with temperature preference. In a series of brief-access tests using a Davis Rig (MS-180), rats reduced licking to cold sucrose compared with 20 °C sucrose, suggesting that unlike water, cold temperature reduced the palatability of sucrose.
Keywords: sucrose preference, taste, temperature, water-restriction
Introduction
The role of temperature on the acceptability of food is poorly understood but may play a larger role than is credited. There is increasing evidence to suggest a role for temperature in ingestive behavior and taste. First, there are behavioral data to suggest that temperature modifies intake and preference for water alone. In 1-bottle short-term tests, rats drank more water when it was 30–35 °C and less when cool (12 °C) or warm (48 °C, Kapatos and Gold 1972). Interestingly, water-restricted rats preferred cool over warm water in short term 2-bottle tests (12 > 36 °C: Deaux and Engstrom 1973, 12 > 40 °C: Ramsauer et al. 1974, 10 > 40 °C and 25 > 40 °C: Smith et al. 2010) and bar pressed more for cold than warm water (12 > 40 °C: Ramsauer et al. 1974) suggesting that cold has a rewarding quality. Similarly, humans made thirsty by exercise reduced cold water intake in comparison to warm water intake (Boulze et al. 1983) but reported increased positive ratings for colder water (Boulze et al. 1983; Sandick et al. 1984). The effect of temperature on intake and the rewarding value of cold suggest that temperature influences ingestive behavior.
In addition, there is evidence that temperature alone evokes taste quality sensations in humans and neural responses from taste nerves in rats. For example, Cruz and Green (2000) found that a subset of their human subjects were thermal tasters and reported a weak sweet taste sensation when the tip of the tongue was warmed and a sour or salty taste when the tongue was cooled (Cruz and Green 2000). Thermal tasters were also more sensitive to taste stimulation in general. Perhaps it is their increased sensitivity that allows them to report thermal taste (Green and George 2004). These psychophysical findings are consistent with electrophysiological findings in rodents where thermal stimulation alone elicited neural responses from taste neurons (Fishman 1957; Ogawa et al. 1968; Lundy and Contreras 1999; Breza et al. 2006).
There is also evidence that temperature influences taste sensation. For example, in humans, sweet intensity ratings were greater at warmer temperatures (Bartoshuk et al. 1982) and taste detection thresholds varied with temperature (McBurney et al. 1973). In rats, temperature influenced taste nerve responsiveness to chemical stimulation of the tongue (Nakamura and Kurihara 1991; Lundy and Contreras 1997; Breza et al. 2006). For example, chorda tympani nerve responses were maximal when stimuli were delivered at 30–35 °C and deviations from that temperature reduced responding (Yamashita and Sato 1965). Furthermore, sucrose-specialist neurons from the geniculate ganglion increased their responses to sucrose while acid-generalist neurons increased their responses to NaCl, citric acid and quinine as stimulus temperature increased from 10 to 40 °C (Breza et al. 2006).
Lastly, Smith et al. (2010) demonstrated that temperature can serve as a conditioned stimulus and influence solution preference. The investigators paired LiCl injections with cold water and cold saccharin solutions. Rats conditioned against cold water generalized the aversion to cold saccharin solutions. Furthermore, rats conditioned to cold saccharin generalized the aversion to both cold water and warm saccharin solutions. This work highlights the strength of temperature as a cue to solution preference. While this work utilized the conditioned aversion paradigm to demonstrate the importance of temperature as a cue in preference, we chose to examine unconditioned preferences for temperature.
We chose to address 3 questions: 1) What is the role of water-restriction in cold water preference? We addressed this through a series of 10-min preference tests, in water-restricted and unrestricted rats, comparing cold water (10 °C) to not only warmer water (20–40 °C) but varying temperatures and concentrations of sucrose, using custom-designed equipment that allowed us to maintain and monitor solution temperatures during testing. We chose sucrose as our chemical stimulus because it is a well-studied taste stimulus and is highly preferred over water at room temperature (22 °C). 2) Does temperature contrast alter licking in a brief-access test? Many experiments have relied on a contrast between 2 temperatures either in a 2-bottle preference or with temperatures presented in succession. To assess the effect of absolute temperature or contrasted temperature on intake, we designed brief access tests to either contrast temperature or to contrast sucrose concentration within a test session. 3) Does temperature change the orosensory-driven responses to water or sweet solutions? We addressed this question by measuring licking responses to both water and 3 concentrations of sucrose at varying temperatures in a series of brief-access tests that minimize post ingestive feedback.
General materials and methods
Animals and housing
Male Sprague–Dawley rats (Charles River Breeding Laboratory) weighing 200–250 g at the start of the studies were individually housed in standard Plexiglas, shoebox cages. There was a 12 h light:dark cycle; lights were on from 0700 to 1900, and ambient room temperature was approximately 22 °C. Rats were maintained on Purina rat chow 5001 (St. Louis, MO) and deionized (DI) water ad libitum except where otherwise noted. All experimental protocols were approved by the Florida State University Animal Care and Use Committee (protocol number 9237).
Statistical analyses
All data presented represent 2-day averages. Data were first analyzed with 3-way repeated measures analyses of variance (ANOVAs) with water-restriction status, temperature, and concentration as factors. To increase our interpretive power, in instances where water-restriction status was significantly different, we followed these analyses with 2-way repeated measures ANOVAs for each water-restriction condition separately, using temperature and concentration as factors. In the case of clusters of licking, which are based on measures of total intake, these analyses were conducted as a 2-way repeated measures ANOVAs for each water-restriction condition separately if the 3-way repeated measures ANOVAs suggested that the water-restriction state effected total intake. Significant differences were followed up using a Tukey HSD test or Bonferroni-corrected planned comparisons.
Experiment 1
Materials and methods
Apparatus
Testing chambers were plastic cages (17-cm wide, 28-cm long, and 13-cm high). On the front of each cage, behind slots 0.8-cm wide and 2.5-cm tall, sipper tubes were mounted and secured in an aluminum block 1.5 cm by 6 cm and 5-cm thick. A diagonal hole was drilled through the block to accommodate a stainless steel sipper tube approximately 11.5 cm in length. A Peltier device was attached to each block. The Peltier device could be heated or cooled to various temperatures between 10 and 40 °C by altering the polarity and the magnitude of the DC current, therefore heating and cooling the aluminum block and the sipper tube contained within. The tubes were held snugly in the blocks by thumb screws which thermally couple the block and tube allowing the liquid in the sipper tube to be cooled and warmed. To calibrate the device, a temperature probe was placed approximately 1.5 cm inside the sipper tube, and the equipment was considered calibrated when the difference between the probe and the controller reading was reliably ≤0.1 °C. Therefore, the setting of the temperature on the central processor is based on the fluid temperature, not the block temperature. Calibration was conducted at the start of the experiment, but the equipment was tested at regular intervals to confirm that the temperatures were reliable. A small fan attached to a heat sink on each Peltier device dissipated the excess temperature off the block.
In addition to temperature controls, each block was equipped with an infrared light emitting diode and a photo detector. Each time a rat licked the sipper tube its tongue protrusion broke the beam between the diode and the detector providing a means for counting the number of licks from each of the 2 tubes in the test cage. Interlick intervals (ILI) were computed and stored on a computer by software titled Temperature Control Lickometer V1.0 (FSU custom software). The microstructure of licking was examined in addition to gross measures of intake. Data were filtered and divided into clusters of licking in Quicklick for Windows (DiLog Instruments). A cluster was defined as 3 or more licks with ILI < 500 ms (Davis and Perez 1993).
Training
During testing, the sipper tubes were partially retracted from the slots in the front of the cage, therefore rats (n = 12) had to be adapted to the test cages and trained to drink from the retracted sipper tubes. Training took place over 4 days. On all training days, rats were 20 h water-restricted and were given 1 h water access in addition to the fluid consumed in the described training. Rats were presented with DI water at 22 °C for 15 min. On the first day, rats were presented with both bottles extended into the cage. In all cases, the rats showed a preference for a single bottle. On the following day, the bottle that was not preferred was presented alone. When rats were observed to have licked for at least 30 s from the available bottle, the second bottle was replaced. On the last 2 days of training, rats were given both bottles in the retracted position.
Testing
In the first series of tests, the rats were water-restricted (20 h). Rats were placed in test cages and given access to 2 bottles for 10 min daily. They were presented with 10 °C deionized (DI) water in one bottle and increasing concentrations of sucrose (0.0, 0.03, 0.06, 0.125, 0.25, 0.5, 1.0 M) in the alternate bottle at 40 °C. Each concentration was presented for 2 consecutive days. Bottles were alternated daily to control for side preference. Water was compared with water at the temperature of interest once a week to ensure stability in the rats’ intakes across the testing period. Each day during testing, rats were given an additional 1 h access to room temperature water approximately 3 h after testing. At the conclusion of the series, the entire sucrose concentration curve was presented at each of the following temperatures in descending order (30, 20, and 10 °C) again compared against the 10 °C DI water. Immediately following the 10 °C sucrose trials, rats were given a second preference test with an abbreviated concentration curve of sucrose (0.0, 0.06, 0.125, 0.25, 0.5 M) at 40 °C to ensure there was no order effect in the data. In this abbreviated curve, solutions were presented once per concentration.
To explore the effect of water repletion on preference, the same rats were tested in a second series of preference tests while unrestricted. This series was identical to the preference tests described above except we did not repeat the abbreviated concentration curve of sucrose in this group. Lastly, to measure the role of water-restriction in the absence of temperature manipulations, all rats were tested in a final series of 2-bottle preference tests for increasing concentrations of sucrose (0.0, 0.03, 0.06, 0.125, 0.25, 0.5, 1.0 M) over water at room temperature (22 °C), water-restricted, and unrestricted.
Results
Total intake
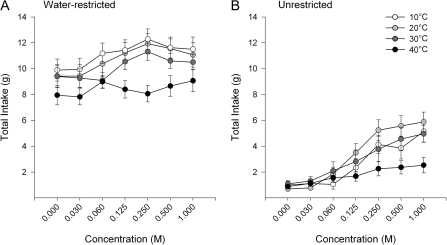
Rats consumed significantly more fluid while water-restricted than when they were unrestricted (Table 1, Figure 1). In both conditions, there was a significant main effect of concentration (Table 1). Figure 1 suggests that higher sucrose concentrations were associated with higher total intake of fluids. In the water-restricted condition, there was a significant effect of temperature on total fluid intake (Table 1), but there was no effect of temperature in the unrestricted rats (Table 1).
Table 1.
Summary of ANOVAs for intake and preference
| Source of variation | 3-way ANOVA |
Water-restricted 2-way ANOVA |
Tukey HSD (Ps < 0.05), water-restricted | Unrestricted 2-way ANOVA |
Tukey HSD (Ps < 0.05), unrestricted | ||||||
| F | df | P | F | df | P | F | df | P | |||
| Total intake (Figure 1) | |||||||||||
| Water-restriction status | 306.9 | 1,88 | <0.001 | ||||||||
| Temperature | 4.17 | 3,88 | 0.01 | 3.01 | 3,44 | 0.04 | 10, 20 > 40 at ≥0.125 M; 30 > 40 at 0.125 and 0.25 M | 1.36 | 3,44 | 0.03 | ns |
| Concentration | 56.43 | 6,528 | <0.001 | 14.11 | 6,264 | <0.001 | 1 > 0 M at 10, 20 | 49.23 | 6,264 | <0.001 | 1 > 0 M at 10, 20, 30 |
| Temperature × concentration | 4.42 | 18,528 | <0.001 | 1.67 | 18,264 | 0.04 | 4.7 | 18,264 | <0.001 | ||
| Temperature × restriction | 0.753 | 3,88 | 0.52 | ||||||||
| Concentration × restriction | 9.77 | 6,528 | <0.001 | ||||||||
| Concentration × restriction × temperature | 2.19 | 18,528 | 0.003 | ||||||||
| Sucrose preference (Figure 2A,B) | |||||||||||
| Water-restriction status | 51.56 | 1,88 | <0.001 | ||||||||
| Temperature | 20.54 | 3,88 | <0.001 | 19.17 | 3,44 | <0.001 | 10, 20 > 30, 40 at ≥0.25 M; 30 > 40 at ≥0.06 M | 2 | 3,44 | 0.12 | ns |
| Concentration | 62.19 | 6,528 | <0.001 | 19.24 | 6,264 | <0.001 | 1 > 0 M at 10, 20, 30 | 28.4 | 6,264 | <0.001 | 1 > 0 M at 10, 20, 30 |
| Temperature × concentration | 3.58 | 18,528 | <0.001 | 2.34 | 18,264 | <0.001 | 10.36 | 18,264 | <0.001 | ||
| Temperature × restriction | 12.60 | 3,88 | <0.001 | ||||||||
| Concentration × restriction | 3.57 | 6,528 | 0 | ||||||||
| Concentration × restriction × temperature | 1.15 | 18,528 | 0.30 | ||||||||
| Sucrose intake (Figure 2C,D) | |||||||||||
| Water-restriction status | 35.11 | 1,88 | <0.001 | ||||||||
| Temperature | 20.35 | 3,88 | 0.008 | 20.5 | 3,44 | <0.001 | 10, 20 > 40 at ≥0.03 M; 30 > 40 at ≥0.125 M; 20, 10 > 30 at ≥0.03 M | 3.8 | 3,44 | 0.02 | ns |
| Concentration | 68.28 | 6,528 | <0.001 | 28.7 | 6,264 | <0.001 | 1 > 0 M at 10, 20, 30 | 56.2 | 6,264 | <0.001 | 1 > 0 M at 10, 20, 30 |
| Temperature × concentration | 6.75 | 18,528 | <0.001 | 3.49 | 18,264 | <0.001 | 4.93 | 18,264 | <0.001 | ||
| Temperature × restriction | 10.45 | 3,88 | <0.001 | ||||||||
| Concentration × restriction | 5.01 | 6,528 | <0.001 | ||||||||
| Concentration × restriction × temperature | 1.27 | 18,528 | 0.2 | ||||||||
| Water intake | |||||||||||
| Water-restriction status | 158.98 | 1,88 | <0.001 | ||||||||
| Temperature | 7.05 | 3,88 | <0.001 | 6.53 | 3,44 | <0.001 | 10, 20 > 40, 30 at ≥0.03 M; 10 > 30 at ≥0.06 M; 20 > 30 at ≥0.03 | 1.66 | 3,44 | 0.19 | ns |
| Concentration | 17.37 | 6,528 | <0.001 | 15.74 | 6,264 | <0.001 | 1 > 0 M at 10, 20 | 2.88 | 6,264 | 0.01 | ns |
| Temperature × concentration | 4.21 | 18,528 | <0.001 | 3.67 | 18,264 | <0.001 | 1.93 | 18,264 | 0.01 | ||
| Temperature × restriction | 5.82 | 3,88 | 0.001 | ||||||||
| Concentration × restriction | 13.21 | 6,528 | <0.001 | ||||||||
| Concentration × restriction × temperature | 3 | 18,528 | <0.001 | ||||||||
df, degrees of freedom; ns, not significant.
Figure 1.
Data represent mean ± standard error of the mean of total fluid intake (g of solution + g of water consumed) during 2-bottle preference tests. Panel A represents total intake when rats were water-restricted. Panel B represents total intake for rats in the unrestricted condition.
Sucrose intake/preference
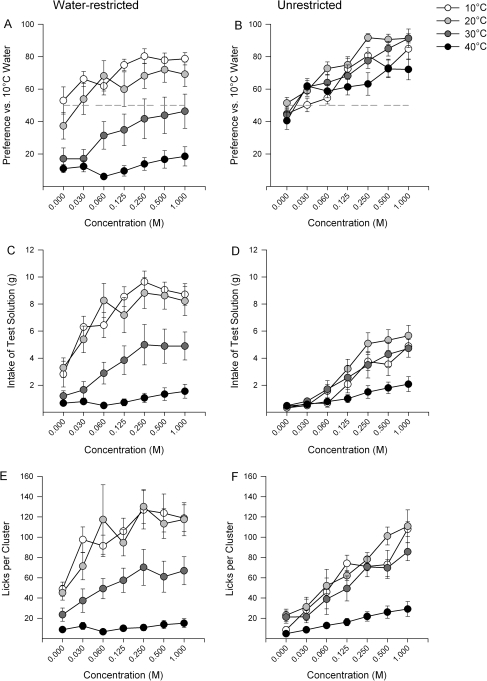
As illustrated in Figure 2A, water-restricted rats did not display a preference (>50% of the total intake) for 30 or 40 °C sucrose over 10 °C water at any concentration but showed a preference when sucrose was 20 °C or lower. Conversely, when the rats were unrestricted, they displayed a preference for sucrose over 10 °C at all temperatures (Figure 2B).
Figure 2.
Data represent mean ± standard error of the mean. Panel A and B represent the average preference score for sucrose over 10 °C water. The horizontal dotted line highlights 50% preference. Panels C and D represent the average intake of sucrose at each temperature. Panels E and F represent the number of licks/cluster of licking, that is, cluster size. Panels on the left (A, C, E) are data collected in the water-restricted condition, whereas panels on the right (B, D, F) represent the unrestricted condition.
There were significant differences between the preference scores and intakes of sucrose across water-restriction states (Table 1). In water-restricted rats, there were significant main effects of concentration and temperature on both the preference for and intake of sucrose (Table 1) such that rats displayed higher preference scores for sucrose and consumed more sucrose as the concentration increased (Figure 2A,C) and the temperature decreased. Post hoc tests revealed 2 exceptions. First, there was no effect of concentration at 40 °C (i.e., they displayed uniformly low preference scores), and second, there was no effect of temperature between 10 and 20 °C (i.e., both were equally preferred at all concentrations). Both concentration and temperature driven increases in intake were concurrent with increases in cluster size (Figure 2E) and cluster number (Table 2).
Table 2.
Summary of ANOVAs for cluster size and cluster number
| Source of variation | Water-restricted 2-way ANOVA | Tukey HSD (Ps < 0.05), water-restricted | Unrestricted 2-way ANOVA | Tukey HSD (Ps < 0.05), unrestricted | ||||
| F | df | P | F | df | P | |||
| Cluster size sucrose (Figure 2E,F) | ||||||||
| Temperature | 17.88 | 3,44 | <0.001 | 10, 20 > 40 at ≥0.03 M; 30 > 40 at ≥0.125 M; 10 > 30 at 0.03, 0.25–1 M; 20 > 30 at 0.06, 0.25–1 M | 23.3 | 3,19 | <0.001 | 10 > 40 at 0.03–0.125 and 1 M; 20 > 40 at ≥0.06 M; 30 > 40 at ≥0.125 M |
| Concentration | 15.5 | 6,264 | <0.001 | 1 > 0 M at 10, 40 | 24 | 6,114 | <0.001 | 1 > 0 M at 10, 20, 30 |
| Temperature × concentration | 2.41 | 18,264 | <0.001 | 3.46 | 18,114 | <0.001 | ||
| Cluster number sucrose | ||||||||
| Temperature | 5.63 | 3,44 | 0.002 | 10 > 40 at 0.03–0.5 M; 10 > 30 at 0.125–0.5 M | 0.42 | 3,44 | 0.73 | ns |
| Concentration | 7.9 | 6,264 | <0.001 | 1 > 0 M at 10, 20 | 46.2 | 6,264 | <0.001 | 1 > 0 M at 10, 20, 30, 40 |
| Temperature × concentration | 2.34 | 18,264 | 0.002 | 1.12 | 18,264 | 0.33 | ||
| Cluster size water | ||||||||
| Temperature | 0.49 | 3,43 | 0.07 | ns | 0.05 | 3,16 | 0.9 | ns |
| Concentration | 1.06 | 6,258 | 0.38 | ns | 3.97 | 5,80 | 0.003 | ns |
| Temperature × concentration | 1.46 | 18,258 | 0.1 | 1.57 | 15,80 | 0.2 | ||
| Cluster number water | ||||||||
| Temperature | 12.23 | 3,44 | <0.001 | 40 > 10, 20 at ≥0.0 M; 10 > 30 at 0.03–0.25 and 1 M; 40 > 30 at ≥0.06 M; 30 > 20 at 0.03–0.06 and 0.25–0.5 M | 3.27 | 3,44 | 0.03 | ns |
| Concentration | 13.51 | 6,264 | <0.001 | 1 < 0.0 M at 10, 20 | 1.94 | 6,264 | 0.07 | ns |
| Temperature × concentration | 2.67 | 18,264 | <0.001 | 1.37 | 18,264 | 0.15 | ||
df, degrees of freedom.
In unrestricted rats, there was a significant main effect of concentration but no effect of temperature (Table 1) on sucrose preference, but both concentration and temperature effected sucrose intake (Table 1). Post hoc tests revealed that rats showed concentration-dependent increases in sucrose intake at 10, 20, and 30 °C. These concentration-dependent increases in sucrose intake were due to increases in cluster size (Table 2, Figure 2F) and number (Table 2). Additionally, cluster size was altered by temperature, but there was no effect of temperature on cluster number (Table 2).
Water intake
There was a main effect of water-restriction status on 10 °C water intake during the 2-bottle tests (Table 1). Water-restricted rats drank more than unrestricted rats. In the water-restricted condition, there were significant main effects of both temperature and concentration on water intake (Table 1). Intake of 10 °C water was decreased by the presentation of increasing concentrations of sucrose and decreasing temperature. This change in intake was due to a decrease in number of clusters but not the size of the cluster (Table 2). In the unrestricted condition, there was a significant main effect of concentration on water intake but no effect of temperature (Table 1).
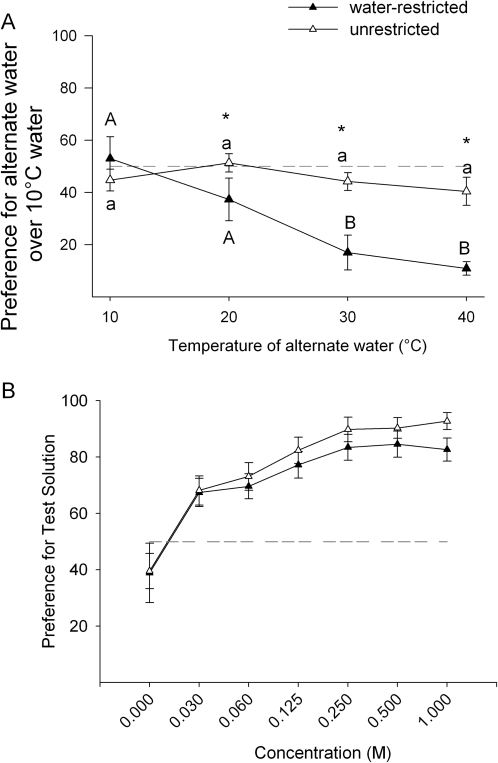
Trials in which temperature was the only variable, that is, where water was presented in both bottles (10 °C vs. 0.0 M concentration of sucrose, Figure 3A), preference scores were significantly higher in the water-restricted condition compared with the unrestricted condition (F1,88 = 19.48, P < 0.001). Rats preferred 10 °C water over warmer water (20–40 °C) in all cases when the rats were water-restricted (Figure 3A). Unrestricted rats weakly preferred 10 °C water or showed indifference (Figure 3A). There was a main effect of temperature in the alternate bottle (F3,88 = 10.40, P < 0.001) and a significant interaction between water-restriction state and temperature (F3,88 = 5.97, P = 0.001). Bonferroni corrected planned comparisons reveal that there are no statistically significant differences between temperatures in the water-restricted condition but in the unrestricted condition the preference for 10 °C water is strongest when the alternative water is 30 or 40 °C water.
Figure 3.
Panel A data represent the mean ± standard error of the mean (SEM) of the preference for 10 °C water over water presented at other temperatures. The horizontal dotted line highlights 50% preference. Different letters represent statistically significant differences within the water-restriction treatments and * represent statistically significant differences between the water-restriction treatments based on Tukey HSD (P < 0.05). Panel B data represent mean ± SEM average preference score for sucrose over water while both test solutions are maintained at 22 °C. The horizontal dotted line highlights 50% preference. Filled triangles represent the preference score measured when rats were water-restricted. The open triangles represent the preference score measured when rats were unrestricted.
Control for order effects
To ensure preferences were not being affected by long-term exposure to sucrose and to control for any potential order effects, water-restricted rats were presented with a second sucrose series at 40 °C compared with 10 °C water. Although there was a main effect of concentration (F4,88 = 4.26, P = 0.003), there was no effect of order (F1,22 = 1.0, P < 0.33) on preference at 40 °C and no interaction (F4,88 = 1.35, P = 0.26) despite being separated by 9 weeks of testing.
Sucrose preference
To understand the role of water-restriction on sucrose preference in the absence of temperature variables, rats were given room temperature (22 °C) sucrose and water in the water-restricted and unrestricted conditions. As illustrated in Figure 3B, there was a significant effect of concentration (F6,132 = 29.97, P < 0.001) but no effect of water-restriction condition on preference (F1,22 = 1.5, P = 0.2) and no significant interaction between water-restriction and concentration (F6,132 = 0.32, P < 0.92) when both bottles were maintained at 22 °C.
Materials and methods
Apparatus
In the prior experiment, both postingestive and orosensory cues may have influenced intake. In the second experiment, we used brief-access tests to minimize the effects of postingestive cues on intake. Rats were tested in a Davis rig (MS-180) modified to control fluid temperature. This behavioral testing apparatus consisted of a Plexiglas chamber with wire mesh floor and an opening that allowed access to 1 of 4 sipper tubes, which were alternated by a motorized sliding platform. A mechanical shutter controlled access to each of the tubes for a programmed length of time. The computer controlled the shutter and the order of stimulus presentation. Each sipper tube was held snugly in an aluminum block containing a Peltier device as described above. Additionally, each individual lick on the sipper tube was detected by a contact lickometer and recorded to a computer installed with the DavisRig3 collection software (FSU custom software). Data were then analyzed in DavisPro (Dialog Instruments).
Training
Rats (n = 8) were adapted to the test chamber and trained to drink from the sipper tubes for 11 days. On the first day of training, rats were 20 h water-restricted. The rat was presented with a single tube containing 0.25 M sucrose at 22 °C. When the rat found the tube and licked it at least 50 times, the training program began. At the start of the program, the shutter closed for 10 s before a new tube containing 0.25 M sucrose was presented. The rat was given 180 s to initiate licking, and once licking was recorded by the computer, the rat was given 30 s access to the tube. At the conclusion of either the 30 s of access or the 180 s limit, the shutter was closed again for 10 s. Each of the 4 tubes, all containing 22 °C 0.25 M sucrose, was presented 5 times. The entire training program took an average of 15 min. On the remaining days of training, rats were not water-restricted and were given the training program as described above for 3 days. During the final stage of training (7 days), rats were presented with varying concentrations of sucrose (0.0, 0.05, 0.1, 0.2 M) at room temperature (22 °C). This series was presented to reduce the novelty of the test situation and to train the animals using variable concentrations. Solutions were presented to the rats at random, and each tube was presented 4 times per session.
Testing
Unrestricted rats were given varying concentrations of sucrose (0.0, 0.05, 0.1, 0.2 M) in each of the 4 bottles. All bottles were held at a constant temperature during testing, and each concentration was presented in 4 trials per day. Each temperature was presented for 2 days in the following order: 40, 10, 30, and 20 °C. Identical to training; the rat was given 180 s to initiate licking, 30 s access once a lick was recorded, and a 10 s delay between stimulus presentations. Presented data represent an average response to each stimulus presentation.
At the conclusion of this series, rats were presented with bottles at varying temperatures (10, 20, 30, and 40 °C) but with a constant concentration of sucrose each day. Concentrations of sucrose (0.0, 0.05, 0.1, 0.2 M) were presented at random; all concentrations were presented for 2 days. The data represent the average number of licks of all 30 s presentations of any given stimulus.
To explore the role of restriction, immediately following the unrestricted testing, the same rats were 20 h water-restricted and tested in the same protocols described above. All rats were given 1 h access to supplemental water 3 h following testing.
Lastly, to determine if there was a contrast effect between the stimuli driven by the warmest solution presented, unrestricted rats were given access to a single concentration of sucrose (0.1 M) for 4 days at various temperatures. On days 1 and 2, rats were presented with 10, 20, 30, and 35 °C. On days 3 and 4, rats were presented with 10, 20, 25, and 30 °C. Once again, tubes were presented at random and the presentation parameters were identical to those described above. These data were compared with the data collected in the previous tests at 10, 20, 30 and 40 °C.
Results
Temperature constant, solution concentration varied
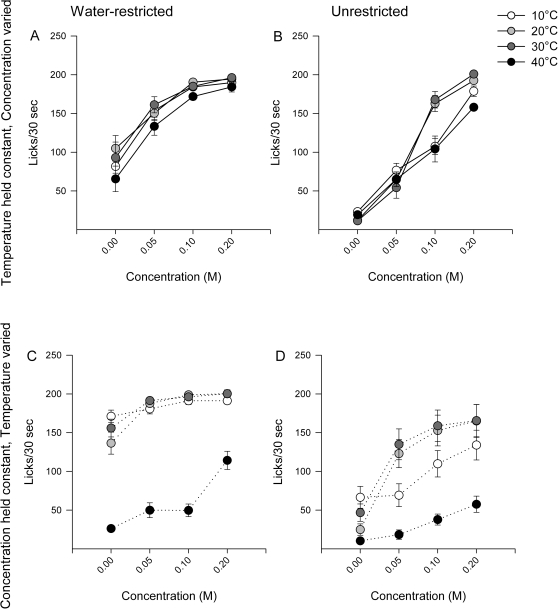
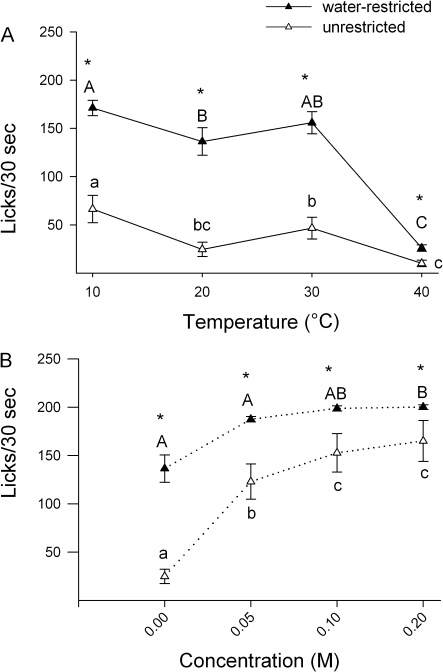
Rats were presented with 4 different concentrations of sucrose at a single temperature each day. There was a significant effect of water-restriction state (Table 3, Figure 4A,B) as water-restricted rats licked more than unrestricted rats. There was also a significant main effect of concentration in both water-restriction conditions (Figure 4A,B, Table 3), and temperature significantly affected licking in the unrestricted condition. Post hoc tests revealed that unrestricted rats licked less to 10 and 40 °C than to 20 and 30 °C of both 0.1 and 0.2 M sucrose. There was no effect of temperature in the water-restricted condition (Table 3).
Table 3.
Summary of ANOVAs for brief-access licking
| Source of variation | 3-way ANOVA |
Water-restricted 2-way ANOVA |
Tukey HSD (Ps < 0.05), water-restricted | Unrestricted 2-way ANOVA |
Tukey HSD (Ps < 0.05), unrestricted | ||||||
| F | df | P | F | df | P | F | df | P | |||
| Temperature constant (Figure 4A,B) | |||||||||||
| Water-restriction status | 137.96 | 1,56 | <0.001 | ||||||||
| Temperature | 6.38 | 3,56 | 0.001 | 1.86 | 3,28 | 0.17 | ns | 6.57 | 2,28 | 0 | 20, 30 > 40, 10 at ≥0.1 M |
| Concentration | 481.75 | 3,168 | <0.001 | 117.74 | 3,84 | <0.001 | 0.2 > 0 M at 10, 20, 30, 40 | 531.57 | 3,84 | <0.001 | 0.2 > 0 M at 10, 20, 30, 40 |
| Temperature × concentration | 2.36 | 9,168 | 0.02 | 0.55 | 9,84 | 0.83 | 8.68 | 9,84 | <0.001 | ||
| Temperature × restriction | 0.32 | 3,56 | 0.81 | ||||||||
| Concentration × restriction | 31.43 | 3,168 | <0.001 | ||||||||
| Concentration × restriction × temperature | 4.19 | 9,168 | <0.001 | ||||||||
| Temperature variable (Figure 4C,D) | |||||||||||
| Water-restriction status | 136.8 | 1,56 | <0.001 | ||||||||
| Temperature | 169.77 | 3,56 | <0.001 | 203.97 | 3,28 | <0.001 | 10 > 20 at 0.0 M; 10, 20, 30 > 40 at ≥0.0 M | 45.67 | 3,28 | <0.001 | 20, 30 > 10, 40 at ≥0.03 M |
| Concentration | 217.1 | 3,168 | <0.001 | 44.42 | 3,84 | <0.001 | 0.2 >0 M at 20, 30, 40 | 210.13 | 3,84 | <0.001 | 0.2 > 0 M at 10, 20, 30, 40 |
| Temperature × concentration | 15.49 | 9,168 | <0.001 | 5.9 | 9,84 | <0.001 | 18.98 | 9,84 | <0.001 | ||
| Temperature × restriction | 6.57 | 3,56 | <0.001 | ||||||||
| Concentration × restriction | 27.61 | 3,168 | <0.001 | ||||||||
| Concentration × restriction × temperature | 8.65 | 9,168 | <0.001 | ||||||||
df, degrees of freedom.
Figure 4.
Data represent mean ± standard error of the mean of licks/30 s. Panels A and B represent the trials in which the temperature was maintained constant and solution concentration varied within a session. Panels C and D represent the trials in which the test solution concentration was maintained constant and temperature varied within a session. Although the stimuli were presented with the concentration constant within a session, the data are graphed across concentration to allow for comparison with panel A.
Temperature varied, solution concentration constant
In a second round of testing, rats were presented with a single concentration of sucrose at 4 different temperatures each day. There was a significant main effect of water-restriction (Table 3); rats licked more when water-restricted than when unrestricted (Figure 4C,D). There were significant main effects of temperature and concentration in both water-restriction conditions (Table 3). Post hoc tests revealed the pattern of results to be similar to the pattern in the “temperature held constant” paradigm except all the effects were exaggerated. Licking to 40 °C sucrose was decreased compared with all other temperatures in the water-restricted condition at every concentration. Likewise, unrestricted rats reduced licking when presented with 10 or 40 °C sucrose compared with 20 or 30 °C.
Examination of the water data alone, where temperature was the only variable (Figure 5A), demonstrates a significant effect of deprivation on licking to water (F1,56 = 136.32, P < 0.001) because water-restricted animals lick more at each temperature. There was also a significant main effect of temperature (F3,56 = 43.06, P < 0.001). Post hoc tests demonstrate that rats lick more to 10 °C than warmer waters (Figure 5A). Finally, there was also a significant interaction between water-restriction and temperature (F3,56 = 10.31, P < 0.001).
Figure 5.
Panel A data represent the mean ± standard error of the mean (SEM) of licks/30 s. Different letters represent statistically significant differences within the water-restriction treatments and * represent statistically significant differences between the water-restriction treatments based on Bonferroni-corrected planned comparisons (P < 0.05). Panel B data represent mean ± SEM average of licks/30 s for water and sucrose while both solutions are maintained at 20 °C. Filled triangles represent licking recorded when rats were water-restricted. The open triangles represent licking recorded when rats were unrestricted.
To parallel the room temperature comparison of water-restriction status in Experiment 1, we have regraphed the licking behavior of water-restricted and unrestricted trials on 20 °C. In this case however, water-restriction status significantly altered the rate of licking (F1,12 = 54.06, P < 0.001) as water-restricted rats lick more than unrestricted rats (Figure 5B). There was also a significant main effect of concentration (F3,36 = 97.34, P < 0.001) and a significant interaction between temperature and water-restriction status (F3,36 = 20.85, P < 0.001).
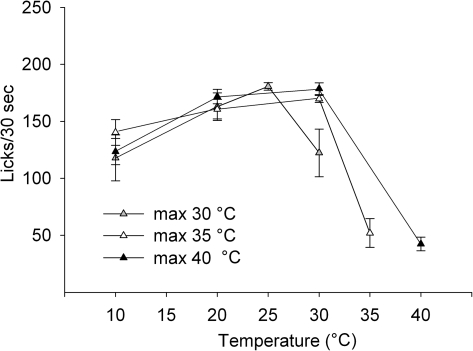
Temperature variants
To determine the role of contrast on unconditioned licking, we varied temperature such that the highest temperature the rats received was 30, 35, or 40 °C (Figure 6) while the sucrose concentration was constant at 0.1 M. The highest temperature presented, in any test session, produced the least amount of licking. Ten and 20 °C were common to all test sessions and did not differ in the amount of licking across the trials (10 °C: F2,14 = 2.57, P = 0.11, 20 °C: F2,14 = 1.4, P = 0.28). Licking to 30 °C was reduced when it was the highest available temperature compared with the test sessions with higher temperatures presented (F2,14 = 7.73, P = 0.005). However, the decrease at 30 °C was not as large as the decrease at 35 or 40 °C (F2,21 = 9.0, P = 0.001).
Figure 6.
Data represent mean ± standard error of the mean of licks/30 s. Each line represents a series of trials with a different temperature as the maximum temperature available. All trials were conducted with 0.1 M sucrose and with unrestricted rats.
Discussion
We posed 3 “simple” questions in our research: 1) What is the role of water-restriction in cold water preference? 2) Does temperature contrast alter licking in a brief access test? and 3) Does temperature change the orosensory driven responses to water or sweet solutions? We have demonstrated that water-restriction plays a large role in cold water preference, that cold is rewarding to water-restricted rats, and conversely, warm is aversive in both water-restricted and unrestricted rats. We have found that temperature contrast exaggerates the effects of temperature on licking but is not necessary to demonstrate temperature/taste interaction. We have also concluded that deviations from room temperature alter orosensory driven responses to sucrose in brief-access tests.
Water-restriction strengthens cold preference
These studies have demonstrated that cold water is rewarding, 5 lines of evidence supporting this hypothesis. First, cold motivates intake, water-restricted rats drink more as solutions are presented at lower temperatures (Figure 1). Second, cold water is preferred over warmer water (Figure 3A). Third, cold water is isopreferred to sucrose solutions in water-restricted rats, that is, water at 10 °C is isopreferred to 0.03 M sucrose (Figure 2A). In fact, each 10 °C reduction in sucrose temperature resulted in a greater increase in sucrose preference than doubling sucrose concentration did (except between 10 and 20 °C where preference seemed to be limited by a ceiling effect). Fourth, cold increases cluster size, a measure of palatability in both water-restriction states (Davis 1989, Figure 2E,F), and lastly, cold increased licking to water in brief-access tests (Figure 5). There are a handful of previous studies in agreement including several preference tests (Deaux and Engstrom 1973; Ramsauer et al. 1974; Smith et al. 2010) and a study demonstrating that rats will bar press more for cold than warm water (Ramsauer et al. 1974).
Although the evidence for a rewarding value of cold came from water-restricted and unrestricted animals, it is clear that the rewarding value of cold is much stronger in water-restricted animals, as unrestricted rats drank much less than water-restricted rats and prefer the cold less. It should be highlighted that water-restriction does not change preference for sucrose but rather changes preference for solution temperature as demonstrated by the fact that the water-restriction state had no effect on sucrose preference when water and sucrose were both presented at room temperature (Figure 3B). Water-restriction seems necessary to maintain the strength of the preference in a long test, however, unrestricted rats display increased licking to cold water in the brief-access test.
Possible value of cold water
We can only speculate on what aspect of cold water is rewarding, but since water-restriction strengthens the preference and the preference has been demonstrated during both volemic and osmotic thirst (Gold et al. 1977), one can consider the physiological changes a rat experiences during water-restriction as starting points for the hypotheses. During water-restriction, there are changes that we believe are potentially altered by cold: a decrease in saliva production (Walsh et al. 2004) and an alteration of the internal fluid balance (Daniels and Fluharty 2009).
Cold water preference may be related to returning the mouth to its normal salivary state. There is evidence that after drying the mouth, rinsing with cold water produces more saliva than rinsing with warm water (Brunstrom et al. 1997). This is consistent with much of the early literature that suggested that the rewarding value of cold water was due to orolingual cooling (Gold 1973; Gold and Prowse 1974). Water-restricted rats emit behaviors in response to orolingual cooling in the absence of water intake such as licking metal (Mendelson and Chillag 1970) or cool jets of air (Hendry and Rasche 1961). Secondly, cold evokes sour perception (Cruz and Green 2000) and stimulates sour responsive neurons (Breza et al. 2006). This is particularly interesting because sour stimuli are known to increase salivation. However, there is to date, no evidence that salivation is rewarding.
A perceived enhancement of rehydration is another possible explanation for the rewarding value of cold. Cold water has differential postingestive effects compared with warm water, and it has been demonstrated that although there is a strong preference for cold water, rats drink less cold water than warm in 1-bottle short-term tests (Kapatos and Gold 1972). This effect, which has been coined “cold water suppression,” suggests that cold is more satiating than warm under these term conditions. Sawchenko et al. (1977) demonstrated that bilateral gastric vagotomy increased the gastric emptying rate of warm water to mirror that of cold water, and this procedure abolished the cold water suppression effect. They did not however explore the cold water preference in these animals.
Although, we cannot currently distinguish between these hypotheses, they are all consistent with our findings. Unrestricted animals do not require either quick hydration (as suggested by the increased gastric emptying) or increased saliva production (orolingual cooling). Either or both of these potential mechanisms could contribute to the rewarding value of cold.
By the same measures that cold seems rewarding, conversely, warm seems aversive. Warm reduces intake (Figure 1), it is avoided in both the 2-bottle and brief-access tests, and there was no effect of concentration on intake or preference of sucrose at 40 °C (Figure 2A), that is, a 1 M increase in sucrose concentration could not elicit intake. Warm decreases cluster size in both water-restriction states (Davis 1989; Figure 2E,F), and lastly, warm decreased licking to water in brief-access tests (Figure 5). Unlike the rewarding value of cold, the aversive properties of warm seem to be independent of water-restriction status. There are several possible ecological reasons a rat may reduce licking to warm solutions or more generally to water above body temperature. For example, warm water is more capable of holding solutes and of growing contaminants than cold water. Perhaps there is an evolutionary history of temperature preference and water safety.
Temperature contrast exaggerates differences in unconditioned licking
To address contrast, we conducted the brief-access testing in 2 ways; we held the temperature constant and contrasted the solution concentration within a session (Figure 4A,B) or we varied the temperature and held the solution concentration constant (Figure 4C,D). Tubes were presented at random in both protocols. Within a session, temperature contrast maximized differences in magnitude of licking to solutions, although the relative responses to different temperatures were similar in both unrestricted protocols, for example, licking to 10 and 40 °C was reduced in both unrestricted series but with greater amplitude when the 2 temperatures were presented in the same test (Figure 4B,D). This relationship suggests that holding the concentration constant highlights the temperature as the salient cue but contrast was not required for differential responses to temperature.
To explore contrast further, we presented rats with 3 brief-access scenarios where the warmest temperature available was 30, 35, or 40 °C (Figure 6). Under these conditions, rats licked significantly less to the highest temperature available compared with the lower temperatures. When 30 °C was the highest temperature, it was consumed less than when it was presented as an intermediate temperature. However, the licking at 30 °C was significantly more than that at 35 or 40 °C, which suggests a relationship between absolute temperature and the acceptability of the stimulus as well as a role for contrast. Interestingly, the break point for stimulus acceptability seemed to be around body temperature.
Temperature alters orosensory responses to sweet solutions
Water-restriction induced near maximal licking making it difficult to detect differential responses to the stimuli. Furthermore, temperature effects were exaggerated when temperatures were contrasted within the brief-access test. Together, these comparisons suggest that the unrestricted condition, which contrasted temperatures, allows us the most information about temperature and taste interactions (Figure 4D).
Consistent with the cold water preference demonstrated in the 2-bottle test, rats increased licking to cold water over warmer water (Figure 3A). However, rats increased licking to warmer sucrose over cold (10 °C) sucrose, except for 40 °C where licking was greatly reduced (Figure 4D). Thus, the rank order of licking elicited from lowest to highest stimuli was 40 °C solutions < warm water < cold water = cold sucrose < warm sucrose. It is important to note that although water-restriction was an important factor in temperature preference, this experiment, which relies mainly on orosensory feedback, demonstrates clearly that temperature alters the behavior of unrestricted rats as well.
The reduction in licking to sucrose at 10 °C could be due to either a trigeminally mediated aversion or a reduction in sweet taste intensity. We use the term “trigeminally mediated aversion” to imply that there is something unpleasant about the stimulus temperature, not that it causes pain or discomfort, as we do not believe any of the temperatures used in the study were capable of causing pain. With that said, it is unlikely that the reduction in licking at 10 °C is due to an aversion to the temperature. Cold water is preferred to warmer water in both experiments suggesting that 10 °C does not decrease licking. The reduction in licking could be indicative of the latter hypothesis that cold reduces sweet taste perception. Although these data do not test this hypothesis directly, it is consistent with both electrophysiological data which demonstrate that cold temperature reduces taste-mediated activity in peripheral gustatory neurons in rats (Breza et al. 2006) and psychophysical evidence that humans report decreased sweet intensity ratings as a solution is cooled (Bartoshuk et al. 1982).
Lastly, licking was also greatly reduced when solutions were presented at 40 °C. As with the decrease in licking recorded at 10 °C, this could be due to a trigeminally mediated aversion or a reduction in sweet taste intensity. Unlike the 10 °C response, there is little evidence to suggest that the decreased licking at 40 °C is due to a reduction in sweet taste intensity. Both 20 and 30 °C produced maximal and identical licking patterns suggesting that 30 °C was as motivating as 20 °C and both produced greater licking than at 10 °C. In fact, human psychophysical subjects report warm sweet solutions to be sweeter than cold sweet solutions (Bartoshuk et al. 1982). A trigeminal aversion seems a likely possibility.
Conclusions
Although the mediation of cold preference in the water-restricted rat remains unclear, temperature is clearly a motivating cue that shapes ingestion. The relationship between temperature and concentration of a solution is variable. Water and temperature have an interaction that is independent of the interaction between temperature and the taste solution. In the future, understanding how temperature interacts with different taste modalities will be important for understanding the role temperature plays in ingestive behavior.
Funding
This work was supported by grants from the National Institute on Deafness and Communication Disorders at the National Institutes of Health [RO1DC-004785 and DC-010110 to R.J.C., NIDCD 04607 to T.A.H. and J.C.S., 1F31DC-009920 to J.M.B., and DC000044 to A.-M.T.].
Acknowledgments
We thank Paul Hendrick, Fred Fletcher, Kyle Wells, Ross Henderson, Te Tang, and Alisa Millet for their excellent technical assistance and Lisa Eckel for her thoughtful comments on this manuscript. A portion of this work was presented at the Association for Chemoreception Sciences, in St. Pete Beach, FL, 2010.
References
- Bartoshuk LM, Rennert K, Rodin J, Steven JC. Effects of temperature on the perceived sweetness of sucrose. Physiol Behav. 1982;28:905–910. doi: 10.1016/0031-9384(82)90212-8. [DOI] [PubMed] [Google Scholar]
- Boulze D, Montastruc P, Cabanac M. Water intake, pleasure and water temperature in humans. Physiol Behav. 1983;30:97–102. doi: 10.1016/0031-9384(83)90044-6. [DOI] [PubMed] [Google Scholar]
- Breza JM, Curtis KS, Contreras RJ. Temperature modulates taste responsiveness and stimulates gustatory neurons in the rat geniculate ganglion. J Neurophysiol. 2006;95:674–685. doi: 10.1152/jn.00793.2005. [DOI] [PubMed] [Google Scholar]
- doi: 10.1016/s0031-9384(96)00517-3. Brunstrom JM, Macrae AW, Roberts B. 1997. Mouth-State dependent changes in the judged pleasantness of water at different temperatures. Physiol Behav. 61:667–669. [DOI] [PubMed] [Google Scholar]
- Cruz A, Green GG. Thermal stimulation of taste. Nature. 2000;403:889–892. doi: 10.1038/35002581. [DOI] [PubMed] [Google Scholar]
- Daniels D, Fluharty SJ. Neuroendocrinology of body fluid homeostasis. Horm Brain Behav. 2009;1:259–288. [Google Scholar]
- Davis JD. The microstructure of ingestive behavior. Ann N Y Acad Sci. 1989;575:106–121. doi: 10.1111/j.1749-6632.1989.tb53236.x. [DOI] [PubMed] [Google Scholar]
- Davis JD, Perez MC. Food deprivation- and palatability-induced microstructural changes in ingestive behavior. Am J Physiol Regul Integr Comp Physiol. 1993;264:R97–R103. doi: 10.1152/ajpregu.1993.264.1.R97. [DOI] [PubMed] [Google Scholar]
- Deaux E, Engstrom R. The temperature of ingested water: preference for cold water as an associative response. Physiol Psychol. 1973;1:257–260. [Google Scholar]
- Fishman IY. Single fiber gustatory impulses in rat and hamster. J Cell Comp Physiol. 1957;49:319–334. doi: 10.1002/jcp.1030490213. [DOI] [PubMed] [Google Scholar]
- Gold RM. Cool water suppression of water intake: one day does not a winter make. Bull Psychon Soc. 1973;1:385–386. [Google Scholar]
- Gold RM, Laforge RC, Sawchenko PE, Fraser JC, Pytko D. Cool water’s suppression of water intake: persistence across deprivation conditions, ages, sexes and osmolarities. Physiol Behav. 1977;18:1047–1053. doi: 10.1016/0031-9384(77)90010-5. [DOI] [PubMed] [Google Scholar]
- Gold RM, Prowse J. Water temperature preference shifts during hydration. Physiol Behav. 1974;13:291–296. doi: 10.1016/0031-9384(74)90047-x. [DOI] [PubMed] [Google Scholar]
- Green BG, George P. ‘Thermal taste’ predicts higher responsiveness to chemical taste and flavor. Chem Senses. 2004;29:617–628. doi: 10.1093/chemse/bjh065. [DOI] [PubMed] [Google Scholar]
- Hendry DP, Rasche RH. Analysis of a new nonnutritive positive reinforcer based on thirst. J Comp Physiol Psychol. 1961;54:477–483. doi: 10.1037/h0047941. [DOI] [PubMed] [Google Scholar]
- Kapatos G, Gold RM. Tongue cooling during drinking: a regulator of water intake in rats. Science. 1972;176:685–686. doi: 10.1126/science.176.4035.685. [DOI] [PubMed] [Google Scholar]
- Lundy RF, Contreras RJ. Temperature and amiloride alter taste nerve responses to Na+, K+, and NH4+ salts in rats. Brain Res. 1997;744:309–317. doi: 10.1016/S0006-8993(96)01118-3. [DOI] [PubMed] [Google Scholar]
- Lundy RF, Contreras RJ. Gustatory neuron types in rat geniculate ganglion. J Neurophysiol. 1999;82:2970–2988. doi: 10.1152/jn.1999.82.6.2970. [DOI] [PubMed] [Google Scholar]
- Mendelson J, Chillag D. Tongue cooling: a new reward for thirsty rodents. Science. 1970;170:1418–1421. doi: 10.1126/science.170.3965.1418. [DOI] [PubMed] [Google Scholar]
- McBurney DH, Collings VB, Glanz LM. Temperature dependence of human taste response. Physiol Behav. 1973;11:89–94. doi: 10.1016/0031-9384(73)90127-3. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Kurihara K. Differential temperature dependence of taste nerve responses to various taste stimuli in dogs and rats. Am J Physiol Regul Integr Comp Physiol. 1991;261:R1402–R1408. doi: 10.1152/ajpregu.1991.261.6.R1402. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Sato M, Yamashita S. Multiple sensitivity of chordat typani fibres of the rat and hamster to gustatory and thermal stimuli. J Physiol. 1968;199:223–240. doi: 10.1113/jphysiol.1968.sp008650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsauer S, Mendelson J, Freed WJ. Effects of water temperature on the reward value and satiating capacity of water in water-deprived rats. Behav Biol. 1974;11:381–393. doi: 10.1016/s0091-6773(74)90667-1. [DOI] [PubMed] [Google Scholar]
- Sandick BL, Engell DB, Maller O. Perception of drinking water temperature and effects for humans after exercise. Physiol Behav. 1984;32:851–855. doi: 10.1016/0031-9384(84)90205-1. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Gold RM, Ferrazano PA. Abolition by selective gastric vagotomy of the influence of water temperature on water intake: mediation via enhanced gastric clearance. Physiol Behav. 1977;18:1055–1059. doi: 10.1016/0031-9384(77)90011-7. [DOI] [PubMed] [Google Scholar]
- Smith PL, Smith JC, Houpt TA. Interactions of temperature and taste in conditioned aversions. Physiol Behav. 2010;99:324–333. doi: 10.1016/j.physbeh.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh NP, Laing SJ, Oliver SJ, Montague JC, Walters R, Bilzon JLJ. Saliva parameters as potential indices of hydration status during acute dehydration. Med Sci Sports Exerc. 2004;36:1535–1542. doi: 10.1249/01.mss.0000139797.26760.06. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Sato M. The effects of temperature on gustatory response of rats. J Cell Comp Physiol. 1965;66:1–17. doi: 10.1002/jcp.1030660102. [DOI] [PubMed] [Google Scholar]