Abstract
The pattern recognition molecules H-ficolin, L-ficolin and M-ficolin bind to micro-organisms. They activate the lectin pathway of complement through mannan-binding lectin (MBL)-associated serine proteases (MASPs). Association between low MBL levels and infections in patients undergoing chemotherapy for haematological diseases has been observed previously. We now examine for MASP-2, MASP-3 and ficolin levels. We assessed the concentration of lectin pathway molecules as risk factors for infection in patients with haematological malignancy undergoing chemotherapy. Samples taken before the initiation of chemotherapy covering 117 chemotherapy cycles in 105 patients were available. MASPs and ficolins were measured by time-resolved immunoflourometric assays and the levels related to parameters of infections. End-points included febrile neutropenia, documented infections, bacteraemia or severe infections. Lower M-ficolin concentrations were found in patients who developed a severe infection: median 0·27 µg/ml compared to 0·47 µg/ml in patients who did not develop a severe infection (P = 0·01). Conversely, MASP-2 was higher in these patients: median 0·53 µg/ml compared to 0·37 µg/ml, respectively (P = 0·008). When considering M-ficolin levels below 0·36 µg/ml as deficient, the time to development of severe infection was shorter in the M-ficolin deficient group: the hazard ratio was 2·60 (95% confidence interval: 1·23–5·49). No associations were revealed between infections and H-ficolin, L-ficolin or MASP-3. Patients with low M-ficolin are more likely to develop severe infections, whereas MASP-2 showed the opposite.
Keywords: chemotherapy infections, ficolin, mannan-binding lectin, MASP, pattern recognition molecules
Introduction
Patients with haematological cancer often receive myelosuppressive chemotherapy. This treatment induces the risk of developing infectious complications. A number of studies have addressed the influence of deficiency of components of the innate immune system on the tendency to develop infections after chemotherapy.
The innate immune system makes use of pattern recognition molecules (PRMs), which are able to differentiate between self- and infectious agents through the recognition of pathogen-associated molecular patterns (PAMPs) [1]. The PRMs may be cell-bound, as the Toll-like receptors, or they are soluble (humoral) proteins. Mannan-binding lectin (MBL) and the three ficolins, H-ficolin, L-ficolin and M-ficolin (also termed ficolin 3, 2 and 1, respectively) form a group of soluble PRMs, characterized by their collagenous structures and their complement-activating capability [2]. These proteins are oligomers made up of three or more subunits which, in turn, are built of three identical polypeptides. The subunits comprise an N-terminal region responsible for cross-linking the chains and the subunits, followed by a collagenous region responsible for trimerization and for mediating complement activation, and end in three globular regions displaying the recognition sites. The oligomers thus present nine, 12 or more recognition sites, which endow the molecules with high avidities even though each recognition site only binds to its ligand with low affinity. This ensures binding to foreign surfaces while ignoring self-surfaces with low density of ligands. The recognition structure of MBL is a C-type lectin domain, while those of the ficolins are fibrinogen-like domains. MBL and the ficolins make use of the MBL-associated serine proteases (MASPs) to activate the complement system [3,4]. MASPs are present in the circulation in complexes with MBL and ficolins in their proenzyme form, but upon binding to PAMPs the MASPs self-activate. MASP-2 appears to be the main component responsible for the further downstream activation of the complement system. The roles of MASP-1 and MASP-3 remain unresolved. Activation of the complement system promotes the killing of the target as well as the release of mediators of inflammatory reactions [2].
MBL deficiency was found to be associated strongly with opsonizing deficiency in children with frequent and severe infections [5]. Deficiency in MBL is linked to certain allotypes [6] occurring at high frequency among Caucasians and even higher among subSaharan Africans [7]. Numerous epidemiological studies have since reported associations between increased risk of infections and low MBL levels or the genotypes determining low MBL levels [8]. Because most people with low MBL levels present no problems, MBL may be regarded as a disease modifier, as also indicated by reports of increased risk of infections in patients with leukaemia when undergoing chemotherapy [9,10]. We engaged previously in a prospective multi-centre study on the relationship between MBL levels and infections in leukaemia patients, the results of which supported a role for MBL in protection against severe infections during chemotherapy [11]. We have now extended this investigation through probing for possible associations between infections and the ficolins or with MASP-2 and MASP-3. MASP-1 levels were not addressed, as no assay for this component had been developed. Because MASP-2 is the component largely responsible for the downstream activation of further complement components in this activation pathway, termed the lectin pathway of complement activation [12], it was anticipated that susceptibility to infections might correlate especially with low MASP-2 levels. Although the present investigation is of retrospective nature, it benefits from the dedicated monitoring of the patients enrolled in the original prospective trial.
Methods
Patients and samples
We were able to retrieve the plasma samples of 105 patients who underwent chemotherapy for haematological cancer and who were followed for the development of infections in the context of the already published clinical trial comprising 255 patients [11]. The samples were obtained on the day of the start of chemotherapy or during the 2 days preceding chemotherapy and used for assessment of H-ficolin, L-ficolin, M-ficolin, MASP-2 and MASP-3. In 93 patients one sample was used, and in 12 patients two samples (two different chemotherapy cycles) could be used, resulting in 117 samples. The only element of selection applied was the availability of the archival samples taken before the initiation of chemotherapy.
End-points
The end-points were the development of febrile neutropenia, documented infections, bacteraemia or severe infections. Neutropenia was defined as an absolute neutrophil count <500 cells/ml. Fever was defined as temperature ≥38·5°C recorded on at least one occasion or as temperature ≥38°C noted on at least two occasions during a 12-h period. Febrile neutropenia was defined as fever that occurred during neutropenia, regardless of what may have been the cause of the fever. Severe infection was defined as the presence of pneumonia, septicaemia, invasive fungal infection or sepsis. Further details were published previously [11].
Assays
All the assays (except for the L-ficolin assay) were performed as time-resolved immunofluorometric assays (TRIFMAs), and have been described previously. This assay type proceeds much like traditional enzyme-linked immunosorbent assay (ELISA). However, instead of enzyme-substrate reactions as reporters, use is made of the long-lasting fluorescence of the europium ion, which allows for counting the fluorescence photons without interference from the rapidly fading background fluorescence. In brief, the M-ficolin assay [13] is carried out by coating microwells with the monoclonal anti-M-ficolin antibody (MAb) 7G1. Dilutions of plasma samples and standard plasma are then applied and, following incubation, bound M-ficolin is determined by applying biotin-labelled MAb 7G1 (the use of the same MAb for coating and development is possible due to the oligomeric structure of M-ficolin), followed by incubation with europium-labelled streptavidin, and finally the wells are read by time-resolved fluorometry. This assay type shows higher sensitivity, a broader dynamic range and greater reproducibility than similarly conducted ELISAs. For H-ficolin [14] the wells were coated with anti-H-ficolin MAb (4H5), and after incubation with samples, developed with biotinylated 4H5 and europium-labelled streptavidin as above. L-ficolin was measured using an ELISA kit (HK 336–148) from HyCult Biotech, Uden, the Netherlands, according to the manufacturer's instructions. For the MASP-2 assay [15] the wells were coated with MAb 8B5 directed against the C-terminal protease domain, and after incubation with diluted samples bound MASP-2 was detected with biotinylated MAb 6G12 directed against the N-terminal part of MASP-2, followed by europium-labelled streptavidin as above. For the MASP-3 assay wells were coated with MAb 1E2 ([16]; Hycult Biotech), which is directed against parts of the A-chain, shared by MASP-1 and MASP-3. After incubation with dilutions of samples and standard plasma, the bound MASP was determined using a biotinylated MAb specific for the MASP-3 B-chain (MAb 38:12–3 raised against the C-terminal region of MASP-3, [17]), followed by europium-labelled streptavidin as above.
Statistical analysis
The Mann–Whitney U-test was used to test the statistical significance of differences in the six markers (H-ficolin, L-ficolin, M-ficolin, MASP-2, MASP-3) in patients with and without severe infection, febrile neutropenia, documented infection and bacteraemia.
To estimate the predictive performance of the markers, the areas under the receiver operating curve [with 95% confidence interval (CI)] have been calculated. A receiver operating characteristic (ROC) plot is obtained by calculating the sensitivity and specificity of every observed data value, and plotting sensitivity against 1 – specificity. A global assessment of the predictive performance is given by the area under the ROC curve. This area is equal to the probability that a random patient with, e.g. a severe infection has a higher value of H-ficolin than a random person who did not develop a severe infection [18].
In order to characterize time to severe infection of the first chemotherapy cycle, Kaplan–Meier estimates were used and hazard ratios calculated together with 95% confidence intervals. Regarding time to event data, these (event = severe infection) have a specific characteristic: at the end of the follow-up period, not all patients have developed a severe infection. For these patients the time to severe infection is said to be censored, indicating that the observation period was cut off before the event (i.e. severe infection) occurred. We did not know when the patient would experience the event, only that he or she has not done so by the end of the observation period [19]. The hazard ratio of, e.g. MASP-2 is the effect of MASP-2 on the hazard or risk of developing a severe infection.
The Spearman correlation was used to assess the correlation between two markers. All reported P-values are two-tailed.
Results
Baseline characteristics of the patients are presented in Table 1. The median age was 54 years. Sixty-one (58%) men and 44 (42%) women were included in the analysis. Thirty three per cent (35 of 105) of the patients had acute leukaemia; 35% (37 of 105) had lymphoma/Hodgkin's disease. Thirty-four patients (32%) developed a severe infection during chemotherapy (38 of the 117 chemotherapy cycles).
Table 1.
Baseline characteristics and prevalence of development of infectious complications
| Age – year | |
| Median | 54 |
| Interquartile range | 40–63 |
| Female sex – no. (%) | 44 (42) |
| Underlying cancer – no. (%) | |
| Acute leukaemia | 35 (33) |
| Lymphocytic | 8 (6) |
| Myelogenous | 26 (25) |
| Promyelocytic | 1 (1) |
| Lymphoma/Hodgkin's disease | 37 (35) |
| Hodgkin's disease | 6 (6) |
| Low-grade lymphoma | 3 (3) |
| Intermediate-grade lymphoma | 16 (15) |
| High-grade lymphoma | 12 (11) |
| Other haematological | 33 (31) |
| Febrile neutropenia – no. (%)* | 62 (53) |
| Documented infection – no. (%)* | 61 (52) |
| Bacteraemia – no. (%)* | 26 (22) |
| Severe infection – no. (%)* | 38 (32) |
Prevalence of febrile neutropenia, documented infection, bacteraemia and severe infection in the 117 chemotherapy cycles.
Lower M-ficolin concentration was found in patients who developed a severe infection: median 0·27 µg/ml compared to 0·47 µg/ml in patients who did not develop a severe infection (P = 0·01) (Table 2). The area under the ROC curve of M-ficolin for discriminating between patients with and without severe infection was 0·65 (95% CI: 0·54–0·75). Lower M-ficolin concentration was also detected in patients with febrile neutropenia compared to patients without (median 0·28 µg/ml versus 0·49 µg/ml, P = 0·01), in patients with documented infection (median 0·28 µg/ml versus 0·47 µg/ml, P = 0·02) or in patients with bacteraemia (0·26 µg/ml versus 0·45 µg/ml, P = 0·02). The area under the ROC curve of M-ficolin for discriminating between patients with and without febrile neutropenia, documented infection or bactaeremia was 0·64 (95% CI: 0·54–0·74), 0·63 (0·53–0·73) and 0·65 (0·54–0·77), respectively.
Table 2.
H-ficolin, L-ficolin, M-ficolin, mannan-binding lectin (MBL)-associated serine protease 2 (MASP-2) and MASP-3 concentrations (µg/ml plasma) in patients with infectious complications
| Febrile neutropenia | Documented infection | Bacteraemia | Severe infection | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | No | Yes | No | Yes | No | Yes | No | Yes | |||||
| (n = 117) | (n = 55) | (n = 62) | P-value | (n = 56) | (n = 61) | P-value | (n = 91) | (n = 26) | P-value | (n = 79) | (n = 38) | P-value | |
| H-ficolin (µg/ml) | |||||||||||||
| Median | 28·5 | 27·4 | 29·2 | 0·42 | 27·2 | 29·7 | 0·16 | 27·8 | 32·4 | 0·34 | 27·4 | 30·6 | 0·41 |
| Interquartile range | 24·1–34·8 | 21·6–35·3 | 24·9–34·8 | 21·7–33·7 | 24·9–38·1 | 24·2–34·6 | 22·9–38·8 | 23·8–34·6 | 24·1–38·8 | ||||
| L-ficolin (µg/ml) | |||||||||||||
| Median | 1·57 | 1·69 | 1·55 | 0·65 | 1·56 | 1·57 | 0·95 | 1·55 | 1·68 | 0·76 | 1·54 | 1·68 | 0·47 |
| Interquartile range | 1·29–2·20 | 1·18–2·72 | 1·29–1·90 | 1·13–2·22 | 1·33–2·05 | 1·21–2·20 | 1·29–2·25 | 1·09–2·13 | 1·33–2·72 | ||||
| M-ficolin (µg/ml) | |||||||||||||
| Median | 0·39 | 0·49 | 0·28 | 0·01 | 0·47 | 0·28 | 0·02 | 0·45 | 0·26 | 0·02 | 0·47 | 0·27 | 0·01 |
| Interquartile range | 0·19–0·75 | 0·24–0·95 | 0·15–0·57 | 0·24–0·94 | 0·12–0·57 | 0·23–0·85 | 0·12–0·45 | 0·23–0·90 | 0·12–0·45 | ||||
| MASP-2 (µg/ml) | |||||||||||||
| Median | 0·41 | 0·37 | 0·44 | 0·23 | 0·34 | 0·47 | 0·007 | 0·37 | 0·57 | 0·01 | 0·37 | 0·53 | 0·008 |
| Interquartile range | 0·29–0·58 | 0·28–0·48 | 0·31–0·59 | 0·27–0·45 | 0·34–0·60 | 0·28–0·51 | 0·35–0·67 | 0·27–0·48 | 0·35–0·64 | ||||
| MASP-3 (µg/ml) | |||||||||||||
| Median | 4·64 | 4·66 | 4·58 | 0·91 | 4·63 | 4·69 | 0·91 | 4·66 | 4·46 | 0·70 | 4·66 | 4·46 | 0·88 |
| Interquartile range | 3·43–5·64 | 3·21–5·81 | 3·84–5·56 | 3·40–5·67 | 3·69–5·64 | 3·34–5·64 | 3·92–6·29 | 3·37–5·64 | 3·73–5·76 | ||||
There was no evidence for an association between the H-ficolin, L-ficolin or the MASP-3 concentration and any of the infection parameters investigated.
Higher MASP-2 concentration was present in patients who developed severe infection: median 0·53 µg/ml compared to 0·37 µg/ml in patients who did not develop severe infection (P = 0·008). High MASP-2 was also associated with documented infection and bacteraemia. The area under the ROC curve of MASP-2 for discriminating between patients with and without severe infection was 0·66 (95% CI: 0·55–0·76), for febrile neutropenia 0·56 (0·46–0·67), for documented infection 0·65 (0·55–0·75) and for bacteraemia 0·66 (0·53–0·79).
Patients with a diagnosis of acute leukaemia had lower M-ficolin concentrations compared to patients with lymphoma or Hodgkin's disease (0·24 µg/ml versus 0·70 µg/ml, P < 0·001); but there was no statistical evidence for a difference of MASP-2 concentration (P = 0·13), MASP-3 or H- and L-ficolin (Table 3).
Table 3.
H-ficolin, L-ficolin, M-ficolin, mannan-binding lectin (MBL)-associated serine protease 2 (MASP)-2, MASP-3 and MBL concentration by underlying cancer
| Total | Acute leukaemia | Lymphoma/Hodgkin's disease | Other | ||
|---|---|---|---|---|---|
| (n = 117) | (n = 41) | (n = 42) | (n = 34) | P-value‡ | |
| H-ficolin† | |||||
| Median | 28·5 | 27·8 | 29·1 | 28·6 | 0·77 |
| Interquartile range | 24·1–34·8 | 22·9–34·5 | 23·8–36·8 | 25·4–33·8 | |
| L-ficolin | |||||
| Median | 1·57 | 1·54 | 1·75 | 1·50 | 0·08 |
| Interquartile range | 1·29–2·20 | 1·27–1·79 | 1·37–2·85 | 1·05–2·13 | |
| M-ficolin | |||||
| Median | 0·39 | 0·24 | 0·70 | 0·35 | <0·001 |
| Interquartile range | 0·19–0·75 | 0·06–0·45 | 0·39–1·15 | 0·21–0·55 | |
| MASP-2 | |||||
| Median | 0·41 | 0·48 | 0·38 | 0·34 | 0·13 |
| Interquartile range | 0·29–0·58 | 0·34–0·60 | 0·28–0·55 | 0·27–0·51 | |
| MASP-3 | |||||
| Median | 4·64 | 4·40 | 4·23 | 5·13 | 0·74 |
| Interquartile range | 3·43–5·64 | 3·14–5·73 | 3·24–5·46 | 4·07–5·76 | |
All values represent µg/ml plasma.
Acute leukaemia versus lymphoma/Hodgkin's disease.
M-ficolin values in haematological cancer patients who did not develop documented infection were lower than in blood donors: median 0·47 µg/ml (95% CI: 0·36–0·68) compared to median 1·41 µg/ml (1·31–1·50), P < 0·001. In this study we define low M-ficolin levels based on the lower limit of the 95% CI in haematological cancer patients who did not develop documented infection, i.e. <0·36 µg/ml. Fifty-two of the 105 patients (50%) could be considered low in M-ficolin. The time to development of severe infection was shorter in the M-ficolin deficient group: hazard ratio of 2·60 (95% CI: 1·23–5·49) (Fig. 1).
Fig. 1.
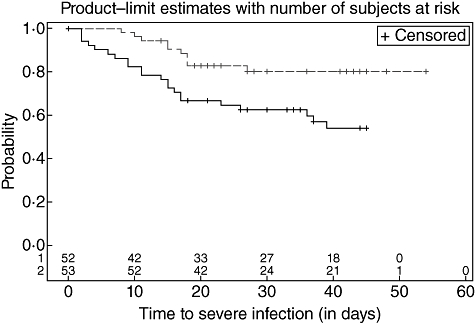
Time to severe infection stratified by M-ficolin levels. The 105 patients were divided into two groups according to the levels before chemotherapy as described in the result section: solid line: patients with M-ficolin below 360 ng/ml serum and dashed line: patients with more than 360 ng/ml.
If we define high MASP-2 as >0·43 µg/ml (upper limit of 95% CI in blood donors), time to development of severe infection was shorter in the high MASP-2 group: hazard ratio of 2·12 (95% CI: 1·04–4·28) (Fig. 2). Forty-four patients (42%) were considered as having an high MASP-2 concentration.
Fig. 2.
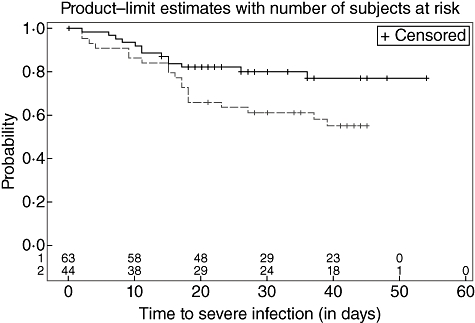
Time to severe infection stratified by mannan-binding lectin (MBL)-associated serine protease 2 (MASP-2) levels. The 105 patients were divided into two groups according to the levels before chemotherapy, as described in the Results section: solid line: patients with MASP-2 below 430 ng/ml serum and dashed line: patients with more than 430 ng/ml.
M-ficolin was not statistically significantly correlated to MASP-2: Spearman's correlation – 0·12 (P = 0·20).
Discussion
Haematological patients undergoing chemotherapy were chosen for analyses of MASP-2, MASP-3 and the three ficolins: H-ficolin, L-ficolin and M-ficolin as these patients suffer from severe depletion of neutrophils, thus rendering them highly susceptible to infections. One may expect concomitant defects or low levels of activity of other elements of the innate immune system to be revealed as disease modifiers.
We therefore revisited plasma samples collected in a prospective study aimed at investigating the possible correlation between the levels of MBL and infections in haematological patients undergoing chemotherapy [11]. The results of this first study found no correlation with the primary parameter, febrile neutropenia and MBL levels. However, significant correlation between lower levels of MBL and infections was revealed when addressing serious infections. When retrieving the archival samples from the −80°C freezers we were able to collect samples covering 117 chemotherapy cycles.
Conversely, low levels of M-ficolin were found to be associated significantly with infections according to all four definitions. Because M-ficolin was shown only recently to be present at significant levels in plasma [13] and not, as thought previously, present only as a monocyte and granulocyte-associated molecule, it was quite unexpected to observe such a robust association of the plasma levels with risk of infection. While the levels in plasma levels are similar to other members of the lectin pathway of complement activation, indicating an independent role for soluble M-ficolin, it is still possible that our finding reflects the activity of monocyte and granulocyte-associated M-ficolin.
Unexpectedly, we found generally lower levels of M-ficolin in the cancer patients than we find in blood donor controls [13]. For the other proteins no difference was seen.
It may be noted that another group reports the presence of only 60 ng of M-ficolin per ml of plasma [20]. We have discussed possible reasons for the discrepancy extensively [13].
MBL and the ficolins are thought to exert their activity mainly through activating the lectin pathway via the associated MASP-2. We thus expected to see a more profound association of low MASP-2 with infections than that of any of the other proteins. Surprisingly, the direct opposite was revealed, i.e. infections were associated significantly with higher MASP-2 levels. This was true no matter what definition was applied for infection. Currently we have no hypothesis to offer for explaining this observation. Previously, in a cohort of colon cancer patients, higher MASP-2 levels were associated with a higher risk for death [21].
Patients with M-ficolin deficiency who undergo chemotherapy are more likely to develop severe infection. Our study was limited by the availability of plasma samples and by the limited sample size. This result needs to be confirmed prospectively with adjustment for other risk factors for the development of severe infection.
Acknowledgments
The authors thank the Danish Council for Independent Research, Medical Sciences and Novo Nordic Foundation.
Disclosure
All authors: no conflicts.
References
- 1.Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RAB. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–18. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 2.Holmskov U, Thiel S, Jensenius JC. Collectins and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol. 2003;21:547–78. doi: 10.1146/annurev.immunol.21.120601.140954. [DOI] [PubMed] [Google Scholar]
- 3.Matsushita M, Endo Y, Fujita T. Cutting edge, complement-activating complex of ficolin and mannose-binding lectin-associated serine protease. J Immunol. 2000;164:2281–4. doi: 10.4049/jimmunol.164.5.2281. [DOI] [PubMed] [Google Scholar]
- 4.Petersen SV, Thiel S, Jensenius JC. The mannan-binding lectin pathway of complement activation: biology and disease association. Mol Immunol. 2001;38:133–49. doi: 10.1016/s0161-5890(01)00038-4. [DOI] [PubMed] [Google Scholar]
- 5.Super M, Thiel S, Lu J, Levinsky RJ, Turner MW. Association of low levels of mannan-binding protein with a common defect of opsonisation. Lancet. 1989;2:1236–9. doi: 10.1016/s0140-6736(89)91849-7. [DOI] [PubMed] [Google Scholar]
- 6.Sumiya M, Super M, Tabona P, et al. Molecular basis of opsonic defect in immunodeficient children. Lancet. 1991;337:1569–70. doi: 10.1016/0140-6736(91)93263-9. [DOI] [PubMed] [Google Scholar]
- 7.Lipscombe RJ, Sumiya M, Hill AV, et al. High frequencies in African and non-African populations of independent mutations in the mannose binding protein gene. Hum Mol Genet. 1992;1:709–15. doi: 10.1093/hmg/1.9.709. [DOI] [PubMed] [Google Scholar]
- 8.Møller-Kristensen M, Thiel S, Jensenius JC. Mannan-binding lectin polymorphisms and infectious diseases. In: Vasta GR, Ahmed H, editors. Animal lectins: a functional view. Boca Raton, FL: CRC Press; 2009. pp. 303–32. [Google Scholar]
- 9.Peterslund NA, Koch C, Jensenius JC, Thiel S. Association between deficiency of mannose-binding lectin and severe infections after chemotherapy. Lancet. 2001;358:637–8. doi: 10.1016/S0140-6736(01)05785-3. [DOI] [PubMed] [Google Scholar]
- 10.Neth O, Hann I, Turner MW, Klein NJ. Deficiency of mannose-binding lectin and burden of infection in children with malignancy: a prospective study. Lancet. 2001;358:614–18. doi: 10.1016/S0140-6736(01)05776-2. [DOI] [PubMed] [Google Scholar]
- 11.Vekemans M, Robinson J, Georgala A, et al. Low mannose-binding lectin concentration is associated with severe infection in patients with hematological cancer who are undergoing chemotherapy. Clin Infect Dis. 2007;44:1593–601. doi: 10.1086/518171. [DOI] [PubMed] [Google Scholar]
- 12.Thiel S. Complement activating soluble pattern recognition molecules with collagen-like regions, mannan-binding lectin, ficolins and associated proteins. Mol Immunol. 2007;44:3875–88. doi: 10.1016/j.molimm.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Wittenborn T, Thiel S, Jensen L, Nielsen HJ, Jensenius JC. Characteristics and biological variations of the pattern recognition molecule M-ficolin in plasma. J Innate Immun. 2010;2:167–80. doi: 10.1159/000218324. [DOI] [PubMed] [Google Scholar]
- 14.Krarup A, Sorensen UB, Matsushita M, Jensenius JC, Thiel SL. Effect of capsulation of opportunistic pathogenic bacteria on binding of the pattern recognition molecules mannan-binding lectin, L-ficolin, and H-ficolin. Infect Immun. 2005;73:1052–60. doi: 10.1128/IAI.73.2.1052-1060.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moller-Kristensen M, Jensenius JC, Jensen L, et al. Levels of mannan-binding lectin-associated serine protease-2 in healthy individuals. J Immunol Methods. 2003;282:159–67. doi: 10.1016/j.jim.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Terai I, Kobayashi K, Matsushita M, Fujita T. Human serum mannose-binding lectin (MBL)-associated serine protease- 1 (MASP- 1): determination of levels in body fluids and identification of two forms in serum. Clin Exp Immunol. 1997;110:317–23. doi: 10.1111/j.1365-2249.1997.tb08334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Degn SE, Jensen L, Gál P, et al. Biological variations of MASP-3 and MAp44, two splice products of the MASP1 gene involved in regulation of the complement system. J Immunol Methods. 2010;361:37–50. doi: 10.1016/j.jim.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Altman DG, Bland JM. Diagnostic tests 3: receiver operating characteristic plots. BMJ. 1994;309:188. doi: 10.1136/bmj.309.6948.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altman DG, Bland JM. Time to event (survival) data. BMJ. 1998;317:468–9. doi: 10.1136/bmj.317.7156.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honoré C, Rorvig S, Munthe-Fog L, et al. The innate pattern recognition molecule ficolin-1 is secreted by monocytes/ macrophages and is circulating in human plasma. Mol Immunol. 2008;45:2782–9. doi: 10.1016/j.molimm.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Ytting H, Christensen IJ, Thiel S, Jensenius JC, Nielsen HJ. Pre- and postoperative levels in serum of mannan-binding lectin associated serine protease-2 – a prognostic marker in colorectal cancer. Hum Immunol. 2008;69:414–20. doi: 10.1016/j.humimm.2008.05.005. [DOI] [PubMed] [Google Scholar]


