Abstract
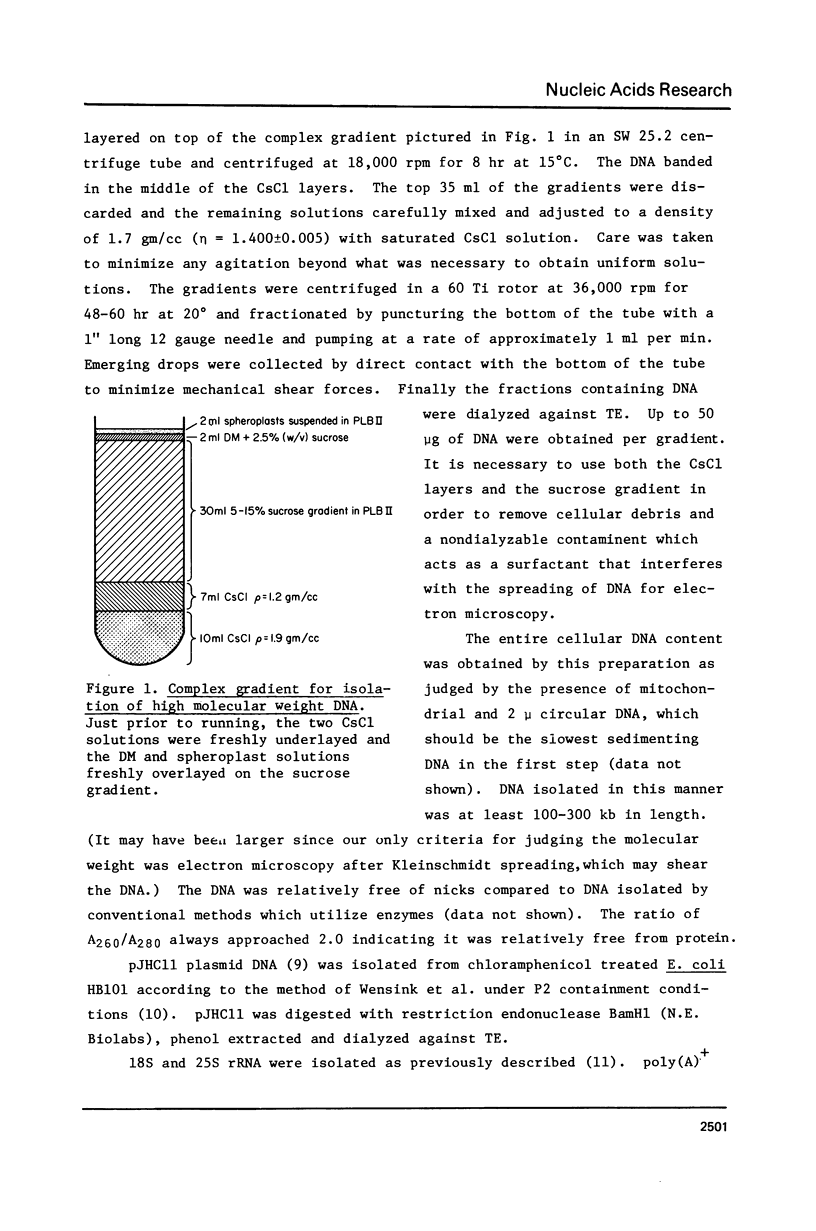
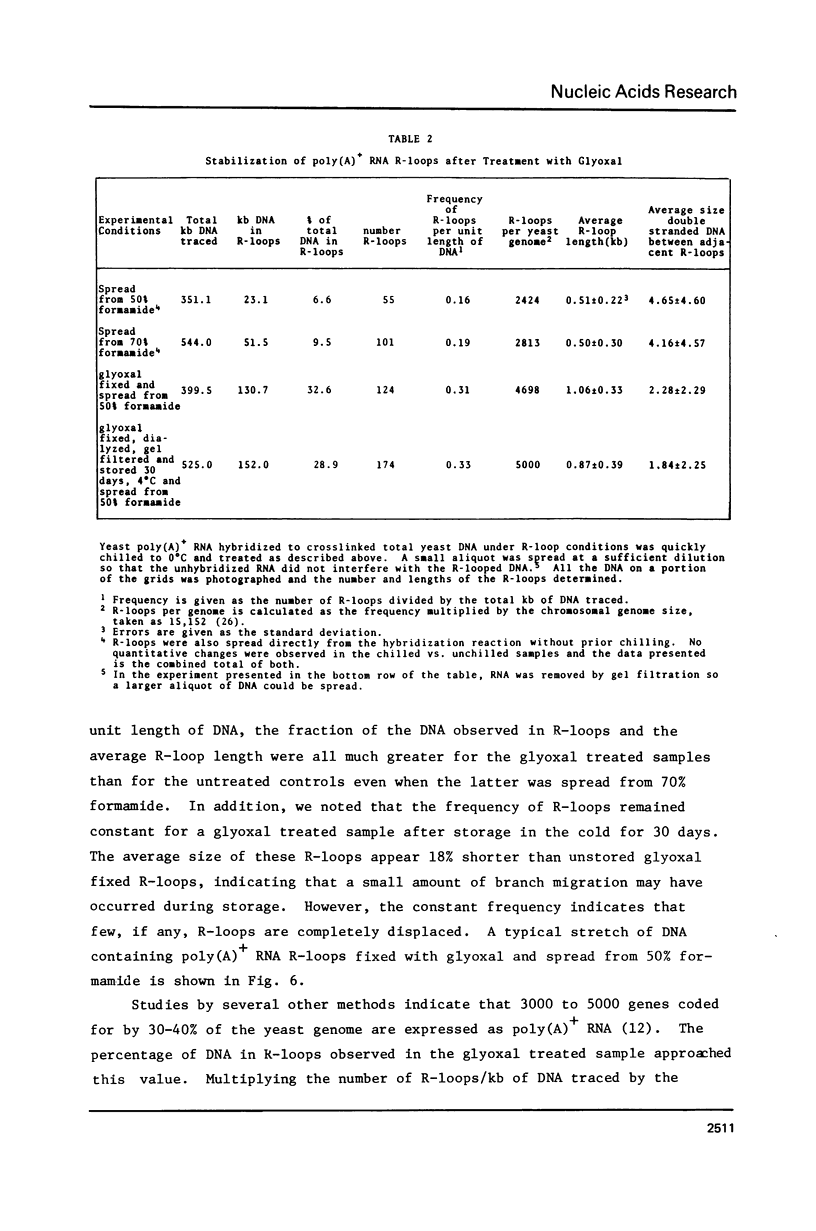
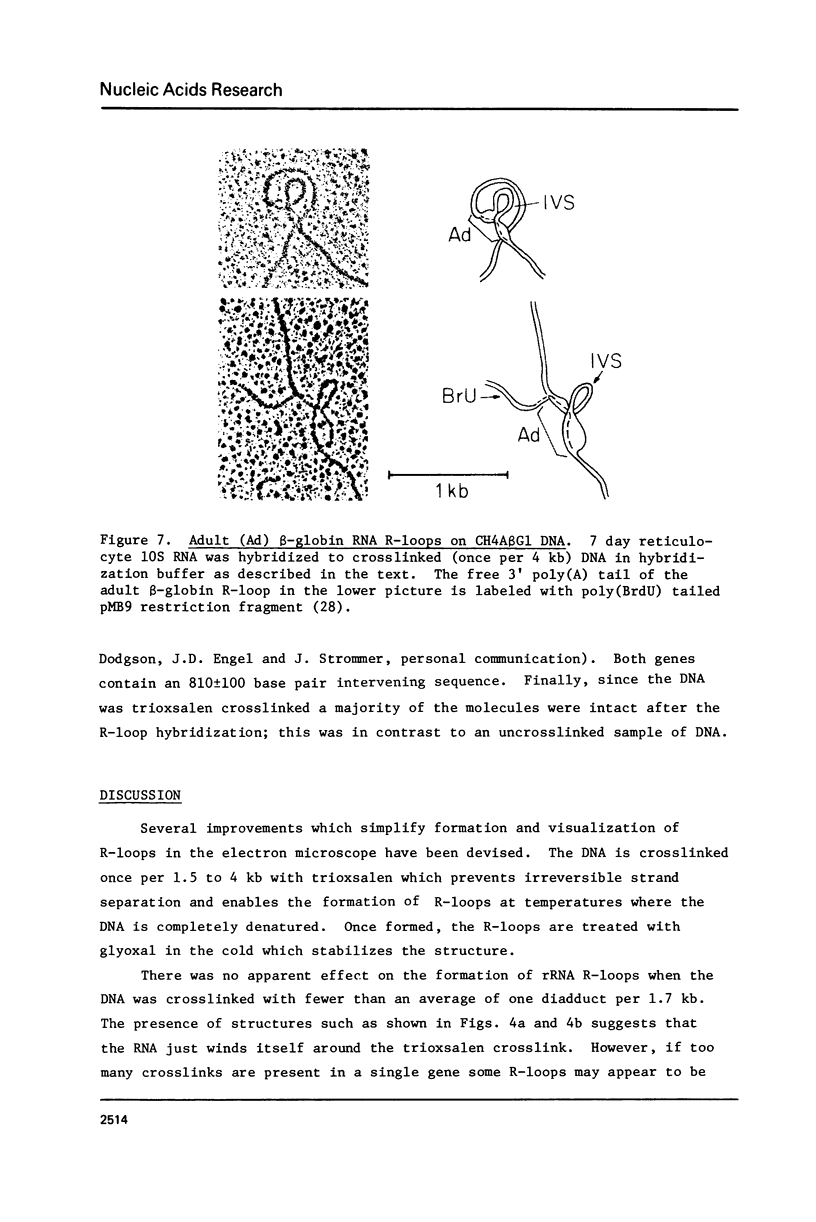
Improved methods for the formation and stabilization of R-loops for visualization in the electron microscope are presented. The two complementary strands of a duplex DNA are photochemically crosslinked once every 1 to 3 kb using 4, 5', 8 trimethylpsoralen. R-loops are then formed by incubation with RNA in 70% formamide at a temperature above the DNA melting temperature. Finally, the R-loops are stabilized by modifying the free single strand of DNA with glyoxal, thus minimizing the displacement of the hybridized RNA by branch migration. In this manner R-loops can be formed and visualized at a high frequency irrespective of the base composition of the nucleic acid of interest.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., DeGennaro L. J., Gelfand D. H., Bishop R. J., Valenzuela P., Rutter W. J. Ribosomal RNA genes of Saccharomyces cerevisiae. I. Physical map of the repeating unit and location of the regions coding for 5 S, 5.8 S, 18 S, and 25 S ribosomal RNAs. J Biol Chem. 1977 Nov 25;252(22):8118–8125. [PubMed] [Google Scholar]
- Blamire J., Cryer D. R., Finkelstein D. B., Marmur J. Sedimentation properties of yeast nuclear and mitochondrial DNA. J Mol Biol. 1972 Jun 14;67(1):11–24. doi: 10.1016/0022-2836(72)90382-8. [DOI] [PubMed] [Google Scholar]
- Broude N. E., Budowsky E. I. The reaction of glyoxal with nucleic acid components. 3. Kinetics of the reaction with monomers. Biochim Biophys Acta. 1971 Dec 30;254(3):380–388. doi: 10.1016/0005-2787(71)90868-9. [DOI] [PubMed] [Google Scholar]
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R., Pardue M. L. Electron microscopy of DNA crosslinked with trimethylpsoralen: test of the secondary structure of eukaryotic inverted repeat sequences. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2644–2648. doi: 10.1073/pnas.73.8.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. T., Gelinas R. E., Broker T. R., Roberts R. J. An amazing sequence arrangement at the 5' ends of adenovirus 2 messenger RNA. Cell. 1977 Sep;12(1):1–8. doi: 10.1016/0092-8674(77)90180-5. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Roberts J. M., Lewis J. B., Broker T. R. A map of cytoplasmic RNA transcripts from lytic adenovirus type 2, determined by electron microscopy of RNA:DNA hybrids. Cell. 1977 Aug;11(4):819–836. doi: 10.1016/0092-8674(77)90294-x. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Light-induced cross-linking of DNA in the presence of a furocoumarin (psoralen). Studies with phage lambda, Escherichia coli, and mouse leukemia cells. Biochim Biophys Acta. 1970 Sep 17;217(1):30–39. doi: 10.1016/0005-2787(70)90119-x. [DOI] [PubMed] [Google Scholar]
- Cramer J. H., Bhargava M. M., Halvorson H. O. Isolation and characterization of DNA of Saccharomyces cerevisiae. J Mol Biol. 1972 Oct 28;71(1):11–20. doi: 10.1016/0022-2836(72)90396-8. [DOI] [PubMed] [Google Scholar]
- Cramer J. H., Farrelly F. W., Barnitz J. T., Rownd R. H. Construction and restriction endonuclease mapping of hybrid plasmids containing Saccharomyces cerevisiae ribosomal DNA. Mol Gen Genet. 1977 Mar 16;151(3):229–244. doi: 10.1007/BF00268786. [DOI] [PubMed] [Google Scholar]
- Early P. W., Davis M. M., Kaback D. B., Davidson N., Hood L. Immunoglobulin heavy chain gene organization in mice: analysis of a myeloma genomic clone containing variable and alpha constant regions. Proc Natl Acad Sci U S A. 1979 Feb;76(2):857–861. doi: 10.1073/pnas.76.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. D., Davidson N. Addition of poly(adenylic acid) to RNA using polynucleotide phosphorylase: an improved method for electron microscopic visualization of RNA-DNA hybrids. Biochemistry. 1978 Sep 5;17(18):3883–3888. doi: 10.1021/bi00611a032. [DOI] [PubMed] [Google Scholar]
- Engel J. D., Dodgson J. B. Analysis of the adult and embryonic chicken globin genes in chromosomal DNA. J Biol Chem. 1978 Nov 25;253(22):8239–8246. [PubMed] [Google Scholar]
- Forsheit A. B., Davidson N., Brown D. D. An electron microscope heteroduplex study of the ribosomal DNAs of Xenopus laevis and Xenopus mulleri. J Mol Biol. 1974 Dec 5;90(2):301–314. doi: 10.1016/0022-2836(74)90375-1. [DOI] [PubMed] [Google Scholar]
- Glover D. M., Hogness D. S. A novel arrangement of the 18S and 28S sequences in a repeating unit of Drosophila melanogaster rDNA. Cell. 1977 Feb;10(2):167–176. doi: 10.1016/0092-8674(77)90212-4. [DOI] [PubMed] [Google Scholar]
- Hereford L. M., Rosbash M. Number and distribution of polyadenylated RNA sequences in yeast. Cell. 1977 Mar;10(3):453–462. doi: 10.1016/0092-8674(77)90032-0. [DOI] [PubMed] [Google Scholar]
- Karijord O., Oyen T. B. Study of a haploid yeast strain with an unusually high rDNA content. II. Fractionation of the DNA in preparative Ag+-Cs2SO4 density gradients. Biochim Biophys Acta. 1975 Mar 21;383(3):255–265. doi: 10.1016/0005-2787(75)90054-4. [DOI] [PubMed] [Google Scholar]
- Kramer R. A., Philippsen P., Davis R. W. Divergent transcription in the yeast ribosomal RNA coding region as shown by hybridization to separated strands and sequence analysis of cloned DNA. J Mol Biol. 1978 Aug 15;123(3):405–416. doi: 10.1016/0022-2836(78)90087-6. [DOI] [PubMed] [Google Scholar]
- Lauer G. D., Roberts T. M., Klotz L. C. Determination of the nuclear DNA content of Saccharomyces cerevisiae and implications for the organization of DNA in yeast chromosomes. J Mol Biol. 1977 Aug 25;114(4):507–526. doi: 10.1016/0022-2836(77)90175-9. [DOI] [PubMed] [Google Scholar]
- Nath K., Bollon A. P. Characterization of yeast ribosomal DNA fragments generated by EcoR1 restriction endonuclease. Mol Gen Genet. 1976 Aug 19;147(2):153–168. doi: 10.1007/BF00267567. [DOI] [PubMed] [Google Scholar]
- Philippsen P., Thomas M., Kramer R. A., Davis R. W. Unique arrangement of coding sequences for 5 S, 5.8 S, 18 S and 25 S ribosomal RNA in Saccharomyces cerevisiae as determined by R-loop and hybridization analysis. J Mol Biol. 1978 Aug 15;123(3):387–404. doi: 10.1016/0022-2836(78)90086-4. [DOI] [PubMed] [Google Scholar]
- Rubin G. M. Preparation of RNA and ribosomes from yeast. Methods Cell Biol. 1975;12:45–64. doi: 10.1016/s0091-679x(08)60951-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Schweizer E., MacKechnie C., Halvorson H. O. The redundancy of ribosomal and transfer RNA genes in Saccharomyces cerevisiae. J Mol Biol. 1969 Mar 14;40(2):261–277. doi: 10.1016/0022-2836(69)90474-4. [DOI] [PubMed] [Google Scholar]
- Shen C. J., Hearst J. E. Detection of long-range sequence order in Drosophila melanogaster satellite DNA IV by a photochemical crosslinking reaction and denaturation microscopy. J Mol Biol. 1977 May 25;112(3):495–507. doi: 10.1016/s0022-2836(77)80195-2. [DOI] [PubMed] [Google Scholar]
- Thomas M., White R. L., Davis R. W. Hybridization of RNA to double-stranded DNA: formation of R-loops. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2294–2298. doi: 10.1073/pnas.73.7.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman S. M., Tiemeier D. C., Seidman J. G., Peterlin B. M., Sullivan M., Maizel J. V., Leder P. Intervening sequence of DNA identified in the structural portion of a mouse beta-globin gene. Proc Natl Acad Sci U S A. 1978 Feb;75(2):725–729. doi: 10.1073/pnas.75.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udem S. A., Warner J. R. Ribosomal RNA synthesis in Saccharomyces cerevisiae. J Mol Biol. 1972 Mar 28;65(2):227–242. doi: 10.1016/0022-2836(72)90279-3. [DOI] [PubMed] [Google Scholar]
- Wensink P. C., Finnegan D. J., Donelson J. E., Hogness D. S. A system for mapping DNA sequences in the chromosomes of Drosophila melanogaster. Cell. 1974 Dec;3(4):315–325. doi: 10.1016/0092-8674(74)90045-2. [DOI] [PubMed] [Google Scholar]








