Abstract
In recent years galectin-3 has gained attention as a signalling molecule, mainly in inflammatory diseases. Data on galectin-3 expression in neonates, however, are limited, and expression of this lectin in cord blood has not yet been reported. The aim of this study was to determine galectin-3 levels in cord blood of term and preterm neonates as well as galectin-3 levels in cord blood of term neonates after stimulation with the prevalent pathogen Streptococcus agalactiae. Cord blood samples were incubated for 24 h and galectin-3 levels were assessed by enzyme-linked immunosorbent assay. There is a positive correlation between gestational age and galectin-3 levels in cord blood. Expression of galectin-3 is significantly higher in cord blood of small-for-gestational-age infants compared to appropriate-for-gestational-age infants. Stimulation with an invasive but not with a colonizing strain of S. agalactiae induced expression of galectin-3. Galectin-3 is expressed constitutively in cord blood of neonates and seems to play a role in the innate immunity of this population.
Keywords: cord blood, galectin-3, Group B Streptococcus
Introduction
The family of galectins is a subgroup of lectins defined by their affinity to galactoside sugars as well as a conserved sequence of approximately 130 amino acids in their carbohydrate recognition domain (CRD). Galectin-3 is the only representative of chimera-type galectins containing one CRD and an extended N-terminus through which pentamers can be formed [1,2]. Galectin-3 is expressed by virtually all immunocompetent cells, i.e. monocytes, macrophages, neutrophils, eosinophils, basophils, mast cells and dendritic cells, and fulfils manifold immunological functions [3–6]. While predominantly being an intracellular protein localized in the cytosol or nucleus, galectin-3 can also be secreted or expressed as a cell surface protein with differential ligands and functions, depending on its localization [7–9]. In numerous studies predominantly proinflammatory properties of galectin-3 have been established [10]. Apart from playing an important role in recruitment of macrophages and neutrophils, and promoting phagocytosis as well as adhesion of granulocytes to endothelium, galectin-3 acts as relevant receptor for Candida albicans[11–14]. In contrast, anti-inflammatory function is exhibited through binding of lipopolysaccharide (LPS) with subsequent impairment of its function as a potent proinflammatory stimulant and blocking of LPS-induced inflammatory cytokine production [15]. The role of galectin-3 in apoptosis is ambivalent, as the intracellular protein has anti-apoptotic effects while extracellular galectin-3 promotes apoptosis of miscellaneous cells [10,16,17]. In recent studies, Doverhag et al. demonstrated that galectin-3 levels in amniotic fluid were elevated in women with chorioamnionitis and in brain tissue of mice after hypoxic or ischaemic insult [18]. The latter observation is supported by in-vitro experiments showing that galectin-3, among other proteins, is hypoxia inducible factor 1 (HIF-1), up-regulated dependently during hypoxia [19]. However, galectin-3 expression levels in placentas of children with intrauterine growth restriction (IUGR), often a result of placental insufficiency with consecutive nutrient and oxygen deficiency, were not altered significantly [20]. It was the aim of this study to determine galectin-3 levels in whole cord blood of preterm and term neonates depending on gestational age and birth weight as well as galectin-3 levels in whole cord blood using a well-established in-vitro sepsis model with viable Group B Streptococcus (GBS).
Materials and methods
Cord blood samples
After obtaining parents' informed consent, cord blood samples of 21 healthy term and 125 preterm infants (Table 1) born in the Department of Women's Health and Obstetrics at the University of Lübeck, Germany, were collected immediately after delivery in lithium–heparin tubes (Sarstedt, Nürnbrecht, Germany) and stored at room temperature before processing. Infants with early-onset sepsis were excluded from the study. The study was approved by the local ethical committee at the University of Lübeck.
Table 1.
Study group of preterm infants
| Characteristics | Data |
|---|---|
| n | 125 |
| Gestational age (weeks), mean (s.d.) | 29·9 (2·8) |
| SGA infants | 27·6 (3·1) |
| AGA infants | 30·1 (2·7) |
| Birth weight (g), mean (s.d.) | 1385 (535) |
| SGA infants | 689 (294) |
| AGA infants | 1439 (512) |
| Male gender (%) | 44·8 |
| Maternal descendence (%) | |
| Germany | 83·2 |
| Turkey | 8·8 |
| Middle East | 7·2 |
| Other | 0·8 |
| Umbilical artery pH, mean (s.d.) | 7·19 (0·08) |
| Cause of preterm delivery (%) | |
| Preterm rupture of membranes | 21·6 |
| Amniotic infection | 22·4 |
| Pre-eclampsia | 7·2 |
| Pathological CTG | 10·4 |
| Placental abruption | 3·2 |
| Other | 35·2 |
AGA: appropriate-for-gestational-age; CTG: pattern of cardiotocography; SGA: small-for-gestational-age; s.d.: standard deviation.
GBS strains
Two GBS strains, one obtained from a blood culture of a neonate with early-onset sepsis, the other from a skin swab of a healthy neonate, were used as stimulating agents. GBS strains were stored at −20°C in culture broth. Before use as stimulant an aliquot of each isolate was plated on sheep blood agar (Oxoid Ltd, Hampshire, UK) and incubated for 24 h. Single colonies were then transferred to NaCl 0·9% solution with an inoculation loop. The resulting suspension was mixed thoroughly, optical density was assessed by photometry at 595 nm (Jenway Ltd, Gransmore Green, Felsted, Dunmow, Essex, UK) and adjusted to an optical density corresponding to 1 × 108 colony forming units (CFU)/ml as determined in preceding experiments by adding more GBS colonies or NaCl 0·9%, respectively.
Whole blood assay
A whole blood assay was performed within 24 h after blood collection as described previously [21]. Heparinized whole cord blood was suspended in RPMI-1640 (PAA Laboratories GmbH, Pasching, Austria) supplemented with 1% penicillin/streptomycin, 2 mM glutamine, 1 mM pyruvate and non-essential amino acids (Biochrom AG, Berlin, Germany) on a six-well plate (Nunc A/S, Roskilde, Denmark) at a concentration of 1 × 106 leucocytes/ml. In addition, 21 specimens of term infants were stimulated at the same time with the two GBS strains at a concentration of 1 CFU/white blood cell (CFU/WBC) and 10 CFU/WBC, respectively. As negative control no stimulant was added. After an incubation period of 24 h at 37°C and 5%CO2 the supernatants of the cell cultures were collected and stored at −20°C until analysis. Galectin-3 levels in supernatants were assessed with human galectin-3 Quantikine enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Inc., Minneapolis, MN, USA), according to the manufacturer's instructions.
Statistical analysis
The Mann–Whitney U-test was applied for statistical analysis of differences between small-for-gestational-age (SGA) infants and non-SGA infants as well as differences between gestational age groups. Statistical differences between groups stimulated with different GBS strains were tested for paired data by the Wilcoxon test (two tailed). The level of significance was defined as P < 0·05 in single comparisons. Spearman's rho correlation coefficient was used to analyse the correlation between gestational age and galectin-3 levels. Statistical analyses were performed using spss 17·0 statistical software (SPSS Inc, Chicago, IL, USA).
Results
Correlation between galectin-3 expression and gestational age
As illustrated in Fig. 1a, significant differences in galectin-3 expression were detected in culture supernatants stratified to gestational age groups. Galectin-3 levels were significantly lower in preterm infants born before 27 weeks gestational age as well as preterm infants born between 29 and 34 weeks gestational age compared to term infants. Within the group of preterm infants, galectin-3 expression of infants born before 27 weeks gestational age was significantly lower than of infants born after at least 27 weeks gestational age. In the whole cohort, galectin-3 levels correlated with gestational age (Fig. 1b).
Fig. 1.
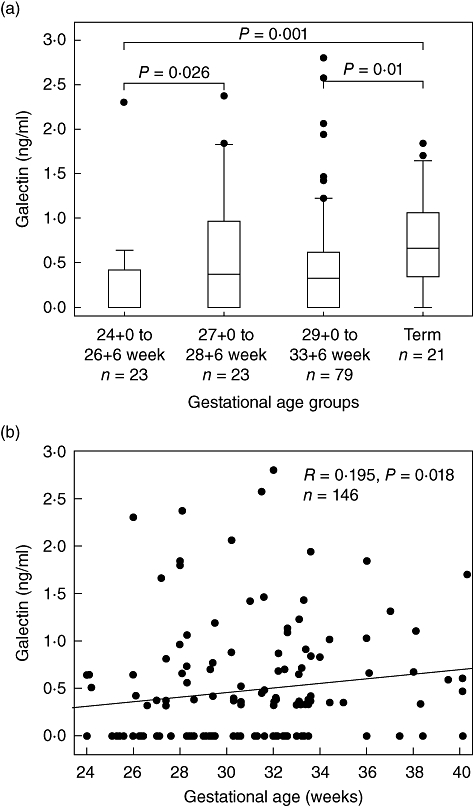
Galectin-3 expression is dependent upon gestational age. Whole cord blood cultures were incubated for 24 h and galectin-3 levels in culture supernatants were assessed by enzyme-linked immunosorbent assay (ELISA). A significant increase in galectin-3 expression is correlated with increasing gestational age. Results for gestational age groups (Fig. 1a) are displayed as Box-and-Whisker plots with median, lower and upper quartile as well as 95% confidence interval. Outliers are represented by dots. (b) Data shown as scatterplot. Spearman's correlation coefficient (R) was calculated.
Galectin-3 expression and intrauterine growth restriction
Galectin-3 levels in whole cord blood culture supernatants of 125 infants were assessed by ELISA. Figure 2 shows that cord blood of infants with a birth weight below the 10th Voigt percentile for their gestational age had higher galectin-3 levels than appropriate-for-gestational-age (AGA) infants [22].
Fig. 2.
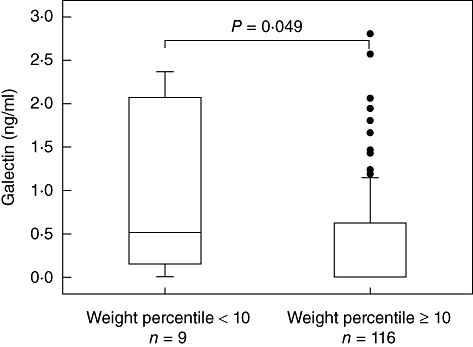
Galectin-3 expression is associated with small-for-gestational-age (SGA). Whole cord blood cultures were incubated for 24 h and galectin-3 levels in culture supernatants were assessed by enzyme-linked immunosorbent assay (ELISA). Significantly higher galectin-3 levels were detected in culture supernatants of SGA infants. Results are displayed as Box-and-Whisker plots with median, lower and upper quartile as well as 95% confidence interval. Outliers are represented by dots.
Induction of galectin-3 by GBS
Whole cord blood cultures of 21 healthy term neonates were stimulated at the same time with asepsis and a colonizing GBS strain. An unstimulated culture served as negative control. Results are displayed in Fig. 3. Stimulation with the sepsis strain at a concentration of 10 CFU/WBC resulted in a significant increase of galectin-3 levels in culture supernatants. Neither stimulation at a concentration of 1 CFU/WBC nor stimulation with the colonizing strain induced a significant increase of galectin-3 in supernatants.
Fig. 3.
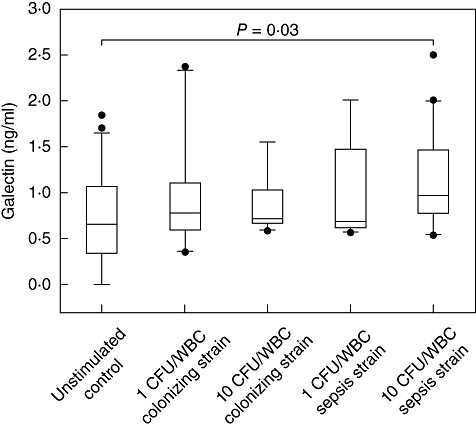
Galectin-3 expression is inducible by Group B Streptococcus (GBS). Whole cord blood samples of healthy neonates were incubated for 24 h at the same time with a colonizing strain or a sepsis strain of S. agalactiae at a concentration of 0, 1 and 10 colony-forming units/white blood cells (CFU/WBC). Galectin-3 levels were then determined by enzyme-linked immunosorbent assay (ELISA). Significantly higher galectin-3 expression is induced after stimulation with 10 CFU/WBC of the sepsis strain compared with no stimulation. Data are presented as Box-and-Whisker plots with median, lower and upper quartile as well as 95% confidence interval. Outliers are represented by dots.
Discussion
This is the first investigation of galectin-3 levels in cord blood of neonates. The role of extracellular galectin-3 has been the subject of several studies, and mainly the proinflammatory properties of this lectin have been demonstrated [10]. The role in innate immunity comprises the promotion of activation, adhesion, chemotaxis and phagocytosis. These effects of galectin-3 on neutrophils and macrophages have been demonstrated in vitro and in vivo.
Galectin-3 is expressed constitutively in humans with mean serum levels of approximately 2–4 ng/ml in healthy adults [23,24]. In our whole cord blood assay, levels of 0–1·8 ng/ml were measured in cord blood samples of term neonates. Increased galectin-3 levels have been reported in a variety of conditions, e.g. infectious, other inflammatory or malignant diseases [9,10]. The human embryo expresses galectin-3 predominantly in epithelium already in the first trimester and the function in cell–cell interaction as well as cell differentiation during embryogenesis might be the reason for this finding [25]. Galectin-3 levels in cord blood of preterm and term infants, however, have not yet been reported. The data presented here show that there is galectin-3 secretion in cord blood of term and preterm neonates and we also noted a positive correlation between gestational age and galectin-3 levels. It has long been recognized that certain protein serum levels correlate positively with gestational age. The same seems to be true for galectin-3 [26–28]. Considering the effects of extracellular galectin-3 on innate immunity as discussed below, it is tempting to speculate that the impaired galectin-3 expression in neonates may contribute in part to the high susceptibility of preterm infants to infection as opposed to term infants or adults. Comparability of galectin-3 levels determined in our experiments with serum levels reported in the literature, however, is restricted due to experimental conditions. We used a whole blood assay in order to approximate in-vivo conditions and experimental settings were standardized to 1 × 106 white blood cells/ml. With these dilution steps included, a significant number of infants had galectin-3 levels below the detection limit of 0·016 ng/ml. Our approach is limited further by the restricted use of cord blood and the exclusion of infected infants. To elucidate further the role of galectin-3 for early- and late-onset infection, we currently investigate the dynamics of galectin-3 expression in the first days of life by serial tests. In addition, the German Neonatal Network, which is led by our group, will also provide a platform to study the genetic impact on galectin-3 levels in a multi-centre setting.
Our data showed higher galectin-3 levels in cord blood of SGA infants compared to AGA infants. SGA is a descriptive term designating infants whose body weight is below the 10th percentile for their gestational age without reference to the underlying condition. Often, however, IUGR, i.e. fetal growth restriction due to fetal, maternal or placental factors inhibiting the full growth potential, is the reason for SGA [29]. IUGR can be caused by a variety of conditions (e.g. poor nutrition, smoking, alcohol consumption, infections, pre-eclampsia) that lead eventually to chronic shortage of oxygen and/or nutrients in the fetus [30]. The induction of inflammation by hypoxia via HIF-1 in interaction with nuclear factor (NF)-κB is a well-recognized phenomenon and has been demonstrated in vitro as well as in humans in vivo (reviewed in [31]). Indeed, increased levels of inflammatory markers in cord blood of SGA infants compared to AGA infants have been reported recently suggesting that chronic fetal hypoxia induces inflammatory mechanisms in fetuses [32]. Furthermore, it has been demonstrated in several studies that galectin-3 expression is regulated by HIF-1 as well as NF-κB [19,33,34]. Considering these findings along with our results, as well as the proinflammatory role of galectin-3, the higher expression of this lectin in SGA infants might be a reflection of inflammation due to chronic hypoxia of the fetus.
Despite great advances in perinatal prophylaxis with subsequent decrease of GBS-related disease in neonates, GBS remains one of the leading causes of early-onset disease [35]. However, it is largely unknown why some neonates acquire early-onset disease while others are being colonized and do not develop symptoms. It has been reported that GBS strains from septic infants induce a stronger interleukin (IL)-6 expression in cord blood monocytes than colonizing strains and that GBS strain COH1, a sepsis strain used in most studies investigating GBS and innate immunity, exhibits an extraordinary ability to stimulate Toll-like receptor (TLR)-2 and cytokine production [36,37]. It is therefore reasonable to presume that both strain-specific and host-specific factors determine the course of GBS host interaction. In this study we analysed galectin-3 levels in whole cord blood cultures and its alteration by sepsis and colonizing strains of GBS. Galectin-3 levels were elevated significantly after incubation with 10 CFU/WBC of the sepsis strain. Neither stimulation with 10 CFU/WBC of the colonizing strain nor stimulation with 1 CFU/WBC of either strain induced significantly elevated galectin-3 levels compared to the unstimulated cell culture. The role of galectin-3 in inflammation and infection has been the subject of several studies during past years uncovering predominantly proinflammatory properties [10]. Exogenous galectin-3 promotes adhesion of neutrophils and migration of macrophages, triggers oxidative burst in macrophages and neutrophils and is a chemoattractant for monocytes and macrophages, all of which are important features of innate immunity [11–13,38]. By augmenting neutrophil function, the severity of pneumococcal pneumonia in mice can be attenuated by galectin-3. Furthermore, galectin-3 acts bacteriostatically on S. pneumoniae and may have similar effects on other Gram-positive bacteria such as GBS [39]. In addition, galectin-3 interacts with LPS of different Gram-negative pathogens, is a receptor for C. albicans and has been proposed to recognize glycoconjugates of Gram-positive bacteria, prompting the possibility that galectin-3 may act as a pathogen recognition receptor (PRR) [15,40,41]. Binding of self-glycans, however, seems to be the preferential way of receptor ligand interaction of galectin-3 [41]. We therefore speculate that galectin-3 may play a pluripotent role for preterm and term neonates, in particular in the first days of life, which are characterized by ‘immune paralysis’ in vulnerable infants prone to infection. Galectin-3 levels are presumably regulated on an individual level, and we were not able to demonstrate any correlation with other surrogate markers of innate immune responses in our cohort, e.g. unstimulated or LPS and S. epidermidis-induced cytokine expression (data not shown). Our data imply that galectin-3 is up-regulated differentially in cord blood cells upon stimulation with GBS possibly depending on strain virulence. This might reflect a stronger need for neutrophil activation by galectin-3 after contact with sepsis strains rather than colonizing strains. GBS factors that induce galectin-3 secretion as well as the role of galectin-3 in GBS sepsis have to be determined by further investigations. Possibly, augmented release of intracellular galectin-3 by increased cell death induced by a sepsis strain and not by a colonizing strain must also be taken into consideration.
The results presented here point to a proinflammatory role of galectin-3 in term and preterm neonates. Several publications have established the role of galectin-3 as signalling molecule in inflammation and revealed mainly proinflammatory effects by promoting recruitment and phagocytosis of macrophages and neutrophils. For the first time, our data show a positive correlation of galectin-3 levels in cord blood with gestational age, significantly elevated galectin-3 levels in cord blood of SGA infants and inducibility of galectin-3 in cord blood of neonates by a GBS sepsis strain. In conclusion, galectin-3 may play a role in innate immunity in term and preterm neonates. Approaches aiming at an increased expression of galectin-3 in neonates might, in future, contribute to new therapeutic strategies in neonatal infection. Further studies are needed to validate these results and to elucidate further the functional relevance of our findings.
Disclosure
No conflict of interest.
References
- 1.Ahmad N, Gabius H-J, André S, et al. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J Biol Chem. 2004;279:10841–7. doi: 10.1074/jbc.M312834200. [DOI] [PubMed] [Google Scholar]
- 2.Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269:20807–10. [PubMed] [Google Scholar]
- 3.Liu FT, Hsu DK, Zuberi RI, Kuwabara I, Chi EY, Henderson WR. Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am J Pathol. 1995;147:1016–28. [PMC free article] [PubMed] [Google Scholar]
- 4.Truong MJ, Gruart V, Kusnierz JP, et al. Human neutrophils express immunoglobulin E (IgE)-binding proteins (Mac-2/epsilon BP) of the S-type lectin family: role in IgE-dependent activation. J Exp Med. 1993;177:243–8. doi: 10.1084/jem.177.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craig SS, Krishnaswamy P, Irani AM, Kepley CL, Liu FT, Schwartz LB. Immunoelectron microscopic localization of galectin-3, an IgE binding protein, in human mast cells and basophils. Anat Rec. 1995;242:211–19. doi: 10.1002/ar.1092420210. [DOI] [PubMed] [Google Scholar]
- 6.Flotte TJ, Springer TA, Thorbecke GJ. Dendritic cell and macrophage staining by monoclonal antibodies in tissue sections and epidermal sheets. Am J Pathol. 1983;111:112–24. [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes RC. Mac-2: a versatile galactose-binding protein of mammalian tissues. Glycobiology. 1994;4:5–12. doi: 10.1093/glycob/4.1.5. [DOI] [PubMed] [Google Scholar]
- 8.Liu F-T, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta. 2002;1572:263–73. doi: 10.1016/s0304-4165(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 9.Perillo NL, Marcus ME, Baum LG. Galectins: versatile modulators of cell adhesion, cell proliferation, and cell death. J Mol Med. 1998;76:402–12. doi: 10.1007/s001090050232. [DOI] [PubMed] [Google Scholar]
- 10.Norling LV, Perretti M, Cooper D. Endogenous galectins and the control of the host inflammatory response. J Endocrinol. 2009;201:169–84. doi: 10.1677/JOE-08-0512. [DOI] [PubMed] [Google Scholar]
- 11.Colnot C, Ripoche MA, Milon G, Montagutelli X, Crocker PR, Poirier F. Maintenance of granulocyte numbers during acute peritonitis is defective in galectin-3-null mutant mice. Immunology. 1998;94:290–6. doi: 10.1046/j.1365-2567.1998.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sano H, Hsu DK, Apgar JR, et al. Critical role of galectin-3 in phagocytosis by macrophages. J Clin Invest. 2003;112:389–97. doi: 10.1172/JCI17592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuwabara I, Liu FT. Galectin-3 promotes adhesion of human neutrophils to laminin. J Immunol. 1996;156:3939–44. [PubMed] [Google Scholar]
- 14.Jouault T, Abed-El ME, Martínez-Esparza M, et al. Specific recognition of Candida albicans by macrophages requires galectin-3 to discriminate Saccharomyces cerevisiae and needs association with TLR2 for signaling. J Immunol. 2006;177:4679–87. doi: 10.4049/jimmunol.177.7.4679. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Komai-Koma M, Gilchrist DS, et al. Galectin-3 is a negative regulator of lipopolysaccharide-mediated inflammation. J Immunol. 2008;181:2781–9. doi: 10.4049/jimmunol.181.4.2781. [DOI] [PubMed] [Google Scholar]
- 16.Moon BK, Lee YJ, Battle P, Jessup JM, Raz A, Kim HR. Galectin-3 protects human breast carcinoma cells against nitric oxide-induced apoptosis: implication of galectin-3 function during metastasis. Am J Pathol. 2001;159:1055–60. doi: 10.1016/S0002-9440(10)61780-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci USA. 1996;93:6737–42. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doverhag C. 2010. Inflammatory mechanisms in experimental neonatal brain injury and in a clinical study of preterm birth; involvement of galectin-3 and free radical formation.
- 19.Greijer AE, der PV, Kemming D, et al. Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1) J Pathol. 2005;206:291–304. doi: 10.1002/path.1778. [DOI] [PubMed] [Google Scholar]
- 20.Jeschke U, Mayr D, Schiessl B, et al. Expression of galectin-1, -3 (gal-1, gal-3) and the Thomsen–Friedenreich (TF) antigen in normal, IUGR, preeclamptic and HELLP placentas. Placenta. 2007;28:1165–73. doi: 10.1016/j.placenta.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Härtel C, Osthues I, Rupp J, et al. Characterisation of the host inflammatory response to Staphylococcus epidermidis in neonatal whole blood. Arch Dis Child Fetal Neonatal Ed. 2008;93:F140–F145. doi: 10.1136/adc.2007.124685. [DOI] [PubMed] [Google Scholar]
- 22.Voigt M, Rochow N, Straube S, Briese V, Olbertz D, Jorch G. Birth weight percentile charts based on daily measurements for very preterm male and female infants at the age of 154–223 days. J Perinat Med. 2010;38:289–95. doi: 10.1515/jpm.2010.031. [DOI] [PubMed] [Google Scholar]
- 23.Weigert J, Neumeier M, Wanninger J, et al. Serum galectin-3 is elevated in obesity and negatively correlates with glycosylated hemoglobin in type 2 diabetes. J Clin Endocrinol Metab. 2010;95:1404–11. doi: 10.1210/jc.2009-1619. [DOI] [PubMed] [Google Scholar]
- 24.Frol'ová L, Smetana K, Borovská D, et al. Detection of galectin-3 in patients with inflammatory bowel diseases: new serum marker of active forms of IBD? Inflamm Res. 2009;58:503–12. doi: 10.1007/s00011-009-0016-8. [DOI] [PubMed] [Google Scholar]
- 25.van den Brule FA, Fernandez PL, Buicu C, et al. Differential expression of galectin-1 and galectin-3 during first trimester human embryogenesis. Dev Dyn. 1997;209:399–405. doi: 10.1002/(SICI)1097-0177(199708)209:4<399::AID-AJA7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 26.Hyvarinen M, Zeltzer P, Oh W, Stiehm ER. Influence of gestational age on serum levels of alpha-1 fetoprotein, IgG globulin, and albumin in newborn infants. J Pediatr. 1973;82:430–7. doi: 10.1016/s0022-3476(73)80116-7. [DOI] [PubMed] [Google Scholar]
- 27.Jacobsen BB, Peitersen B, Andersen HJ, Hummer L. Serum concentrations of thyroxine-binding globulin, prealbumin and albumin in healthy fullterm, small-for-gestational age and preterm newborn infants. Acta Paediatr Scand. 1979;68:49–55. doi: 10.1111/j.1651-2227.1979.tb04429.x. [DOI] [PubMed] [Google Scholar]
- 28.Galinier A, Périquet B, Lambert W, et al. Reference range for micronutrients and nutritional marker proteins in cord blood of neonates appropriated for gestational ages. Early Hum Dev. 2005;81:583–93. doi: 10.1016/j.earlhumdev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Gardosi J. New definition of small for gestational age based on fetal growth potential. Horm Res. 2006;65(Suppl. 3):15–8. doi: 10.1159/000091501. [DOI] [PubMed] [Google Scholar]
- 30.Scifres CM, Nelson DM. Intrauterine growth restriction, human placental development and trophoblast cell death. J Physiol. 2009;587(Pt 14):3453–8. doi: 10.1113/jphysiol.2009.173252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–65. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amarilyo G, Oren A, Mimouni FB, Ochshorn Y, Deutsch V, Mandel D. Increased cord serum inflammatory markers in small-for-gestational-age neonates. J Perinatol. 2011;31:30–2. doi: 10.1038/jp.2010.53. [DOI] [PubMed] [Google Scholar]
- 33.Zeng Y, Danielson KG, Albert TJ, Shapiro IM, Risbud MV. HIF-1 alpha is a regulator of galectin-3 expression in the intervertebral disc. J Bone Miner Res. 2007;22:1851–61. doi: 10.1359/jbmr.070620. [DOI] [PubMed] [Google Scholar]
- 34.Dumic J, Lauc G, Flögel M. Expression of galectin-3 in cells exposed to stress-roles of jun and NF-kappaB. Cell Physiol Biochem. 2000;10:149–58. doi: 10.1159/000016345. [DOI] [PubMed] [Google Scholar]
- 35.Centres for Disease Control and Prevention (CDC) Perinatal group B streptococcal disease after universal screening recommendations – United States, 2003–2005. Morb Mortal Wkly Rep. 2007;56:701–5. [PubMed] [Google Scholar]
- 36.Berner R, Csorba J, Brandis M. Different cytokine expression in cord blood mononuclear cells after stimulation with neonatal sepsis or colonizing strains of Streptococcus agalactiae. Pediatr Res. 2001;49:691–7. doi: 10.1203/00006450-200105000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Henneke P, Berner R. Interaction of neonatal phagocytes with group B streptococcus: recognition and response. Infect Immun. 2006;74:3085–95. doi: 10.1128/IAI.01551-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu DK, Yang RY, Pan Z, et al. Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am J Pathol. 2000;156:1073–83. doi: 10.1016/S0002-9440(10)64975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farnworth SL, Henderson NC, Mackinnon AC, et al. Galectin-3 reduces the severity of pneumococcal pneumonia by augmenting neutrophil function. Am J Pathol. 2008;172:395–405. doi: 10.2353/ajpath.2008.070870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fradin C, Poulain D, Jouault T. beta-1,2-linked oligomannosides from Candida albicans bind to a 32-kilodalton macrophage membrane protein homologous to the mammalian lectin galectin-3. Infect Immun. 2000;68:4391–8. doi: 10.1128/iai.68.8.4391-4398.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato S, Nieminen J. Seeing strangers or announcing ‘danger’: galectin-3 in two models of innate immunity. Glycoconj J. 2004;19:583–91. doi: 10.1023/B:GLYC.0000014089.17121.cc. [DOI] [PubMed] [Google Scholar]


