Abstract
Up-regulation of interleukin (IL)-17 in small intestinal mucosa has been reported in coeliac disease (CD) and in peripheral blood in type 1 diabetes (T1D). We explored mucosal IL-17 immunity in different stages of CD, including transglutaminase antibody (TGA)-positive children with potential CD, children with untreated and gluten-free diet-treated CD and in children with T1D. Immunohistochemistry was used for identification of IL-17 and forkhead box protein 3 (FoxP3)-positive cells and quantitative polymerase chain reaction (qPCR) for IL-17, FoxP3, retinoic acid-related orphan receptor (ROR)c and interferon (IFN)-γ transcripts. IL-1β, IL-6 and IL-17 were studied in supernatants from biopsy cultures. Expression of the apoptotic markers BAX and bcl-2 was evaluated in IL-17-stimulated CaCo-2 cells. The mucosal expression of IL-17 and FoxP3 transcripts were elevated in individuals with untreated CD when compared with the TGA-negative reference children, children with potential CD or gluten-free diet-treated children with CD (P < 0·005 for all IL-17 comparisons and P < 0·01 for all FoxP3 comparisons). The numbers of IL-17-positive cells were higher in lamina propria in children with CD than in children with T1D (P < 0·05). In biopsy specimens from patients with untreated CD, enhanced spontaneous secretion of IL-1β, IL-6 and IL-17 was seen. Activation of anti-apoptotic bcl-2 in IL-17-treated CaCo-2 epithelial cells suggests that IL-17 might be involved in mucosal protection. Up-regulation of IL-17 could, however, serve as a biomarker for the development of villous atrophy and active CD.
Keywords: coeliac disease, FoxP3, gluten-free diet, IL-17, type 1 diabetes
Introduction
Coeliac disease (CD) and type 1 diabetes (T1D) are immune-mediated diseases sharing a predisposing genetic background: human leucocyte antigen (HLA)-DQ2 and HLA-DQ8. In both CD and T1D intestinal inflammation has been observed as altered mucosal cytokine expression and increased activation of intestinal T lymphocytes [1–3]. Intestinal inflammation in CD is characterized by villous atrophy and crypt hyperplasia, which is not seen in T1D. In CD, dietary wheat gliadin has been identified as an environmental trigger of the intestinal inflammation. CD can be divided into two forms: the active CD with villous atrophy and a latent form of the disease, which in this study we call potential CD. In potential CD the normal mucosal architecture exists, but a higher density of γδT cell receptor (TCR)+ intraepithelial lymphocytes and CD-associated antibodies against tissue transglutaminase (TGA) are found [4–6]. CD is regarded as a T helper type 1 (Th1) disease because mucosal up-regulation of the interferon (IFN)-γ pathway is seen [7–9]. We reported recently that mucosal up-regulation of IFN-γ pathway remained elevated even 1 year after gluten-free diet (GFD), suggesting that activation of the Th1 response is triggered not only by dietary gliadin, but is associated more fundamentally with CD, being already present in potential CD and in treated CD [10]. The role of interleukin (IL)-17 immunity in CD is not fully understood. In CD, the IL-17 response has been associated with dietary exposure to wheat gliadin [11]. However, T cell clones reactive with deamidated gliadin peptide did not show IL-17 secretion [12].
Forkhead box protein 3 (FoxP3)-expressing regulatory T cells (Treg) play an important role in the homeostasis of the intestinal immune system by controlling the proinflammatory effector T cells. Recent studies suggest, however, that FoxP3-positive Tregs may convert into pathogenic Th17 cells in inflammatory conditions [13–15].
In T1D, autoreactive T cells destroy insulin-secreting pancreatic islet β cells resulting in insulin deficiency and elevated plasma glucose levels [16]. Previously increased small intestinal immune activation seen as increased numbers of HLA class II-, CD25-, MadCAM-1-, IL-1α- and IL-4-positive cells has been reported in T1D [1–3]. Accumulating evidence suggests intestinal inflammation as part of the disease pathogenesis [17,18]. Animal studies suggest that alterations of the gut immune system, such as increased permeability and enteropathy, are key regulators of autoimmune insulitis and development of T1D [19,20]. Up-regulation of IL-17 immunity in peripheral blood has been reported in T1D [21], but no studies of intestinal IL-17 immunity in T1D have been published. However, stimulation of peripheral blood mononuclear cells from patients with T1D with wheat gliadin resulted in secretion of IL-17 [22].
In this study we aimed to evaluate the activation of IL-17 pathway together with the Treg marker FoxP3 in intestinal inflammation in CD and T1D. We explored mucosal IL-17 immunity in different stages of CD, including transglutaminase antibody (TGA)-positive children with potential CD, children with untreated and gluten-free diet-treated CD and in children with T1D. Immunohistochemistry was used for identification of IL-17 and FoxP3-positive cells and quantitative polymerase chain reaction (qPCR) for IL-17, FoxP3, retinoic acid-related orphan receptor (ROR)c and IFN-γ transcripts. IL-1β, IL-6 and IL-17 were studied in supernatants from biopsy cultures. To evaluate the effects of IL-17 on epithelium, the expression of the apoptotic markers BAX and bcl-2 was studied in IL-17 stimulated CaCo-2 cells.
Methods
Study subjects
In this study we collected distal duodenal biopsy samples taken with the capsule method from patients in Finland and Sweden for different immunological analyses (see Table 1).
Table 1.
Description of the study population
| Method/population | Ref | Pot CD | CD | GFD-CD | T1D | T1D+CD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Age/gender | n = | Age/gender | n = | Age/gender | n = | Age/gender | n = | Age/gender | n = | Age/gender | |
| IHC and qPCR/Fin | 10 | 11·0 | – | – | 15 | 10·0 | – | – | 13 | 9·3 | – | – |
| 2 M/8 F | 5 M/10 F | 11 M/2 F | ||||||||||
| qPCR/Swe | 17 | 4·3 | 10 | 11·6 | 13 | 9·3 | 16 | 6·4 | – | – | – | – |
| 8 M/9 F | 4 M/6 F | 4 M/9 F | 5 M/11 F | |||||||||
| In vitro biopsy culture/Fin | 5 | 10·8 | 5 | 11·8 | 17 | 7·8 | – | – | – | – | 6 | 11·1 |
| 1 M/4 F | 2 M/3 F | 6 M/11 F | 3 M/3 F | |||||||||
IHC: immunohistochemistry; qPCR: quantitative real-time polymerase chain reaction; Ref: reference children who have undergone small intestinal biopsy, but who do not have diagnosed coeliac disease (CD) or other diagnoses; pot CD: children positive for transglutaminase antibodies (TGA), but who do not show villous atrophy and clinical CD: children with untreated CD; GFD-CD: children with CD, after 1 year of a strict gluten free-diet (GFD); T1D: children with type 1 diabetes (T1D), but without CD; M: male; F: female; Fin: Finnish; Swe: Swedish. Mean duration of T1D: 4·5 years (range: 0·25–11·7); T1D + CD = children with T1D and untreated CD.
Immunoenzymatic labelling
The intestinal biopsy specimens were cut into 7-µm sections and stored at −80°C prior to immunohistochemical staining. The lamina propria lymphocytes on frozen sections were stained with the avidin–biotin immunoperoxidase system according to Vectastain ABC Elite kit instructions (Vector Laboratories, Burlingame, CA, USA). After acetone fixation the slides were blocked in normal serum for 30 min. The slides were incubated for 1 h with the following primary antibodies: mouse monoclonal antibodies for FoxP3 (clone 236 A/E7; Abcam, Cambridge, UK), CD4 (clone RPA-T4; BD Pharmingen, San Jose, CA, USA) or rabbit polyclonal antibody for IL-17 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Methanol–hydrogen peroxidase was used to quench endogenous peroxidase activity. The slides were incubated with a biotinylated antibody for 30 min and thereafter in avidin–biotin complex (ABC) reagent for 30 min; 9-amino-ethyl-cardatzole was used as a chromogen. Harris haematoxylin was used to counterstain the slides. Between the staining protocol steps the slides were washed in phosphate-buffered saline (PBS). Negative controls were performed by omission of the primary antibodies.
Microscopic evaluation
The slides were evaluated blinded to the clinical data. The number of positively stained cells in the lamina propria was counted systematically under a Leica DM4000B light microscope through a calibrated eyepiece graticule: positive cells in approximately 30 fields were counted using an objective of × 100 and an eyepiece at × 10. The cell densities were expressed as the mean number of positive cells/mm2 which were used in the statistical analysis.
Quantitative reverse transcription–polymerase chain reaction (RT–PCR)
Remaining biopsies from Finnish subjects were dissected from matrix of optimal cutting temperature (OCT) compound (Miles Laboratories, Elkhart, IN, USA) and homogenized using a pestle (Starlab, Ahrensburg, Germany) in a 0·5 ml Eppendorf tube containing 250 µl of lysis buffer (Sigma, St Louis, MO, USA). Total RNA was isolated with a GenElute mammalian total RNA miniprep kit (Sigma). RNA concentration and purity was measured by a spectrophotometer (ND-1000; NanoDrop Technologies Inc., Wilmington, DE, USA). Reverse transcription was performed with TaqMan reverse transcription reagents (Applied Biosystems, Foster City, CA, USA) with additional treatment of 200 ng of total RNA with DNAse I (0·01 U/ul) (Roche Diagnostics, Mannheim, Germany) to eliminate genomic DNA.
Total RNA, from cryopreserved and homogenized biopsies from the Swedish children, was isolated with RNeasy® Mini Kit (Qiagen, Hilden, Germany) and DNase treated with RNase-Free DNase Set (Qiagen) as described in detail previously [10]. The RNA concentration and purity was measured by a spectrophotometer (ND-1000; NanoDrop Technologies Inc.). Reverse transcription was performed with TaqMan reverse transcription reagents (Applied Biosystems).
Quantitative PCR was performed using StepOnePlus instrumentation (Applied Biosystems) with TaqMan Fast Universal PCR Master Mix and predesigned FAM-labelled gene expression assay reagents (Applied Biosystems). Selected cytokines and transcription factors were IL-17A (cat. no. Hs00174383_m1), FoxP3 (Hs00203958_m1), RORc (cat. no. Hs01076112_m1) and IFN-γ (Hs00174143_m1). Ribosomal 18 s RNA served as the endogenous control (Hs99999901_s1).
The quantities of target gene expression were analysed by a comparative threshold cycle (Ct) method (as recommended by Applied Biosystems). An exogenous cDNA pool calibrator was collected from phytohaemagglutinin (PHA)-stimulated peripheral blood mononuclear cells (PBMC) and considered as an interassay standard to which normalized samples were compared. ΔCt stands for the difference between the Ct of the marker gene and Ct of the 18S gene, whereas ΔΔCt is the difference between the ΔCt of the sample and ΔCt of the calibrator. Calculation of 2−ΔΔCt then gives the relative amount of target gene in the sample compared with the calibrator, both normalized to an endogenous control (18S). For presentations the relative amounts (2−ΔΔCt) of target genes were multiplied by a factor 1000 and expressed as relative units. If the samples Ct value for target gene did not reach quantitative level, then an artificial value that was half the lowest quantitative value in relative units was given to the sample.
Cytokine secretion from in-vitro cultured small intestinal biopsies
We cultured small intestinal biopsy samples from 23 patients with untreated CD (of which six also had T1D) and 10 reference children (five positive for TGA) for 72 h in RPMI-5% human AB serum and measured the concentration of IL-17, Il-1β and IL-6 secreted in the culture supernatants by using flow-cytometric bead array (Bender Medsystems, Vienna, Austria). Samples below the detection limit (or the cut-off level) of the method were considered as undetectable, but were given half the cut-off value to enable statistical analyses.
IL-17 treatment of CaCo-2 cells
The human colon adenocarcinoma cell line (CaCo-2) was obtained from American Type Culture Collection (ATCC) (Teddington, UK). Cells were grown in Eagle's minimal essential medium (Sigma) containing 10% heat-activated and sterile filtered fetal bovine serum (FBS) supplemented with penicillin (0·1 g/l) and streptomycin (0·15 g/l) at + 37°C and 5% CO2. CaCo-2 cells were grown in a 75 cm2 flask for 6 days and were thereafter plated into sterile 48-well plates (Greiner Bio-One GmbH, Frickenhausen, Germany) and grown for 4 days at a density of 1·5 × 105 cells per well and a final volume of 0·5 ml/well. The cells were incubated for 5 h with recombinant human IL (rhIL)-17 (1 or 50 pg/ml; cat. no. 11340176) or rhIL-17 in combination with tumour necrosis factor (TNF)-α in + 37°C, 5% CO2 and were then collected for quantitative real-time reverse transcription–polymerase chain reactions (RT–PCR).
For the analyses of target gene expression in the CaCo-2 cells with quantitative RT–PCR, total RNA was isolated (Sigma), reverse transcription was performed with added DNAse treatment, and qPCR analyses were performed as described above for biopsy samples. Markers of apoptosis were bcl-2 (Hs00608023_m1) and BAX (Hs00180269_m1). Ribosomal 18 s RNA was used as an endogenous control (Hs99999901_s1).
Statistical analysis
The data analysis was performed with SPAW statistics version 17·0 for Windows (SPSS Inc., Chigaco, IL, USA) and GraphPad prism software (San Diego, CA, USA). For comparisons between the groups, the non-parametric Kruskal–Wallis test and Mann–Whitney U-test were used. The Spearman's rank correlation test was applied to analyse correlations between different parameters. P-values < 0·05 were considered significant.
Ethical considerations
The Ethics Committee of the Hospital for Children and Adolescents, Helsinki University Central Hospital, Finland and the Regional Ethics Committee for Human Research at the University Hospital of Linköping, Sweden approved the study plans and written informed consent was obtained from parents and children.
Results
The results of the immunohistochemistry and qPCR analyses of the small intestinal biopsies from the Finnish study population consisting of children with untreated CD, children with T1D and reference children are shown in Fig. 1. The expression of IL-17-positive cells and IL-17-specific mRNA levels differed significantly between the groups (P = 0·029 and P < 0·001, respectively, Kruskal–Wallis test). The density of intestinal IL-17-positive cells was increased in untreated CD compared to the T1D patients (P = 0·039, Mann–Whitney U-test) (Fig. 1a). Additionally, the IL-17 mRNA level was higher in untreated CD than in subjects with T1D or reference children (P < 0·001 for both comparisons, Mann–Whitney U-test) (Fig. 1b). In T1D, no difference in the number of IL-17-positive cells or transcripts was seen in comparison to the reference children. In children with untreated CD the expression of IL-17-positive cells correlated positively with the IL-17 mRNA expression levels (R = 0·444; P = 0·111, Spearman), whereas no such correlation was seen in the reference group (R = −0·247; P = 0·555, Spearman) or in children with T1D (R = −0·104; P = 0·775, Spearman).
Fig. 1.
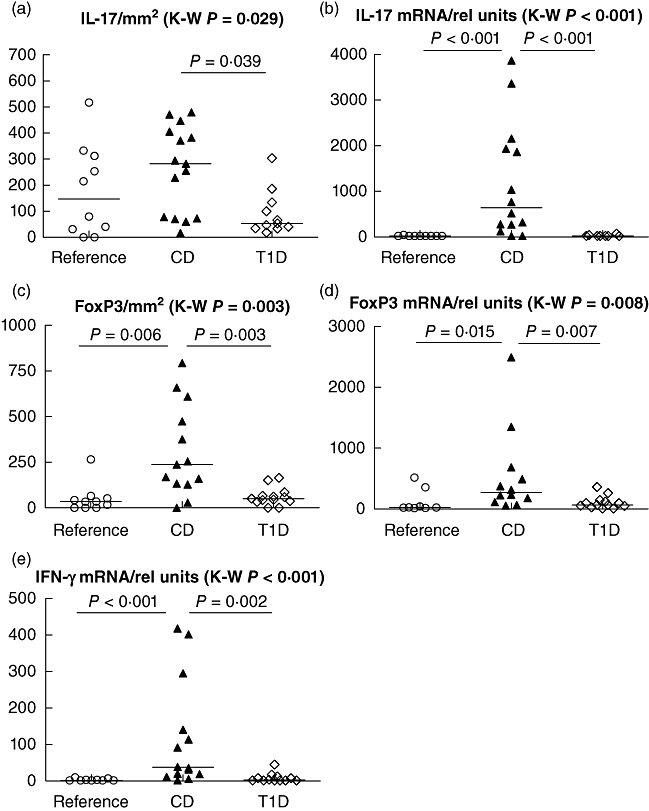
The number of interleukin (IL)-17+ cells differed between the groups in the Finnish study cohort (P = 0·029, Kruskal–Wallis test), and were higher in untreated coeliac disease (CD) than in subjects with type 1 diabetes (T1D) (P = 0·039, Mann–Whitney U-test) (a); IL-17 mRNA expression differed between the groups (P < 0·0001 Kruskal–Wallis test) and was increased in untreated CD compared to T1D and to the reference children (P < 0·0001 in both, Mann–Whitney U-test) (b), the number of forkhead box protein 3 (FoxP3+) cells differed between the groups (P = 0·003, Kruskal–Wallis test) and were higher in untreated CD than in subjects with T1D and reference children (P = 0·003 and P = 0·006, respectively, Mann–Whitney U-test) (c), FoxP3 mRNA expression differed between the groups (P = 0·008, Kruskal–Wallis test) and was higher in untreated CD than in subjects with T1D and references (P = 0·007 and P = 0·015, respectively, Mann–Whitney U-test) (d), interferon (IFN)-γ mRNA expression differed between the groups (P < 0·0001 Kruskal–Wallis test) and was increased in untreated CD compared to T1D and to the references (P < 0·0001 and P = 0·001, respectively, Mann–Whitney U-test) (e). The mRNA levels are expressed as relative units and the number of cells as number of positive cells/mm2.
The number of FoxP3-positive cells and FoxP3-specific mRNA differed significantly between the groups (P = 0·003 and P = 0·008, respectively, Kruskal–Wallis test) (Fig. 1c,d). Increased numbers of FoxP3-positive cells were found in untreated CD when compared to T1D and reference children (P = 0·003 and P = 0·006, respectively, Mann–Whitney U-test) (Fig. 1c). Additionally, untreated CD had higher FoxP3 mRNA levels than subjects with T1D and reference children (P = 0·007 and P = 0·015, respectively, Mann–Whitney U-test) (Fig. 1d). No difference in the number of FoxP3-positive cells or transcripts was seen between T1D and the reference children. The expression of IFN-γ mRNA differed between the groups (P < 0·001, Kruskal–Wallis test) (Fig. 1e). Increased expression of IFN-γ was observed in the children with CD when compared to children with T1D or reference children (P = 0·002 and P < 0·001, respectively, Mann–Whitney U-test).
In the Swedish children we had the possibility to study the effect of a strict GFD on the expression levels of intestinal IL-17 FoxP3 and RORc mRNA. The mucosal IL-17 and FoxP3 mRNA expression differed between the four study groups: TGA-negative reference children, TGA-positive children with potential CD, children with untreated CD and GFD-treated CD (P < 0·001 for both genes, Kruskal–Wallis test). Both the IL-17 and FoxP3 transcripts were higher in the children with untreated CD when compared to GFD-treated children, children with potential CD and TGA-negative children (IL-17A: P = 0·003, P = 0·004 and P = 0·001, FoxP3: P = 0·002, P = 0·001 and P = 0·006, respectively, Mann–Whitney U-test ) (Fig. 2). The IL-17 and FoxP3 mRNA expression levels did not differ between children with treated CD and TGA-negative reference children. The expression of RORc mRNA did not differ between the study groups.
Fig. 2.
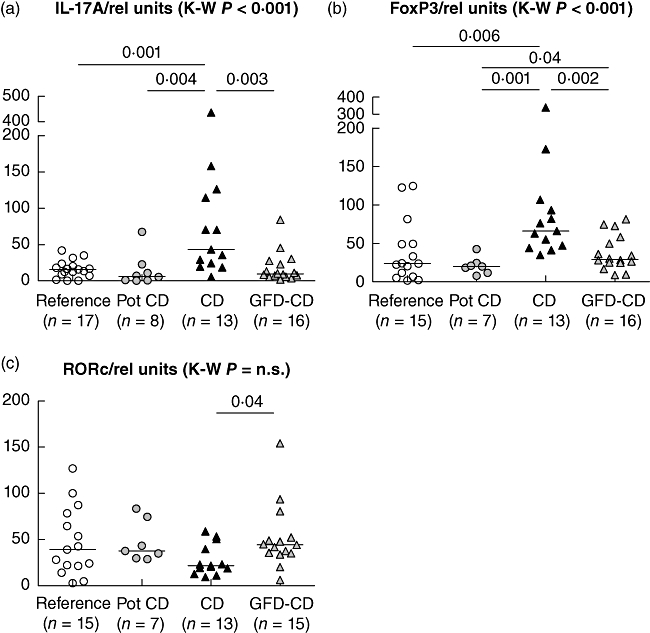
The interleukin (IL)-17A and forkhead box protein 3 (FoxP3) mRNA expression levels in the Swedish study population differed significantly between the groups (P < 0·0001 for both, Kruskal–Wallis test) (a,b), while no difference was seen in the retinoic acid-related orphan receptor (RORc) levels (c). Children with untreated coeliac disease (CD) had higher IL-17A and FoxP3 mRNA levels than gluten-free diet (GFD)-treated children (GFD-CD) (P = 0·003 and P = 0·002, Mann–Whitney U-test) and also compared to the transglutaminase antibody (TGA)-positive children with potential CD (pot CD) and TGA-negative reference children (P = 0·004 and P = 0·001 for IL-17A and P = 0·006 and P = 0·001 for FoxP3, Mann–Whitney U-test). The mRNA levels are expressed as relative units.
The expression levels of IL-17A and FoxP3 correlated positively in children with untreated CD (R = 0·60, P = 0·03 Spearman). We found no correlation between the IL-17A and RORc mRNA levels [R = −0·24, P = not significant (n.s.), Spearman].
The levels of transcripts detected differed between Swedish and Finnish series of samples due to the difference in the RNA isolation steps between the Finnish and Swedish samples, as described in the Methods. Finnish samples were collected in OCT and used primarily for immunohistochemistry, and RNA isolation was performed in samples from OCT matrix. Therefore RNA isolation was more effective in Swedish samples and low-copy genes, such as IL-17A, could be detected from almost all the samples. In Finnish samples, IL-17A transcripts were below the detection limit (or the cut-off level) of the method in 10 of 13 children with T1D, in eight of nine reference children, but only in two of 14 children with untreated CD, as shown in Fig. 1. In Swedish samples, undetectable IL-17A transcripts were seen in two of 17 reference children, in one of eight children with potential CD, and in none of the children with untreated or GFD-treated CD.
The Swedish reference children were younger than the children with CD, as seen in Table 1. We tested the correlation of IL-17 mRNA expression with age and did not find a correlation (R = 0·193; P = 0·16).
IL-1beta, IL-6 and IL-17 secretion from intestinal biopsies
Spontaneous IL-1β and IL-6 secretion in supernatants from small intestinal biopsy cultures were increased in children with untreated CD (with or without T1D) when compared to children with potential CD and TGA-negative reference children (Fig. 3) (Kruskal–Wallis P = 0·001 and Mann–Whitney U-test P = 0·009 and P = 0·004 respectively for IL-1β, Kruskal-Wallis P < 0·001 and Mann–Whitney U-test, P = 0·002 and P = 0·001 for IL-6). The secretion of IL-17 was above the detection limit of the assay in eight of 23 intestinal biopsy samples from CD patients, but in none of five reference samples.
Fig. 3.
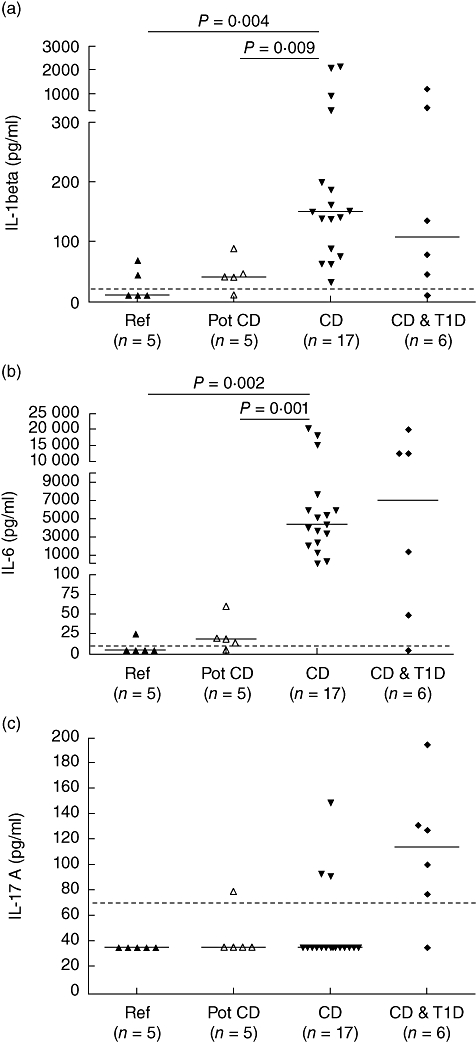
Spontaneous interleukin (IL)-1β, IL-6 and IL-17A secretion in culture supernatants from small intestinal biopsies. The secretion of IL-1β (a) and IL-6 (b) was increased in the small intestinal biopsies from children with untreated coeliac disease (CD) with or without type 1 diabetes (T1D) compared to the levels in children with potential CD and transglutaminase antibody (TGA)-negative reference children (Kruskal–Wallis P = 0·001 and Mann–Whitney U-test, P = 0·009 and P = 0·004, respectively, for IL-1β, Kruskal–Wallis P < 0·001 and Mann–Whitney U-test, P = 0·002 and P = 0·001 for IL-6). IL-17A secretion (c) was detectable in none of the biopsy samples from five reference children, in one sample from five children with potential CD and in eight samples from 23 children with CD with or without T1D. The horizontal line shows the detection limit of the assay.
IL-17 up-regulated anti-apoptotic bcl-2 in Caco2-cells
We examined the apoptotic effects of IL-17 on Caco2-cells in vitro, alone or in combination with TNF-α, which is known to be apoptotic for epithelial cells. IL-17 receptor A mRNA transcripts were highly expressed in CaCo-2 cells (Ct was 24 for IL-17RA and 13 for 18S; n = 8). Furthermore, incubation with IL-17 increased the transcription of the anti-apoptotic gene bcl-2 but did not up-regulate the expression of BAX, which is activated in the apoptosis (Fig. 4).
Fig. 4.
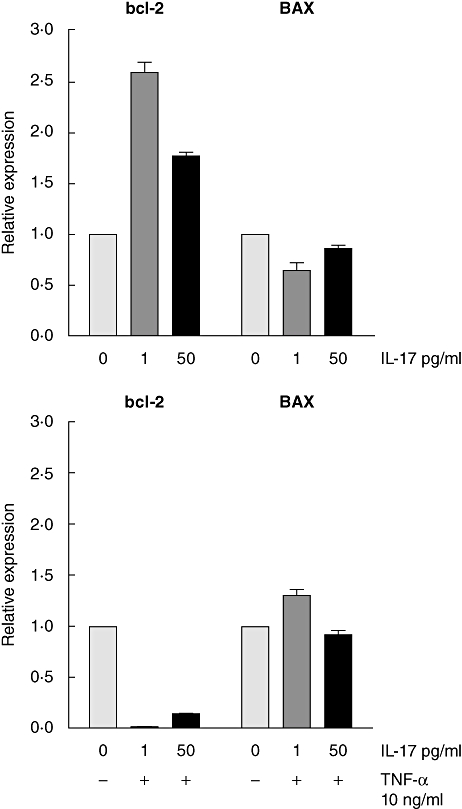
Real-time polymerase chain reaction (PCR) expression level of the anti-apoptotic gene BCL-2 increased (> twofold) and the apoptotic gene BAX transcripts did not change significantly in CaCo-2 epithelial cells treated with interleukin (IL)-17 alone (upper panel) at the concentration of 0 ( ), 1 (
), 1 ( ) and 50 pg/ml (
) and 50 pg/ml ( ), whereas the treatment with apoptosis-trigger tumour necrosis factor (TNF)-α at the concentration of 10 ng/ml in combination of the above-mentioned concentrations of IL-17 resulted in the decreased BCL-2 expression (< 0·03-fold) and in a slight increase of BAX expression (lower panel). The levels of the target gene mRNA transcripts were normalized with ribosomal RNA (18S) and an in-house calibrator and the target gene expression level was related to expression levels in the CaCo-2 cells cultured without IL-17 (i.e. 1·0). The results are from three replicates.
), whereas the treatment with apoptosis-trigger tumour necrosis factor (TNF)-α at the concentration of 10 ng/ml in combination of the above-mentioned concentrations of IL-17 resulted in the decreased BCL-2 expression (< 0·03-fold) and in a slight increase of BAX expression (lower panel). The levels of the target gene mRNA transcripts were normalized with ribosomal RNA (18S) and an in-house calibrator and the target gene expression level was related to expression levels in the CaCo-2 cells cultured without IL-17 (i.e. 1·0). The results are from three replicates.
Discussion
We did not find evidence supporting an up-regulation of intestinal IL-17 immunity in T1D-related intestinal inflammation or in potential CD, but in CD the IL-17 response was linked to untreated CD characterized by villous atrophy and IL-17 immunity was down-regulated after GFD. Our results point out that up-regulation of mucosal IL-17 immunity is seen at the late stage of CD, when villous atrophy has developed. We found up-regulation of IL-17 immunity only in children with untreated CD, as demonstrated in independent patient series from Finland and Sweden. Elevation of duodenal IL-17A transcripts was observed and the small intestinal biopsies of untreated CD patients seemed to spontaneously secrete more IL-17A in vitro compared to reference children. However, the numbers of IL-17-positive cells in Finnish children with untreated CD were not increased significantly compared to reference children. This might indicate up-regulation of Il-17A production without remarkable expansion of Th17 cells at the time of villous atrophy. Our findings of the effect of GFD on the normalization of intestinal IL-17 up-regulation is in agreement with Italian studies showing an association of mucosal IL-17 activation in untreated but not in GFD-treated CD [23,24]. We also studied healthy children with and without TGA, and showed that up-regulation of IL-17 immunity does not occur in children with TGA, who are at high risk of CD and are considered as having potential CD. In potential CD the inflamed intestinal mucosa is characterized by increased numbers of γ/δ T cells and up-regulation of the IFN-γ pathway. Accordingly, our findings in children with potential CD indicate that wheat gliadin induced mucosal inflammation, which is already present in potential CD, does not include IL-17 immunity. Our findings of the activation of IL-17 immunity at only a late stage of the disease could explain the discrepant reports of IL-17 secretion by gliadin-specific T cells [12,25]. Bodd et al. showed that T cells reactive to deamidated gliadin do not secrete IL-17 [12]. A recent study, however, reported that gliadin-specific Th17 cells are present in the mucosa of untreated CD patients [25]. These cells secrete proinflammatory and anti-inflammatory cytokines simultaneously indicating plasticity, e.g. changes in the profile of secreted cytokines.
We found up-regulation of intestinal FoxP3 in children with untreated CD in association with the enhanced IL-17 immunity. It has been suggested that FoxP3-expressing Tregs show plasticity and may develop into Th17 cells in the tissue inflammation [13–15]. In our study, the activation of intestinal FoxP3, similar to IL-17 immunity, seems to occur only in the late phase of disease progression, and up-regulation of FoxP3 was not present in potential CD. Treatment with a strict GFD normalized the expression of both FoxP3 and IL-17. The expression of RORc mRNA did not correlate with IL-17 mRNA, which instead correlated positively with FoxP3 mRNA in CD. This could be an indicator of plasticity reported between Tregs and Th17 cells [13–15]. The IL-1β and IL-6 cytokine environment supports the conversion from FoxP3-expressing Tregs to IL-17-secreting cells. In our study a remarkably high secretion of both IL-1β and IL-6 was demonstrated in the active CD mucosa. Thus, on one hand the mucosal cytokine environment in CD supports IL-17 differentiation and on the other hand it may lead to impaired suppressive function of FoxP3-expressing cells [26]. A recent study suggested that Th17 cell clones also may change their phenotype when RORc is down-regulated and FoxP3 up-regulated upon repeated TCR engagement [27]. This kind of plasticity might explain the low RORc mRNA expression in association with IL-17 and FoxP3 expression demonstrated in the mucosa of untreated CD.
To evaluate the role of IL-17 in the induction of epithelial cell apoptosis and villous atrophy [28], we treated the epithelial cell line, CaCo-2, with IL-17 to study the induction of apoptosis. CaCo-2 cells showed expression of IL-17RA, and IL-17 potentiated the expression of the anti-apoptotic gene bcl-2. The expression of the apoptotic signalling gene, BAX, decreased slightly. These findings suggest that IL-17 is not contributing to the apoptosis of enterocytes. On the contrary, it may instead activate protective anti-apoptotic mechanisms in epithelial cells.
The dualistic role of IL-17 immunity in tissue inflammation has been reported to depend at least partly on the response of the target tissue on IL-17. In a murine model of autoimmune diabetes, the induction of IL-17 immunity contributed to the progression of autoimmune diabetes during the effector phase of the disease [29] and IL-17 also induced apoptotic mechanisms in human islet cells [21]. Conversely, a recent study showed that a commensal bacteria strain which mediated protection from autoimmune diabetes in a rodent model caused induction of mucosal IL-17 immunity [30]. Our results suggest that the up-regulation of intestinal IL-17 immunity is related to the mechanisms of protection from tissue damage in the inflamed mucosa, as also suggested by others [31], and could thus explain the beneficial effects of mucosal IL-17 up-regulation in autoimmune diabetes.
In this study, we did not see evidence for the up-regulation of small intestinal IL-17 immunity in children with T1D who did not have CD, although we have reported enhanced activation of IL-17 immunity in peripheral blood T cells in children with T1D [21]. The IL-17-positive CD4-cells from children with T1D expressed CCR6, which indicates mucosal homing properties. Despite this, only in the series of children with both T1D and CD was IL-17 immunity associated with the subclinical small intestinal inflammation in T1D. Intestinal biopsies of T1D patients with CD seemed to have more spontaneous release of IL-17 in vitro compared to patients with CD alone (see Fig. 3). This indicates that T1D might induce IL-17 production under certain conditions, such as at high-grade mucosal inflammation associated with villous atrophy. Interestingly, IL-17A transcripts were elevated in the Langerhans islets from a newly diagnosed patient with T1D when compared to the samples from non-diabetic individuals [32]. It is thus possible that IL-17-positive cells infiltrate the islets and are absent from the intestine. In non-obese diabetic (NOD)-mice, up-regulation of IL-17 immunity was reported in the colon [33], and our samples are from small intestine.
In summary, our results support the view that up-regulation of IL-17 immunity is associated with untreated CD and especially villous atrophy, whereas mucosal IL-17 immunity is not present in potential, GFD-treated CD or in T1D. IL-17 may not act as a direct trigger of villous atrophy and tissue destruction because it did not promote apoptotic mechanisms in the CaCo-2 epithelial cell line. IL-17 up-regulation was a marker of active CD and its role as a predictive biomarker of villous atrophy and the need for small intestinal biopsy in subjects with TGA positivity should be evaluated.
Acknowledgments
We thank all the children and adolescents who participated in the study. We thank Anneli Suomela for technical assistance. Lars Stenhammar, Pia Laurin, Louise Forslund and Maria Nordwall at the Paediatric Clinics in Linköping, Norrköping and Motala are acknowledged for the clinical support. The research nurses at the Division of Paedatrics in Linköping, Norrköping and Motala and the laboratory technicians Gosia Konefal and Ingela Johansson are also thanked for theie help with the sample collection. This work was generously supported by the Sigrid Juselius Foundation, the Academy of Finland, the Diabetes Research Foundation, the County Council of Östergötland, the Swedish Child Diabetes Foundation (Barndiabetesfonden) and the Swedish Research Council.
Disclosure
The authors have no conflicts of interest to declare.
References
- 1.Westerholm-Ormio M, Vaarala O, Pihkala P, Ilonen J, Savilahti E. Immunologic activity in the small intestinal mucosa of pediatric patients with type 1 diabetes. Diabetes. 2003;52:2287–95. doi: 10.2337/diabetes.52.9.2287. [DOI] [PubMed] [Google Scholar]
- 2.Tiittanen M, Westerholm-Ormio M, Verkasalo M, Savilahti E, Vaarala O. Infiltration of forkhead box P3-expressing cells in small intestinal mucosa in coeliac disease but not in type 1 diabetes. Clin Exp Immunol. 2008;152:498–507. doi: 10.1111/j.1365-2249.2008.03662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auricchio R, Paparo F, Maglio M, et al. In vitro-deranged intestinal immune response to gliadin in type 1 diabetes. Diabetes. 2004;53:1680–3. doi: 10.2337/diabetes.53.7.1680. [DOI] [PubMed] [Google Scholar]
- 4.Holm K, Maki M, Savilahti E, Lipsanen V, Laippala P, Koskimies S. Intraepithelial gamma delta T-cell-receptor lymphocytes and genetic susceptibility to coeliac disease. Lancet. 1992;339:1500–3. doi: 10.1016/0140-6736(92)91262-7. [DOI] [PubMed] [Google Scholar]
- 5.Arranz E, Bode J, Kingstone K, Ferguson A. Intestinal antibody pattern of coeliac disease: association with gamma/delta T cell receptor expression by intraepithelial lymphocytes, and other indices of potential coeliac disease. Gut. 1994;35:476–82. doi: 10.1136/gut.35.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaukinen K, Collin P, Holm K, Karvonen AL, Pikkarainen P, Maki M. Small-bowel mucosal inflammation in reticulin or gliadin antibody-positive patients without villous atrophy. Scand J Gastroenterol. 1998;33:944–9. doi: 10.1080/003655298750026967. [DOI] [PubMed] [Google Scholar]
- 7.Kontakou M, Przemioslo RT, Sturgess RP, et al. Cytokine mRNA expression in the mucosa of treated coeliac patients after wheat peptide challenge. Gut. 1995;37:52–7. doi: 10.1136/gut.37.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kontakou M, Sturgess RP, Przemioslo RT, Limb GA, Nelufer JM, Ciclitira PJ. Detection of interferon gamma mRNA in the mucosa of patients with coeliac disease by in situ hybridisation. Gut. 1994;35:1037–41. doi: 10.1136/gut.35.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nilsen EM, Jahnsen FL, Lundin KE, et al. Gluten induces an intestinal cytokine response strongly dominated by interferon gamma in patients with celiac disease. Gastroenterology. 1998;115:551–63. doi: 10.1016/s0016-5085(98)70134-9. [DOI] [PubMed] [Google Scholar]
- 10.Lahdenpera A, Ludvigsson J, Falth-Magnusson K, Hogberg L, Vaarala O. The effect of gluten-free diet on Th1–Th2–Th3-associated intestinal immune responses in celiac disease. Scand J Gastroenterol. 2011;46:538–49. doi: 10.3109/00365521.2011.551888. [DOI] [PubMed] [Google Scholar]
- 11.Castellanos-Rubio A, Santin I, Irastorza I, Castano L, Carlos Vitoria J, Ramon Bilbao J. TH17 (and TH1) signatures of intestinal biopsies of CD patients in response to gliadin. Autoimmunity. 2009;42:69–73. doi: 10.1080/08916930802350789. [DOI] [PubMed] [Google Scholar]
- 12.Bodd M, Raki M, Tollefsen S, et al. HLA-DQ2-restricted gluten-reactive T cells produce IL-21 but not IL-17 or IL-22. Mucosal Immunol. 2010;3:594–601. doi: 10.1038/mi.2010.36. [DOI] [PubMed] [Google Scholar]
- 13.Ayyoub M, Deknuydt F, Raimbaud I, et al. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc Natl Acad Sci USA. 2009;106:8635–40. doi: 10.1073/pnas.0900621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komatsu N, Mariotti-Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci USA. 2009;106:1903–8. doi: 10.1073/pnas.0811556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou X, Bailey-Bucktrout SL, Jeker LT, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–7. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atkinson MA, Maclaren NK. The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med. 1994;331:1428–36. doi: 10.1056/NEJM199411243312107. [DOI] [PubMed] [Google Scholar]
- 17.Vaarala O. Leaking gut in type 1 diabetes. Curr Opin Gastroenterol. 2008;24:701–6. doi: 10.1097/MOG.0b013e32830e6d98. [DOI] [PubMed] [Google Scholar]
- 18.Vaarala O, Atkinson MA, Neu J. The ‘perfect storm’ for type 1 diabetes: the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes. 2008;57:2555–62. doi: 10.2337/db08-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neu J, Reverte CM, Mackey AD, et al. Changes in intestinal morphology and permeability in the biobreeding rat before the onset of type 1 diabetes. J Pediatr Gastroenterol Nutr. 2005;40:589–95. doi: 10.1097/01.mpg.0000159636.19346.c1. [DOI] [PubMed] [Google Scholar]
- 20.Graham S, Courtois P, Malaisse WJ, Rozing J, Scott FW, Mowat AM. Enteropathy precedes type 1 diabetes in the BB rat. Gut. 2004;53:1437–44. doi: 10.1136/gut.2004.042481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honkanen J, Nieminen JK, Gao R, et al. IL-17 immunity in human type 1 diabetes. J Immunol. 2010;185:1959–67. doi: 10.4049/jimmunol.1000788. [DOI] [PubMed] [Google Scholar]
- 22.Mojibian M, Chakir H, Lefebvre DE, et al. Diabetes-specific HLA-DR-restricted proinflammatory T-cell response to wheat polypeptides in tissue transglutaminase antibody-negative patients with type 1 diabetes. Diabetes. 2009;58:1789–96. doi: 10.2337/db08-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sapone A, Lammers KM, Mazzarella G, et al. Differential mucosal IL-17 expression in two gliadin-induced disorders: gluten sensitivity and the autoimmune enteropathy celiac disease. Int Arch Allergy Immunol. 2010;152:75–80. doi: 10.1159/000260087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monteleone I, Sarra M, Del Vecchio Blanco G, et al. Characterization of IL-17A-producing cells in celiac disease mucosa. J Immunol. 2010;184:2211–18. doi: 10.4049/jimmunol.0901919. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez S, Molina IJ, Romero P, et al. Characterization of gliadin-specific Th17 cells from the mucosa of celiac disease patients. Am J Gastroenterol. 2011;106:528–38. doi: 10.1038/ajg.2010.465. [DOI] [PubMed] [Google Scholar]
- 26.Beriou G, Costantino CM, Ashley CW, et al. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113:4240–9. doi: 10.1182/blood-2008-10-183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye J, Su X, Hsueh EC, et al. Human tumor-infiltrating Th17 cells have the capacity to differentiate into IFN-gamma+ and FOXP3+ T cells with potent suppressive function. Eur J Immunol. 2011;41:936–51. doi: 10.1002/eji.201040682. [DOI] [PubMed] [Google Scholar]
- 28.Mazzarella G, Stefanile R, Camarca A, et al. Gliadin activates HLA class I-restricted CD8+ T cells in celiac disease intestinal mucosa and induces the enterocyte apoptosis. Gastroenterology. 2008;134:1017–27. doi: 10.1053/j.gastro.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emamaullee JA, Davis J, Merani S, et al. Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes. 2009;58:1302–11. doi: 10.2337/db08-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lau K, Benitez P, Ardissone A, et al. Inhibition of type 1 diabetes correlated to a Lactobacillus johnsonii N6·2-mediated Th17 bias. J Immunol. 2011;186:3538–46. doi: 10.4049/jimmunol.1001864. [DOI] [PubMed] [Google Scholar]
- 31.Blaschitz C, Raffatellu M. Th17 cytokines and the gut mucosal barrier. J Clin Immunol. 2010;30:196–203. doi: 10.1007/s10875-010-9368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arif S, Moore F, Marks K, et al. Peripheral and islet interleukin-17 pathway activation characterizes human autoimmune diabetes and promotes cytokine-mediated {beta}-cell death. Diabetes. 2011;60:2112–19. doi: 10.2337/db10-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alam C, Valkonen S, Palagani V, Jalava J, Eerola E, Hanninen A. Inflammatory tendencies and overproduction of IL-17 in the colon of young NOD mice are counteracted with diet change. Diabetes. 2010;59:2237–46. doi: 10.2337/db10-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]


