Abstract
The activation of nuclear factor-kappa B (NF-κB) in vascular endothelial cells may be involved in vascular pathogeneses such as vasculitis or atherosclerosis. Recently, it has been reported that some amino acids exhibit anti-inflammatory effects. We investigated the inhibitory effects of a panel of amino acids on cytokine production or expression of adhesion molecules that are involved in inflammatory diseases in various cell types. The activation of NF-κB was determined in human coronary arterial endothelial cells (HCAECs) because NF-κB modulates the production of many cytokines and the expression of adhesion molecules. We examined the inhibitory effects of the amino acids cysteine, histidine and glycine on the induction of NF-κB activation, expression of CD62E (E-selectin) and the production of interleukin (IL)-6 in HCAECs stimulated with tumour necrosis factor (TNF)-α. Cysteine, histidine and glycine significantly reduced NF-κB activation and inhibitor κBα (IκBα) degradation in HCAECs stimulated with TNF-α. Additionally, all the amino acids inhibited the expression of E-selectin and the production of IL-6 in HCAECs, and the effects of cysteine were the most significant. Our results show that glycine, histidine and cysteine can inhibit NF-κB activation, IκBα degradation, CD62E expression and IL-6 production in HCAECs, suggesting that these amino acids may exhibit anti-inflammatory effects during endothelial inflammation.
Keywords: amino acid, coronary arterial endothelial cell, E-selectin (CD62E), IL-6, NF-κB
Introduction
Nuclear factor-kappa B (NF-κB) is an inducible regulatory system involved in endothelial activation [1,2]. In addition, among the cytokines modulating endothelial functions, the proinflammatory tumour necrosis factor (TNF)-α plays a crucial role in activation [3]. The control of processes leading to endothelial dysfunction could be a useful tool for investigating the pathogenesis of some vascular diseases. The activation of NF-κB in vascular endothelial cells may be involved in the vascular pathogenesis of vasculitis or atherosclerosis [4,5]. Recently, studies have shown that certain amino acids, including glycine, histidine, cysteine, glutamine and tryptophan, exhibit anti-inflammatory effects and histidine and glutamine suppress NF-κB activation [6–16]. However, the anti-inflammatory effects of amino acids in coronary endothelial cells are still unclear.
In this study, we focused on cysteine, glycine and histidine – amino acids that have been reported to exhibit anti-inflammatory effects [6–14]– and investigated whether these amino acids exhibit inhibitory effects against TNF-α-induced NF-κB activation in human coronary arterial endothelial cells (HCAECs). Furthermore, we also examined the inhibitory effects of glycine, histidine and cysteine on CD62E (E-selectin) expression and interleukin (IL)-6 production in HCAECs.
Materials and methods
Cell culture and stimulation conditions
HCAECs were obtained from Lonza (Walkersville, MD, USA), and the cells were maintained as stationary cultures at 37°C under humidified 5% CO2. The cells were grown in endothelial cell growth medium-2 (EGM-2) BulletKit (Lonza). The cells were incubated in Dulbecco's modified Eagle's medium (Gibco RBL Life Technologies, Inc., Gaithersburg, MD, USA) without fetal calf serum (FCS) (Gibco RBL Life Technologies, Inc.) for 12 h prior to pretreatment with alanine, glycine, histidine or cysteine (provided by Ajinomoto Pharma Co. Ltd, Tokyo, Japan). After washing, the cells were resuspended in a medium that did not contain amino acids (provided by Ajinomoto Pharma Co. Ltd). The cells were incubated with or without alanine, glycine, histidine or cysteine at concentrations of 0·2 mm, 2 mm and 20 mm for 2 h, prior to stimulation with 2 ng/ml TNF-α (R&D Systems, Minneapolis, MN, USA). We chose these concentrations of amino acids on the basis of the protocols in previous reports [12]. Cell viability was determined by performing trypan blue (Gibco RBL Life Technologies, Inc.) staining.
Nuclear extracts and determination of NF-κB activation
Nuclear extracts were harvested from HCAECs pretreated with or without each amino acid after stimulation with 2 ng/ml of TNF-α for 30 min by using a nuclear extract kit (Active Motif, Carlsbad, CA, USA), according to the manufacturer's instructions. The protein concentrations of the nuclear extracts were determined using the Coomassie Plus Protein Assay Reagent (Pierce, Rockford, IL, USA). The concentrations of NF-κB were determined using an activated NF-κB enzyme-linked immunosorbent assay (ELISA) kit (Active Motif). An oligonucleotide containing the NF-κB consensus site (5′-GGGACTTTCC-3′) was adsorbed onto polystyrene microwells. The activated NF-κB in the nuclear extracts bound specifically to this oligonucleotide and the NF-κB complexes bound to the oligonucleotide were then detected using an anti-NF-κB p65 antibody. Subsequently, a secondary antibody conjugated to horseradish peroxidase was added. The absorbance was measured at 450 nm by spectrophotometry [17].
Western blotting of inhibitor κBα (IκBα)
HCAECs were pretreated with or without each amino acid at a final concentration of 20 mm and then stimulated with 2 ng/ml of TNF-α for 0, 10, 20, 30 and 60 min. Whole cell lysates were obtained by incubating the cell samples in ice-cold lysis buffer [2% sodium dodecyl sulphate, 1 mm ethylenediamine tetraacetic acid (EDTA), 0·2 mm phenylmethylsulphonyl fluoride; Wako Pure Chemical Industries Ltd, Osaka, Japan] with protease inhibitors (1 µm leupeptin and 1 µm pepstatin; Wako Pure Chemical Industries Ltd), followed by centrifugation to remove debris (12 000 g for 10 min at 4°C). Protein concentrations were determined using the Bio-Rad protein concentration reagent (Bio-Rad, Hercules, CA, USA). The samples were stored at −80°C, and samples containing 15 µg of protein were separated on denaturing 10% polyacrylamide (Bio-Rad) gels and then transferred to polyvinylidene difluoride membranes (Millipore Co., Bedford, MA, USA). After three washes in Tris-buffered saline with Tween 20 (TBST; 40 mm Tris-HCl, pH 7·6, 300 mm NaCl and 0·5% Tween 20; Wako Pure Chemical Industries Ltd), the membranes were incubated with 1:1000 diluted rabbit monoclonal anti-IκBα antibodies (Cell Signaling, Beverly, MA, USA) in TBST containing 5% non-fat dry milk (Wako Pure Chemical Industries Ltd) overnight at 4°C. We also used rabbit polyclonal anti-human β-actin antibody (1:200; AnaSpec, Inc., San Jose, CA, USA) as an internal control. After three washes in TBST, the membranes were incubated with 1:2000 diluted horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin (Ig)G (Bio-Rad) for 1 h at room temperature. Immunoreactive proteins were detected by enhanced chemiluminescence (Amersham, Arlington Heights, IL, USA) and analysed by radiography. The quantification of bands was performed using Kodak Digital Science 1D (Eastman Kodak Company, New Haven, CT, USA).
Determination of CD62E expression
HCAECs (2 × 106 cells/ml) were exposed to 2 ng/ml of TNF-α with or without pretreatment with alanine, glycine, histidine or cysteine for 2 h. The cells were collected 2 h after the incubation with or without TNF-α, after which the cells were harvested and the expression of CD62E was determined by flow cytometric analysis, using a phycoerythrin (PE)-conjugated anti-CD62E antibody (BD Pharmingen, San Diego, CA, USA). PE-conjugated mouse IgG1 (BD Pharmingen) was also used as the isotype-matched control. Immunofluorescence staining was analysed with a fluorescence-activated cell sorter (FACS), FACScalibur flow cytometer equipped with CellQuest software (Becton-Dickinson Biosciences, San Diego, CA, USA). In each assay, 10 000 cells were analysed.
Determination of IL-6 concentrations
The concentration of IL-6 in the supernatant of HCAECs after incubation with or without TNF-α (2 × 106 cells/ml, stimulation for 6 h) was determined using a sandwich-type ELISA kit (R&D Systems, Minneapolis, MN, USA). The detection limit for IL-6 was 3·1 pg/ml.
Statistical analysis
The data are presented as the mean ± standard error of the mean (s.e.m.). Statistical analysis was performed using a one-way analysis of variance (anova), with P-values less than 0·05 considered as significant. Analyses and calculations were performed using spss version 12·0 (SPSS, Inc., Chicago, IL, USA).
Results
Prior to these experiments, we determined cell viability by trypan blue staining and found that there were no significant differences in cell viability with or without treatment with each amino acid (data not shown). Figure 1 presents the NF-κB activation and inhibitory effects of amino acids in HCAECs stimulated with TNF-α. NF-κB activation was induced significantly by stimulation with 2 ng/ml TNF-α for 30 min in non-treated HCAECs (#P < 0·001). Pretreatment with glycine (20 mm), histidine (0·2 mm, 2 mm and 20 mm) and cysteine (0·2 mm, 2 mm and 20 mm) inhibited NF-κB activation significantly in TNF-α-stimulated HCAECs (**P < 0·01, **P < 0·01, **P < 0·01, **P < 0·01, ***P < 0·001, ***P < 0·001, and ***P < 0·001, respectively), whereas alanine did not affect NF-κB activation. It has been reported previously that alanine does not exhibit any anti-inflammatory effects [12]; therefore, we used alanine as an amino acid control in subsequent experiments. The inhibitory effect of cysteine was significant compared to that of alanine, glycine and histidine at all concentrations.
Fig. 1.
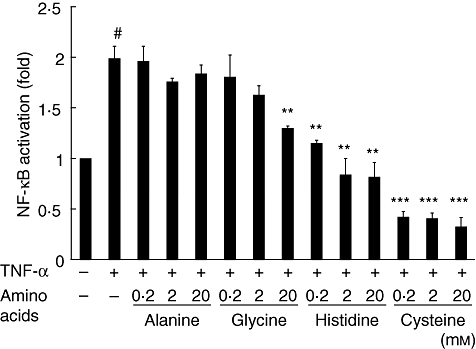
Inhibitory effect of amino acids on nuclear factor-kappa B (NF-κB) activation measured using enzyme-linked immunosorbent assay (ELISA) in human coronary arterial endothelial cells (HCAECs) stimulated with tumour necrosis factor (TNF)-α (2 ng/ml) for 30 min. Data (n = 6) are presented as mean ± standard error of the mean. *P < 0·05; **P < 0·01; ***P < 0·001, compared to cells stimulated with TNF-α. #P < 0·001, compared to non-treated cells.
As shown in Fig. 2, TNF-α stimulation induced degradation of IκBα significantly, with a peak at 10 min after stimulation in HCAECs, compared to β-actin, which was used as a housekeeping protein control. Pretreatment with 20 mm histidine or cysteine inhibited IκBα degradation significantly at 10 min. Pretreatment with glycine (20 mm), histidine (2 mm and 20 mm) or cysteine (0·2 mm, 2 mm and 20 mm) inhibited significantly the TNF-α-induced expression of CD62E (***P < 0·001, **P < 0·01, ***P < 0·001, ***P < 0·001, ***P < 0·001 and ***P < 0·001, respectively), as shown in Fig. 3. Alanine did not suppress TNF-α-induced expression of CD62E. Among these groups, the effects of cysteine and the inhibitory effects of NF-κB activation were the most significant. The addition of amino acids did not affect the expression of CD62E.
Fig. 2.
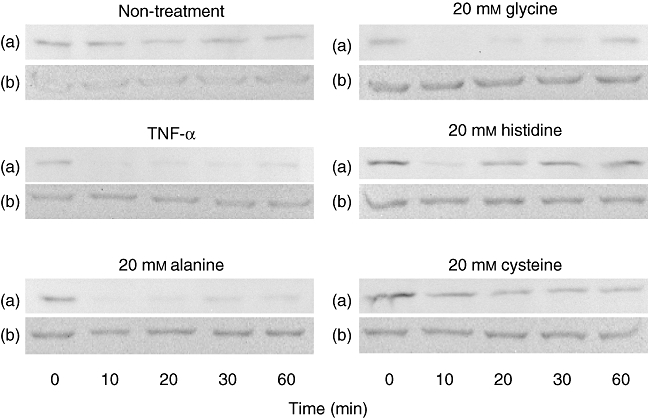
Effects of amino acids on tumour necrosis factor (TNF)-α-induced inhibitor κBα (IκBα) degradation analysed by Western blotting of human coronary arterial endothelial cells (HCAECs) (a). β-Actin was used as an internal control (b). TNF-α stimulation induced degradation of IκBα, and the peak occurred 10 min after stimulation in HCAECs. Pretreatment with 20 mm of histidine or cysteine inhibited IκBα degradation compared to TNF-α-stimulated cells at 10 min compared to non-treated cells. Similar results were obtained in three independent experiments. Representative blots are shown.
Fig. 3.
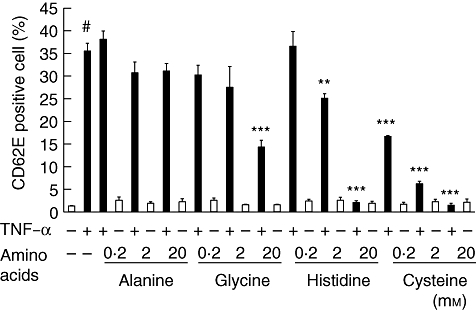
Inhibitory effects of amino acids on CD62E (E-selectin) expression analysed by flow cytometry in human coronary arterial endothelial cells (HCAECs) stimulated with (black column) or without (white column) tumour necrosis factor (TNF)-α (2 ng/ml) for 2 h. Data (n = 6) are presented as the mean ± standard error of the mean. *P < 0·05; **P < 0·001, compared to cells stimulated with TNF-α. #P < 0·001, compared to non-treated cells.
Pretreatment with glycine (20 mm), histidine (2 mm and 20 mm) and cysteine (0·2 mm, 2 mm and 20 mm) reduced IL-6 production significantly from TNF-α-treated HCAECs (*P < 0·05, **P < 0·01, ***P < 0·001, ***P < 0·001, ***P < 0·001 and ***P < 0·001, respectively), while IL-6 production was not affected by alanine (Fig. 4). Histidine (20 mm) and cysteine (2 mm and 20 mm) without TNF-α stimulation reduced IL-6 production.
Fig. 4.
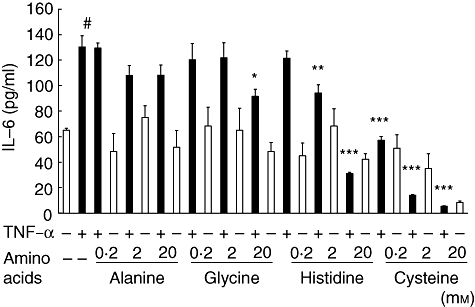
Inhibitory effects of amino acids on interleukin (IL)-6 production analysed by enzyme-linked immunosorbent assay (ELISA) in human coronary arterial endothelial cells (HCAECs) stimulated with (black column) or without (white column) tumour necrosis factor (TNF)-α (2 ng/ml) for 6 h. Data (n = 6) are presented as mean ± standard error of the mean. *P < 0·001, compared to cells stimulated with TNF-α. #P < 0·001, compared to culture fluid from non-treated cells.
Discussion
We have studied NF-κB activation previously in various cell types, including HCAECs in vitro[18–20]. We have also reported that histidine and cysteine exhibit anti-inflammatory effects on THP-1 cells, a human acute monocytic leukaemia cell line [21]. In this study, we showed that glycine, histidine and cysteine, but not alanine, inhibited NF-κB activation and IκBα degradation by stimulation with TNF-α. Further, these amino acids also inhibited CD62E expression, E-selection expression and IL-6 production induced by NF-κB activation in HCAECs stimulated with TNF-α. Glycine (20 mm), histidine (0·2 mm, 2 mm and 20 mm) and cysteine (0·2 mm, 2 mm and 20 mm) inhibited NF-κB activation significantly. IκBα degradation was inhibited by 20 mm histidine and cysteine. In addition, 20 mm glycine, 2 mm and 20 mm histidine and 0·2 mm, 2 mm and 20 mm cysteine TNF-α-induced CD62E expression and IL-6 production significantly in our study. Glycine and histidine exhibit anti-oxidant effects [7,12], and it has been reported that glycine exhibits anti-inflammatory effects by suppressing intracellular Ca++ levels via activation of the glycine-gated chloride channels [9–11]. Further, some reports have shown that histidine exhibits anti-oxidant effects such as scavenging free radicals, binding divalent metal ions and glycating actions [22–24]. Interestingly, our results suggest that cysteine, a sulphur-containing amino acid, also exhibits an inhibitory effect against NF-κB activation, and this inhibition is greater than that exerted by glycine or histidine. N-acetyl-l-cysteine (NAC), a derivative of cysteine and glutathione, which is synthesized by cysteine and glycine, are known to exhibit anti-oxidant effects [25]. Cysteine has a sulphhydryl residue (–SH) that may protect cells against oxidants, as for NAC and glutathione. Regarding the strong anti-inflammatory effects of cysteine, we speculate that the sulphhydryl residue may promote anti-oxidant effects and suppress TNF-α-induced NF-κB activation and IκBα degradation.
We also showed that among all the amino acids used in our study, pretreatment with cysteine exerted the highest inhibitory effects on CD62E expression and IL-6 production induced by NF-κB activation in HCAECs after TNF-α stimulation. CD62E is responsible for the adhesiveness of endothelial cells with respect to leucocytes and platelets [26,27]. IL-6 was identified originally as a factor that induces the synthesis of Igs in activated B cells but has now been found to exhibit a wide range of biological functions [28,29]. Previous reports have shown that IL-6 and CD62E play an important role in the pathogenesis of vascular inflammatory conditions such as atherosclerosis and vasculitis [30,31].
Cysteine at a concentration of 0·2 mm exhibited inhibitory effects on TNF-α-induced NF-κB activation, CD62E expression and IL-6 expression in HCAECs in our study. Regarding the concentration of amino acids in previous reports, the anti-inflammatory effects of glycine were studied at concentrations of less than 10 mm[32,33]. One report showed that 45 mm glycine inhibited NF-κB activation induced by HgCl2[34]. Some studies have investigated histidine at high concentrations (above 20 mm), which show anti-inflammatory effects [12,13]. Because cysteine is reportedly toxic to hepatocytes at a concentration of 4 mm[35], the effects of 20 mm cysteine used in our study might lead to cell toxicity. It has been reported that 5% dietary histidine increased plasma or tissue concentrations to more than 2 mm in a mouse model of colitis [9]. We speculated that oral ingestion of less than 5% dietary amino acid would elevate the amino acid levels to more than 2 mm in the vascular endothelium. Thus, our results suggest that sufficient concentrations of amino acids can be achieved in the plasma or tissues by consuming daily meals, which can induce anti-inflammatory effects in vitro. We believe that appropriate dietary supplements containing these amino acids may exert inhibitory effects against vasculitis and atherosclerosis in vivo.
TNF-α activates not only the NF-κB pathway but also the activator protein-1 (AP-1) pathway. It is well known that AP-1 is also an important molecule in endothelial inflammation [36]. It has been reported that NAC inhibits AP-1 activation induced by TNF-α in gastric epithelial cells [25]. In this study, histidine and cysteine strongly inhibited CD62E expression and IL-6 production. It is possible that, as for NAC, histidine and cysteine also inhibit AP-1 activation because histidine and cysteine showed strong anti-inflammatory effects in our study.
In conclusion, our results show that glycine, histidine and cysteine can each inhibit NF-κB activation, CD62E expression and IL-6 production in HCAECs, suggesting that these amino acids may exhibit anti-inflammatory effects during endothelial inflammation.
Disclosure
None of the authors have any conflicts of interest.
References
- 1.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 2.Read MA, Whitley MZ, Williams AJ, et al. NF-kappa B and I kappa B alpha: an inducible regulatory system in endothelial activation. J Exp Med. 1994;179:503–12. doi: 10.1084/jem.179.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sedgwick JD, Riminton DS, Cyster JG, et al. Tumor necrosis factor: a master-regulator of leukocyte movement. Immunol Today. 2000;21:110–13. doi: 10.1016/s0167-5699(99)01573-x. [DOI] [PubMed] [Google Scholar]
- 4.Pober JS, Cotran RS. Cytokines and endothelial cell biology. Physiol Rev. 1990;70:427–51. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- 5.Gimbrone MA, Jr, Cybulsky MI, Kume N, et al. Vascular endothelium. An integrator of pathophysiological stimuli in atherogenesis. Ann NY Acad Sci. 1995;748:122–31. discussion 131–2. [PubMed] [Google Scholar]
- 6.Tsune I, Ikejima K, Hirose M, et al. Dietary glycine prevents chemical-induced experimental colitis in the rat. Gastroenterology. 2003;125:775–85. doi: 10.1016/s0016-5085(03)01067-9. [DOI] [PubMed] [Google Scholar]
- 7.Wheeler MD, Thurman R. Production of superoxide and TNF-α from alveolar macrophages is blunted by glycine. Am J Physiol. 1999;277:L952–9. doi: 10.1152/ajplung.1999.277.5.L952. [DOI] [PubMed] [Google Scholar]
- 8.Stachlewitz RF, Li X, Smith S, et al. Glycine inhibits growth of T lymphocytes by an IL-2-independent mechanism. J Immunol. 2000;164:176–82. doi: 10.4049/jimmunol.164.1.176. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Bradford BU, Wheeler MD, et al. Dietary glycine prevents peptidoglycan–saccharide-induced reactive arthritis in the rats: role for glycine-gated chloride channel. Infect Immun. 2001;69:5883–91. doi: 10.1128/IAI.69.9.5883-5891.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wheeler MD, Rose ML, Yamashima S, et al. Dietary glycine blunts lung inflammatory cell influx following acute endotoxin. Am J Physiol Lung Cell Mol Physiol. 2000;279:L390–8. doi: 10.1152/ajplung.2000.279.2.L390. [DOI] [PubMed] [Google Scholar]
- 11.Wheeler MD, Ikejema K, Enomoto N, et al. Glycine: a new anti-inflammatory immunonutrient. Cell Mol Life Sci. 1999;56:843–56. doi: 10.1007/s000180050030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andou A, Hisamatsu Okamoto S, et al. Dietary histidine ameliorates murine colitis by inhibition of proinflammatory cytokine production from macrophage. Gastroenterology. 2009;136:564–74. doi: 10.1053/j.gastro.2008.09.062. [DOI] [PubMed] [Google Scholar]
- 13.Son DO, Satsu H, Shimizu M. Histidine inhibits oxidative stress- and TNF-α-induced interleukin-8 secretion in intestinal epithelial cells. FEBS Lett. 2005;579:4671–7. doi: 10.1016/j.febslet.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 14.Kim CJ, Kovacs-Nolan J, Yang C, et al. L-cysteine supplementation attenuates local inflammation and restores gut homeostasis in a porcine model of colitis. Biochem Biophys Acta. 2009;1790:1161–9. doi: 10.1016/j.bbagen.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 15.Liboni KC, Li N, Scumpia PO, et al. Glutamine modulates LPS-induced IL-8 production through IκB/NF-κB in fetal and adult intestinal epithelium. J Nutr. 2005;135:245–51. doi: 10.1093/jn/135.2.245. [DOI] [PubMed] [Google Scholar]
- 16.Kim CJ, Kovacs-Nolan JA, Yang C, et al. l-Tryptophan exhibits therapeutic function in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J Nutr Biochem. 2010;21:468–75. doi: 10.1016/j.jnutbio.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 17.Renard P, Ernest I, Houbion A, et al. Development of a sensitive multi-well colorimetric assay for active NF-κB. Nucleic Acids Res. 2001;29:e21. doi: 10.1093/nar/29.4.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ichiyama T, Hasegawa S, Umeda M, et al. Pranlukast inhibits NF-kappa B activation in human monocytes/macrophages and T cells. Clin Exp Allergy. 2003;33:802–7. doi: 10.1046/j.1365-2222.2003.01673.x. [DOI] [PubMed] [Google Scholar]
- 19.Ichiyama T, Ueno Y, Hasegawa M, et al. Intravenous immunoglobulin inhibits NF-kappaB activation and affects Fcgamma receptor expression in monocytes/macrophages. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:428–33. doi: 10.1007/s00210-004-0877-x. [DOI] [PubMed] [Google Scholar]
- 20.Ichiyama T, Ueno Y, Isumi H, et al. An immunoglobulin agent (IVIG) inhibits NF-kappaB activation in cultured endothelial cells of coronary arteries in vitro. Inflamm Res. 2004;53:253–6. doi: 10.1007/s00011-004-1255-3. [DOI] [PubMed] [Google Scholar]
- 21.Hasegawa S, Ichiyama T, Sonaka I, et al. Amino acids exhibit anti-inflammatory effects in human monocytic leukemia cell line, THP-1 cells. Inflamm Res. 2011;60:1013–19. doi: 10.1007/s00011-011-0362-1. [DOI] [PubMed] [Google Scholar]
- 22.Ukeda H, Hasegawa Y, Harada Y, et al. Effect of carnosine and related compounds on the inactivation of human Cu, Zn-superoxide dismutase by modification of fructose and glycolaldehyde. Biosci Biotechnol Biochem. 2002;66:36–43. doi: 10.1271/bbb.66.36. [DOI] [PubMed] [Google Scholar]
- 23.Guiotto A, Calderan A, Ruzza P, et al. Carnosine and carnosine-related antioxidants: a review. Curr Med Chem. 2005;12:2293–315. doi: 10.2174/0929867054864796. [DOI] [PubMed] [Google Scholar]
- 24.Yan SL, Wu ST, Yin MC, et al. Protective effects from carnosine and histidine on acetaminophen-induced liver injury. J Food Sci. 2009;74:H259–65. doi: 10.1111/j.1750-3841.2009.01330.x. [DOI] [PubMed] [Google Scholar]
- 25.O'Hara AM, Bhattacharyya A, Bai J, et al. Tumor necrosis factor (TNF)-alpha-induced IL-8 expression in gastric epithelial cells: role of reactive oxygen species and AP endonuclease-1/redox factor (Ref)-1. Cytokine. 2009;46:359–69. doi: 10.1016/j.cyto.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boehme MW, Schmitt WH, Youinou P, et al. Clinical relevance of elevated serum thrombomodulin and soluble E-selectin in patients with Wegener's granulomatosis and other systemic vasculitides. Am J Med. 1996;101:387–94. doi: 10.1016/S0002-9343(96)00230-6. [DOI] [PubMed] [Google Scholar]
- 27.Zakeri SM, Meyer H, Meinhardt G, et al. Effects of trovafloxacin on the IL-1-dependent activation of E-selectin in human endothelial cells in vitro. Immunopharmacology. 2000;48:27–34. doi: 10.1016/s0162-3109(99)00191-5. [DOI] [PubMed] [Google Scholar]
- 28.Schuett H, Luchtefeld M, Grothusen C, et al. How much is too much? Interleukin-6 and its signalling in atherosclerosis. Thromb Haemost. 2009;102:215–22. doi: 10.1160/TH09-05-0297. [DOI] [PubMed] [Google Scholar]
- 29.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 30.Cid MC, Cebrián M, Font C, et al. Cell adhesion molecules in the development of inflammatory infiltrates in giant cell arteritis: inflammation-induced angiogenesis as the preferential site of leukocyte–endothelial cell interactions. Arthritis Rheum. 2000;43:184–94. doi: 10.1002/1529-0131(200001)43:1<184::AID-ANR23>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 31.Ohta N, Fukase S, Aoyagi M. Serum levels of soluble adhesion molecules ICAM-1, VCAM-1 and E-selectin in patients with Wegener's granulomatosis. Auris Nasus Larynx. 2001;28:311–14. doi: 10.1016/s0385-8146(01)00097-9. [DOI] [PubMed] [Google Scholar]
- 32.Okoko T, Awhin EP. Glycine reduces cadmium-induced alterations in the viability and activation of macrophage U937 cells. Food Chem Toxicol. 2010;48:536–8. doi: 10.1016/j.fct.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 33.Xu FL, You HB, Li XH, Chen XF, Liu ZJ, Gong JP. Glycine attenuates endotoxin-induced liver injury by downregulating TLR4 signaling in Kupffer cells. Am J Surg. 2008;196:139–48. doi: 10.1016/j.amjsurg.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 34.Pal PB, Pal S, Das J, Sil PC. Modulation of mercury-induced mitochondria-dependent apoptosis by glycine in hepatocytes. Amino Acids. 2011. DOI: 10.1007/s00726-011-0869-3. Online publication. [DOI] [PubMed]
- 35.Viña J, Saez GT, Wiggins D, Roberts AF, Hems R, Krebs HA. The effect of cysteine oxidation on isolated hepatocytes. Biochem J. 1983;212:39–44. doi: 10.1042/bj2120039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–15. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]


