Abstract
Renal allograft survival is related directly to cell senescence. In the transplantation scenario many cellular events – participating as immunological and non-immunological factors – could contribute to accelerate this biological process, responsible for the ultimate fate of the graft. Mechanisms concerned in tolerance versus rejection are paramount in this outcome. For this reason, immunosuppressive treatment constitutes an extremely important decision to prevent organ dysfunction and, finally, graft loss. This study was conducted to document the proportion of CD4+/interleukin (IL)-17A+-, CD16+/indoleamine 2, 3-dioxygenase (IDO+)-, forkhead box protein P3 (FoxP3+)-expressing cells, senescent cells (p16INK4α) and the percentage of interstitial fibrosis (IF) in graft biopsies of kidney transplant recipients participating in the BENEFIT (Bristol-Myers Squibb IM103008) study. CD4+/IL-17A+, CD16+/IDO+, FoxP3+ and p16INK4α+ cells were evaluated by immunohistochemistry, and the percentage of IF by morphometry on graft biopsies obtained at time 0 (pre-implantation) and at 12 months post-transplant. Senescent cells and CD4+/IL-17A+ cells were increased among graft biopsies in subjects receiving cyclosporin A (CsA) compared to those under belatacept treatment. Meanwhile, CD16+/IDO+ and FoxP3+-expressing cells were lower in biopsies from CsA treatment compared to patients treated with Belatacept. Histological morphometric analyses disclosed more IF in 12-month CsA-treated patients in comparison to pre-implantation biopsy findings. Summing up, renal biopsies from patients receiving belatacept showed greater amounts of FoxP3+ cells and lower amounts of CD4+/IL-17A+ and senescent cells compared to patients under CsA treatment. Along with these findings, an increase in IF in annual CsA-treated-patients biopsies compared to pre-implantation and belatacept-treated patients were observed.
Keywords: belatacept, cyclosporin A, FoxP3+ expressing cells, kidney transplant, senescense
Introduction
Belatacept, a selective co-stimulation blocker, is a human fusion protein combining a modified extracellular portion of cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) with the constant-region fragment (Fc) of human immunoglobulin (Ig)G1[1]. Belatacept binds surface co-stimulatory ligands CD80 and CD86 of the antigen-presenting cells, preventing their interaction with CD28 on T cells (signal 2). Blockade of signal 2 inhibits T cell activation, promoting anergy and apoptosis [2]. Belatacept is designed to provide effective immunosuppression and avoid both renal and non-renal toxicities associated with calcineurin inhibitors.
Previous studies have demonstrated that kidney transplant recipients (KTR) immunosuppressed with belatacept showed a significantly higher glomerular filtration rate (GFR) at 12 and 36 months post-transplant and a lower incidence of chronic allograft nephropathy (IF/TA) compared with KTR receiving cyclosporin A (CsA) at 12 months [3,4].
Several mechanisms could be participating in the preservation that belatacept confers to renal function, certainly one of the most pursued objectives in KTR. These mechanisms involved the chronic nephrotoxic effect imposed to the graft by CsA with the known detrimental effect in renal function, even in the absence of acute rejection [5]. In contrast, belatacept is devoid of nephrotoxicity. Also, an intriguing mechanism could be offered to explain, at least in part, the superiority in graft function of belatacept-treated patients by the degree of cell senescence. Previous works shed some light on some of the CsA toxicity mechanisms involving accelerated cell senescence [6,7]. Using very sensitive laboratory methods, it was demonstrated that even at low doses CsA causes a significantly increased rate of cell apoptosis as well as up-regulation of cellular expression of p53. Consequently, it was concluded that CsA nephrotoxicity is caused by progressive apoptotic cell loss [6]. p53 expression is considered to be one of the genetically determined mechanisms of cell senescence [8,9]. If immunosuppression can cause stimulation of p53 expression, it might as well trigger the accelerated cell senescence.
Another possible mechanism that could explain the superiority in graft function of belatacept compared to CsA relates to the role in immune regulation of the intracellular enzyme indoleamine 2, 3-dioxygenase (IDO). IDO is an interferon (IFN)-γ-inducible enzyme which catalyses tryptophan. The effect of its activity is tryptophan deficiency. Tryptophan deficiency and kynurenine excess (breakdown product) have immunomodulatory effects, including suppressing lymphocyte responses, particularly by sensitizing them to apoptosis [10]. Increased IDO activities in transplanted cells have been demonstrated to have anti-rejection properties both in vitro and in vivo. Recently, CTLA4Ig was determined to have much of its effect via increased IDO activity in dendritic cells, which in turn induces T regulatory cells (Tregs), suggesting a possible peripheral tolerogenic pathway [11]. Thus, by ligating CD80 and CD86, belatacept induces expression of the tryptophan-degrading enzyme, IDO. In previous studies we have found that the percentages of IDO-expressing peripheral blood cells were comparable in patients receiving belatacept or CsA, except for a subpopulation of CD16+ monocytes, which were increased significantly in the group receiving belatacept [12].
Relevant to graft cellular expression is the increase proportion of forkhead box protein 3 (FoxP3+) Tregs in rejecting allografts in belatacept-treated patients. This finding has been proposed as a mechanism whereby belatacept can mitigate the severity of acute rejection and improve graft outcome [13]. Furthermore, GFR was significantly higher at 12 months post-transplant in the belatacept patients with history of acute rejection compared to the CsA patients without acute rejection events during the first post-transplant year [3]. This is in keeping with the concept that all immune responses involve both effector and Tregs, and that it is the balance between these two populations that determines the outcome of the response [14].
In this study we examined the proportion of senescence marker p16INK4α+-producing cells, CD16+/IDO+ cells, CD4+/IL-17A+ subset, and FoxP3+-expressing cells, as well as the percentage of IF in graft biopsies performed per protocol at time 0 (pre-implantation), and 12 months post-kidney transplantation (post-KT), from KT recipients (KTR) participating in the BENEFIT study, in three Mexican centres. The study was intended to elucidate possible mechanisms involved in the favourable renal function observed in belatacept-treated KTR, beyond the absence of vasoconstrictive properties of this biological product.
Materials and methods
Kidney transplant graft biopsies
All graft biopsies tissue included for analysis in this study are from primary KTR enrolled in the BENEFIT study in Hospital Miguel Hidalgo, Aguascalientes, Instituto Mexicano de Trasplantes, Morelos and Hospital Ignacio Morones Prieto, San Luis Potosí, in Mexico. Principal investigators from these centres (RRA, GMR, ACR) were invited to gather all the blocks of paraffin embedded tissue that remained from pre-implantation biopsies (baseline), and 12-month biopsies performed per protocol from every participant patient in the study at their centres. One hundred and four blocks of paraffin-embedded tissue were received for analysis; 56 corresponded to pre-implantation biopsies from an equal number of patients and 48 corresponded to 12-month graft biopsies. All the blocks were processed as described further.
Demographic relevant data from all the donors and recipients were gathered from the clinical files of every patient participating in the BENEFIT study, included in this analysis from the three centres. Similarly, data regarding donor prenephrectomy renal function and recipient renal function at 1- and 12-month post-KT was obtained. Renal function was evaluated by calculated GFR (cGFR) using the modification of diet in renal disease (MDRD) formula. Calculated delta GFR (Δ cGFR) 12 months – 1 month post-KT was also obtained for the KTR. Other information gathered included pretransplant %panel-reactive antibodies (PRA), and biopsy confirmed acute rejection (BCAR) episodes occurring during the first 12 months post-KT, classified according to Banff97.
Briefly, the BENEFIT is a 3-year, randomized, Phase III study conducted in approximately 100 sites globally in adults receiving a kidney transplant from a living or deceased donor with an anticipated cold ischaemia time <24 h. Patients (n = 666) were randomized 1 : 1 : 1 to a more or less intensive regimen of belatacept or CsA; all patients received basiliximab induction, mycophenolate mofetil and corticosteroids [3,4]. Co-primary endpoints were composite patient/graft survival, composite renal function [measured GFR (mGFR) < 60 ml/min/1·73 m2 at month 12 or a decrease in mGFR ≥ 10 ml/min/1·73 m2 from month 3 to month 12 and incidence of acute rejection].
This study was conducted with authorization of Bristol-Myers Squibb. The protocol was approved by the Committee of Medical Ethics of the participating institutions. All patients have given informed consent to participate in the study.
Histology and morphometric evaluation of interstitial fibrosis
Double-blinded histological analysis was performed on formalin-fixed paraffin-embedded tissue. In order to evaluate tissue architecture samples were stained by periodic acid Schiff (PAS) technique. To determine IF, 4-µm sections were stained with Picro-Sirius Red, a specific stain for collagen. Morphological analysis was performed with the Leica QUIPS image and analysis system (Leica Imaging systems Ltd, Cambridge, UK). Total area and fibrotic area were measured and the percentage of fibrotic area was calculated.
Immunohistochemistry
In order to determine senescence and FoxP3-expressing cells, 4-µm-thick sections of available formalin-fixed paraffin-embedded tissue – both pre-implantation and 12 months post-transplant – were placed on positively charged slides. Sections were deparaffinized and rehydrated through a series of xylene and graded alcohols. Endogenous peroxidase was blocked with 3% H2O2 for 20 min. A 3% normal serum was employed for 30 min as protein blocker. Tissues were incubated for 18 h at 4°C with mouse monoclonal anti-human p16INK4α IgG2a antibody at 10 µg/ml (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or with goat polyclonal anti-human FoxP3 IgG antibody at 10 µg/ml (Santa Cruz Biotechnology). Binding was detected by incubating sections for 60 min at room temperature with biotinylated donkey anti-goat IgG antibody or goat anti-mouse IgG antibody (ABC Staining System; Santa Cruz Biotechnology). Slides were incubated with horseradish peroxidase (HRP)–streptavidin for 45 min, followed by incubation with the peroxidase substrate 3,3′-diaminobenzidine (DAB) (Sigma, St Louis, MO, USA) for 10 min. The sections were counterstained with haematoxylin, dehydrated with alcohol and xylene and mounted in resin. Negative control staining was performed with normal human serum diluted 1:100, instead of primary antibody. The reactive blank was incubated with phosphate buffer saline–egg albumin (Sigma) instead of the primary antibody. Both controls excluded non-specific staining or endogenous enzymatic activities. At least two different sections were examined for each biopsy. FoxP3– and p16INK4α-expressing cells were assessed by estimating positively staining cells in two fields of cortex and two fields of medulla, and it was reported as the percentage of immunoreactive cells. Results are expressed as the mean ± standard error of the mean (s.e.m.) of cells quantified by the program Image Pro Plus version 5·1.1.
Double-staining procedure
To determine the subpopulation of CD16+/IDO+– expressing monocytes and CD4+/IL-17A+ cell subpopulation a simultaneous detection was performed. A second generation of EnVision™ G|2 Doublestain System (Dako, Glostrup, Denmark) was used for the simultaneous detection of two antigens present at low concentrations within one biopsy. The procedure is a sequential double staining where the first antigen was visualized using horseradish peroxidase (HRP)/3′3′-diaminobenzidine (DAB) and the second antigen was visualized using alkaline phosphatase (AP)/Permanent Red. Briefly, incubation of samples with 200 µl of dual endogenous enzyme block was performed for 5 min. This procedure inhibited endogenous AP, peroxidase, and pseudoperoxidase activity present in tissues. After blocking, tissues were incubated with 200 µl of normal serum as negative control, primary mouse monoclonal anti-CD4 IgG1 antibody or rabbit polyclonal anti-IDO IgG antibody (Santa Cruz Biotechnology) at 10 µg/ml for 10 min at room temperature. The samples were then incubated with 200 µl polymer/HRP reagent for 10 min. This reagent is an HRP-conjugated dextran polymer that also carries antibodies to mouse and rabbit immunoglobulins. The reaction was visualized by incubation of 200 µl DAB plus chromogen for 5–15 min. Tissues were incubated with 200 µl Doublestain Block reagent for 3 min and were then incubated with a normal serum as negative control or second primary mouse monoclonal anti-CD16 IgG1 antibody or rabbit polyclonal anti-Il-17A IgG antibody (Santa Cruz Biotechnology) at 10 µg/ml for 10 min at room temperature. In the next step 200 µl rabbit/mouse LINK was added for 10 min. This reagent is a dextran polymer carrying antibodies to mouse and rabbit immunoglobulins. Finally, tissues were incubated with 200 µl of polymer/AP reagent for 10 min. Reaction was visualized by incubation with 200 µl Permanent Red chromogen for 5–20 min. Tissues were counterstained with haematoxylin and mounted in aqueous mounting medium. At least two different sections were examined for each biopsy. Double-positive CD16/IDO- and CD4/IL-17-expressing cells were assessed by estimating positively staining cells in two fields of cortex and two fields of medulla, and was reported as the percentage of immunoreactive cells. Results are expressed as the mean ± s.e.m. of cells quantified by the program Image Pro Plus version 5·1·1.
Statistics
Statistical analysis was performed using the SigmaStat version 3·2 program using the one-way analysis of variance on ranks and by the Holm–Sidak method for all pairwise multiple comparison procedures. Data were expressed as the median, range and mean ± s.e.m. P-values smaller than or equal to 0·05 were considered as significant.
Results
Graft biopsies analysed
Once all the paraffin-embedded tissue were processed it was evident that not all the blocks contained enough tissue to perform all the immunohistochemistry markers planned for the study. Thus, we selected those specimens with sufficient tissue that allowed the programmed stainings. Thirty-six pre-implantation biopsies were analysed (belatacept n = 27; CsA n = 9), and 12-month graft biopsies were 23 (belatacept n = 15; CsA n = 8). It is worth mention that all 12-month biopsies analysed (n = 23) also had their corresponding pre-implantation biopsy analysed (Fig. 1).
Fig. 1.
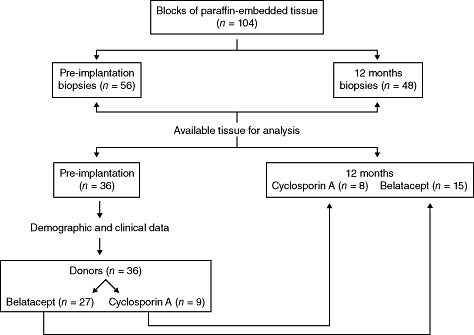
Kidney graft biopsies analysed.
The observer was blind to the corresponding biopsies evaluated regarding the treatment, i.e. belatacept or CsA, and whether the biopsy corresponded to pre-implantation or 12 months post-KT.
Demographic and clinical data
Table 1 summarizes the demographic, clinical characteristics and graft function data of donors and recipients. Data corresponded to all the donors and KTR patients for whom pre-implantation biopsies were analysed, n = 36 (belatacept n = 27; CsA n = 9).
Table 1.
Demographic and clinical data of donors and kidney transplant patients
| Variables | Belatacept n = 27 | CsA n = 9 | P value |
|---|---|---|---|
| Donor's age (years); mean ± s.d. | 33·8 ± 11·2 | 29·8 ± 9·6 | n.s. |
| Female donor, n (%) | 12 (44·4) | 4 (44·4) | n.s. |
| Living donor, n (%) | 23 (85·2) | 8 (88·9) | n.s. |
| Donor pre-implantation cGFR (MDRD) (ml/min); mean ± s.d. | 100·7 ± 23·9 | 108·2 ± 17·8 | n.s. |
| Recipient age (years); mean ± s.d. | 32·1 ± 10·2 | 30·8 ± 11·7 | n.s. |
| Female recipient, n (%) | 11 (40·7) | 3 (33·3) | n.s. |
| PRA (%) | 1·81 (0–37) | 1·08 (0–27) | n.s. |
| Acute rejection during 1st year | 2 (Banff IA, Banff III) | 1 (Banff IA) | n.s. |
| Borderline | 0 | 1 | |
| Recipient cGFR (MDRD) at 1 month (ml/min); mean ± s.d. | 70·9 ± 21·2 | 76·8 ± 15·8 | n.s. |
| Recipient cGFR (MDRD) at 12 months (ml/min); mean ± s.d. | 77·9 ± 29·7 | 69·8 ± 18·0 | n.s. |
| Δ cGFR (MDRD) [12–1 months] (ml/min); mean ± s.d. | 6·2 ± 17·2 | −6·2 ± 20·3 | 0·029 |
CsA: cyclosporin A; cGFR: glomerular filtration rate calculated by modification of diet in renal disease (MDRD) formula; PRA: panel-reactive antibodies; s.d.: standard deviation; n.s.: not significant.
No differences were found in donor and recipient characteristics; however, cGFR at 12 months showed improvement in belatacept patients compared to CsA-treated patients; moreover, calculated Δ GFR (12 months–1 month, post-KT) demonstrated a mean gain of 6·2 ± 17·2 ml/min for belatacept-treated patients in contrast to a slope in this parameter in CsA-treated patients (−6·2 ± 20·3 ml/min), P = 0·029.
As depicted in Table 1, the number of BCAR was low for both groups and none of them conditioned graft loss. Therefore, the possible influence that BCAR events occurring during the first year post-KT could have had in the 12-month graft function difference between patients under belatacept versus CsA treatment was probably null.
Similarly, in order to define the possible impact imposed by acute rejection on the degree of IF at month 12, the number of BCAR events during the first year post-KT was evaluated for the belatacept and CsA patients included in the 12-month graft biopsies analysis (belatacept n = 15, CsA n = 8), and none of these patients experienced BCAR during this period.
Interstitial fibrosis and inflammatory infiltrates
Fibrosis was evident in both treated groups. However, as shown in Fig. 2, the percentage of fibrosis in belatacept-treated patients was significantly lower than in the CsA-treated group (P = 0·024).
Fig. 2.
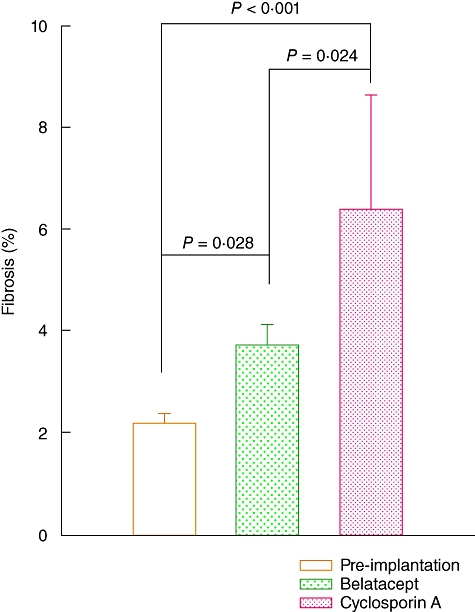
Interstitial fibrosis in graft biopsies from kidney transplant recipients (KTR) under belatacept or cyclosporin A (CsA) treatment. Pre-implantation: n = 36 biopsies; belatacept: n = 27 biopsies (only 15 samples had their corresponding pre-implantation biopsy); CsA: n = 9 biopsies (only eight samples had their corresponding pre-implantation biopsy). Results are expressed as mean ± standard error.
Fibrosis was related to glomerulosclerosis, interstitial fibrosis, tubular atrophy, arteriolar hyalinosis and fibrointimal thickening and interstitial infiltrates. They were distributed in two patterns, diffuse infiltrates and periglomerular/perivascular aggregates. The composition was mainly of mononuclear cells (Fig. 3b,c).
Fig. 3.
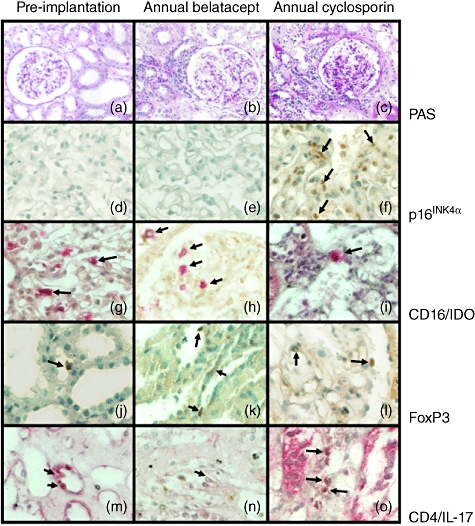
Differences in senescence and immunological characteristics in graft biopsies from kidney transplant recipients (KTR) under belatacept or cyclosporin A (CsA) treatment. (a) Photomicrograph of tissue architecture of a representative kidney biopsy at pre-implantation, stained by periodic acid Schiff (PAS technique) (×200). (b) Histological image of kidney transplant recipient biopsy from a patient receiving belatacept at 12 months post-transplant, stained by PAS technique (×200). (c) Photomicrograph of kidney transplant recipient biopsy from a patient under CsA treatment at 12 months post-transplant, stained by PAS technique (×200). (d) p16INK4a-expression in kidney biopsy at pre-implantation (×520). (e) p16INK4a-expression in transplant recipient biopsy from a patient receiving belatacept at 12 months post-transplant (×520). (f) p16INK4a- expressing cells in transplant recipient biopsy from a patient under CsA treatment at 12 months post-transplant (×520). (g) CD16+ (in pink)/indoleamine 2, 3-dioxygenase (IDO+) (in brown) expression in kidney biopsy at pre-implantation (×520). (h) CD16+/IDO+-expressing cells in transplant recipient biopsy from a patient receiving belatacept at 12 months post-transplant (×520). (i) CD16+/IDO+ expression in transplant recipient biopsy from a patient under CsA treatment at 12 months post-transplant (×520). (j) Forkhead box protein 3 (FoxP3) expression in kidney biopsy at pre-implantation (×520). (k) FoxP3+-expressing regulatory T cells in transplant recipient biopsy from a patient receiving belatacept at 12 months post-transplant (×520). (l) FoxP3 expression in transplant recipient biopsy from a patient under CsA treatment at 12 months post-transplant (×520). (m) Interleukin (IL)-17A-producing CD4 T cells [CD4+ (in brown)/IL-17A+ (in pink)] cells in kidney biopsy at pre-implantation (×520). (n) IL-17A-producing CD4 T cells in transplant recipient biopsy from a patient receiving belatacept at 12 months post-transplant (×520). (o) IL-17A-producing CD4 T cells in transplant recipient biopsy from a patient under CsA treatment at 12 months post-transplant (×520). Arrows depict immunoreactive cells.
Effect of belatacept compared with CsA on senescence
In order to determine the effect of CsA and belatacept on cell senescence, p16INK4α+ immunoreactive cells were quantitated in renal graft biopsies. A higher percentage of p16INK4α-expressing tissue cells were observed in KTR under CsA treatment compared to belatacept and pre-implantation (18·1 ± 3·0 versus 10·3 ± 1·0 versus 10·8 ± 1·9 immunoreactive cells; P ≤ 0·021; Figs 3d–f and 4).
Fig. 4.
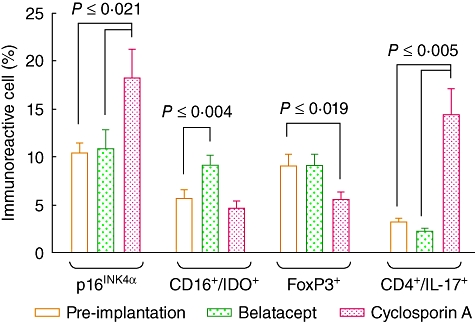
Percentage of senescent cells, monocyte-derived indoleamine 2, 3-dioxygenase (IDO)-, forkhead box protein 3 (FoxP3)- and CD4/interleukin (IL)-17A-expressing cells in graft biopsies from kidney transplant recipients (KTR) under belatacept or cyclosporin A (CsA) treatment. Pre-implantation: n = 36 biopsies; belatacept: n = 27 biopsies (only 15 samples had their corresponding pre-implantation biopsy); CsA: n = 9 biopsies (only eight samples had their corresponding pre-implantation biopsy). Results are expressed as mean ± standard error. Statistical analysis: one-way analysis of variance; Holm–Sidak test.
Effects of belatacept compared with CsA in CD16+/IDO+ cells
Evaluation of tissue from KTR receiving belatacept showed a statistically significant higher percentage of IDO-expressing CD16+ cells than those under calcineurin inhibitor treatment and pre-implantation (9·6 ± 0·6 versus 4·6 ± 0·8 versus 5·6 ± 0·9 immunoreactive cells; P ≤ 0·04; Figs 3g–i and 4).
Effects of belatacept compared with CsA in FoxP3-expressing tissue cells
Regarding to FoxP3-expressing cells, it is noteworthy that renal graft biopsies from patients receiving CsA had a significant decrease in this subset compared to belatacept and pre-implantation (5·5 ± 0·8 versus 9·1 ± 1·1 versus 9·0 ± 1·2 immunoreactive cells; P ≤ 0·019; Figs 3j–l and 4).
Effects of belatacept compared with CsA in IL-17A-producing CD4 T cells
A low IL-17A-expressing CD4+ T cell numbers were found at pre-implantation and belatacept-treated groups compared to calcineurin inhibitor treatment (3·2 ± 0·4 versus 2·2 ± 0·3 versus 14·2 ± 2·6 immunoreactive cells; P ≤ 0·005; Figs 3m–o and 4).
Discussion
The introduction of the immunosuppressive calcineurin inhibitor CsA in the 1980s dramatically improved organ transplantation outcome. Later, however, CsA was found to be nephrotoxic, causing stripped tubulointerstitial fibrosis, tubular atrophy and afferent arteriolopathy [15,16]. The new selective co-stimulation blocker belatacept lacks nephrotoxicity and seems to act more as an immunomodulatory, rather than an immunosuppressive agent.
Herein, we evaluated biochemical and immunological aspects in renal graft biopsies from patients under belatacept or CsA treatment.
Several reports have shown that calcineurin inhibitors induce p21WAF and p16INK4α, proteins related to senescence phenotype [7]. The former is associated with telomeric shortening, while the latter has an independent mechanism of telomere shortening [17,18]. Thus, it was important to compare a senescence biomarker in belatacept or CsA-treated patients. CsA was a more potent inductor of p16INK4α by far than was belatacept at 12 months post-transplant. It is worth mention that p16INK4α expression in the belatacept group was comparable to pre-implantation graft values, while CsA almost doubles the pre-implantation expression. These data confirm previous reports that showed senescence induced phenotype by CsA, and in this regard clearly suggest an innocuous effect of belatacept which translates into a plausible mechanism of graft function preservation with this therapy. It is interesting to note that, as a group, at 12 months cGFR improved in the belatacept patients compared to the CsA-treated patients. Also the calculated Δ GFR was significantly better. This difference in graft function between belatacept and CsA patients has been reported previously in the BENEFIT study (which included 666 patients), although to a lesser extent to that found in this substudy. In this regard, it is tempting to speculate that the reasons for this finding relate to a higher proportion of living donors as well as younger donor age (∼10 years), and recipient age participating in this substudy compared to the whole patient population included in the BENEFIT study [3].
Another biochemical marker involved in graft tolerance is IDO expression [19]. We have shown previously the presence of a peripheral CD16+/IDO+ monocyte subpopulation in patients under belatacept treatment and proposed that this peculiar cell population may be a systemic manifestation of the in-situ graft quiescence induced by this biological treatment [12]. Our present results complement this initial observation. Indeed, a significantly higher proportion of this IDO+/CD16+ cell subpopulation was found in renal biopsies at annual evaluation when compared to pre-implantation values in the belatacept specimens, while no differences were found in the CsA specimens. These results support the notion that belatacept has a more prominent immunomodulatory, rather than immunosuppressive, role inducing a tolerance profile through up-regulation of functional IDO expression in organ transplantation [20].
It has been shown that IL-17A-expressing CD4+ T cells, more than IFN-γ-producing CD4+ Th1 cells, play an important role in rejection and vasculopathy in clinical solid organ transplantation [21–23]. Twelve-month transplant specimens from CsA-treated patients showed important mononuclear cell infiltrates in which IL-17A-producing CD4+ T cells were clearly evident, even in the absence of acute rejection criteria; this process was accompanied by fibrosis and hyaline material. By contrast, in the belatacept treatment arm a scarce infiltrate of mononuclear cells was noticed with extremely few IL-17A-producing CD4+ T cells and a better preserved tissue architecture in terms of less fibrosis compared to those biopsies from CsA-treated patients. Thus, it is reasonable to speculate that belatacept might show a more prominent immunomodulatory rather than immunosuppressive role, allowing a certain degree of inflammation. It may act to modulate the turnover of extracellular matrix avoiding fibrosis, thus mitigating renal function deterioration and a lower incidence of interstitial fibrosis at 12 months among belatacept- versus CsA-treated patients [3,24]. The limited number of biopsies evaluated makes it difficult to speculate about the origin of increased interstitial fibrosis observed in 12-month graft biopsies from patients under CsA. However, the related increased interstitial inflammation by IL-17A-producing CD4+ T cells observed in these biopsies suggests an ongoing persistent chronic alloimmune response, resulting in increased presence of inflammatory infiltrates and interstitial fibrosis and more senescence. Previous studies demonstrated that concomitant interstitial fibrosis and inflammation in protocol biopsies obtained in the first year correlates with later allograft dysfunction and loss [25].
Finally, belatacept treatment allowed maintaining a stable number of FoxP3+ cells at tissue level which, in turn, could contribute to preserve in-situ tolerance mechanisms [26]. Although the levels of FoxP3+-expressing cells were not statistically different at 12 months between both group specimens, CsA was associated with a significant decrease of FoxP3+ cell percentages when compared to pre-implantation levels, which is in agreement with a previous study where CsA and rapamycin were compared [27].
Overall, our results show that belatacept elicit a clear-cut different immunological scenario at the tissue level than that induced by CsA. While the latter leads to cellular senescence and a CD4+/IL-17A+ proinflammatory infiltrate, belatacept seems to have fewer deleterious effects on the cellular fate and do not tend to favour a conspicuous inflammatory infiltrate. Even though these data do not enable us to establish a cause-and-effect relationship between the infiltrates and clinical improvement, the link between them is amply suggestive that this indeed could be the case.
Nevertheless, our study suggests that belatacept confers a better tissue profile at 12 months post-transplantation in terms of senescence and immune regulation than CsA. Certainly, belatacept has to be considered with other new immunomodulatory/immunosuppressive agents in order to find its right place in the current therapeutic armamentarium for organ transplantation.
Acknowledgments
The study was supported partially by Bristol-Myers Squibb, and by a research grant of CONACYT (Mexico) SALUD-2009-C01-115268.
Disclosure
The authors have no financial conflicts of interest.
References
- 1.Larsen CP, Pearson TC, Adams AB, et al. Rational development of LEA29Y (belatacept), a high affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5:443–53. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 2.Sayegh MH, Turka LA. The role of T-cell costimulatory activation pathways in transplant rejection. N Engl J Med. 1998;338:1813–21. doi: 10.1056/NEJM199806183382506. [DOI] [PubMed] [Google Scholar]
- 3.Vincenti F, Charpentier B, Vanrenterghem Y, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study) Am J Transplant. 2010;10:535–46. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 4.Vincenti F, Larsen CP, Alberú J, et al. Three-year outcomes from BENEFIT: a phase III study of belatacept vs. cyclosporine in kidney transplant recipients. Am J Transplant. 2011;11(Suppl. s2):227. Abstract. [Google Scholar]
- 5.Snanoudj R, Royal V, Elie C, et al. Specificity of histological markers of long-term CNI nephrotoxicity in kidney-transplant recipients under low-dose cyclosporine therapy. Am J Transplant. 2011 doi: 10.1111/j.1600-6143.2011.03718.x. doi: 10.1111/j.1600-6143.2011.03718.x. [DOI] [PubMed] [Google Scholar]
- 6.Amore A, Emancipator SN, Cirina P, et al. Nitric oxide mediates cyclosporine-induced apoptosis in cultured renal cells. Kidney Int. 2000;57:1549–59. doi: 10.1046/j.1523-1755.2000.00999.x. [DOI] [PubMed] [Google Scholar]
- 7.Horike K, Takeda A, Yamaguchi Y, et al. Is arteriolar vacuolization a predictor of calcineurin inhibitor nephrotoxicity? Clin Transpl. 2011;25(Suppl. s23):23–7. doi: 10.1111/j.1399-0012.2011.01474.x. [DOI] [PubMed] [Google Scholar]
- 8.Kohsaka S, Sasai K, Takahashi K, et al. A population of BJ fibroblasts escaped from Ras-induced senescence susceptible to transformation. Biochem Biophys Res Commun. 2011;410:878–84. doi: 10.1016/j.bbrc.2011.06.082. [DOI] [PubMed] [Google Scholar]
- 9.Duncan EL, Wadhwa R, Kaul SC. Senescence and immortalization of human cells. Biogerontology. 2000;1:103–21. doi: 10.1023/a:1010000132671. [DOI] [PubMed] [Google Scholar]
- 10.Mulley WR, Nikolic-Paterson DJ. Indoleamine 2,3-dioxygenase in transplantation. Nephrology. 2008;13:204–11. doi: 10.1111/j.1440-1797.2007.00921.x. [DOI] [PubMed] [Google Scholar]
- 11.Grohmann U, Orabona C, Fallarino F, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 12.Furuzawa-Carballeda J, Lima G, Uribe-Uribe N, et al. High levels of IDO-expressing CD16+ peripheral cells, and Tregs in graft biopsies from kidney transplant recipients under belatacept treatment. Transplant Proc. 2010;42:3489–96. doi: 10.1016/j.transproceed.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 13.Bluestone JA, Liu W, Yabu JM, et al. The effect of costimulatory and interleukin 2 receptor blockade on regulatory T cells in renal transplantation. Am J Transplant. 2008;8:2086–96. doi: 10.1111/j.1600-6143.2008.02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng XX, Sánchez-Fueyo A, Sho M, et al. Favorably tipping the balance between cytopathic and regulatory T cells to create transplantation tolerance. Immunity. 2003;19:503–14. doi: 10.1016/s1074-7613(03)00259-0. [DOI] [PubMed] [Google Scholar]
- 15.Chapman JR. Chronic calcineurin inhibitor nephrotoxicity – lest we forget. Am J Transplant. 2011;11:693–7. doi: 10.1111/j.1600-6143.2011.03504.x. [DOI] [PubMed] [Google Scholar]
- 16.Jennings P, Koppelstaetter Ch AS, et al. Cyclosporine A induces senescence in renal tubular epithelial cells. Am J Physiol Renal Physiol. 2007;293:F831–8. doi: 10.1152/ajprenal.00005.2007. [DOI] [PubMed] [Google Scholar]
- 17.Gan Q, Huang J, Zhou R, et al. PPAR accelerates cellular senescence by inducing p16INK4α expression in human diploid fibroblasts. J Cell Science. 2008;121:2235–45. doi: 10.1242/jcs.026633. [DOI] [PubMed] [Google Scholar]
- 18.Cammarano MS, Nekrasova T, Noel B, Minden A. Pak4 induces premature senescence via a pathway requiring p16INK4/p19ARF and mitogen-activated protein kinase signaling. Mol Cell Biol. 2005;25:9532–42. doi: 10.1128/MCB.25.21.9532-9542.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ge W, Jiang J, Arp J, Liu W, Garcia B, Wang H. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation. 2010;90:1312–20. doi: 10.1097/TP.0b013e3181fed001. [DOI] [PubMed] [Google Scholar]
- 20.Cook CH, Bickerstaff AA, Wang JJ, et al. Spontaneous renal allograft acceptance associated with ‘regulatory’ dendritic cells and IDO. J Immunol. 2008;180:3103–12. doi: 10.4049/jimmunol.180.5.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan X, Paez-Cortez J, Schmitt-Knosalla I, et al. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J Exp Med. 2008;13:3133–44. doi: 10.1084/jem.20081937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atalar K, Afzali B, Lord G, Lombarde G. Relative roles of Th1 and Th17 effector cells in allograft rejection. Curr Opin Organ Transplant. 2009;14:23–9. doi: 10.1097/MOT.0b013e32831b70c2. [DOI] [PubMed] [Google Scholar]
- 23.Deteix C, Attuil-Audenis V, Duthey A, et al. Intragraft Th17 infiltrate promotes lymphoid neogenesis and hastens clinical chronic rejection. J Immunol. 2010;184:5344–51. doi: 10.4049/jimmunol.0902999. [DOI] [PubMed] [Google Scholar]
- 24.Vincenti F, Larsen CH, Durrbach A, et al. Costimulation blockade with Belatacept in renal transplantation. N Engl J Med. 2005;353:770–81. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 25.Park WD, Griffin MD, Cornell LD, Cosio FG, Stegall MD. Fibrosis with inflammation at one year predicts transplant functional decline. J Am Soc Nephrol. 2010;21:1987–97. doi: 10.1681/ASN.2010010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thebault P, Condamine T, Heslan M, et al. Role of IFN-γ in allograft tolerance mediated by CD4+CD25+regulatory T cells by induction of IDO in endothelial cells. Am J Transplant. 2007;7:2472–82. doi: 10.1111/j.1600-6143.2007.01960.x. [DOI] [PubMed] [Google Scholar]
- 27.Kopf H, de la Rosa GM, Howard OM, Chen X. Rapamycin inhibits differentiation of Th17 cells and promotes generation of Foxp3+ T regulatory cells. Int Immunopharmacol. 2007;7:1819–24. doi: 10.1016/j.intimp.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]


