Abstract
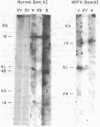
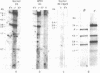
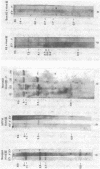
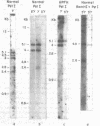
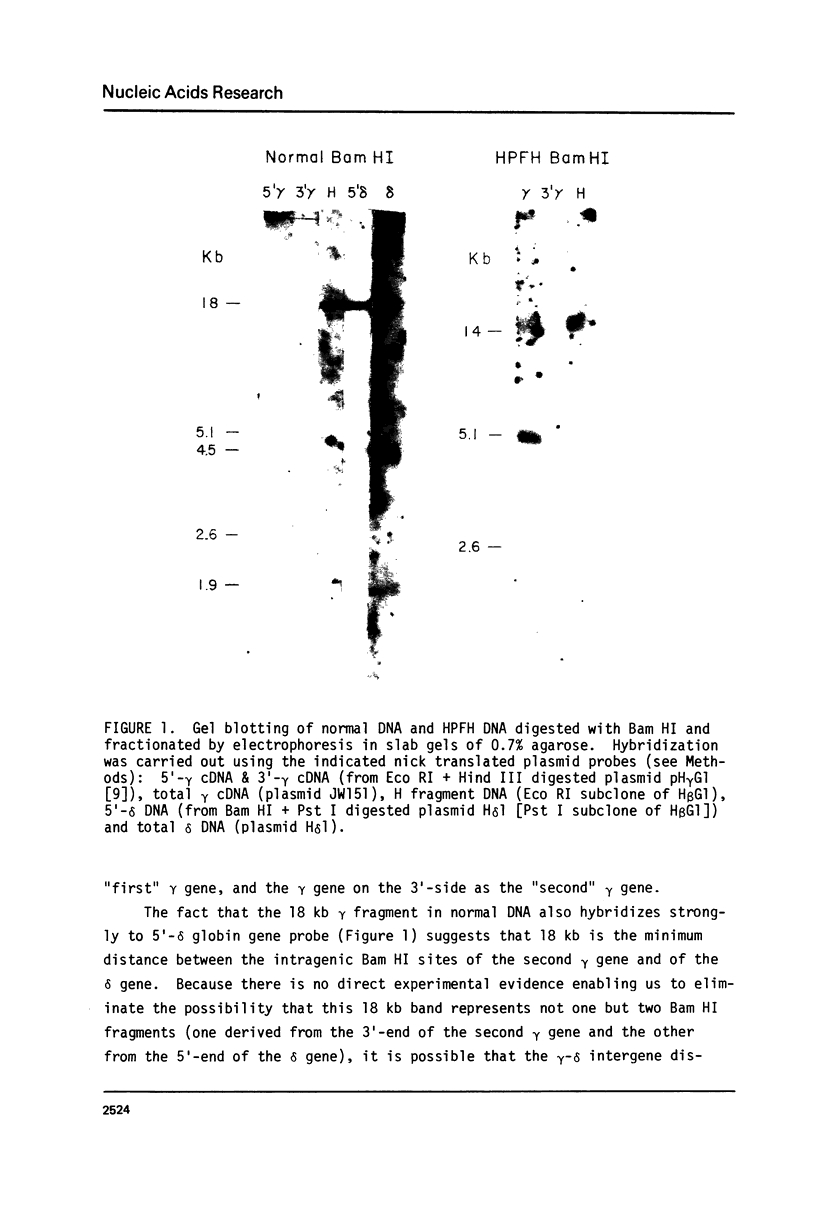
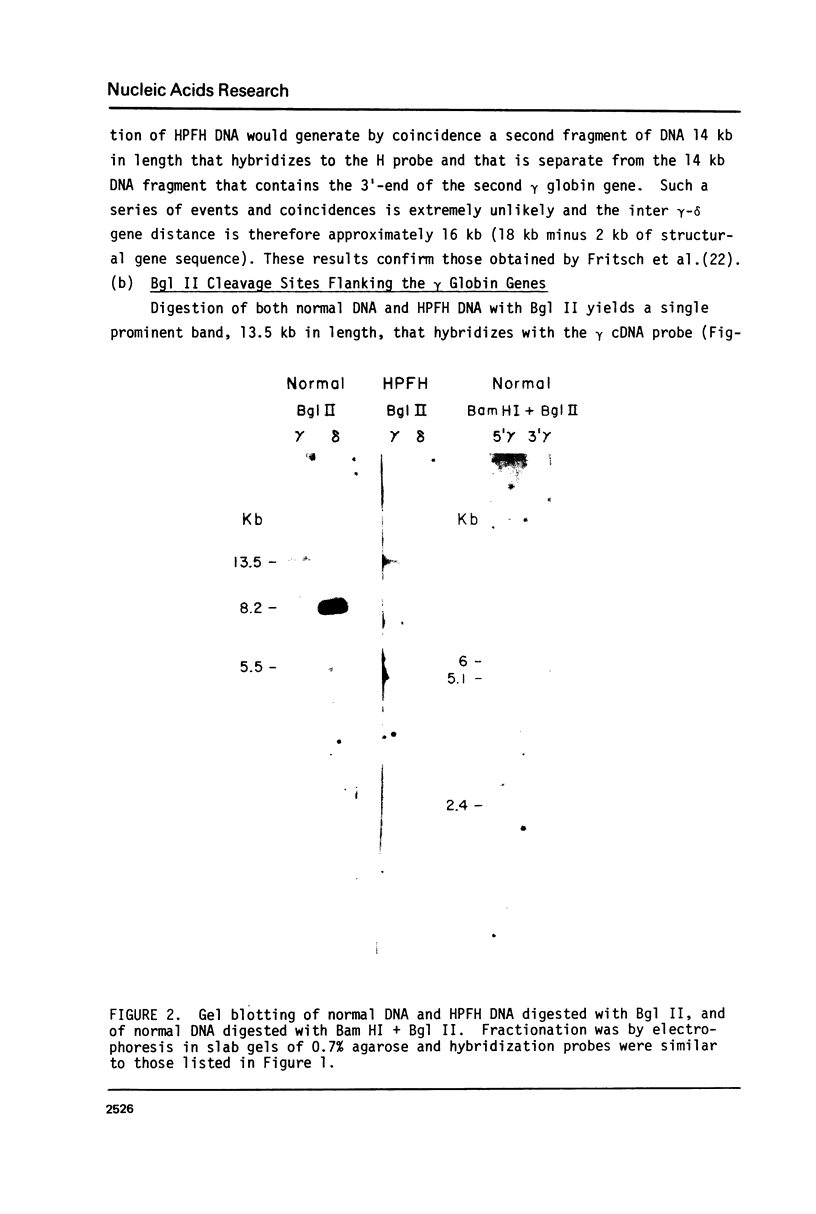
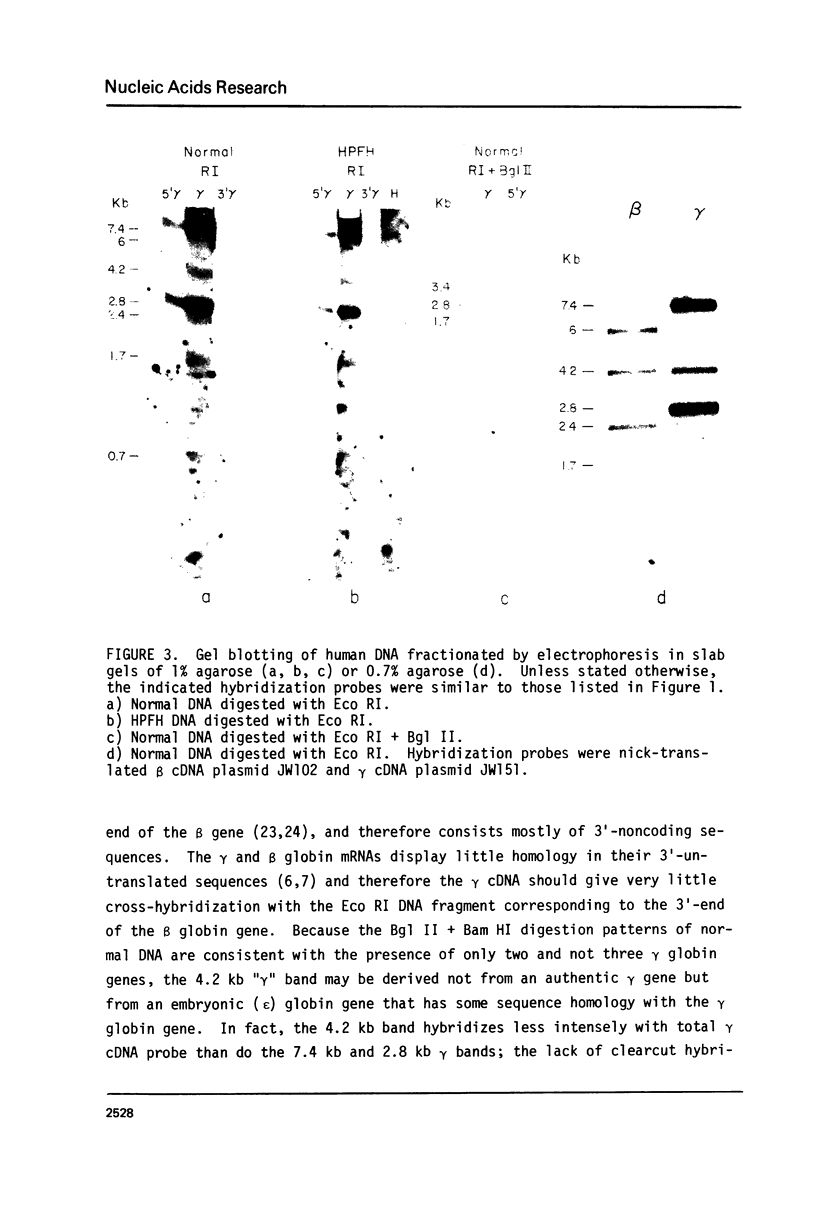
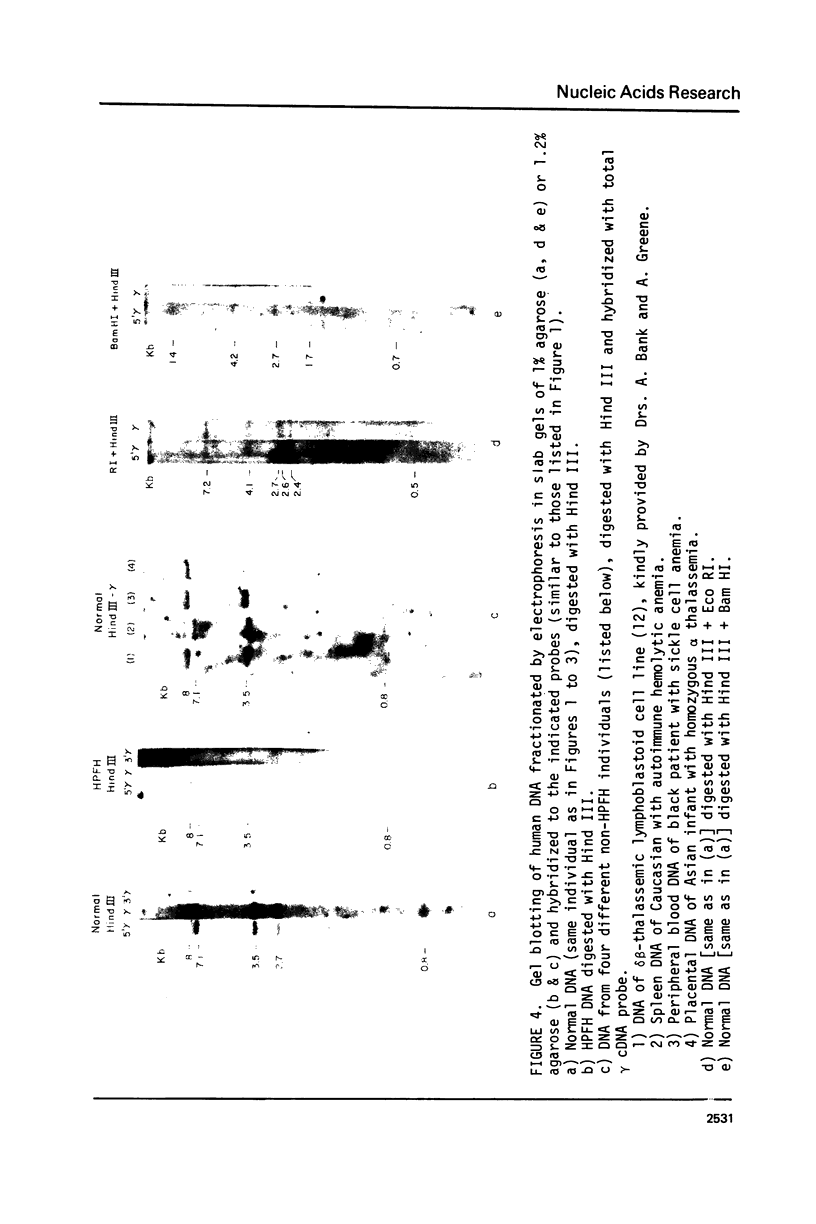
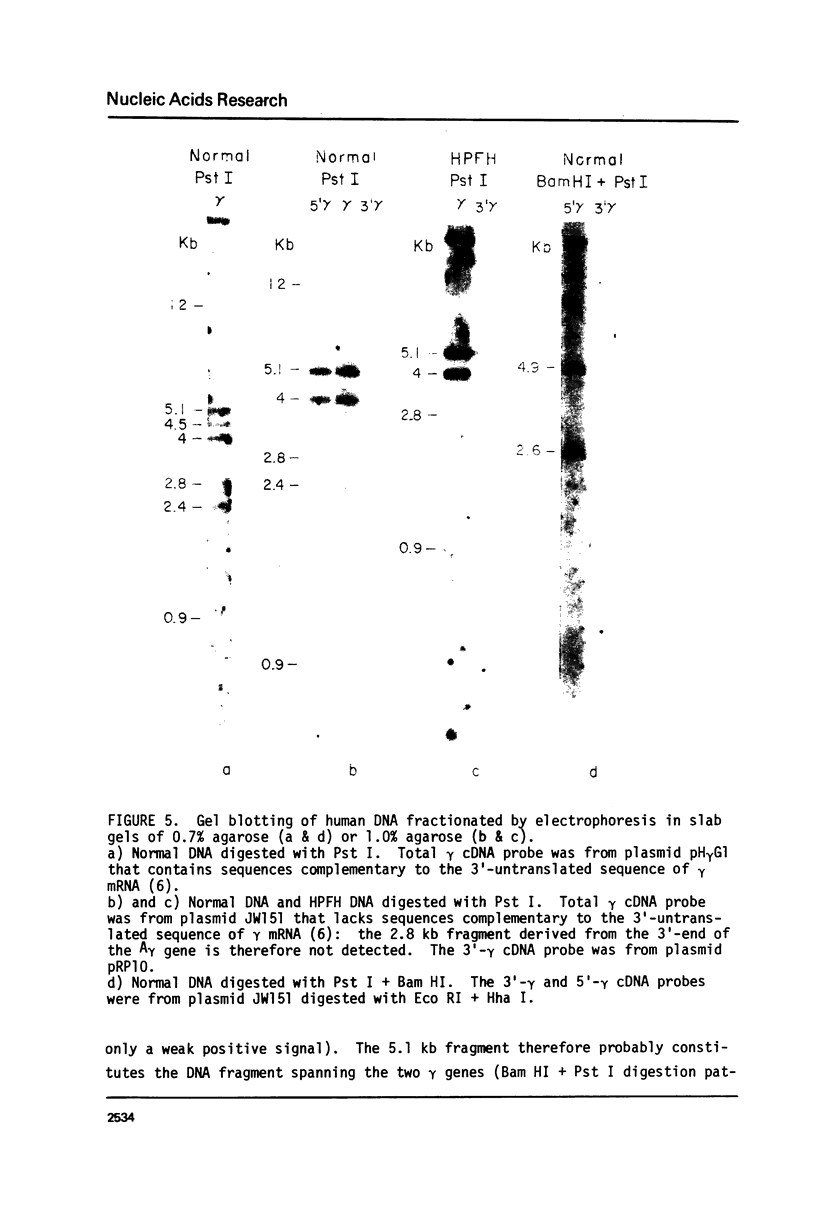
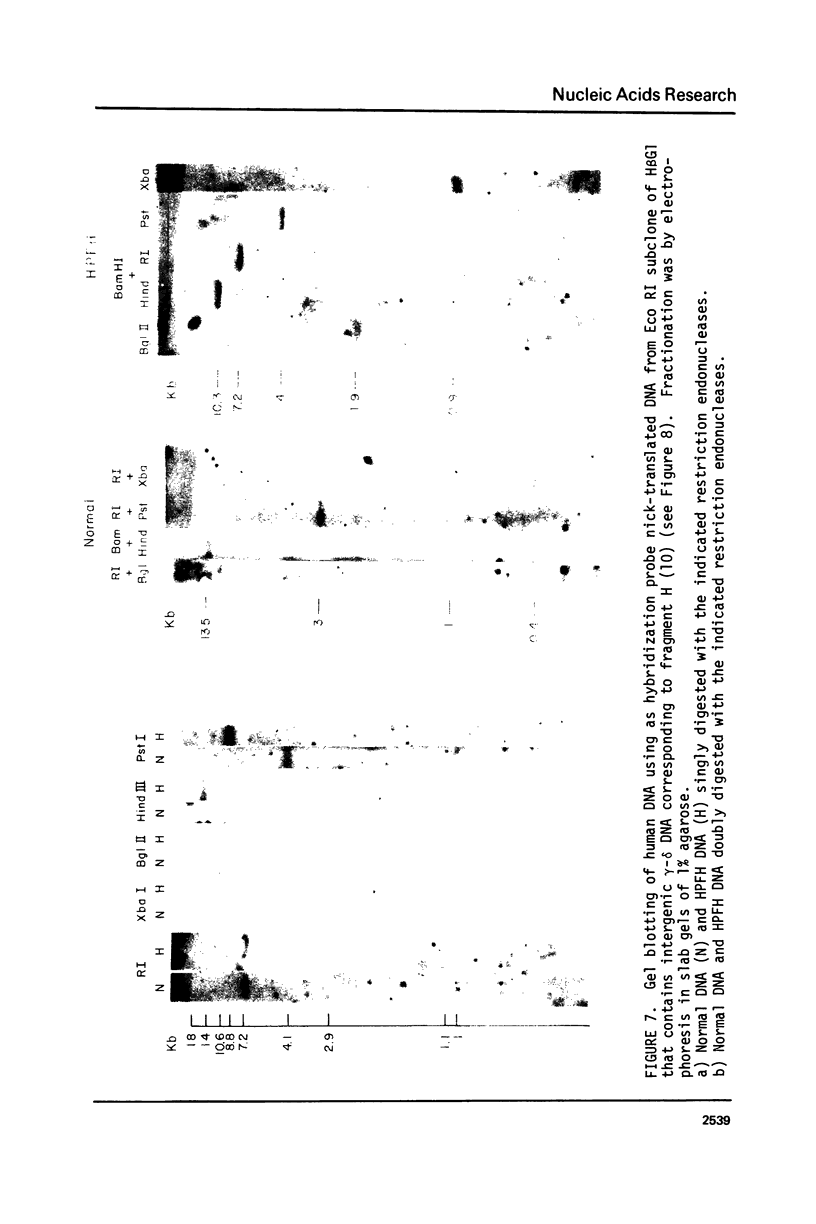
The restriction endonuclease sites in and around the human gamma globin gene loci have been mapped using the gel blotting technique of Southern, in both normal DNA and DNA from an individual with hereditary persistence of fetal hemoglobin (HPFH). In normal DNA, the gamma genes are linked to the delta (and beta) globin genes, and the orientation of these genes with respect to transcription is (5') G gamma leads to A gamma leads to delta leads to beta (3'). The distance between the G gamma and A gamma genes is 3.5 kb and that between the A gamma and delta genes is 16 kb. In both normal DNA and HPFH DNA, the gamma genes are interrupted by an intervening sequence, approximately 1 kb in length that is situated between codon positions 99 and 121 of the coding sequence. In different DNA samples, there is polymorphism for the presence or absence of a Hind III site in the intervening sequence of either gamma golbin gene. In HPFH DNA, a deletion of at least 16 kb of DNA has been detected. This deletion starts at a point approximately 12.5 kb from the 3'-end of A gamma gene and extends through the delta and beta globin genes to a point at least 3 kb beyond the 3'-end of beta globin gene.
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. C., Poon R., Neumann K. H., Kan Y. W. The nucleotide sequence of the 5' untranslated region of human gamma-globin mRNA. Nucleic Acids Res. 1978 Oct;5(10):3515–3522. doi: 10.1093/nar/5.10.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S., Adams J. M. A very large repeating unit of mouse DNA containing the 18S, 28S and 5.8S rRNA genes. Cell. 1977 Aug;11(4):795–805. doi: 10.1016/0092-8674(77)90292-6. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Britten R. J. Organization, transcription, and regulation in the animal genome. Q Rev Biol. 1973 Dec;48(4):565–613. doi: 10.1086/407817. [DOI] [PubMed] [Google Scholar]
- Deisseroth A., Nienhuis A., Lawrence J., Giles R., Turner P., Ruddle F. H. Chromosomal localization of human beta globin gene on human chromosome 11 in somatic cell hybrids. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1456–1460. doi: 10.1073/pnas.75.3.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. A., Kooter J. M., De Boer E., Little P. F., Williamson R. Analysis of the beta-delta-globin gene loci in normal and Hb Lepore DNA: direct determination of gene linkage and intergene distance. Cell. 1978 Sep;15(1):25–41. doi: 10.1016/0092-8674(78)90080-6. [DOI] [PubMed] [Google Scholar]
- Forget B. G., Hillman D. G., Lazarus H., Barell E. F., Benz ej J. R., Caskey C. T., Huisman T. H., Schroeder W. A., Housman D. Absence of messenger RNA and gene DNA for beta-globin chains in hereditary persistence of fetal hemoglobin. Cell. 1976 Mar;7(3):323–329. doi: 10.1016/0092-8674(76)90161-6. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Kan Y. W., Holland J. P., Dozy A. M., Charache S., Kazazian H. H. Deletion of the beta-globin structure gene in hereditary persistence of foetal haemoglobin. Nature. 1975 Nov 13;258(5531):162–163. doi: 10.1038/258162a0. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Little P. F., Flavell R. A., Kooter J. M., Annison G., Williamson R. Structure of the human fetal globin gene locus. Nature. 1979 Mar 15;278(5701):227–231. doi: 10.1038/278227a0. [DOI] [PubMed] [Google Scholar]
- Little P., Curtis P., Coutelle C., Van den Berg J., Dalgleish R., Malcolm S., Courtney M., Westaway D., Williamson R. Isolation and partial sequence of recombinant plasmids containing human alpha-, beta- and gamma-globin cDNA fragments. Nature. 1978 Jun 22;273(5664):640–643. doi: 10.1038/273640a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Mears J. G., Ramirez F., Leibowitz D., Bank A. Organization of human delta--and beta-globin genes in cellular DNA and the presence of intragenic inserts. Cell. 1978 Sep;15(1):15–23. doi: 10.1016/0092-8674(78)90079-x. [DOI] [PubMed] [Google Scholar]
- Mears J. G., Ramirez F., Leibowitz D., Nakamura F., Bloom A., Konotey-Ahulu F., Bank A. Changes in restricted human cellular DNA fragments containing globin gene sequences in thalassemias and related disorders. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1222–1226. doi: 10.1073/pnas.75.3.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old J., Clegg J. B., Weatherall D. J., Ottolenghi S., Comi P., Giglioni B., Mitchell J., Tolstoshev P., Williamson R. A direct estimate of the number of human gamma-globin genes. Cell. 1976 May;8(1):13–18. doi: 10.1016/0092-8674(76)90180-x. [DOI] [PubMed] [Google Scholar]
- Ottolenghi S., Giglioni B., Comi P., Gianni A. M., Polli E., Acquaye C. T., Oldham J. H., Masera G. Globin gene deletion in HPFH, delta (o) beta (o) thalassaemia and Hb Lepore disease. Nature. 1979 Apr 12;278(5705):654–657. doi: 10.1038/278654a0. [DOI] [PubMed] [Google Scholar]
- Poon R., Kan Y. W., Boyer H. W. Sequence of the 3'-noncoding and adjacent coding regions of human gamma-globin mRNA. Nucleic Acids Res. 1978 Dec;5(12):4625–4630. [PMC free article] [PubMed] [Google Scholar]
- Ramirez F., O'Donnell J. V., Natta C., Bank A. Quantitation of human gamma globin genes and gamma globin mRNA with purified gamma globin complementary DNA. J Clin Invest. 1976 Dec;58(6):1475–1481. doi: 10.1172/JCI108604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithies O., Blechl A. E., Denniston-Thompson K., Newell N., Richards J. E., Slightom J. L., Tucker P. W., Blattner F. R. Cloning human fetal gamma globin and mouse alpha-type globin DNA: characterization and partial sequencing. Science. 1978 Dec 22;202(4374):1284–1289. doi: 10.1126/science.725604. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wilson J. T., Wilson L. B., deRiel J. K., Villa-komaroff L., Efstratiadis A., Forget B. G., Weissman S. M. Insertion of synthetic copies of human globin genes into bacterial plasmids. Nucleic Acids Res. 1978 Feb;5(2):563–581. doi: 10.1093/nar/5.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]