Abstract
The immunomodulatory effects of probiotics were assessed following exposure of normal peripheral blood mononuclear cells (PBMC), cord blood cells and the spleen-derived monocyte/macrophage cell line CRL-9850 to Lactobacillus acidophilus LAVRI-A1, Lb. rhamnosus GG, exopolysaccharides (EPS)-producing Streptococcus thermophilus St1275, Bifidobacteriun longum BL536, B. lactis B94 and Escherichia coli TG1 strains. The production of a panel of pro- and anti-inflammatory cytokines by PBMC following bacterial stimulation was measured, using live, heat-killed or mock gastrointestinal tract (GIT)-exposed bacteria, and results show that (i) all bacterial strains investigated induced significant secretion of pro- and anti-inflammatory cytokines from PBMC-derived monocytes/macrophages; and (ii) cytokine levels increased relative to the expansion of bacterial cell numbers over time for cells exposed to live cultures. Bifidobacteria and S. thermophilus stimulated significant concentrations of transforming growth factor (TGF)-β, an interleukin necessary for the differentiation of regulatory T cells (Treg)/T helper type 17 (Th17) cells and, as such, the study further examined the induction of Th17 and Treg cells after PBMC exposure to selected bacteria for 96 h. Data show a significant increase in the numbers of both cell types in the exposed populations, measured by cell surface marker expression and by cytokine production. Probiotics have been shown to induce cytokines from a range of immune cells following ingestion of these organisms. These studies suggest that probiotics' interaction with immune-competent cells produces a cytokine milieu, exerting immunomodulatory effects on local effector cells, as well as potently inducing differentiation of Th17 and Treg cells.
Keywords: cytokines, T helper type 17, immunomodulation, probiotics, regulatory T cells
Introduction
Commensal bacteria in the intestinal lumen play an important role aiding digestion and synthesis of vitamins and nutrients. The composition of the gut bacterial population is relatively stable over time, but this profile can vary considerably between individuals [1]. This balance can be disturbed by dietary changes, stress and antibiotic treatment. However, a healthy balance can be re-established with probiotic supplementation, consisting mainly of Bifidobacterium species and selected lactic acid bacteria (LAB), which protect the host by excluding pathogenic bacteria and promoting immune modulatory responses from the gut epithelia [2].
T helper cell (Th) subsets are regulators of the adaptive immune response against infection. Th1-type cells produce cytokines which include interleukin (IL)-2, tumour necrosis factor (TNF)-α and interferon (IFN)-γ, activate macrophages and promote cell-mediated immunity, protective against intracellular infections. Th2-type cells produce a variety of anti-inflammatory cytokines including IL-1 receptor antagonist (IL-1ra), IL-4, IL-5, IL-6, IL-10 and IL-13 and promote humoral immune responses against extracellular pathogens [3]. Th17 cells are a subset of CD4+ T cells that produce a proinflammatory cytokine IL-17. Th17 cells have been shown recently to play a critical role in clearing pathogens during host defence reactions and in inducing tissue inflammation in autoimmune disease [4]. Regulatory T cells (Treg) are thought to be the master regulators of the immune response in both humans and rodents. Defects in the transcription factor forkhead box protein 3 (FoxP3), which defines the Treg lineage, results in multiple autoimmune diseases and atopy [5,6], demonstrating the central role of FoxP3+ CD4 cells in immune homeostasis.
The probiotic, Lactobacillus (Lb) rhamnosus GG, has been shown to influence Th2-, Th1- and Th17-mediated disorders [7,8]. In addition, increases in FoxP3 mRNA expression in peri-bronchial lymph nodes have been noted upon administration of Bifidobacterium lactis Bb12 and Lb. rhamnosus GG, suggesting the induction of regulatory cells by these strains [9]. The important discovery that transforming growth factor (TGF)-β and IL-6 could promote Th17 differentiation from naive T cells [10] prompted studies that confirmed that Treg can also be generated in vitro by stimulation with TGF-β in the absence of IL-6 [11,12]. The remarkable balancing act of adaptive immunity to facilitate the targeted destruction of pathogens without excessive collateral damage to self is nowhere better exemplified than in the shared use of TGF-β in controlling the newly described Th17 effector lineage and adaptive Treg development. Probiotic bacteria can be potent inducers of cytokines, for example Gram-positive bacteria, have been found to stimulate IL-12, while Gram-negative bacteria tend to stimulate IL-10 production [13]. Several studies have demonstrated that selected probiotics are able to induce the production of proinflammatory cytokines by macrophages and Th1 cytokines by peripheral blood monocytes [14,15]. However, little is known about the effects of exposure time and bacterial state on the stimulation of cytokine production. As such, the aim of this study was to profile pro- and anti-inflammatory cytokines secretion from human peripheral blood mononuclear cells (PBMCs)and the CRL-9850 cell line and the differentiation of Th17 or induced Treg cells following exposure to various strains of live, heat-killed or gastrointestinal tract (GIT)-simulated bacteria.
Materials and methods
Bacteria and cell lines
Lb. acidophilus LAVRI-A1, Bifidobacterium (B.) lactis B94 and Lb. rhamnosus GG (LGG) were kindly provided by DSM Food Specialties (Moorebank, NSW, Australia), and Vaalia Parmalat Australia Ltd (South Brisbane, Queensland, Australia), respectively. Exopolysaccharides-producing Streptococcus (S.) thermophilus St1275, B. longum BL536 and pathogenic Escherichia (E.) coli TG1 used as a Gram-negative control strain were supplied by the culture collection of Victoria University (Melbourne, Australia). Strains were stored at −80°C in 40% glycerol. Sterile 10 ml aliquots of de Man Rogosa and Sharpe (MRS) broth (Sigma Chemical Co., St Louis, USA) were inoculated with 1% (v/v) LAVRI-A1 and LGG. Additionally, sterile 10 ml aliquots of MRS were supplemented with 0·05% L-cystein.HCl and inoculated with 1% (v/v) B94 and BL536 and incubated at 37°C for 18 h. For the propagation of E. coli and St1275, 1% (v/v) of either strain was used to inoculate 10 ml tryptic soy broth (BHI; Difco Laboratories, Sparks, MD, USA) or M17 broth (Amyl Media, Dandenong, Australia), respectively [16]. Following two successive transfers to fresh 10-ml broth preparations, bacteria were grown for 18 h log phase growth. Cultures were harvested at 1360 g for 30 min at 4°C. To heat kill, samples were incubated at 80°C for 30 min. GIT-simulated samples were treated as described below. Following these manipulations, preparations were centrifuged and the pellet resuspended in phosphate-buffered saline (PBS). Strains were washed three times in PBS and subsequently frozen at −80°C in aliquots of 107 CFU/vial in Iscove's modified Dulbecco's medium (IMDM) supplemented with 1% l-glutamine (Sigma). The spleen-derived human CRL-9850 cell line was purchased from the American Type Culture Collection (ATCC, Manassas VA, USA). Cells were grown in ATCC complete growth medium supplemented with 1% anti-mycotic solution (Sigma).
Simulated gastrointestinal digestion of bacteria
The survival of Gram-positive LAB and Gram-negative bacteria in the gastrointestinal tract was investigated by simulating the physiological secretion of gastric acid and bile, in the stomach and the small intestine, respectively. The method described in previous studies [17,18] was used with some modifications, as described. To simulate bacterial digestion in the stomach, distilled-deionized water (40 ml) was added to 0·3 g of bacterial pellet, and the pH was adjusted to 2·0. Then, 0·25 g of freshly prepared pepsin solution [4% pepsin A (E.C. 3·4.23·1); Sigma, St. Louis, MO, USA] in 0·1 m HCl, pH 2·0 was added and the volume was brought to 100 ml. Following incubation at 37°C for 2 h in a shaking water-bath, the sample was incubated on ice for 10 min to stop pepsin digestion. For the subsequent intestinal digestion the pH of the gastric digests was brought to pH 5·2, then 0·6 g of freshly prepared pancreatin–bile extract mixture (pancreatin 0·04 g, from porcine pancreas, plus bile extract 0·25 g; Sigma) dissolved in 10 ml of 0·1 m NaHCO3, pH 5·2 was added and incubated for an additional 2 h in the 37°C shaking water-bath. After a subsequent 10 min incubation on ice, the pH was adjusted to 7·2 and samples were centrifuged (1360 g for 15 min, 4°C) and the pellets washed in PBS, before resuspending in 30 ml PBS.
Enumeration of bacterial cells
For enumeration of bacterial cell numbers, 1 ml of each freshly prepared culture, live (untreated) GIT and killed, was 10-fold serially diluted and plated onto tryptic soy agar (E. coli), M17 agar (St1275), de Man, Rogosa and Sharpe (MRS) agar (LAVRI-A1 and LGG) and MRS agar supplemented with 0·05% l-cysteine.HCl (bifidobacteria), and incubated anaerobically for 72 h at 37°C [19]. For all bacterial strains, standard growth curves were produced by plotting optical density at 610 nm in MRS broth versus agar plate counts of freshly prepared, serially diluted cultures. These curves were fitted with logarithmic expressions (in order to calculate viable bacterial counts in freshly prepared cultures) of which each yielded r2 values of >0·985 (data not shown).
Isolation of human peripheral mononuclear cells from buffy coat and cord blood using Ficoll gradient
Human peripheral mononuclear cells were isolated from buffy coats [Australian Red Cross Blood Services (ARCBS), Melbourne, Australia] and cord blood (CB; Cord Blood Bank, Royal Children's Hospital, Melbourne, Australia) by Ficoll-Paque gradient. PBMCs were isolated according to the methods described by Hessle et al. [13] and de Roock et al. [20], with minor modifications. Briefly, buffy coats were diluted with an equal volume of PBS and layered on Ficoll-Paque Plus (GE Healthcare, Bio-Sciences, Uppsala, Sweden). Cells at the interphase were collected following centrifugation (680 g, 25 min, 18°C) (Sorvall® RT7 centrifuge; DuPont, Newtown, CT, USA). Blood lymphocytes were washed once in cold PBS, and following centrifugation (680 g, 18°C, 10 min) the pellet was resuspended in 2 ml red blood cell lysis buffer [0·15 m NH4Cl, 0·01 m KHCO3 and 10 µm ethylenediamine tetraacetic acid (EDTA) Na2·2H2O] and incubated for 2 min at room temperature. The volume was then adjusted to 30 ml using sterile PBS and centrifuged. Following two subsequent washes, the cell pellet was resuspended in IMDM (Sigma) supplemented with 10% fetal bovine serum (FBS; Gibco, Mulgrave, Australia) and anti-mycotic solution (10 mg/l; Sigma).
Stimulation of human PBMCs and CRL-9850 cells with bacteria and cytokine quantification
PBMCs/CRL-9850 cells were plated in six-well tissue culture plates (Corning, Sigma) at 5 × 106 cells/well and incubated at 5% CO2, 37°C for 24 h prior to stimulation with bacteria, as described by Amrouche et al. [21]. Briefly, 106 freshly prepared viable (live or GIT) or equivalent (∼106 CFU/ml) heat-killed bacteria were added per 106 cells and co-cultured for 72 h at 5% CO2, 37°C. At 6, 12, 24, 48 and 72 h, 500 µl samples of the culture medium were collected and analysed for cytokine secretion by ELISA (Becton Dickinson, San Jose, CA, USA), in accordance with the manufacturer's instructions. Data are expressed as the mean cytokine response minus background (pg/ml) of each treatment from triplicate wells, plus or minus the standard error of the mean.
Cell staining and flow cytometry analysis
Treg/Th17 populations were characterized following PBMC/bacteria co-culture. Briefly, 106 PBMC were co-cultured with either live or killed bacteria, lipopolysaccharides (LPS; Sigma) or media alone, in a 24-well plate at 37°C in 5% CO2 for 96 h, then cells were washed twice using FACS buffer (PBS + 2% FCS) and centrifuged at 500 g for 10 min. PBMC were resuspended at 106 cells/ml, and surface marker staining was performed using fluorescein isothiocynate (FITC)-labelled anti-human CD4, allophycocyanin-labelled anti-human CD25/CD3 (Becton-Dickinson), peridinin chlorophyll protein (PerCP)-labelled anti-human CD3 (Biolegend, San Diego, CA, USA) and PerCP cyanine (Cy)5·5-labelled anti-human CCR6 (CD196). Intracellular staining was performed using phycoerythrin (PE)-labelled anti-human FoxP3/RORγt (BD Pharmingen and R&D Systems, Minneapolis, MN, USA, respectively), according to the manufacturer's instructions. Samples were read using a BD FACSCalibur, data acquired using CellQuest program (Becton Dickinson Biosciences), and analysis performed using Gatelogic version 3·07 software (Inivai, Victoria, Australia). Absolute numbers of Treg cells and Th17 cells were calculated as a percentage of the total lymphocyte number.
Statistical analysis
All co-cultures were carried out in triplicate. Results obtained were analysed as a split plot in time design with three main factors: strains (six levels) and treatments (three levels) as the main plot and time (five levels) as a subplot. The statistical evaluations of the data were performed using the general linear model (GLM) [22]. Significant differences between treatments were tested by analysis of variance (anova) followed by a comparison between treatments performed by Fisher's least significant difference (LSD) method, with a level of significance of P < 0·05.
Results
Cytokine secretion by PBMCs, cord blood and spleen-derived macrophage cell line following co-culturing with live bacteria
Pooled PBMCs or CRL-9850 cells incubated with selected live bacteria for 48 and 72 h yielded cytokine levels as shown in Figs 1a–c and 2a,b. Also shown are three individual donor cytokine profiles (48 or 72 h) as a representative of the 30 donor PBMCs investigated depicting varying cytokine levels detected between donors (Table 1a–c). A comparison of the 30 individual donor PBMCs with the pooled donor PBMCs, shows significant differences of cytokine levels in line with previous results [23]. Even though some cytokines were not detectable from individual donors, substantial and significant production of all investigated cytokines were recorded from pooled PBMC in response to LAB. All strains of bacteria had the capacity to induce pro- and anti-inflammatory cytokine production from the cell line and PBMCs; however, the magnitude of production of each cytokine varied depending on the strain, as reported similarly by Wu et al. [24]. Generally, buffy coat-sourced PBMC produced significantly higher (P < 0·05) concentrations (100–8800 pg/ml) of cytokines compared to cord blood-derived PBMCs or CRL-9850 cells. In addition, cytokine production in the buffy coat PBMC was detectable from early culture (6 h, data not shown) and maintained up to 72 h, while cord blood PBMC and CRL 9580 cells showed a later appearance of cytokines in culture (48–72 h, Fig. 2a,b), the delayed response due probably to a lack of established adaptive immune responses in cord blood [25]. While proinflammatory cytokines were produced significantly in the supernatants for all treatments, anti-inflammatory cytokines such as TGF-β, IL-6 and IL-10 were also detected. In the majority of cord blood samples, T cell responses show an IL-10 or Th2-like pattern of cytokine production (Fig. 2a) [25,26]. Previous studies have suggested that IL-10 may play a major role in influencing the activity of the placental trophoblast, which has been proposed as a key cell type in regulating fetal immunoprotection [27,28].
Fig. 1.
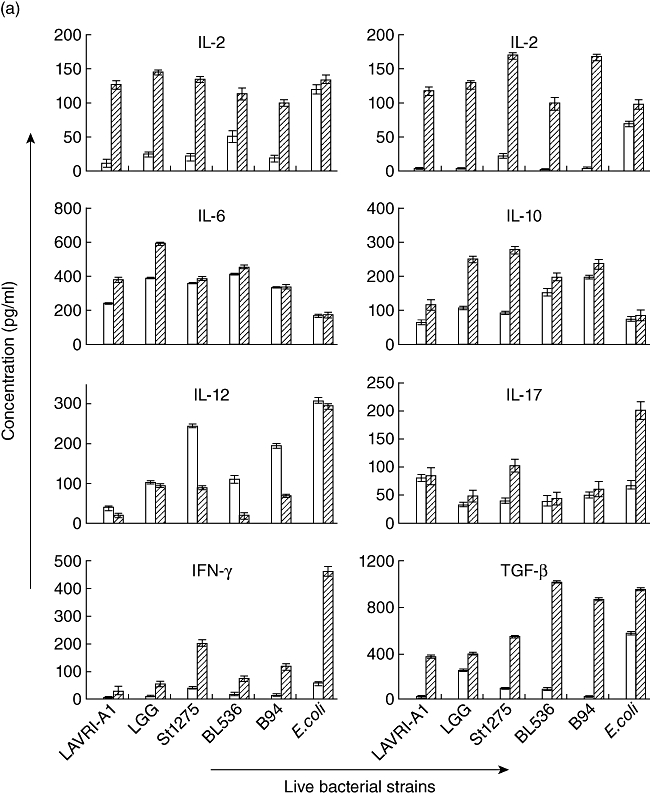
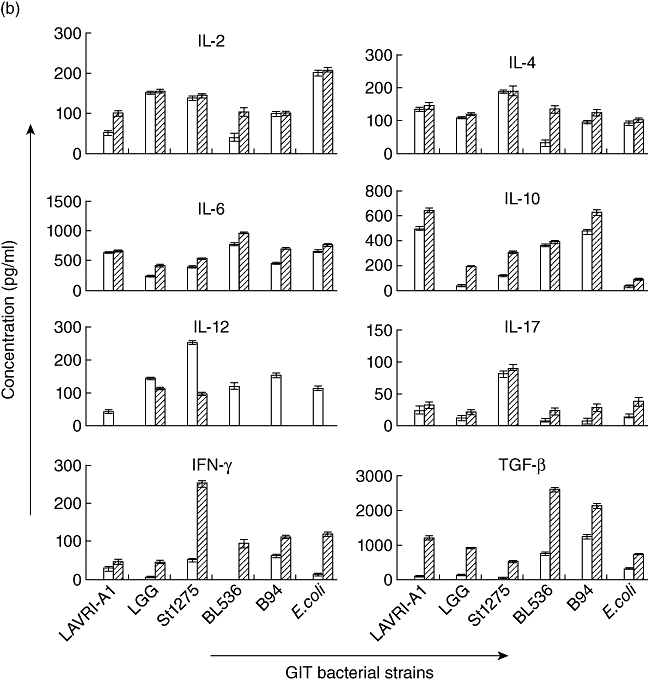
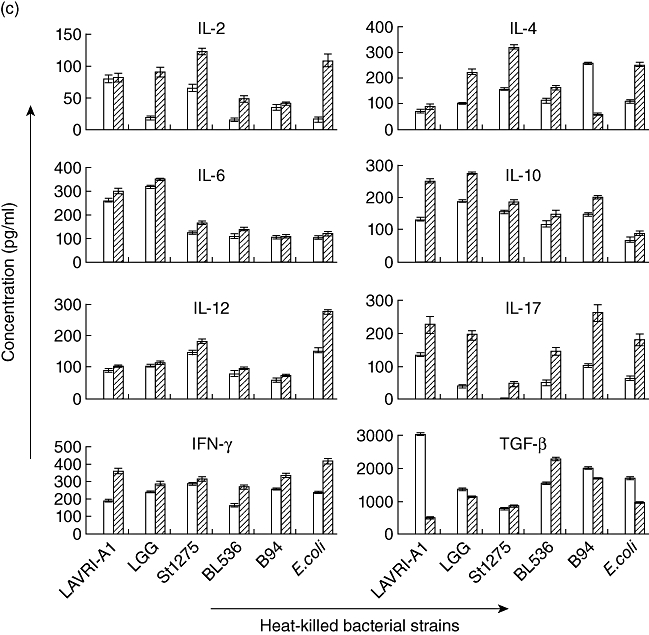
(a–c) In vitro production of interleukin (IL)-2, IL-4, IL-6, IL-10, IL-12, IL-17, interferon (IFN)-γ and transforming growth factor (TGF)-β. Supernatants of □ 48 and  72 co-cultures of pooled buffy coat-derived peripheral blood mononuclear cells (PBMC) with live, gastrointestinal tract (GIT)-simulated and heat-killed Lactobacillus acidophilus LAVRI-A1, Lb. rhamnosus GG, exopolysaccharides (EPS)-producing Streptococcus thermophilus St1275, Bifidobacterium longum BL536, B. lactis B94 or Escherichia coli TG1 or PBMC in medium alone were collected. The concentration of cytokines was subsequently determined using enzyme-linked immunosorbent assay (ELISA) kits. Data are expressed as the mean cytokine response minus controls (pg/ml) of each treatment from triplicate wells, plus or minus the standard error of the mean (s.e.m.).
72 co-cultures of pooled buffy coat-derived peripheral blood mononuclear cells (PBMC) with live, gastrointestinal tract (GIT)-simulated and heat-killed Lactobacillus acidophilus LAVRI-A1, Lb. rhamnosus GG, exopolysaccharides (EPS)-producing Streptococcus thermophilus St1275, Bifidobacterium longum BL536, B. lactis B94 or Escherichia coli TG1 or PBMC in medium alone were collected. The concentration of cytokines was subsequently determined using enzyme-linked immunosorbent assay (ELISA) kits. Data are expressed as the mean cytokine response minus controls (pg/ml) of each treatment from triplicate wells, plus or minus the standard error of the mean (s.e.m.).
Fig. 2.
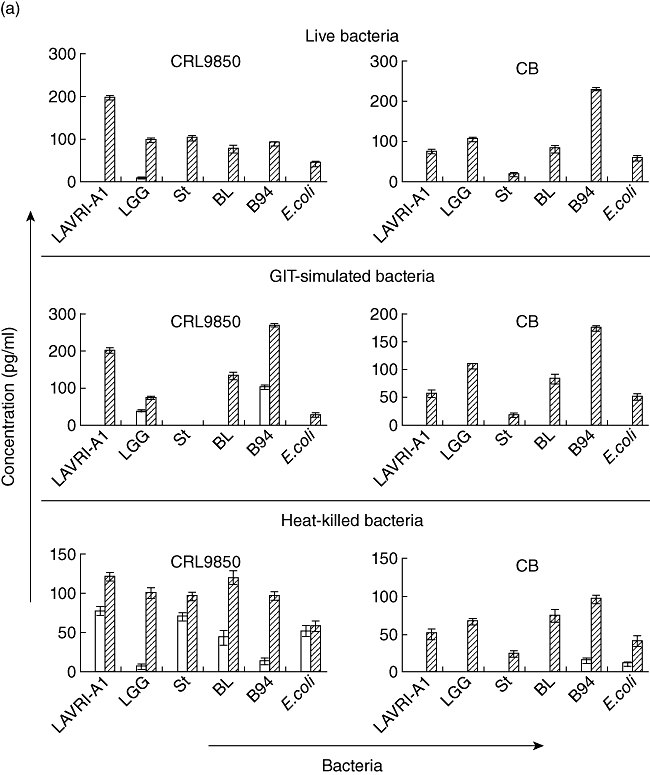
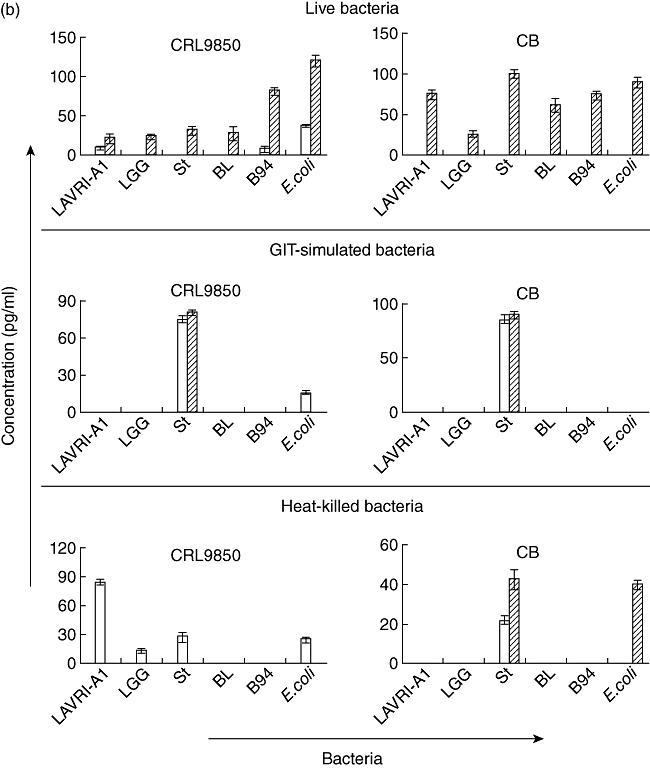
(a,b) In vitro production of interleukin (IL)-10 and IL-17. Supernatants of □ 48 and  72 h co-cultures of CRL9850 or cord blood-derived peripheral blood mononuclear cells (PBMCs) with live, gastrointestinal tract (GIT)-simulated or killed Lactobacillus acidophilus LAVRI-A1, Lb. rhamnosus GG, exopolysaccharides (EPS)-producing Streptococcus thermophilus St1275, Bifidobacterium longum BL536, B. lactis B94 or Escherichia coli TG1 or PBMC in medium alone were collected. The concentration of cytokines was subsequently determined using enzyme-linked immunosorbent assay (ELISA) kits. Data are expressed as the mean cytokine response minus controls (pg/ml) of each treatment from triplicate wells, plus or minus standard error of the mean (s.e.m.).
72 h co-cultures of CRL9850 or cord blood-derived peripheral blood mononuclear cells (PBMCs) with live, gastrointestinal tract (GIT)-simulated or killed Lactobacillus acidophilus LAVRI-A1, Lb. rhamnosus GG, exopolysaccharides (EPS)-producing Streptococcus thermophilus St1275, Bifidobacterium longum BL536, B. lactis B94 or Escherichia coli TG1 or PBMC in medium alone were collected. The concentration of cytokines was subsequently determined using enzyme-linked immunosorbent assay (ELISA) kits. Data are expressed as the mean cytokine response minus controls (pg/ml) of each treatment from triplicate wells, plus or minus standard error of the mean (s.e.m.).
Table 1a.
Cytokine levels of cultured peripheral blood mononuclear cells (PBMCs) in response to live lactic acid bacteria (LAB)
| Cytokine production upon bacterial treatment (pg/ml) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-2 | IL-4 | IL-6 | IL-10 | IL-12 | IL-17 | IFN-γ | TGF-β | ||||||||||
| Donor | Strains | 48 h | 72 h | 48 h | 72 h | 48 h | 72 h | 48 h | 72 h | 48 h | 72 h | 48 h | 72 h | 48 h | 72 h | 48 h | 72 h |
| 1 | La | 4·1 ± 1·7 | n.d. | 5·4 ± 1·5 | 10·7 ± 2·4 | 41 ± 13·6 | 20·6 ± 5·2 | 14·4 ± 3·1 | 15·6 ± 4·4 | n.d. | 56·8 ± 15·7 | 32 ± 5·1 | 325 ± 41·3 | 41·8 ± 10·6 | n.d. | 2264 ± 564 | 7 400 ± 1 264 |
| LGG | 22·4 ± 4·8 | 67·1 ± 20·2 | 1·4 ± 0·4 | n.d. | 59 ± 18·8 | 182 ± 41 | 23·2 ± 2·9 | 34·4 ± 8·5 | n.d. | n.d. | 68 ± 12·3 | 116 ± 20 | 18·4 ± 1·4 | 34·5 ± 5·3 | 1246 ± 287 | 2 350 ± 525 | |
| Bl | 13·5 ± 3·1 | 374 ± 92 | 47·1 ± 12·1 | 5·4 ± 1·7 | 19 ± 8·3 | 213 ± 33 | 6·4 ± 1·3 | 24 ± 8·3 | 135 ± 32 | 29·3 ± 3·5 | n.d. | 18 ± 1·5 | 20·9 ± 5·2 | 60·4 ± 5·8 | 1478 ± 324 | 4 300 ± 423 | |
| B94 | 18·2 ± 2·6 | 32·9 ± 8·1 | 25·7 ± 8·2 | 23 ± 6·1 | 218 ± 45·3 | 503 ± 128 | 22·8 ± 3·4 | 68·4 ± 12·5 | 6·25 ± 1·2 | 32·5 ± 4·9 | 34 ± 6·1 | 26 ± 3·6 | 29·7 ± 4·8 | 35·6 ± 2·6 | 75 ± 4·8 | 250 ± 57 | |
| St | 48·2 ± 12·6 | 37·6 ± 7·5 | 63·6 ± 21·2 | 60·7 ± 12·5 | 332 ± 63·8 | 2314 ± 225 | 46·8 ± 14·3 | 87·2 ± 20·6 | n.d. | n.d. | 1497 ± 312 | 2200 ± 234 | 7·6 ± 1·6 | 86·9 ± 9·8 | 910 ± 154 | 9 750 ± 2 460 | |
| Ec | 420 ± 125 | 338 ± 78 | n.d. | n.d. | 220 ± 52 | 44·3 ± 13·8 | n.d. | n.d. | n.d. | n.d. | 12 ± 0·8 | 18 ± 1·4 | n.d. | n.d. | 850 ± 206 | 550 ± 57 | |
| 2 | La | n.d. | n.d. | n.d. | 10·4 ± 2·9 | 122 ± 33 | 131 ± 26 | n.d. | n.d. | 613 ± 112 | 1170 ± 286 | 8 ± 1·5 | n.d. | 15·1 ± 3·3 | 58·5 ± 6·1 | 205 ± 41 | 5 050 ± 1 263 |
| LGG | 123 ± 29 | 422 ± 102 | 1·4 ± 0·6 | 7·9 ± 2·3 | 147 ± 28 | 972 ± 204 | n.d. | n.d. | 158 ± 32 | 1152 ± 254 | n.d. | n.d. | 24·7 ± 8·1 | 37·6 ± 4·7 | 185 ± 14 | 215 ± 42 | |
| Bl | 25·9 ± 5·6 | n.d. | 39 ± 11 | n.d. | 129 ± 24 | 769 ± 198 | n.d. | 32·4 ± 6·9 | 584 ± 82 | 1267 ± 376 | 25 ± 4·3 | 36 ± 2·8 | 24·6 ± 3·8 | 66·6 ± 5·2 | 4865 ± 1204 | 500 ± 62 | |
| B94 | 8·8 ± 2·3 | n.d. | 11·4 ± 2·9 | 7·1 ± 1·9 | 145 ± 31 | 903 ± 226 | 23·8 ± 6·9 | 187 ± 51 | 384 ± 54 | 1000 ± 154 | 8 ± 2·6 | 42 ± 12·5 | 36·2 ± 6·2 | 49·3 ± 6·7 | 4555 ± 671 | 1 250 ± 429 | |
| St | 9·4 ± 1·8 | 20·6 ± 6·3 | 15·4 ± 3·8 | n.d. | 128 ± 18 | 1682 ± 371 | 73·2 ± 21·3 | 18·4 ± 4·8 | n.d. | n.d. | 80 ± 11·4 | 74 ± 6·8 | 19·2 ± 1·7 | 50 ± 11·2 | 415 ± 41·3 | 18 350 ± 5 410 | |
| Ec | 1128 ± 35 | 338 ± 102 | 26 ± 4 | n.d. | 520 ± 108 | 676 ± 91 | n.d. | n.d. | 925 ± 175 | 415 ± 57 | n.d. | n.d. | 131 ± 22 | n.d. | 300 ± 44 | 24 300 ± 4 630 | |
| 3 | La | n.d. | 22·2 ± 4·7 | 0·7 ± 0·3 | 1·8 ± 0·5 | 21·7 ± 4·9 | 12·7 ± 2·9 | 6·8 ± 1·7 | 23·6 ± 6·6 | 4 ± .0·4 | 50 ± 10·5 | 108 ± 14 | 76 ± 12·1 | 11 ± 2·1 | 12 ± 3·6 | n.d. | 60 ± 5·6 |
| LGG | 1·8 ± 0·4 | n.d. | 5·3 ± 1·8 | 10 ± 2·5 | 23·3 ± 5·7 | 8 ± 3·1 | 13·6 ± 2·8 | 735 ± 183 | 21·8 ± 3·7 | 16·2 ± 2·4 | 20 ± 2·6 | 40 ± 5·3 | 8 ± 1·1 | 31·2 ± 10·8 | 40·5 ± 13·5 | 425 ± 65 | |
| Bl | 5·9 ± 1·2 | 2·9 ± 1·1 | 1 ± 0·3 | 2 ± 0·4 | 2·7 ± 0·5 | 2·3 ± 0·4 | 12·4 ± 2·9 | 541 ± 144 | 8·1 ± 1·7 | 40·6 ± 3·2 | n.d. | n.d. | 25 ± 3·8 | n.d. | 160 ± 28 | 165 ± 26 | |
| B94 | 25·9 ± 12·5 | n.d. | 1·8 ± 0·6 | 2 ± 0·6 | 10 ± 1·4 | 46·7 ± 11·5 | 7·6 ± 1·1 | 22·4 ± 9·8 | 28·7 ± 4·1 | 5·6 ± 1·4 | 410 ± 52 | 86 ± 12 | 20·5 ± 5·6 | 28·4 ± 9·2 | 70 ± 22 | 125 ± 33 | |
| St | 2·4 ± 1·1 | n.d. | 1·4 ± 0·5 | 5 ± 1·1 | 38 ± 11·6 | 13·7 ± 2·9 | 4·4 ± 0·8 | 13·2 ± 2·4 | 10 ± 1·6 | 6·8 ± 1·1 | 28 ± 4·8 | 60 ± 3·8 | 24·6 ± 2·6 | 29·8 ± 7·6 | 80 ± 19·5 | 125 ± 25 | |
| Ec | 34·4 ± 11·4 | 42·2 ± 13·3 | n.d. | n.d. | 12·7 ± 1·4 | 28·5 ± 6·8 | 8·3 ± 1·5 | 4·1 ± 1·1 | 37·5 ± 12·5 | 22·5 ± 7·3 | 38 ± 2·6 | 318 ± 44 | 40 ± 2·9 | 4 ± 1·3 | n.d. | n.d. | |
IL: interleukin; n.d.: not done; IFN: interferon; TGB: transforming growth factor.
Table 1c.
Cytokine levels of cultured peripheral blood mononuclear cells (PBMCs) in response to heat-killed lactic acid bacteria (LAB)
| Cytokine production upon bacterial treatment (pg/ml) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-2 | IL-4 | IL-6 | IL-10 | IL-12 | IL-17 | IFN-γ | TGF-β | ||||||||||
| Donor | Strains | 48 h | 72 h | 48 h | 72 h | 48 h | 72 h | 48 h | 72 h | 48 h | 72 h | 48 h | 72 h | 48 h | 72 h | 48 h | 72 h |
| 1 | La | 178 ± 32 | 84·4 ± 21·4 | n.d. | n.d. | 20 ± 5·6 | 132 ± 24 | 7·1 ± 1·6 | 84·2 ± 21·3 | 12·1 ± 2·6 | 13·4 ± 2·3 | 256 ± 54 | 142 ± 32 | 4·1 ± 1·5 | 10 ± 4·2 | 1200 ± 300 | 1600 ± 288 |
| LGG | 3·8 ± 0·5 | 7·1 ± 1·6 | 51·3 ± 8·5 | 102 ± 16 | 37·1 ± 8·6 | 412 ± 114 | 2·8 ± 0·8 | 9·6 ± 2·4 | 255 ± 33 | 121 ± 23 | 128 ± 32·5 | 74 ± 14 | 40 ± 11·8 | 12·8 ± 3·6 | 3100 ± 1510 | 4100 ± 1885 | |
| Bl | n.d. | n.d. | 351 ± 52 | 182 ± 33 | 135 ± 32·5 | 197 ± 35·6 | 37·1 ± 12·4 | 22·8 ± 4·4 | 12·5 ± 3·5 | 30 ± 4·8 | 12·9 ± 2·6 | 52 ± 12 | 110 ± 24·7 | 63·2 ± 16·4 | 4600 ± 1652 | 1300 ± 365 | |
| B94 | n.d. | n.d. | 13·2 ± 3·4 | 4·8 ± 1·2 | 95·7 ± 24·6 | 127 ± 22·5 | 70 ± 10·5 | 101 ± 15·6 | 15 ± 2·8 | 26·5 ± 6·7 | 112 ± 24 | 42 ± 8·5 | 60 ± 8·9 | 41·8 ± 9·8 | 4150 ± 2150 | 800 ± 210 | |
| St | 6·6 ± 1·5 | 38·8 ± 6·8 | 54·2 ± 11·4 | 5·7 ± 1·6 | 14·2 ± 3·6 | 32·8 ± 6·8 | 31·4 ± 9·5 | 14·7 ± 3·8 | 27·5 ± 4·5 | 11·4 ± 4·2 | 2·8 ± 0·6 | 1·3 ± 0·4 | 130 ± 24 | 12·4 ± 3·7 | 1250 ± 315 | 2450 ± 158 | |
| Ec | 17·7 ± 3·6 | 4·2 ± 0·8 | 27·1 ± 4·6 | 52·8 ± 11·4 | 28·5 ± 4·5 | 14·7 ± 3·4 | 94·2 ± 13·4 | 308 ± 42 | 33·7 ± 8·4 | 51·9 ± 6·3 | n.d. | n.d. | 55·7 ± 12·2 | 14·2 ± 3·9 | 1600 ± 285 | 5400 ± 1425 | |
| 2 | La | 210 ± 35 | 82·3 ± 21·2 | 3·4 ± 0·8 | 1·9 ± 0·6 | 204 ± 36·8 | 192 ± 32·2 | 11·4 ± 4·5 | 40 ± 8 | 4·3 ± 1·2 | 9·6 ± 1·3 | 182 ± 28 | 66 ± 6·6 | 118 ± 28·8 | 188 ± 36 | 650 ± 214 | 2050 ± 380 |
| LGG | 171 ± 25 | 114 ± 22 | 24·2 ± 3·4 | 11·5 ± 2·6 | 55·7 ± 12·5 | 37·1 ± 6·6 | n.d. | n.d. | 95 ± 22·1 | 47·3 ± 6·4 | 10 ± 4 | 106 ± 22 | 14·3 ± 2·9 | 97·1 ± 24·6 | 450 ± 113 | 245 ± 82 | |
| Bl | n.d. | n.d. | 12·8 ± 4·4 | 208 ± 35 | n.d. | n.d. | 431 ± 108 | 554 ± 107 | 35 ± 5·6 | 41·8 ± 10·4 | 84 ± 23·6 | 24 ± 4·6 | 118 ± 24 | 38·5 ± 11·3 | 750 ± 274 | 6050 ± 1450 | |
| B94 | n.d. | 3·7 ± 0·5 | n.d. | n.d. | 127 ± 18·6 | 235 ± 35 | 35·7 ± 6·8 | 23·4 ± 6·3 | 4·3 ± 0·8 | n.d. | 30 ± 6·5 | 14·6 ± 3·8 | 8·5 ± 1·7 | 124 ± 24 | 2700 ± 875 | 1300 ± 278 | |
| St | 5·8 ± 1·7 | 14·1 ± 2·8 | n.d. | n.d. | 54·2 ± 13·6 | 118 ± 12 | 734 ± 124 | 1214 ± 312 | 5 ± 1·2 | 3·8 ± 0·8 | 74 ± 12·5 | 20 ± 3·3 | 165 ± 42 | 32·3 ± 7·8 | 1400 ± 215 | 625 ± 85 | |
| Ec | 4·8 ± 0·6 | n.d. | 27·1 ± 5·2 | 52·8 ± 8·5 | 28·5 ± 8·5 | 42·8 ± 8·4 | 258 ± 52 | 688 ± 198 | n.d. | n.d. | 216 ± 46 | 289 ± 42 | 48·5 ± 12·3 | 192 ± 46 | 8500 ± 2250 | 4300 ± 1250 | |
| 3 | La | 27·7 ± 6·4 | 15·5 ± 4·5 | 27·1 ± 5·5 | 37·4 ± 6·4 | 42·8 ± 11·5 | 38·5 ± 4·8 | 13·3 ± 4·3 | 75 ± 24·7 | 60 ± 7·5 | 115 ± 21·5 | 40 ± 11·2 | 518 ± 78 | 121 ± 18 | 56·7 ± 13·3 | 5850 ± 1815 | 3050 ± 815 |
| LGG | 18·8 ± 3·5 | 8·3 ± 3·4 | n.d. | n.d. | 164 ± 32 | 7·4 ± 2·6 | 96·6 ± 14·6 | 171 ± 52 | 67·5 ± 15·5 | 52·8 ± 8·8 | 60 ± 15·4 | 1140 ± 254 | 287 ± 52 | 132 ± 28 | 1250 ± 235 | 2550 ± 520 | |
| Bl | 45·5 ± 5·9 | 77·7 ± 15·4 | 55·7 ± 16·3 | 231 ± 46 | 52·8 ± 12·6 | 138 ± 25 | 176 ± 28 | 203 ± 45 | 670 ± 125 | 710 ± 124 | 78 ± 13 | 236 ± 44 | 252 ± 46 | 101 ± 21 | 1200 ± 157 | 5250 ± 1728 | |
| B94 | 67·7 ± 9·5 | 121 ± 32 | 25·7 ± 7·5 | 43·2 ± 9·6 | 12·4 ± 2·6 | 35·7 ± 5·6 | 25 ± 6·5 | 32 ± 6 | 35 ± 4·6 | 327 ± 52 | 548 ± 42 | 274 ± 38 | 83·4 ± 17·4 | 177 ± 35 | 2250 ± 385 | 8450 ± 2415 | |
| St | 34·2 ± 4·3 | 53·3 ± 11·5 | 51·4 ± 11·3 | 8·6 ± 2·4 | 170 ± 25 | 112 ± 22 | 343 ± 45 | 391 ± 45 | n.d. | n.d. | 12 ± 4 | 20 ± 4·2 | 288 ± 62 | 34·2 ± 6·8 | 1300 ± 220 | 2450 ± 425 | |
| Ec | 15·5 ± 2·9 | 23·3 ± 6·5 | n.d. | n.d. | 55·7 ± 12·5 | 84·2 ± 21·2 | 23·3 ± 6·5 | 58·8 ± 18·2 | 305 ± 42 | 128 ± 29 | 70 ± 11·6 | 98 ± 21·6 | 14·1 ± 3·7 | 32·8 ± 11·2 | 4100 ± 1100 | 1600 ± 265 | |
Values are means ± standard error of the mean (three healthy donors). Data differ significantly (P < 0·05). Lactobacillus (Lb.) acidophilus LAVRI-A1; Lb. rhamnosus GG (LGG); Bifidobacterium (B.) lactis B94; B. longum BL536; exopolysaccharides (EPS)-producing Streptococcus (S.) thermophilus St1275; Escherichia (E.) coli; TG1; IL: interleukin; IFN: interferon; TGF: transforming growth factor.
Table 1b.
Cytokine levels of cultured peripheral blood mononuclear cells (PBMCs) in response to gastrointestinal tract (GIT) lactic acid bacteria (LAB)
| Cytokine production upon bacterial treatment (pg/ml) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-2 | IL-4 | IL-6 | IL-10 | IL-12 | IL-17 | IFN-γ | TGF-β | ||||||||||
| Donor | Strains | 48 h | 72 h | 48 h | 72 h | 48 h | 72 h | 48 h | 72 h | 48 h | 72 h | 48h | 72 h | 48 h | 72 h | 48 h | 72 h |
| 1 | La | 6·6 ± 1·2 | 30 ± 5·3 | n.d. | n.d. | 52·8 ± 13·2 | 124 ± 22 | 90 ± 12·6 | 78 ± 21·4 | 57·5 ± 13·6 | 525 ± 105 | 8 ± 2·4 | 152 ± 24 | 2·1 ± 0·5 | 12·8 ± 2·6 | 2180 ± 342 | 9400 ± 2546 |
| LGG | 7·5 ± 2·8 | 16·6 ± 4·2 | n.d. | n.d. | 57·1 ± 14·5 | 104 ± 15 | 52·5 ± 8·8 | 32 ± 4·5 | 305 ± 102 | 227 ± 62 | 440 ± 128 | 218 ± 20 | 3·4 ± 0·9 | 9·6 ± 3·2 | 3850 ± 452 | 2400 ± 638 | |
| Bl | 8·8 ± 1·2 | 6·4 ± 1·8 | 10 ± 2 | n.d. | 28·5 ± 5·9 | n.d. | 62·5 ± 9·7 | 41 ± 6·8 | 242 ± 44 | 570 ± 83 | 36 ± 2·9 | n.d. | n.d. | n.d. | 350 ± 41 | 3800 ± 465 | |
| B94 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 87·5 ± 12·6 | 43·2 ± 10·2 | n.d. | n.d. | 2·4 ± 1·1 | 4·7 ± 0·6 | 348 ± 33 | 410 ± 75 | |
| St | 18·8 ± 5·2 | 197 ± 33 | n.d. | n.d. | 1045 ± 225 | 520 ± 132 | 222 ± 45 | 800 ± 156 | n.d. | n.d. | 504 ± 46 | 90 ± 15·6 | 14·2 ± 3·5 | 41·8 ± 12·2 | 1100 ± 204 | 6550 ± 524 | |
| Ec | n.d. | n.d. | 91·4 ± 21·5 | n.d. | 17·1 ± 3·4 | 288 ± 54 | 12 ± 2·2 | 18 ± 4·8 | 42·5 ± 6·4 | 30 ± 5·4 | 24 ± 4·5 | 48 ± 12·2 | 18·7 ± 4·8 | 87·2 ± 14·6 | 5450 ± 1425 | 1300 ± 156 | |
| 2 | La | 28·8 ± 12·3 | 38·8 ± 6·3 | n.d. | n.d. | 2542 ± 643 | 2118 ± 345 | 218 ± 56 | 670 ± 136 | 22·5 ± 6·5 | 8·4 ± 2·3 | n.d. | n.d. | 5·7 ± 2·6 | 15·4 ± 3·4 | 700 ± 133 | 1050 ± 245 |
| LGG | 12·2 ± 2·6 | 24·1 ± 12·5 | n.d. | n.d. | 901 ± 124 | 843 ± 106 | 27·5 ± 6·4 | 100 ± 22 | 132 ± 25 | 53·2 ± 15·4 | n.d. | n.d. | 14·2 ± 3·8 | 27·1 ± 6·2 | 350 ± 62 | 500 ± 85 | |
| Bl | 28·8 ± 11·2 | 26·4 ± 5·8 | n.d. | 62·8 ± 21·1 | 768 ± 129 | 478 ± 56 | 8·5 ± 1·4 | 87·5 ± 32·5 | 225 ± 35 | 30 ± 5·9 | n.d. | n.d. | 7·8 ± 1·9 | 11·4 ± 2·4 | 128 ± 24 | 250 ± 36 | |
| B94 | 508 ± 136 | 412 ± 69 | n.d. | 611 ± 124 | 2504 ± 356 | 1582 ± 314 | 472 ± 78 | 727 ± 212 | n.d. | n.d. | 52 ± 11·6 | 42 ± 5·6 | 20 ± 4·6 | 14·2 ± 3·1 | 650 ± 34 | 700 ± 152 | |
| St | 11·1 ± 1·6 | 8·6 ± 2·6 | 15·7 ± 2·6 | 45·7 ± 12·6 | 905 ± 145 | 818 ± 146 | 13·8 ± 3·6 | 107 ± 17 | 1·8 ± 0·8 | 3·4 ± 0·8 | 270 ± 25 | 226 ± 53 | 22·8 ± 4·6 | 70 ± 10·6 | 4100 ± 654 | 7350 ± 1545 | |
| Ec | n.d. | n.d. | n.d. | 17·1 ± 3·5 | 1558 ± 416 | 957 ± 178 | n.d. | n.d. | 16·4 ± 3·3 | 45 ± 5·6 | 32 ± 8·4 | n.d. | n.d. | 21·44 ± 3·6 | 3150 ± 224 | 1300 ± 362 | |
| 3 | La | 25·5 ± 5·9 | 13·2 ± 3·6 | n.d. | n.d. | 11·4 ± 4·2 | 17·1 ± 13·6 | 5 ± 1·2 | 9·3 ± 2·8 | n.d. | n.d. | 22 ± 4·2 | 48 ± 6·7 | 2·3 ± 0·7 | 1·8 ± 0·4 | 280 ± 25 | 1350 ± 254 |
| LGG | 129 ± 41 | 434 ± 125 | n.d. | n.d. | 7·1 ± 2·4 | 42·8 ± 12·5 | 3·2 ± 0·5 | n.d. | n.d. | n.d. | 8 ± 1·4 | 4·4 ± 1·2 | 4·7 ± 1·3 | 6·3 ± 1·4 | 1475 ± 453 | 3250 ± 245 | |
| Bl | 7·7 ± 2·3 | 3·6 ± 0·9 | n.d. | n.d. | 21·4 ± 3·6 | 19·8 ± 3·4 | 1·8 ± 0·4 | 2·4 ± 0·6 | 1000 ± 212 | 284 ± 44 | n.d. | n.d. | 5·1 ± 1·2 | 2·9 ± 0·6 | 725 ± 45 | 1584 ± 128 | |
| B94 | 166 ± 29 | 42·8 ± 11·3 | 142 ± 35 | 394 ± 67 | 50 ± 8·5 | 78·5 ± 19·4 | n.d. | n.d. | 1107 ± 158 | 382 ± 51 | n.d. | n.d. | 6·7 ± 2·3 | 21·4 ± 3·9 | 1450 ± 275 | 5200 ± 1526 | |
| St | 11·1 ± 2·5 | 4·4 ± 1·1 | 44·2 ± 11·2 | 84·3 ± 15·3 | 58·5 ± 12·6 | 745 ± 135 | 170 ± 32·4 | 187 ± 22 | 1217 ± 324 | 432 ± 58 | 128 ± 26·3 | 106 ± 25·6 | 4·4 ± 1·5 | 12·3 ± 2·4 | 350 ± 34 | 7250 ± 2547 | |
| Ec | 90 ± 32·5 | 475 ± 105 | 12·9 ± 2·5 | 70 ± 21 | 11·4 ± 4·1 | 37·1 ± 6·9 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 423 ± 68 | 284 ± 62 | |
Cytokine secretion induced by mock GIT-subjected bacteria
The survival of bacteria subjected to conditions mimicking those in the GIT (e.g. low pH, exposure to enzymes and bile) was measured and compared to untreated bacteria growth. No significant differences were observed between the two sets of results, indicating that the bacteria are able to withstand the harsh physiological conditions (Table 2) [17,29]. Proinflammatory cytokine production was measured following co-cultured of GIT-simulated bacteria with the different cells as above. In general, results showed cytokine production similar to that observed from live bacteria (Fig. 1a,b). Of all the bacterial strains assessed, St1275 induced the highest production of IL-12 from buffy coat PBMC (Fig. 1b). Conversely, when cultured with cord blood-derived PBMC, St1275 induced significantly (P < 0·05) lower levels of IL-12 compared to other bacteria (data not shown). Again, St1275 appeared to have stimulated significantly higher concentrations of IL-17 in all GIT co-cultured from buffy coat-derived PBMCs but lower concentrations or no production with CRL9850 or cord blood-derived PBMCs (Figs 1b and 2b). E. coli induced IL-10 secretion poorly from buffy coat PBMC. In contrast LAVRI-A1, B94, BL536, ST1275 and LGG were found to stimulate high levels of IL-10 (Fig. 1b). From CRL9850 and cord blood-derived PBMCs, only LAVRI-A1, LGG, Bl536 and B94 induced significant (P < 0·05) levels of IL-10 production (Fig. 2a).
Table 2.
Enumeration of bacteria after 18 h incubation
| Cell count (CFU)/ml | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LAVRI-A1 | LGG | Bl536 | B94 | St1275 | E. coli | |||||||
| Treatment | 0 h | 18 h | 0 h | 18 h | 0 h | 18 h | 0 h | 18 h | 0 h | 18 h | 0 h | 18 h |
| Live | 7·47 | 9·06 | 7·00 | 9·29 | 7·31 | 8·74 | 7·10 | 8·65 | 7·10 | 8·77 | 6·89 | 8·56 |
| GIT simulation | 6·27 | 8·12 | 6·00 | 8·43 | 6·10 | 8·53 | 6·15 | 8·39 | 6·00 | 8·02 | 6·00 | 8·27 |
| Killed | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| s.e.m. | 0·24 | |||||||||||
Results presented as a mean (n = 3) ± pooled standard error of the mean (s.e.m.) (0·243). Lactobacillus (Lb.) acidophilus LAVRI-A1; Lb. rhamnosus GG (LGG); Bifidobacterium (B.) lactis B94; B. longum BL536; exopolysaccharides (EPS)-producing Streptococcus (S.) thermophilus St1275; Escherichia (E.) coli; GIT: gastrointestinal tract; CFU: colony-forming units.
Cytokine secretion induced by heat-killed bacterial cells
Killed bacteria were able to induce substantial levels of all cytokines from buffy coat PBMC (Fig. 1c). Strikingly, only IL-10 was seen to be induced in significant amounts (P < 0·05) when killed bacteria were incubated with the other cell types.
Induction of FoxP3 and ROR-γt expression in PBMCs by live/killed selected bacteria strains
PBMC incubation with LAB resulted in enhanced expression of CD25 on CD4+ T lymphocytes (Fig. 3), in line with Niers et al. [23]. To investigate whether treatment with lactobacilli or bifidobacteria lead to enhanced Th17 or Treg cell differentiation we assessed Th17/Treg populations in PBMC following 72–96 h of treatment with live or heat-killed bacteria. In all cases, following 72–96 h co-culture the number of Treg (CD4+CD25+FoxP3+) cells as a percentage of total PBMC increased substantially compared to untreated control cells, albeit to different levels [Fig. 4a(i) and a(ii)]. BL536 and B94 were found to be the most potent live strains and LAVRI-A1, B94 and St1275 the most potent heat-killed strains at inducing FoxP3 expression. The capacity of live or killed bacteria to induce IL-17-producing cells from PBMC was also investigated. As shown in Fig. 4b, the number of IL-17-expressing CD3+CD4+ cells was increased substantially compared to control. Because Th17 cells typically produce IL-17 in culture, it was therefore likely that these cells were of the Th17 lineage. To confirm Th17 cell identity, extracellular marker CCR6 (CD196) and intracellular marker ROR-γt were subsequently used. The proportion of Th17 cells (CD3+CD4+CCR6+ROR-γt+) induced by live and killed bacteria was increased 2·5-fold above control [Fig. 4b(i) and b(ii)], with Bl536 being the most potent strain (P < 0·01). Interestingly, the induction of Th17 cells by the stimulation of PBMCs with E. coli or LPS were similar.
Fig. 3.
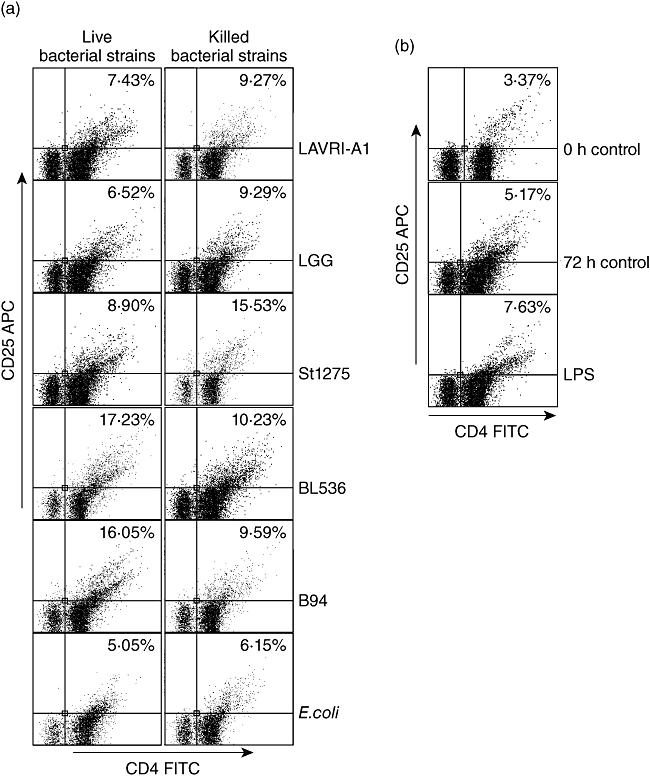
Expression of activation marker CD25 by lymphocytes in response to selected bacteria. peripheral blood mononuclear cells (PBMCs) were cultured with Lactobacillus acidophilus LAVRI-A1, Lb. rhamnosus GG, exopolysaccharides (EPS)-producing Streptococcus thermophilus St1275, Bifidobacterium longum BL536, B. lactis B94 or Escherichia coli TG1 for 72 h and evaluated on the expression of CD25 on T lymphocytes after 72 h of co-culture. Plots were gated on CD3. One representative experiment is shown of three different donors and from six strains (live and killed) used in these experiments.
Fig. 4.
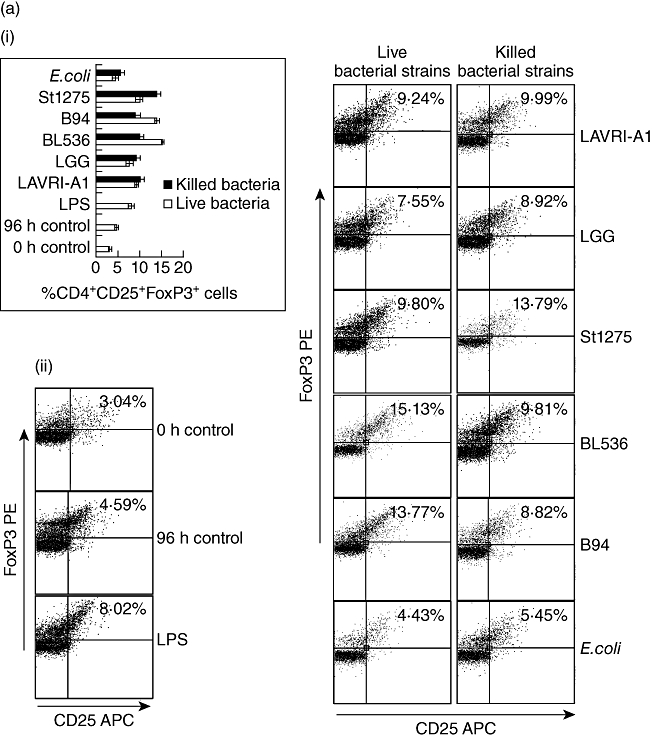
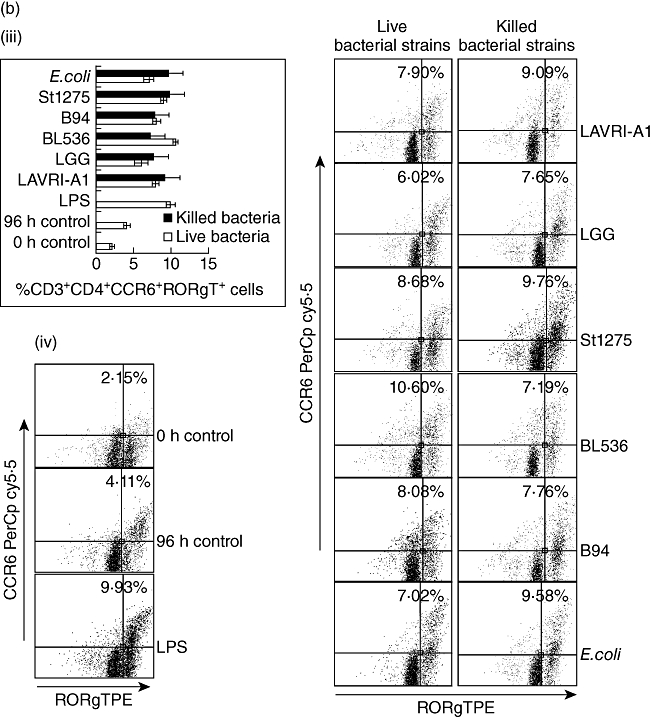
Peripheral blood mononuclear cells (PBMCs) were co-cultured with live or heat killed Lactobacillus acidophilus LAVRI-A1, Lb. rhamnosus GG, exopolysaccharides (EPS)-producing Streptococcus thermophilus St1275, Bifidobacterium longum BL536, B. lactis B94 or Escherichia coli TG1 in a ratio that does not induce apoptosis, lipopolysaccharide (LPS) or cells alone as control. The percentage of induced CD25+forkhead box protein 3 (FoxP3+) cells (a), and the induction of ROR-γt expressing T helper type 17 (Th17) cells (b), were assessed intracellularly by fluorescence activated cell sorter (FACS)Calibur after 96 h of co-culture. (b) Representative FACS plots for cultures described in (a); (d) Representative FACS plots for cultures described in (c). Data are expressed as means plus or minus standard error of the mean (s.e.m.) of three independent experiments.
Discussion
Probiotic bacteria are commonly marketed to aid digestion and optimize microbial balance in the GIT. The current studies assessed the capacity of probiotic bacteria to affect the local cytokine production and regulatory cell populations among different cell types. In addition, the models used in these studies simulated the conditions that ingested micro-organisms face during transit through the GIT such as low pH, bile concentration, and enzymatic digestion to assess their effect on cell survival and the capacity to influence host immunoregulation [17,30]. Our results demonstrated the bacteria were resistant to the extreme conditions faced in the gut, in line with previous reports [17].
The current studies assessed the ability of common probiotics to induce cytokine production from PBMCs, cord blood cells and spleen-derived macrophages. The substantial concentrations of IL-2, IL-12, IL-17 and IFN-γ produced by PBMCs in this study indicate the cells' potential to prevent/fight infection. LGG has been reported to aid in the prevention of atopic dermatitis in infants and as well as alleviate food allergy [31,32]; if these effects are largely IL-12-driven, St1275, B94 and E. coli in our study may probably be as effective in their immunomodulatory effects. Miettinen et al. [15] reported that LGG induced the production of proinflammatory cytokines such as IL-6, IL-12 and IFN-γ but limited IL-10 from human PBMC. Conversely, in our study LAVRI-A1, LGG and bifidobacteria induced significantly higher concentrations of IL-10 from PBMCs compared to the proinflammatory cytokines, which makes these probiotic strains good candidates for management of autoimmune disorders.
In the current study we report that selected probiotics induced significant amounts of proinflammatory cytokines, including IL-2, which is a critical cytokine for clonal expansion of recently antigen-activated T cells and in Treg homeostasis [33]. Macrophage-produced IL-12 stimulates IFN-γ production in T cells and natural killer cells, which accelerates the development of naive CD4+ T cells into Th1-type cells [34]. Therefore, IL-12 is a key immunoregulator favouring Th1-type responses. However, IFN-γ in turn induces IL-12 production, which can cause a positive feedback loop of IFN-γ and IL-12 production and can be detrimental, leading to uncontrolled cytokine production and possible shock [35]. IL-17 has been found recently to be elevated in the intestinal tissue and serum of patients with inflammatory bowel disease (IBD) and other autoimmune disorders [36]. In contrast, anti-inflammatory cytokines IL-4, IL-10 and TGF-β were also found to be produced in significant concentrations by our healthy PBMCs with the co-culture of selected bacteria. These cytokines function to inhibit IL-12 and the production of other proinflammatory cytokines from antigen-presenting cells, including macrophages, as well by inducing expression of other co-stimulatory surface molecules and soluble cytokines [37]. Our findings show that all the selected bacteria, especially LAVRI-A1, LGG and bifidobacteria, induced significant secretion of IL-10 and TGF-β, which was in line with earlier reports on L. acidophilus and bifidobacteria [14,38,39]. In addition to its activity as a Th2 lymphocyte cytokine, IL-10 is also a potent deactivator of monocyte/macrophage proinflammatory cytokine synthesis [40]. TGF-β1 down-regulates monocyte and macrophage activity in a manner similar to IL-10, albeit less potently [41]. It suppresses the proliferation and differentiation of T cells and B cells and limits IL-2, IFN-γ and TNF-α production. The severe and uncontrolled inflammatory reactions observed in the TGF-β1 knock-out mouse attests to the physiological role of TGF-β as an endogenous anti-inflammatory cytokine [42].
Even though in this study Gram-negative E. coli stimulated substantial amount of proinflammatory cytokines, the induction of pro- and anti-inflammatory cytokines with live Gram-positive bacteria (including GIT simulated bacteria), on average, was significantly higher. Hessle et al. [13] reported that Gram-positive bacteria appeared to stimulate IL-12 production and Gram-negative bacteria stimulate IL-10 production preferentially. However, concordant with observations reported in Berg et al. [43] and in our study, Gram-negative E. coli induced the secretion of significant concentrations of proinflammatory cytokines by PBMCs and the CRL-9850 cell line. While the mechanisms by which some bacteria induce the production of IL-10 are unclear, LPS of Gram-negative bacteria may stimulate this anti-inflammatory response [43]. Compounds other than LPS in lactobacilli probably contributed to the ability of these probiotic bacteria to stimulate an anti-inflammatory cytokine response. Probiotic LAVRI-A1, LGG, B94 and BL536 induced substantial amounts of pro-and anti-inflammatory cytokines in line with previous studies [44], with the balance skewed towards the anti-inflammatory response in our study. A demonstration of the utility of this response is the finding that LGG reduced inflammation in Crohn's disease [45]. The human gut microbiota has been estimated recently to consist of at least 400 different species [46], and it is likely that the potency of each of these species to influence immune homeostasis is different. Indeed, cytokine profiles in co-cultures of bacteria with PBMC show marked differences between strains [23]. In addition, the effects of lactobacilli supplementation on experimental autoimmune encephalomyelitis have been shown to be highly strain-dependent [47]. It is therefore conceivable that the contradicting results found in the human trials can be explained partly by differences in the immunomodulatory capacity of the strains used.
The fact that the killed bacteria in our study were inefficient in inducing substantial amounts of pro- and anti-inflammatory cytokines compared to live bacteria suggests that extra- and intracellular bacterial components as well as metabolites probably contribute to cytokine production [48]. Conceivably, a combination of certain bacterial fragments, metabolites produced in situ and particular structural motifs may need to interact with receptors on monocytes to induce optimal cytokine synthesis [21,49]. Cross et al. [50] and Macpherson and Harris [51] reported that live lactobacilli were more potent inducers of cytokine production in mammalian leucocytes compared to killed bacteria, similar to our findings. The results of the present study indicate that differential immunomodulatory effects may exist between Lactobacilli, bifidobateria and S. thermophilus, suggesting that these bacteria may be stronger boosters of host immunity. However in the case of St1275, the presence of EPS might have also influenced its ability to stimulate sustained and substantial levels of cytokines in the co-cultures. Exopolysaccharides from LAB have been claimed to participate in various regulatory processes such as immunomodulatory, cholesterol-lowering and anti-ulcer activities.
This study also investigated the differentiation of Treg and Th17 cells from PBMCs stimulated with the bacteria. TGF-β has been shown to be involved in both Treg and Th17 development. Animal models have demonstrated that at high levels of TGF-β, FoxP3 expression is up-regulated and Treg differentiation is induced, whereas at low levels of TGF-β, IL-6 and IL-21 synergize to promote the differentiation of Th17 cells [52]. In the current studies, we observed elevated levels of TGF-β in the PBMC supernatant following incubation with the probiotics, suggesting a prime environment for Treg differentiation. Indeed, substantially increased numbers of Tregs were identified in these cultures. Similarly, the identification of the transcription factor ROR-γt by intracellular and CCR6 extracellular staining confirmed the presence of Th17 cells. Th17 cells induce a range of proinflammatory mediators that bridge the innate and adaptive immune response enabling the clearance of invading pathogens [53]. The balance between Treg and Th17 cells may be essential for maintaining immune homeostasis. Hence, therapeutic approaches that aim to re-establish homeostasis by increasing the number of Treg, while also controlling effector T cell populations, may prove effective in the treatment of autoimmune diseases, whereas the reverse may also hold true for inflammatory diseases such as allergy.
In the current studies, the bacterial strains that induced high FoxP3 expression also stimulated the highest levels of the suppressive cytokine, IL-10 [20]. The mechanism of FoxP3+ Treg induction in the co-cultures still remains unclear. TGF-β appears to be a key cytokine in this induction, although IL-2 also plays an apparent and important role [54]. This was also apparent in our study, as IL-2 and TGF-β were among the various cytokines released. Furthermore, we have shown that production of cytokines and induction of ROR-γt/FoxP3 cells were strain-dependent, and differed depending on bacterial treatment (i.e. live or killed). Similar findings were reported previously [20], when strains of lactobacilli differed significantly in their capacity to induce FoxP3+ regulatory cells in vitro, independent of the IL-10 production. The overall extent of induction of FoxP3+ (Treg) and ROR-γt+ (Th17) cells by the selected bacteria in our study showed a balance between these cells, representative of that found in a healthy donor [55]. Previously, Lb. acidophilus strain LAVRI-A1 had no clinical effect on eczema [56]; however, this strain may be effective for other inflammatory disorders, as the current study shows a moderate induction of FoxP3/ROR-γt in vitro. Future studies will focus on the difference in cell components, such as cell wall proteins or sugars from these strains, to determine what combination of factors may be responsible for their immune modulating abilities.
Acknowledgments
This research was funded by the Victoria University Research Fellow Grant and the Researcher Development Grants Scheme. We thank the Australian Red Cross Services and the Cord Blood Bank (BMDI, Royal Children Hospital, Melbourne, Australia) for their supply of blood products. The in-kind financial and technical supports by Burnet Institute, and The Walter and Eliza Hall Institute of Medical Research (Parkville, VIC, Australia) are also gratefully acknowledged. Researches at the Walter and Eliza Hall and Burnet Institutes were made possible through Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS.
Disclosure
The authors declare no conflicts of interest.
References
- 1.McCartney AL, Wenzhi W, Tannock GW. Molecular analysis of the composition of the bifidobacterial and lactobacillus microflora of humans. Appl Environ Microbiol. 1996;62:4608–13. doi: 10.1128/aem.62.12.4608-4613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fooks LJ, Gibson GR. Probiotics as modulators of the gut flora. Br J Nutr. 2002;88:S39–S49. doi: 10.1079/BJN2002628. [DOI] [PubMed] [Google Scholar]
- 3.Gill HS. Best practice and research. Clin Gastroenterol. 2003;17:755–73. doi: 10.1016/s1521-6918(03)00074-x. [DOI] [PubMed] [Google Scholar]
- 4.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 5.Lin W, Truong N, Grossman WJ, et al. Allergic dysregulation and hyperimmunoglobulinemia E in FoxP3 mutant mice. J Allergy Clin Immunol. 2005;116:1106–15. doi: 10.1016/j.jaci.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 6.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FoxP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 7.Kalliomaki M, Salminen S, Poussa T, Isolauri E. Probiotics during the first 7 years of life: a cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:1019–21. doi: 10.1016/j.jaci.2006.12.608. [DOI] [PubMed] [Google Scholar]
- 8.Baharav E, Mor F, Halpern M, Weinberger A. Lactobacillus GG bacteria ameliorate arthritis in Lewis rats. J Nutr. 2004;134:1964–9. doi: 10.1093/jn/134.8.1964. [DOI] [PubMed] [Google Scholar]
- 9.Feleszko W, Jaworska J, Rha RD, et al. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin Exp Allergy. 2007;37:498–505. doi: 10.1111/j.1365-2222.2006.02629.x. [DOI] [PubMed] [Google Scholar]
- 10.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–6. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 12.Mangan PR, Harrington LE, O'Quinn DB, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 13.Hessle C, Andersson B, Wold AE. Gram-positive bacteria are potent inducers of monocytic interleukin-12 (IL-12) while Gram-negative bacteria preferentially stimulate IL-10 production. Infect Immun. 2000;68:3581–6. doi: 10.1128/iai.68.6.3581-3586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He F, Morita H, Ouwehand AC, et al. Stimulation of the secretion of pro-inflammatory cytokines by Bifidobacterium strains. Microbiol Immunol. 2002;46:781–5. doi: 10.1111/j.1348-0421.2002.tb02765.x. [DOI] [PubMed] [Google Scholar]
- 15.Miettinen M, Matikainen S, Vuopio-Varkila J, et al. Lactobacilli and streptococci induce interleukin-12 (IL-12), IL-18, and interferon gamma production in human peripheral blood mononuclear cells. Infect Immun. 1998;66:6058–62. doi: 10.1128/iai.66.12.6058-6062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dave RI, Shah NP. Evaluation of media for selective enumeration of Streptococcus thermophilus, Lactobacillus delbrueckii ssp. bulgaricus, Lactobacillus acidophilus, and bifidobacteria. J Dairy Sci. 1996;79:1529–36. doi: 10.3168/jds.S0022-0302(96)76513-X. [DOI] [PubMed] [Google Scholar]
- 17.Marteau P, Minekus M, Havenaar R, Huis in't veld JHJ. Survival of lactic acid bacteria in a dynamic model of the stomach and small intestine: validation and the effects of bile. J Dairy Sci. 1997;80:1031–7. doi: 10.3168/jds.S0022-0302(97)76027-2. [DOI] [PubMed] [Google Scholar]
- 18.Miquel E, Gómez JA, Alegría A, Barberá R, Farré R, Recio I. Identification of casein phosphopeptides after simulated gastrointestinal digestion by tandem mass spectrometry. Eur Food Res Technol. 2006;222:48–53. [Google Scholar]
- 19.Donkor ON, Henriksson A, Singh TK, Vasiljevic T, Shah NP. ACE-inhibitory activity of probiotic yoghurt. Int Dairy J. 2007;17:1321–31. [Google Scholar]
- 20.de Roock S, van Elk M, van Dijk MEA, et al. Lactic acid bacteria differ in their ability to induce functional regulatory T cells in humans. Clin Exp Allergy. 2009;40:103–10. doi: 10.1111/j.1365-2222.2009.03344.x. [DOI] [PubMed] [Google Scholar]
- 21.Amrouche T, Boutinb Y, Prioulta G, Flissa I. Effects of bifidobacterial cytoplasm, cell wall and exopolysaccharide on mouse lymphocyte proliferation and cytokine production. Int Dairy J. 2006;16:70–80. [Google Scholar]
- 22.SAS/STAT. Software: changes and enhancements through release 6.11. Cary, NC: SAS Institute, Inc; 1996. [Google Scholar]
- 23.Niers LEM, Timmerman HM, Rijkers GT, et al. Identification of strong interleukin-10 inducing lactic acid bacteria which down regulate T helper type 2 cytokines. Clin Exp Allergy. 2005;35:1481–9. doi: 10.1111/j.1365-2222.2005.02375.x. [DOI] [PubMed] [Google Scholar]
- 24.Wu CL, Chan MC, Chang GC, et al. Aetiology and cytokine expression in patients requiring mechanical ventilation due to severe community-acquired pneumonia. J Formos Med Assoc. 2006;105:49–55. doi: 10.1016/s0929-6646(09)60108-x. [DOI] [PubMed] [Google Scholar]
- 25.Breitling LP, Fendel R, Mordmueller B, Adegnika AA, Kremsner PG, Luty AJF. Cord blood dendritic cell subsets in African newborns exposed to Plasmodium falciparum in utero. Infect Immun. 2006;74:5725–9. doi: 10.1128/IAI.00682-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malhotra IP, Mungai E, Muchiri J, et al. Distinct Th1- and Th2-type prenatal cytokine responses to Plasmodium falciparum erythrocyte invasion ligands. Infect Immun. 2005;73:3462–70. doi: 10.1128/IAI.73.6.3462-3470.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanna N, Hanna I, Hleb M, et al. Gestational age-dependent expression of IL-10 and its receptor in human placental tissues and isolated cytotrophoblasts. J Immun. 2000;164:5721–8. doi: 10.4049/jimmunol.164.11.5721. [DOI] [PubMed] [Google Scholar]
- 28.Bennet WA, Lagoo-Deendayalan S, Whitworth NS, et al. First trimester human chorionic villi express both immunoregulatory and inflammatory cytokines: a role for interleukin-10 in regulating the cytokine network of pregnancy. Am J Reprod Immunol. 1999;41:70–8. doi: 10.1111/j.1600-0897.1999.tb00077.x. [DOI] [PubMed] [Google Scholar]
- 29.Oozeer R, Leplingard A, Mater DDG, et al. Survival of Lactobacillus casei in the human digestive tract after consumption of fermented milk. Appl Environ Microbiol. 2006;72:5615–17. doi: 10.1128/AEM.00722-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minekus M, Marteau P, Havenaar R, Huis in't Veld JHJ. A multicompartmental dynamic computercontrolled computer controlled model simulating the stomach and small intestine. Altern Lab Anim. 1995;23:197–209. [Google Scholar]
- 31.Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomized placebo-controlled trial. Lancet. 2001;357:1076–9. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- 32.Majamaa H, Isolauri E. Probiotics: a novel approach in the management of food allergy. J Allergy Clin Immunol. 1997;99:179–85. doi: 10.1016/s0091-6749(97)70093-9. [DOI] [PubMed] [Google Scholar]
- 33.Malek TR. The main function of IL-2 is to promote the development of T regulatory cells. J Leuk Biol. 2003;74:961–5. doi: 10.1189/jlb.0603272. [DOI] [PubMed] [Google Scholar]
- 34.Trinchieri G. Proinflammatory and immunoregulatory functions of interleukin-12. Int Rev Immunol. 1998;16:365–96. doi: 10.3109/08830189809043002. [DOI] [PubMed] [Google Scholar]
- 35.Liu Z, Jiu J, Liu S, Fa X, Li F, Du Y. Blockage of tumor necrosis factor prevents intestinal mucosal inflammation through down-regulation of interleukin-23 secretion. J Autoimmun. 2007;29:187–94. doi: 10.1016/j.jaut.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–94. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 37.Meyaard L, Hovenkamp E, Otto SA, Miedma F. IL-12 induced IL-10 production by human T cells as a negative feedback for IL-12 induced immune responses. J Immunol. 1996;156:2776–82. [PubMed] [Google Scholar]
- 38.Morita H, He F, Fuse T, et al. Cytokine production by the murine macrophage cell line J774.1 after exposure to Lactobacilli. Biosc Biotech Biochem. 2002;66:1963–6. doi: 10.1271/bbb.66.1963. [DOI] [PubMed] [Google Scholar]
- 39.Soichi T, Kinuta Y, Saito Y. Bifidobacterium infantis suppresses proinflammatory interleukin-17 production in murine splenocytes and dextran sodium sulfate-induced intestinal inflammation. Int J Mol Med. 2008;22:181–5. [PubMed] [Google Scholar]
- 40.Clarke CJP, Hales A, Hunt A, et al. IL-10 mediated suppression of TNF-alpha production is independent of its ability to inhibit NF-kB activity. Eur J Immunol. 1998;28:1719–26. doi: 10.1002/(SICI)1521-4141(199805)28:05<1719::AID-IMMU1719>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 41.Litterio JJ, Roberts AB. TGF-b: a critical modulator of immune cell function. Clin Immunol Immunopathol. 1997;84:244–50. doi: 10.1006/clin.1997.4409. [DOI] [PubMed] [Google Scholar]
- 42.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–72. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 43.Berg DJ, Kuhn R, Rajewsky K, et al. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J Clin Investig. 1995;96:2339–47. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kitazawa H, Tomioka Y, Matsumura K, et al. Expression of mRNA encoding IFN alpha in macrophages stimulated with Lactobacillus gasseri. FEMS Microbiol Lett. 1994;120:315–21. doi: 10.1111/j.1574-6968.1994.tb07052.x. [DOI] [PubMed] [Google Scholar]
- 45.Gupta P, Andrew H, Kirschner BS, Guandalini S. Is Lactobacillus GG helpful in children with Crohn's disease? Results of a preliminary, open-label study. J Pediatr Gastroenterol Nutr. 2000;31:453–7. doi: 10.1097/00005176-200010000-00024. [DOI] [PubMed] [Google Scholar]
- 46.Zoetendal EG, Rajilic-Stojanovic M, de Vos WM. High-throughput diversity and functionality analysis of the gastrointestinal tract microbiota. Gut. 2008;57:1605–15. doi: 10.1136/gut.2007.133603. [DOI] [PubMed] [Google Scholar]
- 47.Maassen CB, Claassen E. Strain-dependent effects of probiotic lactobacilli on EAE autoimmunity. Vaccine. 2008;26:2056–7. doi: 10.1016/j.vaccine.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 48.Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci USA. 1996;93:2879–83. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lawrence C, Nauciel C. Production of interleukin-12 by murine macrophages in response to bacterial peptidoglycan. Infect Immun. 1998;66:4947–9. doi: 10.1080/00222936700770321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cross ML, Ganner A, Teilab D, Fray LM. Patterns of cytokine induction by Gram-positive and Gram-negative probiotic bacteria. FEMS Immunol Med Microbiol. 2004;42:173–80. doi: 10.1016/j.femsim.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–85. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 52.Zhou L, Lopes JE, Chong MM, et al. TGF-β-induced FoxP3 inhibits Th17 cell differentiation by antagonizing RORγt function. Nature. 2008;453:236–40. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.LeibundGut-Landmann S, Gross O, Robinson MJ, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–8. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 54.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25– cells to CD25+FoxP3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–27. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 55.Eastaff-Leung N, Mabarrack N, Barbour A, Cummins A, Barry S. FoxP3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J Clin Immunol. 2010;30:80–8. doi: 10.1007/s10875-009-9345-1. [DOI] [PubMed] [Google Scholar]
- 56.Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high risk children: a randomized controlled trial. J Allergy Clin Immunol. 2007;119:184–91. doi: 10.1016/j.jaci.2006.08.036. [DOI] [PubMed] [Google Scholar]


