Abstract
One of the promising approaches in the therapy of ulcerative colitis is administration of butyrate, an energy source for colonocytes, into the lumen of the colon. This study investigates the effect of butyrate producing bacterium Clostridium tyrobutyricum on dextran sodium sulphate (DSS)-induced colitis in mice. Immunocompetent BALB/c and immunodeficient severe combined immunodeficiency (SCID) mice reared in specific-pathogen-free (SPF) conditions were treated intrarectally with C. tyrobutyricum 1 week prior to the induction of DSS colitis and during oral DSS treatment. Administration of DSS without C. tyrobutyricum treatment led to an appearance of clinical symptoms – bleeding, rectal prolapses and colitis-induced increase in the antigen CD11b, a marker of infiltrating inflammatory cells in the lamina propria. The severity of colitis was similar in BALB/c and SCID mice as judged by the histological damage score and colon shortening after 7 days of DSS treatment. Both strains of mice also showed a similar reduction in tight junction (TJ) protein zonula occludens (ZO)-1 expression and of MUC-2 mucin depression. Highly elevated levels of cytokine tumour necrosis factor (TNF)-α in the colon of SCID mice and of interleukin (IL)-18 in BALB/c mice were observed. Intrarectal administration of C. tyrobutyricum prevented appearance of clinical symptoms of DSS-colitis, restored normal MUC-2 production, unaltered expression of TJ protein ZO-1 and decreased levels of TNF-α and IL-18 in the descending colon of SCID and BALB/c mice, respectively. Some of these features can be ascribed to the increased production of butyrate in the lumen of the colon and its role in protection of barrier functions and regulation of IL-18 expression.
Keywords: butyrate, Clostridium tyrobutyricum, confocal fluorimetry, dextran sodium sulphate-induced colitis, SCID mice
Introduction
The enteric mucosa of ulcerative colitis (UC) patients is aberrant due to the abnormal interaction between microbiota and the intestinal mucosal immune system, which leads to mucosal inflammation [1,2]. In experimental dextran sodium sulphate (DSS) models of colitis, interaction of components from both pathogenic and commensal microorganisms with the host mucosal immune system can trigger inflammatory responses and alter the colonic function [3–5]. Under germ-free conditions mice develop markedly limited incidence of colitis [6]. One of the widely used experimental mouse models of UC involves addition of DSS to the drinking water, which causes rapid alterations in the inner colon mucus layer, making it permeable to bacteria [7,8]. Protection of colonic mucosa requires normal production of mucins, such as secretory MUC-2, synthesized by goblet cells in healthy colon of humans, rats and mice [9]. However, in experimental ulcerative colitis the mucus production is impaired [10]. Butyrate ameliorates inflammation in experimental ulcerative colitis by up-regulating the expression of mucin genes [11,12] and by protecting mucosal surfaces against increased mucosal permeability [13]. Several probiotics have been reported to enhance epithelial permeability and/or protect against barrier disruption by pathogens in vitro (reviewed recently [14]). Recently a study was performed in human volunteers which showed that perfusion of Lactobacillus plantarum into the duodenum increased the localization (immunofluorescent staining) of occludin and zonula occludens (ZO)-1 in the epithelial tight junctions (TJs) of tissue biopsies [15]. Protection of mice against DSS induced colitis by probiotic E. coli Nissle 1917 has been associated with increased expression of TJ protein zonula occludens (ZO-2) expression in epithelial cells [16].
Ulcerative colitis is associated with an elevated production of inflammatory cytokines such as tumour necrosis factor (TNF)-α and interleukin (IL)-1β, leading to increased intestinal permeability and activation of nuclear factor (NF)-κB and c-Jun N-terminal kinase (JNK)/p38 mitogen-activated protein kinase (MAPK) pathways [17]. Decreased production of proinflammatory cytokines constitutes an important mechanism for the partial amelioration of colitis by probiotics [18,19].
Butyrate enemas have been reported to be effective in therapy of UC. Microbially produced butyrate is considered important for colonic health and in the prevention of colorectal cancer, owing to its use as an energy source for colonocytes and as a modulator of oxidative stress and inflammation [20]. Oral administration of Clostridium butyricum M 588, characterized by high production of butyrate during fermentative growth, has been shown to protect against DSS colitis in the mouse [21]. Our candidate probiotic Clostridium tyrobutyricum (DSM 2637) was isolated from raw cow's milk and is a Gram-positive, rod-shaped, spore-forming obligate anaerobe that can ferment a wide variety of carbohydrates to butyric acid. In a survey of 35 Clostridium species and 243 strains, C. tyrobutyricum was shown not to produce cytotoxins [22]. To verify further the safety of C. tyrobutyricum strain DSM 2637 we assessed its potential to translocate from the gut into the blood and organs of germ-free severe combined immunodeficiency (SCID) mice that were monocolonized for 30 days in a gnotobiotic isolator. All animals colonized with C. tyrobutyricum strain DSM 2637 remained healthy and lacked any signs of pathology. As expected, C. tyrobutyricum was found in the lumen of the jejunum, ileum, colon ascendens and colon descendens, but could not be detected in the blood, mesenteric lymph nodes (MLNs), liver or spleen.
We have shown previously that immunodeficient SCID (T and B cell-independent) and immunocompetent BALB/c mouse strains reared under specific-pathogen-free (SPF) conditions, but not as germ-free mice, develop colitis after 1 week of DSS treatment [6]. The BALB/c mice survive two rounds of DSS treatment, albeit with clear evidence of colonic damage and T and B cell infiltration in the mucus, whereas SCID mice survive only one round of DSS treatment [6]. The main objectives of this study were to investigate the role of the immune response in regulating colitis in SCID and BALB/c mice and to evaluate the potential protective effect of C. tyrobutyricum (DSM 2637) on DSS colitis development. Changes in intestinal mucins, barrier function of TJ and production of the inflammatory cytokines IL-18 and TNF-α were measured during colitis induction. As butyrate enemas and colonic application of faecal bacteria [23] have been shown to be beneficial in clinical studies we also evaluated the therapeutic potential of intrarectal administration of C. tyrobutyricum in the DSS colitis model.
Materials and methods
Animals
BALB/c and SCID (background BALB/cJHanHsd-SCID) mice were reared in SPF conditions. The absence of T lymphocytes in SCID mice was proved with fluorescein isothiocyanate (FITC)-labelled monoclonal anti-CD3 antibody (Serotec, Oxford, UK) using fluorescence activated cell sorter (FACS)Calibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). Two-month-old mice were used for these studies and their body weights were measured before and after each experiment.
Bacterial strain and culture conditions
C. tyrobutyricum (DSM 2637) isolated from raw cow's milk was provided from the Food Research Institute, Prague, Czech Republic and cultured in sterile Bryant Burkey bouillon with resazurin and lactate (Merck KGaA, Darmstadt, Germany) at 37°C under anaerobic conditions. Prior to administration to mice a fresh overnight culture of bacteria was adjusted to 109 colony-forming units (CFU)/ml in saline.
Intrarectal administration of C. tyrobutyricum and induction of acute ulcerative colitis by DSS
The experimental groups of five to 10 mice and their respective treatments are shown in Table 1. Groups 1, 2, 4 and 5 (Table 1) received 2·5% DSS (molecular weight 40 kDa; ICN Biomedicals, Cleveland, OH, USA) in drinking water ad libitum for 1 week. The untreated control groups 3 and 6 received only drinking water. The C. tyrobutyricum-treated groups 1 and 4 received intrarectally (via tubing) a daily dose of 2 × 108 CFU of strain DSM 2637 in 0·2 ml saline for 7 days prior to DSS exposure and also during the 7 days exposure to DSS in the drinking water. Control groups 3 and 6 received 0·2 ml saline [phosphate-buffered saline (PBS)]. The following clinical symptoms were measured or assessed: firmness of faeces, rectal prolapses, rectal bleeding and colon length after the mice were killed. The colon descendens was divided into two pieces, one being used for TNF-α determination after 48 h culture and the other for histological assessment. Animal experiments were approved by the Ethical Committee of the Institute of Microbiology, Academy of Sciences of the Czech Republic, v.v.i.
Table 1.
Development of DSS-colitis with association of Clostridium tyrobutyricum in conventional BALB/c and severe combined immunodeficiency (SCID) mice: clinical and histological gradings and detection of tumour necrosis factor (TNF)-α
| Body weight | Damage score | Mucin secretion | Length of colon | TNF-α | ||||
|---|---|---|---|---|---|---|---|---|
| Strain | Treatment | 2·5% DSS | Mortality/n | (g) | (0–4) | (4–0) | (cm) | (pg/10 mg tissue) |
| 1 BALB/c | C. tyrobutyricum (i.r.) | + | 0/5 | 18·8 ± 1·2 | 1·6 ± 0·5ΔΔΔ | 2·7 ± 0·4ΔΔΔ | 7·6 ± 0·5# | 27·9 ± 12·7## |
| 2 BALB/c | saline (i.r.) | + | 0/5 | 17·6 ± 1·9 | 3·9 ± 0·2ΔΔΔ | 0·1 ± 0·2ΔΔΔ | 6·9 ± 0·6# | 29·3 ± 15·3## |
| 3 BALB/c | saline (i.r.) | – | 0/5 | 19·6 ± 0·5 | 0 | 4 | 9·8 ± 0·7 | 7·8 ± 5·0 |
| 4 SCID | C. tyrobutyricum (i.r.) | + | 1/10 | 21·0 ± 2·1 | 0·5 ± 0·4ΔΔΔ | 3·5 ± 0·5ΔΔΔ | 10·0 ± 1·9* | 25·2 ± 5·2**,## |
| 5 SCID | saline (i.r.) | + | 2/10 | 20·0 ± 2·4 | 3·9 ± 0·1ΔΔΔ | 0·1 ± 0·2ΔΔΔ | 7·8 ± 0·8# | 79·3 ± 8·0## |
| 6 SCID | saline (i.r.) | – | 0/8 | 21·5 ± 1·2 | 0 | 4 | 10·9 ± 0·3 | 11·0 ± 2·2 |
Values are means ± standard deviation
P < 0·05
P < 0·01, significant difference of group 4 versus group 5
P < 0·0001, significant difference of group 1 versus group 2 and group 4 versus group 5 of mice
P < 0·05
P < 0·01, significant difference of groups 1 and 2 versus control group 3 of BALB/c mice and group 4 and 5 versus control group 6 of SCID mice; i.r.: intrarectal administration. DSS: dextran sodium sulphate.
Histological evaluation of inflammation
The tissue was fixed in Carnoy's fluid for 30 min, transferred into 96% ethanol and embedded in paraffin. Five-µm paraffin-embedded sections were cut and stained with haematoxylin and eosin (H&E) and Alcian Blue and post-stained with Nuclear Fast Red (all from Vector, Burlingame, CA, USA) for mucin production. The samples were viewed under an Olympus BX 40 microscope equipped with an Olympus Camedia DP 70 digital camera, and the images were analysed using Olympus DP-Soft. The degree of damage to the surface epithelium, crypt distortion and mucin production in individual colon segments were evaluated according to Cooper et al. [3].
Expression of CD 11b, ZO-1 and MUC-2
Segments of the colon descendens were frozen in liquid nitrogen. Cryosections (5 µm thick) of acetone-fixed colon were used for immunocytochemistry. The membrane marker CD11b was detected directly by fluorescein-labelled monoclonal antibody anti-CD11b FITC (Serotec, Kidlington, UK). The antigen CD11b is known to be expressed on the surface of polymorphonuclear leucocytes, monocytes and natural killer (NK) cells. Expression of ZO-1 was detected by rabbit anti-mouse polyclonal antibody (Zymed Laboratories, Carlsbad, CA, USA) and secondary antibody Cy3 goat anti-rabbit IgG (Biomeda, Burlingame, CA, USA). Production of MUC-2 was detected by primary, polyclonal rabbit anti-mouse IgG, specific for MUC-2, and secondary antibody Cy3 goat anti-rabbit IgG (all from Biomeda).
Measurement of colonic TNF-α production
Pre-weighed colonic fragments were cultured in RPMI-1640 medium enriched with 10% bovine serum albumin in 5% CO2 and 95% air at 37°C, in 24-well flat-bottomed plates (Nunc, Roskilde, Denmark) for 48 h. Quantification of TNF-α level was performed by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's protocols using Infinite 200 apparatus (Tecan Group Ltd, Grödig, Austria).
Measurment of IL-18 in tissues by confocal fluorimetry
Expression of IL-18 in acetone-fixed sections of colon descendens was detected by polyclonal rabbit antibody to mouse IL-18 (Acris Antibodies, Hiddenhausen, Germany) and Cy3-labelled goat anti-rabbit IgG (Biomeda). Relative confocal fluorimetry of IL-18 visualized by Cy3 staining was performed by laser scanning confocal microscopy using Leica SPE and Leica SP-2 microscopes with oil immersion objectives ×20, ×40 and ×63 and an excitation line of 532 nm and emission detection at 550–700 nm; a multiple accumulation of weaker signal was used. Fluorescence intensity of Cy3 in epithelial colonocytes was evaluated (expressed in units on a scale of 0–255) using different regions and sections of at least three mice per group. It was possible to distinguish regions with lower and higher amounts of IL-18 (Table 2) and evaluate them separately, using correct statistical methods.
Table 2.
Evaluation of fluorescence for interleukin (IL)-18 in the colon of mice
| Mouse strain | SCID | BALB/c | ||||
|---|---|---|---|---|---|---|
| DSS | – | + | + | – | + | + |
| C. tyrobutyricum | – | – | + | – | – | + |
| IL-18 (lower intensity) | 29·1 ± 7·2 | 19·2 ± 9·0 | 77·9 ± 15·8 | 34·8 ± 11·7 | 82·7 ± 6·6 | 50·7 ± 6·3 |
| IL-18 (higher intensity) | 76·5 ± 4·8 | 151 ± 25·4 | 161·9 ± 32·3 | 72·2 ± 11·1 | 146·4 ± 15·0 | 98·2 ± 11·8 |
Values are expressed as means ± standard deviation (s.d.) in units of fluorescence intensity on scale 0–255. SCID: severe combined immunodeficiency; DSS: dextran sodium sulphate.
Measurement of short-chain fatty acids (SCFA)
SCFAs were measured in bacterial cultures and in faecal samples of SCID mice. Concentrations of acetic acid, propionic acid, n-butyric acid, iso-butyric acid, valeric acid, iso-valeric acid and 2-methylbutyric acid were measured using gas chromatography on a HP 5890 GC with flame ionization detector (FID) and nitrogen as carrier gas at 1·2 ml/min column flow. The samples were analysed on an Equity-1 column (30 m × 0·32 mm i.d., 1 µm film thickness; Supelco, Prague, Czech Republic). A 1-µl sample was injected into the gas chromatograph. The split ratio was 1:1. The oven temperature was held at 40°C for 1 min, then raised to 230°C at a rate of 10°C/min, and held at 230°C for 15 min; both injector and detector temperatures were 250°C. Peak identification was confirmed by retention times of commercially obtained standards from Sigma-Aldrich.
Statistical analysis
Statistical analyses were performed using Student's t-test. Values of *P ≤ 0·05 were considered significantly different. Levels of Cy3 in colonocytes, expressed in units of fluorescence intensity on a scale of 0–255, were compared in unpaired Student's t-test using statistical significance level *P = 0·001.
Results
Clinical evaluation of BALB/c and SCID mice under the influence of DSS and C. tyrobutyricum
Control BALB/c and SCID mice not exposed to DSS remained healthy during the experiment. In contrast the DSS-treated mice developed clinical signs of colitis, including diarrhoea, rectal prolapses and bleeding. The SCID mice (group 5, Table 1) were more susceptible to DSS than BALB/c mice (group 2, Table 1), resulting in 20% mortality. No significant changes in body weight were observed in BALB/c and SCID mice as a consequence of exposure to DSS. One of the characteristic signs of DSS-induced colitis is shortening of the colon, which was observed in both saline–DSS-treated BALB/c and SCID mice (groups 2 and 5, Table 1) but not in the control mice (groups 3 and 6, Table 1). In SCID mice, treatment with C. tyrobutyricum prevented shortening of the colon (group 4, Table 1), while no significant protection was observed in C. tyrobutyricum-treated BALB/c mice (group 1, Table 1).
Histological colon damage score in DSS-treated BALB/c and SCID mice
The histological colon damage score after DSS treatment assessed according to the scale (0–4) of Cooper et al. [3] is presented in Table 1. The damage score was of grade 0 in control BALB/c and SCID mice (groups 3 and 6). The damage score was of grade 3·9 ± 0·2 in BALB/c and 3·9 ± 0·1 in SCID saline–DSS-treated mice (groups 2 and 5; Fig. 1a). In C. tyrobutyricum-treated BALB/c and SCID mice the damage due to DSS exposure was significantly less, grade 1·6 ± 0·5 (group 1) and grade 0·5 ± 0·4 (group 4, Fig. 1b), respectively.
Fig. 1.
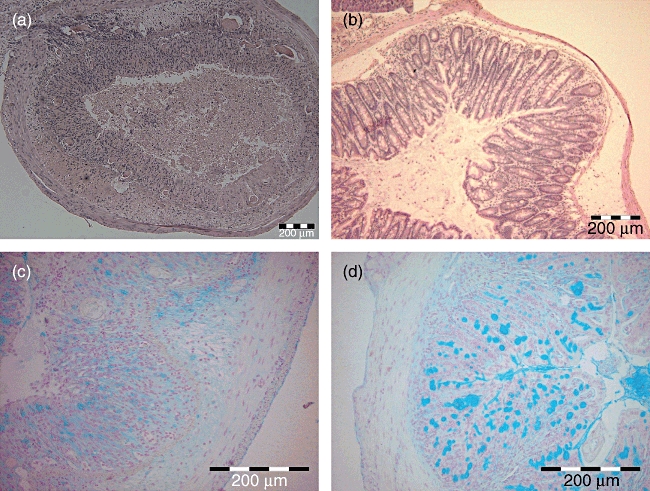
Histological cross-sectional views of colon descendens of severe combined immunodeficiency (SCID) mice in experimental model of acute ulcerative colitis: (a) saline–dextran sodium sulphate (DSS)-treated mice (damage grade 4); (b) C. tyrobutyricum–DSS-treated mice (damage grade 0–1) (haematoxylin and eosin staining); (c) saline–DSS-treated mice, mucin production was decreased to grade 0; (d) C. tyrobutyricum–DSS-treated mice, grades 3–4, mucin production was preserved (Alcian blue staining).
Goblet cell mucins and specific MUC-2 production in the colon
In healthy control mice (groups 3 and 6, Table 1), colonic mucin production (Alcian Blue staining) was grade 4 according to the scale (4–0) of Cooper et al. [3]. In both saline–DSS–treated BALB/c and SCID mice (groups 2 and 5, Table 1, Fig. 1c) it was decreased drastically to grade 0·1 ± 0·2. Treatment with C. tyrobutyricum attenuated the loss of mucin due to DSS exposure in both BALB/c mice (group 1 grade 2·7 ± 0·4) and SCID mice (group 4 grade 3·5 ± 0·5, Table 1, Fig. 1d), with protection being more evident in the SCID mice.
Moreover, changes in the production of the major secreted mucin MUC-2 was verified in SCID mice using immunocytochemistry (Fig. 2). In the saline-treated SCID mice (Fig. 2a) and BALB/c mice (Fig. 2d), the production of MUC-2 was intact. In the saline–DSS-treated SCID (Fig. 2b) and BALB/c mice (Fig. 2e) the production of colonic MUC-2 was depressed, whereas in C. tyrobutyricum–DSS-treated SCID (Fig. 2c) and BALB/c mice (Fig. 2f) MUC-2 secretion was preserved.
Fig. 2.
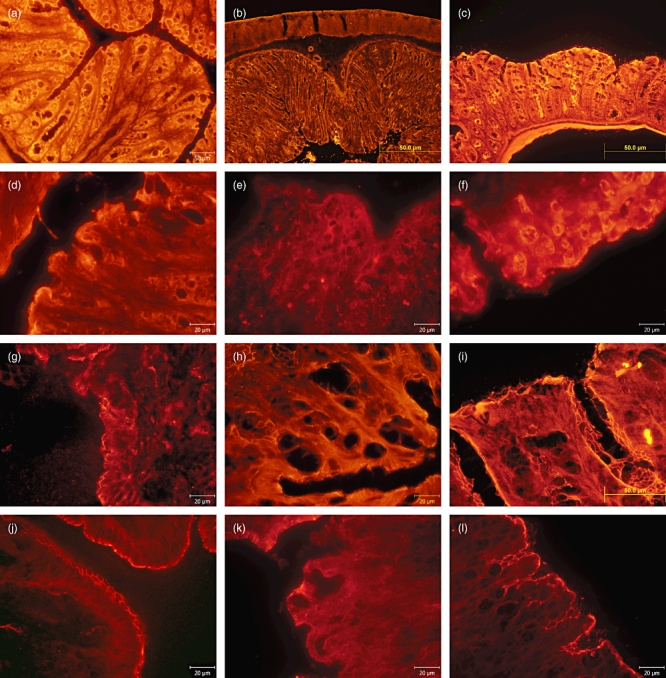
Immunohistochemical evaluation of mucin (MUC)-2 detected with monoclonal antibody MUC-2/CY3 in colon descendens of severe combined immunodeficiency (SCID) and BALB/c mice in experimental model of acute ulcerative colitis: (a) saline-treated SCID mice with intact production of MUC-2; (b) saline–dextran sodium sulphate (DSS)-treated SCID mice with depressed production of MUC-2; (c) C. tyrobutyricum–DSS-treated SCID mice where MUC-2 secretion was preserved; (d) saline-treated BALB/c mice with intact production of MUC-2; (e) saline–DSS-treated BALB/c mice with depressed production of MUC-2; (f) C. tyrobutyricum–DSS-treated BALB/c mice MUC-2 secretion was preserved. Immunohistochemical evaluation of tight junction protein zonula occludens (ZO)-1 detected using monoclonal antibody ZO-1/CY3; (g) saline-treated SCID mice with intact production of ZO-1; (h) saline–DSS-treated SCID mice with markedly reduced ZO–1 production; (i) C. tyrobutyricum–DSS-treated SCID mice with preserved production of ZO-1; (j) saline-treated BALB/c mice with intact production of ZO-1, (k) saline–DSS-treated BALB/c mice with markedly reduced ZO-1 production; (l) C. tyrobutyricum–DSS-treated BALB/c mice with preserved production of ZO-1.
Expression of TJ protein ZO-1
Expression of TJ protein ZO-1 is presented in Fig. 2. In the saline-treated SCID (Fig. 2g) and BALB/c mice (Fig. 2j) ZO-1 production was intact. In saline–DSS-treated SCID (Fig. 2h) and BALB/c mice (Fig. 2k) the production of ZO-1 was markedly reduced, whereas in C. tyrobutyricum–DSS-treated SCID (Fig. 2i) and BALB/c mice (Fig. 2l) the production of ZO-1 was preserved.
Mucosal infiltration of CD11b-positive immune cells
Monoclonal antibody to CD11b, a membrane marker of polymorphonuclear leucocytes, monocytes and natural killer cells, was used (Fig. 3). Saline-treated SCID (Fig. 3a) and BALB/c mice (Fig. 3d) were without infiltration of immune cells. Massive infiltration of inflammatory cells in lamina propria occurred in saline–DSS-treated SCID (Fig. 3b) and BALB/c mice (Fig. 3e), while the colon of C. tyrobutyricum–DSS-treated SCID mice (Fig. 3c) and BALB/c mice (Fig. 3f) did not exhibit infiltration of these cells.
Fig. 3.
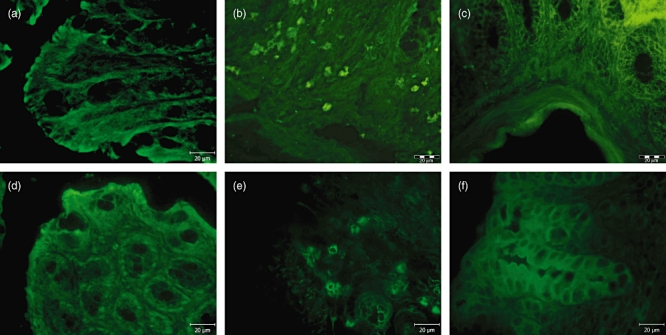
Immunohistochemical detection of infiltrated proinflammatory cells using fluorescein isothiocyanate (FITC)-labelled monoclonal antibody to CD11b in colon-descendens mucosa of mice: (a) saline-treated severe combined immunodeficiency (SCID) mice without infiltration of immune cells; (b) saline–dextran sodium sulphate (DSS)-treated SCID mice with massive infiltration of proinflammatory cells; (c) C. tyrobutyricum–DSS-treated SCID mice, where infiltration of immune cells was not observed; (d) saline-treated BALB/c mice without infiltration of immune cells; (e) saline–DSS-treated BALB/c mice with massive infiltration of proinflammatory cells; (f) C. tyrobutyricum–DSS-treated BALB/c mice, where infiltration of immune cells was not observed.
Release of proinflammtory cytokine TNF-α in colon organ cultures
Spontaneous release of TNF-α into the medium of cultured sections from colon descendens of control mice was very low (groups 3 and 6, Table 1) after 48 h. Colon segments from saline–DSS-treated mice, and especially the SCID mice, released markedly higher amounts of TNF-α (groups 2 and 5, Table 1) than the controls. Compared to the saline–DSS-treated SCID mice (group 5, Table 1), a significantly lower level of TNF-α was detected in the cultured colon segments from C. tyrobutyricum–DSS-treated SCID mice (group 4, Table 1). However, the level of TNF-α released from the colon segments from C. tyrobutyricum–DSS-treated BALB/c mice (group 1, Table 1) did not differ from that of saline–DSS-treated BALB/c mice (group 2, Table 1).
Visualization and quantification of proinflammatory IL-18 in the descending colon
Table 2 shows that DSS-induced colitis in BALB/c mice is associated with increased level of cytokine IL-18 in colon epithelium. C. tyrobutyricum treatment reduced intracolonic IL-18 content significantly, although not to the level in non-inflamed mucosa. In SCID mice the severity of DSS colitis was not associated with IL-18 production. C. tyrobutyricum enhanced significantly the expression of IL-18 in colon epithelium of SCID mice. This might be an effect of butyrate on gene and protein production of IL-18.
C. tyrobutyricum-increased levels of propionic and butyric acids in SCID mice
More than 95% of the SCFA are absorbed and metabolized rapidly by the host [24]. To see any effect of C. tyrobutyricum on SCFA production, we determined the percentage of main short-chain fatty acids (acetic, propionic, isobutyric, n-butyric, isovaleric, 2-methylbutyric and valeric) in bacterial culture and in faecal samples obtained from C. tyrobutyricum–DSS-treated SCID mice and saline–DSS-treated SCID mice at the end of the experiment using gas chromatography (Table 3). Threefold higher percentage of n-butyric acid and twofold higher percentages of propionic acid were measured in C. tyrobutyricum–DSS-treated SCID mice than in the saline–DSS-treated mice. Conversely, a lower percentage of acetic acid was found in the C. tyrobutyricum–DSS-treated than in the saline–DSS-treated mice.
Table 3.
Production of short-chain fatty acids determined as relative concentrations (%) in the bacterial broth of C. tyrobutyricum and in the faeces (taken at the end of the experiments). Clostridium tyrobutyricum dextran sodium sulphate (DSS)-treated and saline DSS-treated severe combined immunodeficiency (SCID) mice
| C. tyrobutyricum | C. tyrobutyricum (i.r.) | Saline (i.r.) | |
|---|---|---|---|
| Acids | (bacterial culture) | (DSS-treated) | (DSS-treated) |
| Acetic | 62·5 | 59·2 | 81·4 |
| Propionic | 17·9 | 18·0 | 7·8 |
| Isobutyric | 10·6 | 9·2 | 4·8 |
| n-Butyric | 9·0 | 13·6 | 2·8 |
| Isovaleric | n.d. | n.d. | 2·1 |
| 2-Methylbutyric | n.d. | n.d. | 0·6 |
| Valeric | n.d. | n.d. | 0·5 |
i.r.: intrarectal administration; n.d.: not detected.
Discussion
In the present study, intrarectal administration of C. tyrobutyricum prior to the onset of experimental colitis protected both immunocompetent BALB/c and immunodeficient SCID mice from histological damage, shortening of the colon and decreased mucin production.
Both BALB/c and SCID mice display comparable symptoms of DSS-induced colitis as judged by the histological damage score, colon shortening and weight loss. However, 20% mortality occurred in the DSS-treated SCID mice suggesting that parameters such as loss of body weight cannot be used as a measure of the severity of inflammation in these two mouse strains. This conclusion is supported by several previous studies [25–27] not showing an apparent body weight loss before day 6 of acute DSS colitis in BALB/c mice. On the basis of the symptoms, the reduction of TJ protein ZO-1 production, depression of MUC-2 mucin production and infiltration of macrophages during inflammation, it appeared that the two mouse strains behave similarly on the qualitative level.
Decreased numbers of goblet cells and reduced levels of MUC-2 protein were measured in the inflamed colonic mucosa of DSS-treated BALB/c and SCID mice, a result similar to that observed in humans with active UC [28]. This is in agreement with other experimental models of inflammatory bowel diseases that demonstrate the critical role of MUC-2 in colonic protection [29]. Changes in mucins, particularly in MUC-2 mRNA, in experimental models are considered to contribute to the development of ulcerative colitis [10,30,31]. Recently it has been observed that DSS in drinking water rapidly affects the biophysical structure of the inner mucus layer, making it permeable to bacteria within 12 h [7]. Thus the increased contact of bacteria with the epithelium is probably the trigger for the inflammatory reactions observed in colitis and would explain why DSS does not induce colitis in germ-free mice. Similarly, loss of TJ protein ZO-1 in the intestinal epithelium, as observed in this study, was interpreted as an early event in DSS-induced colitis and is associated with increased permeability and intestinal inflammation [32].
Crucially important in DSS-colitis is an activation of T lymphocytes via macrophages that have been activated directly by DSS [8]. Indeed, direct evidence for the involvement of CD4+ T cells and their proliferation in pathogenesis of DSS-induced colitis has been described previously [33]. Moreover, the introduction of bacterial flagellin-specific CD4+ T cells into naive SCID mice (T and B cell-independent) results in severe colitis [34]. Similarly, transfer of CD4+CD45RBhigh T cell subpopulation from conventional mice into SCID mice also induces severe inflammation [5].
A major difference between BALB/c and SCID mice in development of DSS-colitis relates to the expression of the proinflammatory cytokine IL-18. In agreement with other published studies [35], we showed significantly increased IL-18 colon content in DSS-induced colitis in BALB/c mice. In BALB/c and C57BL/6 mice DSS-induced colitis induces increased expression of IL-18 in the colonic mucosa, where it polarizes CD4+ T cells toward T helper cell type 1 (Th1)-mediated immune response [35] and thus increased production of the proinflammatory cytokine interferon (IFN)-γ. IFN-γ stimulated secretion of IL-18 from enterocyte-like IEC-6 cells has been correlated with IFN-γ-increased expression of caspase-1 activity [36], an enzyme required for cleavage of the precursor form of IL-18 into mature biologically active IL-18. Indeed, inhibition of caspase-1 by the specific inhibitor pralnacasan [26] attenuated DSS-induced colitis, this effect being mediated by suppression of proinflamatory IL-18 and IFN-γ.
Recent observations highlight the role of Nod-like receptors (NLRs) when stimulated by inflammatory mediators to form inflammasomes, multi-protein complexes that serve for activation of caspase-1 essential for maturation and secretion of IL-18. The highest levels of IL-18 were localized in intestinal epithelial cells, then macrophages and dendritic cells of the lamina propria. Interestingly, deletion of macrophages or use of neutralizing antibodies to IL-18 or inhibition of caspase-1 [37] result in prevention of the inflammatory cascade leading to sustained infiltration of macrophages, neutrophils and activation of lamina propria effector T cells. Thus, it seems that inflammasome activation [38] would have a proinflammatory effect, with IL-18 inducing inflammation in lamina propria mononuclear cells. Conversely, in intestinal epithelial cells the inflammasome would have a compensatory proliferative response, the secreted IL-18 mediating protection, proliferation and cell integrity [27,39,40]. This study shows for the first time that in SCID mice (a T and B cell-independent model) the severity of colitis was associated with limited production of biologically active form of IL-18. It seems that only constitutively expressed precursor form of IL-18 is associated with normal and inflamed SCID mouse colon. This finding led us to the assumption that SCID mice lack some components of inflammasome, pro-IL-18 could not be activated, and display an increased susceptibility to DSS-induced colitis associated with increased lethality, especially in the chronic phase of inflammation [6]. In SCID mouse colon we detected increased surface antigen CD11b, associated with an increased number of infiltrating immune cells, possibly macrophages [4]. Lack of IL-18 secretion is compensated by increased secretion of inflammatory TNF-α from the colon found in organ cultures. In comparison with UC patients it was suggested that macrophages migrating into the inflamed mucosa [41,42] secrete high levels of TNF-α. We observed only moderate expression of TNF-α in BALB/c mice with induced inflammation which was not commensurate with the marked infiltration of CD11b-positive polymorphonuclear leucocytes and monocytes into the mucosa. These mice represent a model of Th1-mediated immune response that contributes to the production of IL-18 and IFN-γ.
Studies of UC in humans have shown a lower availability and diminished capacity to oxidize butyrate [12,43], an energy source for colonocytes and end-product of the fermentation of undigested fibre and complex carbohydrates by the luminal microbiota. Similarly, a decrease in butyrate oxidation was found in the colonocytes of mice with DSS-induced colitis. Recent studies on faecal microflora of UC and IBS (irritable bowel syndrome) patients showed depletion of members of Bacteroidetes and Firmicutes (comprising some Clostridium groups) [44]. In healthy mice synergistic interactions between specific members of these phyla are linked to butyrate formation [45]. Thus, increased production of butyrate in the lumen of the colon was proposed as a treatment to ameliorate the symptoms of UC [46]. However, butyrate enemas in UC patients in remission were shown recently to have only mild effects on inflammation and anti-oxidant status in the colonic mucosa [47]. In our study we expected a higher faecal concentration of butyrate after intrarectal administration of C. tyrobutyricum and protection from DSS-induced colitis. In both BALB/c and SCID mice, intrarectal administration of C. tyrobutyricum prevented the reduction of MUC-2 protein observed in DSS-induced colitis and led to an almost normal level of MUC-2 secretion, most probably to the reported stimulation of MUC-2 gene expression in mouse colon [12]. We further showed that C. tyrobutyricum protects against impairment of the TJs in the colon of BALB/c and SCID mice by preventing dissolution of ZO-1 from the TJ in DSS-treated mice. Bacterial Toll-like receptor (TLR)-2 ligands have also been reported to increase ZO-1 expression and its localization in the TJs [15,48], and cannot be ruled out as a mechanism for the effects of C. tyrobutyricum on ZO-1.
Several in vitro and ex vivo studies reviewed by Hamer et al. [20] assessed the effect of butyrate which, at low concentrations, induces a decrease in permeability associated with increased expression of TJ proteins. However, overproduction of butyrate might be toxic for maturation of the intestine in premature infants and also in newborn rats [49].
TNF-α at concentrations found in inflamed mucosa may reduce oxidation of butyrate and decrease energy supply to colonocytes, as shown in vitro in the mucosa of human colonic biopsies [50]. As intrarectal administration of C. tyrobutyricum increased production of butyrate in the colon lumen it could overcome any insufficiencies resulting from the increased production of TNF-α. C. tyrobutyricum can be considered as a candidate human probiotic due to its beneficial effects in mouse colitis model. In BALB/c mice the expression of TNF-α in inflamed colon was lower in comparison with TNF-α production in the inflamed colon of SCID mice. Additionally, we found that treatment with C. tyrobutyricum had no effect on regulation of TNF-α production in BALB/c, but a strongly attenuating effect on TNF-α production in SCID mice.
Our work on IL-18 expression in DSS-induced colitis and its regulation by C. tyrobutyricum helped to differentiate between distinct responsiveness of BALB/c and SCID mice. Remarkably, C. tyrobutyricum treatment significantly reduced intracolonic IL-18 protein content in the inflamed mucosa of BALB/c mice, although not down to the level in non-inflamed mucosa. In contrast to BALB/c mice, C. tyrobutyricum enhanced significantly the expression of IL-18 in colon epithelium of SCID mice which lacked inflammation-associated expression of IL-18. This could be due to the fact that butyrate enhances gene expression and protein production of IL-18 in epithelial cells (HT-29 and Caco-2) in vitro, and in vivo in butyrate-treated mice [51]. This enhanced effect of butyrate at the transcription level seems to be hidden in BALB/c mice expressing increased IL-18 production in DSS-treatment.
This study demonstrates that in the DSS model, the severity of inflammatory symptoms depends largely but not exclusively on host immune functions. Thus, C. tyrobutyricum protection against destruction of mucosal barrier is equally effective in immunodeficient SCID mice and immunocompetent BALB/c mice. Manifestation of cytokines IL-18 and TNF-α in acute DSS-colitis depends largely on immune cell repertoire of the host mouse. As a typical product of macrophages, TNF-α expression increased significantly in the colon epithelium in SCID mice, while mature IL-18, a Th1 cytokine, important for systemic balance between Th1 and Th2 signalling [52], played a key role in immunocompetent BALB/c mice. The combined effect of C. tyrobutyricum in suppressing high levels of both cytokines appears promising in treatment of acute experimental colitis.
Acknowledgments
We thank Mrs I. Grimova, B. Drabonova, J. Jarkovska and A. Smolova for excellent technical assistance. This study was supported by grants 303/08/0367, 303/09/0449 and 304/11/1252 of the Science Foundation of the Czech Republic, grants 2B06155 and ME10017 of the Ministry of Education, Youth and Sports and by Institutional Research Concepts AV0Z50200510 and AV0Z50110509. Zhengyu Du is a Marie Curie Research Fellow in the EC FP7 Cross-talk project (PITN-GA-2008-215553) and gratefully acknowledges their financial support.
Disclosure
The authors declare that they have no conflict of interest related to the publication of this manuscript.
References
- 1.Tlaskalova-Hogenova H, Tuckova L, Stepankova R, et al. Involvement of innate immunity in the development of inflammatory and autoimmune diseases. Ann NY Acad Sci. 2005;1051:787–98. doi: 10.1196/annals.1361.122. [DOI] [PubMed] [Google Scholar]
- 2.Frolova L, Drastich P, Rossmann P, Klimesova K, Tlaskalova-Hogenova H. Expression of Toll-like receptor 2 (TLR2), TLR4, and CD14 in biopsy samples of patients with inflammatory bowel diseases: upregulated expression of TLR2 in terminal ileum of patients with ulcerative colitis. J Histochem Cytochem. 2008;56:267–74. doi: 10.1369/jhc.7A7303.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–49. [PubMed] [Google Scholar]
- 4.Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107:1643–52. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- 5.Stepankova R, Powrie F, Kofronova O, et al. Segmented filamentous bacteria in a defined bacterial cocktail induces intestinal inflammation in SCID mice reconstituted with CD45RBhigh CD4+ T cells. Inflamm Bowel Dis. 2007;13:1202–11. doi: 10.1002/ibd.20221. [DOI] [PubMed] [Google Scholar]
- 6.Hudcovic T, Stepankova R, Cebra J, Tlaskalova-Hogenova H. The role of microflora in the development of intestinal inflammation: acute and chronic colitis induced by dextran sulfate in germ-free and conventionally reared immunocompetent and immunodeficient mice. Folia Microbiol. 2001;46:565–72. doi: 10.1007/BF02818004. [DOI] [PubMed] [Google Scholar]
- 7.Johansson ME, Gustafsson JK, Sjoberg KE, et al. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS ONE. 2010;5:e12238. doi: 10.1371/journal.pone.0012238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 9.Tytgat KM, Bovelander FJ, Opdam FJ, Einerhand AW, Buller HA, Dekker J. Biosynthesis of rat MUC2 in colon and its analogy with human MUC2. Biochem J. 1995;309:221–9. doi: 10.1042/bj3090221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renes IB, Boshuizen JA, Van Nispen DJ, et al. Alterations in Muc2 biosynthesis and secretion during dextran sulfate sodium-induced colitis. Am J Physiol. 2002;282:G382–9. doi: 10.1152/ajpgi.00229.2001. [DOI] [PubMed] [Google Scholar]
- 11.Araki Y, Fujiyama Y, Andoh A, Koyama S, Kanauchi O, Bamba T. The dietary combination of germinated barley foodstuff plus Clostridium butyricum suppresses the dextran sulfate sodium-induced experimental colitis in rats. Scand J Gastroenterol. 2000;35:1060–7. doi: 10.1080/003655200451180. [DOI] [PubMed] [Google Scholar]
- 12.Gaudier E, Rival M, Buisine MP, Robineau I, Hoebler C. Butyrate enemas upregulate Muc genes expression but decrease adherent mucus thickness in mice colon. Physiol Res. 2009;58:111–19. doi: 10.33549/physiolres.931271. [DOI] [PubMed] [Google Scholar]
- 13.Venkatraman A, Ramakrishna BS, Pulimood AB, Patra S, Murthy S. Increased permeability in dextran sulphate colitis in rats: time course of development and effect of butyrate. Scand J Gastroenterol. 2000;35:1053–9. doi: 10.1080/003655200451171. [DOI] [PubMed] [Google Scholar]
- 14.Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr. 2011;141:769–76. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- 15.Karczewski J, Troost FJ, Konings I, et al. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol. 2010;298:G851–9. doi: 10.1152/ajpgi.00327.2009. [DOI] [PubMed] [Google Scholar]
- 16.Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, Schmidt MA. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCzeta redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol. 2007;9:804–16. doi: 10.1111/j.1462-5822.2006.00836.x. [DOI] [PubMed] [Google Scholar]
- 17.Desreumaux P, Colombel JF. Intestinal microflora and chronic inflammatory bowel diseases. Gastroenterol Clin Biol. 2001;25:C89–93. [PubMed] [Google Scholar]
- 18.Nanda Kumar NS, Balamurugan R, Jayakanthan K, Pulimood A, Pugazhendhi S, Ramakrishna BS. Probiotic administration alters the gut flora and attenuates colitis in mice administered dextran sodium sulfate. J Gastroenterol Hepatol. 2008;23:1834–9. doi: 10.1111/j.1440-1746.2008.05723.x. [DOI] [PubMed] [Google Scholar]
- 19.Peran L, Camuesco D, Comalada M, et al. A comparative study of the preventative effects exerted by three probiotics, Bifidobacterium lactis, Lactobacillus casei and Lactobacillus acidophilus, in the TNBS model of rat colitis. J Appl Microbiol. 2007;103:836–44. doi: 10.1111/j.1365-2672.2007.03302.x. [DOI] [PubMed] [Google Scholar]
- 20.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–19. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 21.Okamoto T, Sasaki M, Tsujikawa T, Fujiyama Y, Bamba T, Kusunoki M. Preventive efficacy of butyrate enemas and oral administration of Clostridium butyricum M588 in dextran sodium sulfate-induced colitis in rats. J Gastroenterol. 2000;35:341–6. doi: 10.1007/s005350050358. [DOI] [PubMed] [Google Scholar]
- 22.Schallehn G, Wolff MH. Morphological changes in human embryonic lung fibroblasts caused by cytotoxins of various Clostridium species. Zentralbl Bakteriol Mikrobiol Hyg. 1988;267:367–78. [PubMed] [Google Scholar]
- 23.Khoruts A, Sadowsky MJ. Therapeutic transplantation of the distal gut microbiota. Mucosal Immunol. 2011;4:4–7. doi: 10.1038/mi.2010.79. [DOI] [PubMed] [Google Scholar]
- 24.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–64. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 25.Melgar S, Karlsson A, Michaelsson E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am J Physiol. 2005;288:G1328–38. doi: 10.1152/ajpgi.00467.2004. [DOI] [PubMed] [Google Scholar]
- 26.Bauer C, Loher F, Dauer M, et al. The ICE inhibitor pralnacasan prevents DSS-induced colitis in C57BL/6 mice and suppresses IP-10 mRNA but not TNF-alpha mRNA expression. Dig Dis Sci. 2007;52:1642–52. doi: 10.1007/s10620-007-9802-8. [DOI] [PubMed] [Google Scholar]
- 27.Elinav E, Strowig T, Kau AL, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–57. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs LR, Huber PW. Regional distribution and alterations of lectin binding to colorectal mucin in mucosal biopsies from controls and subjects with inflammatory bowel diseases. J Clin Invest. 1985;75:112–18. doi: 10.1172/JCI111662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van der Sluis M, De Koning BA, De Bruijn AC, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–29. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 30.Amit-Romach E, Reifen R, Uni Z. Mucosal function in rat jejunum and ileum is altered by induction of colitis. Int J Molecular Med. 2006;18:721–7. [PubMed] [Google Scholar]
- 31.Einerhand AW, Renes IB, Makkink MK, van der Sluis M, Buller HA, Dekker J. Role of mucins in inflammatory bowel disease: important lessons from experimental models. Eur J Gastroenterol Hepatol. 2002;14:757–65. doi: 10.1097/00042737-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Poritz LS, Garver KI, Green C, Fitzpatrick L, Ruggiero F, Koltun WA. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J Surg Res. 2007;140:12–19. doi: 10.1016/j.jss.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 33.Shintani N, Nakajima T, Okamoto T, Kondo T, Nakamura N, Mayumi T. Involvement of CD4+ T cells in the development of dextran sulfate sodium-induced experimental colitis and suppressive effect of IgG on their action. Gen Pharmacol. 1998;31:477–81. doi: 10.1016/s0306-3623(98)00004-4. [DOI] [PubMed] [Google Scholar]
- 34.Friswell M, Campbell B, Rhodes J. The role of bacteria in the pathogenesis of inflammatory bowel disease. Gut Liver. 2010;4:295–306. doi: 10.5009/gnl.2010.4.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegmund B, Fantuzzi G, Rieder F, et al. Neutralization of interleukin-18 reduces severity in murine colitis and intestinal IFN-gamma and TNF-alpha production. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1264–73. doi: 10.1152/ajpregu.2001.281.4.R1264. [DOI] [PubMed] [Google Scholar]
- 36.Kolinska J, Lisa V, Clark JA, et al. Constitutive expression of IL-18 and IL-18R in differentiated IEC-6 cells: effect of TNF-alpha and IFN-gamma treatment. J Interferon Cytokine Res. 2008;28:287–96. doi: 10.1089/jir.2006.0130. [DOI] [PubMed] [Google Scholar]
- 37.Bauer C, Duewell P, Mayer C, et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59:1192–9. doi: 10.1136/gut.2009.197822. [DOI] [PubMed] [Google Scholar]
- 38.Siegmund B. Interleukin-18 in intestinal inflammation: friend and foe? Immunity. 2010;32:300–2. doi: 10.1016/j.immuni.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–91. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dupaul-Chicoine J, Yeretssian G, Doiron K, et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32:367–78. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 41.Mahida YR. The key role of macrophages in the immunopathogenesis of inflammatory bowel disease. Inflamm Bowel Dis. 2000;6:21–33. doi: 10.1097/00054725-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Reinecker HC, Steffen M, Witthoeft T, et al. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993;94:174–81. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thibault R, Blachier F, Darcy-Vrillon B, de Coppet P, Bourreille A, Segain JP. Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: a transport deficiency. Inflam Bowel Dis. 2010;16:684–95. doi: 10.1002/ibd.21108. [DOI] [PubMed] [Google Scholar]
- 44.Noor SO, Ridgway K, Scovell L, et al. Ulcerative colitis and irritable bowel patients exhibit distinct abnormalities of the gut microbiota. BMC Gastroenterol. 2010;10:134. doi: 10.1186/1471-230X-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willing BP, Finlay BB. Gut microbiology: fitting into the intestinal neighbourhood. Curr Biol. 2009;19:R457–9. doi: 10.1016/j.cub.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 46.Wachtershauser A, Stein J. Rationale for the luminal provision of butyrate in intestinal diseases. Eur J Nutr. 2000;39:164–71. doi: 10.1007/s003940070020. [DOI] [PubMed] [Google Scholar]
- 47.Hamer HM, Jonkers DM, Vanhoutvin SA, et al. Effect of butyrate enemas on inflammation and antioxidant status in the colonic mucosa of patients with ulcerative colitis in remission. Clin Nutr. 2010;29:738–44. doi: 10.1016/j.clnu.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology. 2004;127:224–38. doi: 10.1053/j.gastro.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 49.Nafday SM, Chen W, Peng L, Babyatsky MW, Holzman IR, Lin J. Short-chain fatty acids induce colonic mucosal injury in rats with various postnatal ages. Pediatr Res. 2005;57:201–4. doi: 10.1203/01.PDR.0000150721.83224.89. [DOI] [PubMed] [Google Scholar]
- 50.Nancey S, Moussata D, Graber I, Claudel S, Saurin JC, Flourie B. Tumor necrosis factor alpha reduces butyrate oxidation in vitro in human colonic mucosa: a link from inflammatory process to mucosal damage? Inflam Bowel Dis. 2005;11:559–66. doi: 10.1097/01.mib.0000161918.04760.f3. [DOI] [PubMed] [Google Scholar]
- 51.Kalina U, Koyama N, Hosoda T, et al. Enhanced production of IL-18 in butyrate-treated intestinal epithelium by stimulation of the proximal promoter region. Eur J Immunol. 2002;32:2635–43. doi: 10.1002/1521-4141(200209)32:9<2635::AID-IMMU2635>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 52.Kolinska J, Zakostelecka M, Schwarzer M, Stepankova R, Hudcovic T, Kozakova H. Effect of nonpathogenic Escherichia coli monoassociation on small intestinal brush-border glycoconjugate moieties and cytokine production after colonization in ex-germ-free rats and piglets. Int J Interferon Cytokine Mediat Res. 2010;2:73–84. [Google Scholar]


