SUMMARY
Biophysical signaling is required for both embryonic polarity and regenerative outgrowth. Exploiting endogenous ion transport for regenerative therapies will require direct regulation of membrane voltage. Here, we develop a pharmacological method to target ion transporters, uncovering a novel role for membrane voltage as a key regulator of anterior polarity in regenerating planaria. Utilizing the highly specific inhibitor, SCH-28080, our data reveal that H+,K+-ATPase-mediated membrane depolarization is essential for anterior gene expression and brain induction. H+,K+-ATPase-independent manipulation of membrane potential with ivermectin confirms that depolarization drives head formation, even at posterior-facing wounds. Using this chemical genetics approach, we demonstrate that membrane voltage controls head-vs.-tail identity during planarian regeneration. Our data suggest well-characterized drugs (already approved for human use) might be exploited to control adult stem cell-driven pattern formation during the regeneration of complex structures.
INTRODUCTION
Developing regenerative therapies for limb injuries and organ replacement requires we understand not only the processes that induce stem cell proliferation and new tissue growth, but also the complex machinery that properly aligns new tissues with the original polarity. This machinery must integrate adult stem cell behavior with the morphogenesis required to replace only the missing tissue. The planarian model system is perfectly suited for such investigations, possessing the ability to regenerate any lost or damaged tissues. They have a complex central nervous system (CNS) with a true brain (Sarnat and Netsky, 1985), a well-defined adult stem cell population comprising roughly 30% of all cells, a clear anterior-posterior (A/P) polarity that is maintained during regeneration, and over a hundred years of regenerative analyses under a wide variety of conditions (Reddien and Sanchez Alvarado, 2004; Forsthoefel and Newmark, 2009; Salo et al., 2009). Moreover, planarians share more genes in common with vertebrates than either Drosophila or C. elegans (Sanchez Alvarado et al., 2002).
Certain molecular methods, such as transgenesis, still remain to be perfected for planaria. However, as a model system for pharmacological studies they have proved exceptional (Oviedo and Levin, 2008; Pagan et al., 2008). Developing chemical approaches to disease treatment is essential as a much-needed alternative to gene therapy in which targeted delivery in human patients is problematic. Chemical genetics (CG), the application of bioactive small molecules to alter protein activity (Yeh and Crews, 2003; Smukste and Stockwell, 2005; Wheeler and Brandli, 2009), is an especially powerful tool for probing the role of biophysical processes (Adams and Levin, 2006a). Alongside the rapid action potentials of excitable cells, and the calcium waves that initiate development (Ducibella et al., 2006), there operate a complex network of long-term, steady-state membrane voltage gradients and ion flows (Forrester et al., 2007; Levin, 2007). These long-range bioelectrical signals are essential for regenerative outgrowth, regulating cell proliferation, differentiation, and migration (Blackiston et al., 2009; Levin, 2009; McCaig et al., 2009). Biophysical regulation of polarity and regeneration is of special interest, since the well-characterized drugs that exist for manipulating most ion transporters offer the possibility of quick translation into therapies. However, we do not yet understand the role of biophysical signals in influencing polarity and mediating in vivo stem cell behavior.
Using CG, we uncovered a role for biophysical signaling during adult stem cell-mediated regeneration in planarians. As part of a CG screen (Nogi et al., 2005), we identified the H,K-ATPase as a candidate for regulating regenerative A/P polarity. A P2C class plasma membrane ATPase best known for regulating stomach pH, the H,K-ATPase is also important in many other tissues (Jaisser and Beggah, 1999). The H,K-ATPase is electroneutral, transferring one H+ ion out of, and one K+ ion into, the cell where it is expressed. However, it can work in tandem with the K+ efflux channels KCNQ1/KCNE2 (Lambrecht et al., 2005; Shin et al., 2009) and KCNJ10 (Kir4.1, Fujita et al., 2002; Shibata et al., 2006) to control membrane voltage, as it does in both the adult mammalian gut and inner ear (Shibata et al., 2006; Kaufhold et al., 2008) and in Xenopus embryonic left/right patterning (Aw et al., 2008; Morokuma et al., 2008a).
Like humans, planarians have multiple H,K-ATPase genes; the Schmidtea mediterranea planarian genome (Robb et al., 2008) contains at least seven potential H,K-ATPase genes (the genome of the Dugesia japonica used here has not been sequenced). Functional redundancies mean single gene knockdown with RNA interference (RNAi) are unlikely to produce informative phenotypes. For instance, RNAi against the one non-gastric H,K-ATPase alpha subunit identified in D. japonica [which is expressed ubiquitously in intact worms and at the dorsal-ventral border of the anterior wound in regenerates (Nogi et al., 2005)] produced no effect. While RNAi of 2–3 genes together has been reported (Rink et al., 2009; Oviedo et al., 2010), RNAi is not practical for simultaneous inhibition of greater numbers. It was also important to consider the development of drug-based strategies for future biomedical use. Therefore, a pharmacological approach was an ideal method for loss-of-function studies, to ask whether H,K-ATPase activity was involved in regeneration, as well as gain-of-function approaches to explore biophysical modulation of regenerative patterning at the plasma-membrane level. We chose to use D. japonica, as the asexual strain of S. mediterranea are much less tolerant of chemical exposure (high toxicity), and the larger D. japonica are more amenable to complex amputation assays.
The experiments revealed an exciting new regulator of anterior polarity during planarian regeneration—membrane voltage. Our data show that H,K-ATPase activity is required for depolarization of the regenerating anterior blastema, anterior gene expression, and head formation. H,K-ATPase-independent modulation of membrane potential (via chloride flux) demonstrated that depolarization itself is sufficient to drive ectopic anterior regeneration (even in posterior blastemas), highlighting membrane voltage as a key initiator of head formation during regeneration.
RESULTS
H,K-ATPase Activity is Required to Regenerate Head
SCH-28080 (SCH) was identified in a CG screen to uncover roles for ion transport during regeneration. The 1,2-α-imidazopyridine SCH is a specific, potent, and reversible inhibitor of the H,K-ATPase that does not inhibit the closely related Na,K-ATPase (Lyu and Farley, 1997; Vagin et al., 2002) and blocks invertebrate H,K-ATPases (Klaassen et al., 1997; Mukherjee et al., 2001; Hibino et al., 2006). Previous investigation of the H,K-ATPase in planarians reported exposure to 70 µM SCH resulted in abnormal and/or deficient anterior regeneration, specifically in eye formation (Nogi et al., 2005). Many other pharmacological blockers were tested and found to have no effect on anterior regeneration, while two other H,K-ATPase inhibitors (omeprazole and prodigiosin) induced similar anterior defects (Nogi et al., 2005). This suggested H,K-ATPase activity specifically may be required for normal head regeneration.
Using freshly-reconstituted solutions, a significantly lower SCH concentration (18 µM) results in planarian fragments that regenerate normally following amputation but do not form head structures (Figure 1). pH analyses confirm that SCH-treated fragments are more acidic (as expected if H+ efflux is blocked), suggesting H,K-ATPase activity has been targeted (Supplemental Figure S1). Modulation of extracellular medium pH (pH=4, n=50; or pH=9, n=60) was not sufficient to affect regenerative polarity. As previously shown, SCH had no effect on intact worms (Nogi et al., 2005), ruling out toxicity or inhibition of stem cell maintenance. Transverse worm fragments were amputated so that both head and tail regeneration could be assayed in the same regenerate. Importantly, SCH-treated regenerates formed anterior blastemas (Supplemental Figure S2), suggesting H,K-ATPase activity is not required for stem cell proliferation or wound healing. Additionally, SCH treatment did not induce lesions or necrosis associated with toxicity in regenerating fragments.
Figure 1. H,K ATPase inhibition blocks head regeneration.
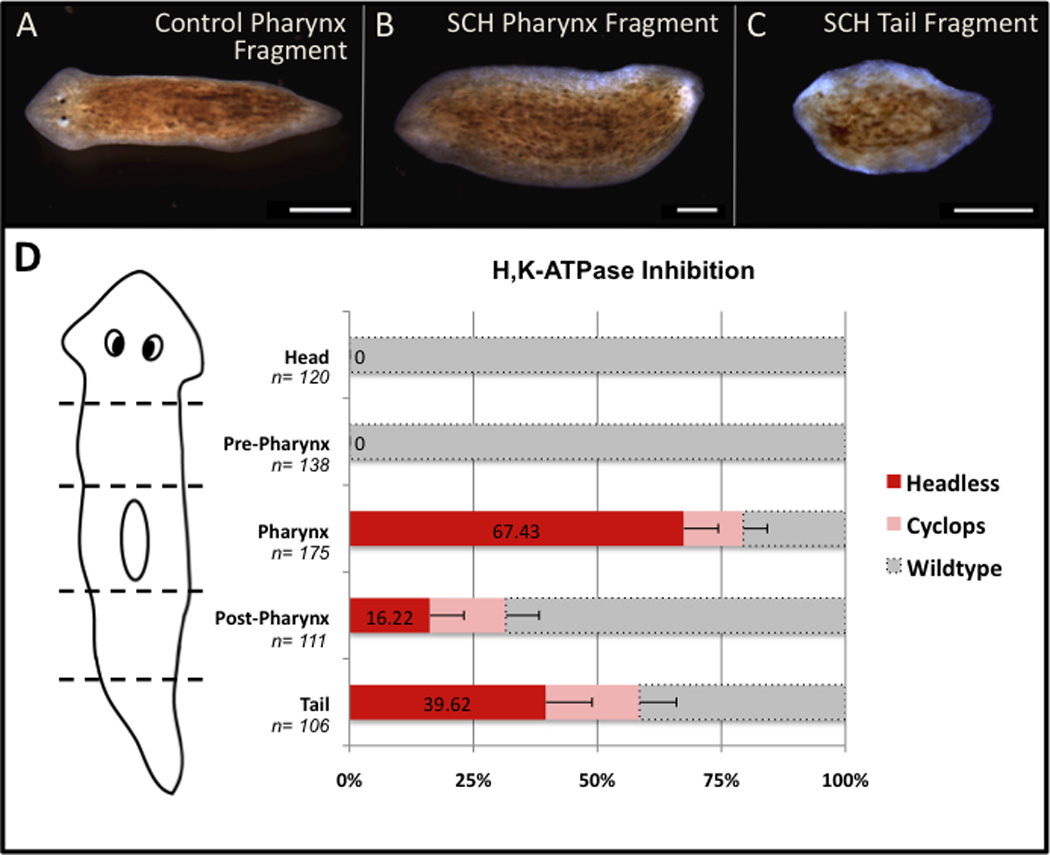
Effects of 18µM SCH-28080 (SCH). (A) Control pharynx, (B) SCH-treated pharynx, and (C) SCH-treated tail fragments at 14 days regeneration. Anterior to the left. Scale bars=500µm. (D) Phenotype distribution by anatomical region. Error bars=95% confidence intervals (CI). See also Figures S1 and S2.
H,K-ATPase inhibition during the first 72 hours of regeneration blocked regeneration of head structures in posterior fragments (Figure 1A–C). Exposure yielded some cyclopic (reduced anterior) animals, while most regenerates lacked any visible anterior structures producing a “headless” phenotype (Figure 1D). Even after 2 months, H,K-ATPase-inhibited worms remained headless. Treatment for <72 hours resulted in a dose response effect (where cyclopia increased and headless-ness decreased), with SCH treatment for only 24 hours producing no effect (data not shown). We conclude that H,K-ATPase activity is required for normal anterior morphogenesis, but that patterning mechanisms are able to recover unless inhibition is maintained past a critical point. Based on these findings, we choose to focus our analyses solely on pharynx fragments, which are most affected by H,K-ATPase inhibition.
H,K-ATPase Inhibition Prevents Heads Without Duplicating Tails
While some headless worms possessed one pointed (posterior morphology) and one rounded (non-posterior morphology) end, most H,K-ATPase-inhibited regenerates had two pointed ends, suggestive of a two-tailed phenotype (see Figure 1B–C). To determine the true polarity, we performed molecular analyses of tissue morphology and A/P markers (Figure 2). Surprisingly, headless worms had a single posterior end, with the second end neither posterior nor anterior. This could be seen in the gastrovascular (GV) tract of H,K-ATPase-inhibited regenerates, where the double-branched posterior morphology was present but the single-branched anterior morphology was not (Figure 2A). Instead, the two posterior GV branches connected around the pharynx, but failed to extend anteriorly (Figure 2A2). Additionally, the GV tract ectopically extended into the “anterior” margin. Examination of the CNS revealed headless worms lack cephalic ganglia and therefore have no brain (Figure 2B). Instead, the ventral nerve cords running along each side ectopically connected, producing a single nerve cord looped around the pharynx, which retained its correct A/P polarity (Figure 2B2). The pharynx also failed to undergo morphallaxis—in headless worms the pharynx did not re-center and shrink in relation to the fragment’s smaller size.
Figure 2. Blocking H,K ATPase activity prevents anterior gene expression.
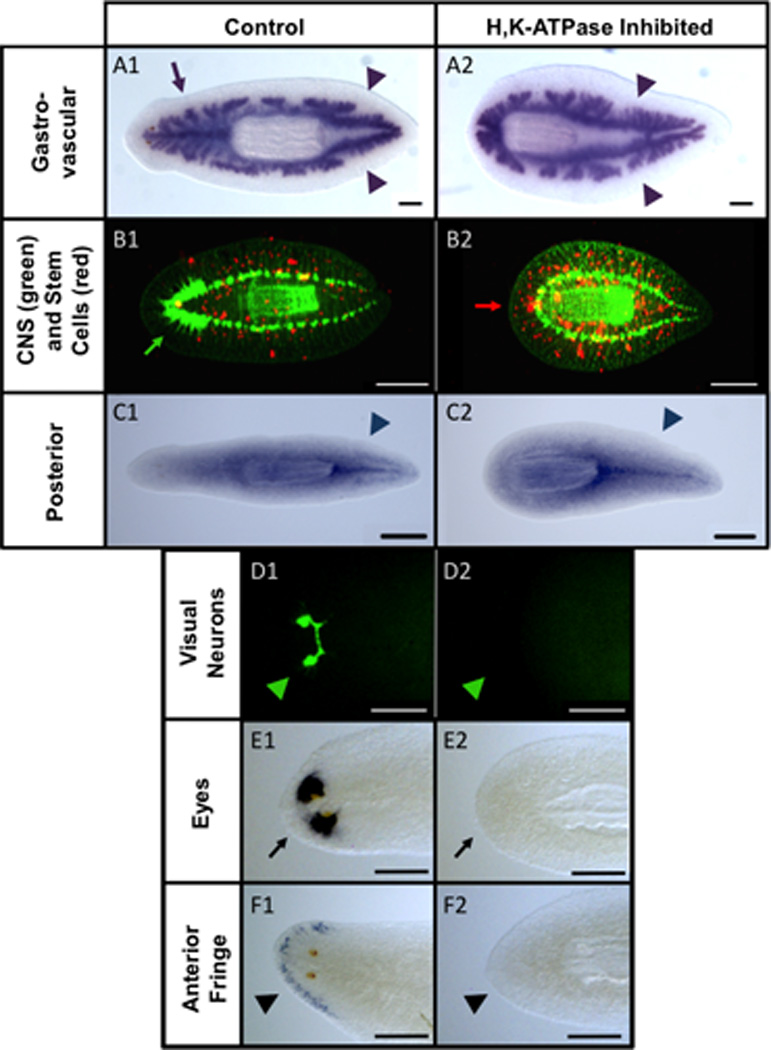
Anterior-Posterior and tissue-specific marker analyses at 14 days regeneration. (A) Innexin7. Arrow=single anterior branch (lost in A2). Arrowheads=double posterior branches. (B) Synapsin (green), Phospho-Histone H3 (red). Green arrow=brain (lost in B2). Red arrow=neoblasts atypically at “anterior” end (B2). (C) Hox9. Arrowheads=tail staining (not duplicated in C2). (D) Arrestin. Arrowhead=optic nerves (lost in D2). (E) Eye marker 0251_HH, and (F) anterior marker 0821_HN. Arrow=eye staining (E1) and arrowhead=anterior fringe staining (F1), (both lost in E2, F2). n≥5 for each. Anterior to the left. Scale bars=250µm.
The region anterior to the eyes in planaria is devoid of stem cells (neoblasts). However, headless worms had neoblasts right up to their “anterior” margin (Figure 2B2). Despite lacking anterior structures, there was no duplication of posterior markers in H,K-ATPase-inhibited regenerates (Figure 2C). H,K-ATPase inhibition prevented optical nerve (Figure 2D) and photosensitive cell (Figure 2E) formation, corroborating morphological observations that SCH treatment blocks eye formation. Headless worms also failed to induce anterior genes, such as those expressed by the sensory cells in the anterior fringe (Figure 2F). We conclude that headless worms regenerate tail but fail to regenerate head. Together these data reveal that H,K-ATPase activity is required for anterior gene expression and head morphogenesis, without affecting posterior genes or reversing blastema polarity.
In spite of having a complete pharynx, H,K-ATPase-inhibited regenerates did not eat even when placed directly on food (data not shown). This is consistent with a requirement for brain in order to feed (Sheiman et al., 2002). Similarly, although headless worms moved when touched by a pipette tip, they otherwise remained stationary. This contrasts with controls, which even at 24 hours of regeneration still move about the dish. Therefore, we performed quantitative behavioral analyses of H,K-ATPase-inhibited planarians (Figure 3A) using a custom-made computer vision system for tracking behavior (Hicks et al., 2006). When placed in new environments, planarians typically undergo a short exploration period; however, H,K-ATPase-inhibited regenerates completely lost exploratory behavior. At 2 weeks of regeneration, control pharynx fragments (Figure 3A1) explored roughly 27% of the dish, whereas comparable H,K-ATPase-inhibited pharynx fragments (Figure 3A2) explored 0% (Figure 3A3, p=0.0015). In comparison, regenerates lacking mature brains, such as 24-hour untreated pharynx fragments, move significantly more than 2 week-old H,K-ATPase-inhibited regenerates (Figure 3A3, p<0.0001). This suggests H,K-ATPase-inhibited worms do not process neural inputs sufficient to coordinate exploratory behavior, which control fragments can by 24 hours of regeneration, highlighting an early role for H,K-ATPase activity during brain regeneration.
Figure 3. H,K ATPase activity is required for brain induction and ectopic head formation.
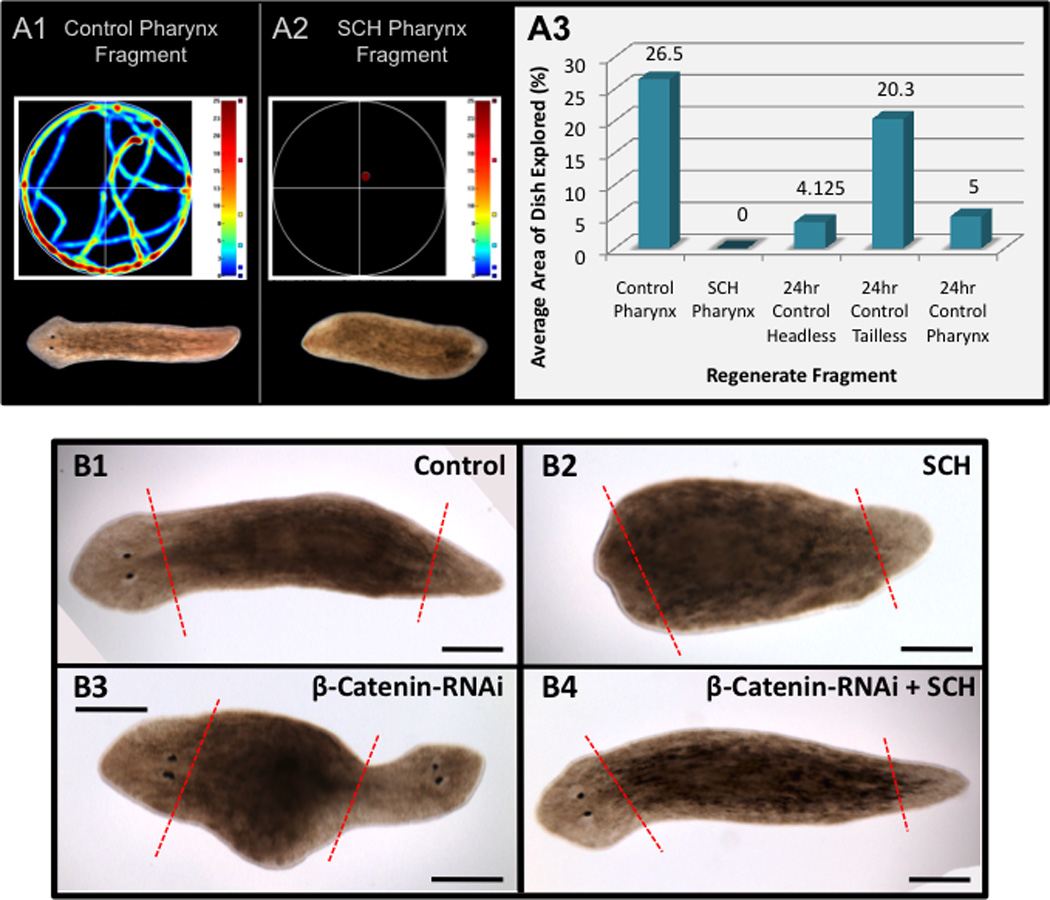
(A) Behavioral analyses. Heat maps track movement for worms shown. Red indicates most time spent in area. (A1) Control and (A2) SCH-treated pharynx fragments at 14 days regeneration. (A3) Average area of dish covered by each regenerate type. 95% CI: Control pharynx (n=8, CI=0.31); SCH pharynx (n=9, CI=0); 24-hour control headless (n=8, CI=0.14); 24-hour control tailless (n=10, CI=0.25); 24-hour control pharynx (n=10, CI=0.14). (B) β-catenin-RNAi assay with pharynx regenerates. (B1) Control injected. (B2) SCH-treated control-injected. (B3) β-catenin-RNAi injected (66.7% double-heads, CI=0.27). (B4) SCH-treated β-catenin-RNAi injected (25% double-heads, CI=0.25). Red dashed lines=amputation planes. Anterior to the left. Scale bars=250µm.
These data suggest H,K-ATPase activity is an initial step in regenerating anterior structures, during or prior to the signaling that patterns the anterior blastema. To position ion transport relative to known molecular pathways, we investigated Wnt signaling (Figure 3B), which regulates A/P polarity during planarian regeneration (Kobayashi et al., 2007; Petersen and Reddien, 2009). Inhibition of nuclear β-catenin signaling by RNAi causes ectopic posterior head formation, producing two-headed worms (Figure 3B3) (Gurley et al., 2008; Iglesias et al., 2008; Petersen and Reddien, 2008; Yazawa et al., 2009; Oviedo et al., 2010). However, H,K-ATPase inhibition prevents ectopic head formation (Figure 3B4, p<0.05). The inability of β-catenin-RNAi to ectopically induce anterior signaling when H,K-ATPase is also inhibited suggests H,K-ATPase activity is necessary to initiate ectopic anterior signals.
Anterior Signaling Rescues H,K-ATPase Inhibition
Unlike posterior fragments, pre-pharyngeal fragments regenerate heads despite SCH exposure (Figure 1D), although eye development is delayed by several days. One possibility is that the presence of anterior signals in this region compensates for lost H,K-ATPase activity, inducing head regeneration despite H,K-ATPase inhibition. To test this, increasing amounts of pre-pharyngeal tissue were amputated, allowing us to compare a gradient of regeneration responses to SCH treatment—from regenerates retaining the entire pre-pharynx region to those without any pre-pharyngeal tissue (Figure 4A). The data reveal that as more anterior tissue is removed, regenerates are more affected by H,K-ATPase inhibition. This suggests the presence of anterior tissue is sufficient to rescue anterior morphogenesis from H,K-ATPase inhibition.
Figure 4. Anterior CNS rescues H,K-ATPase inhibition.
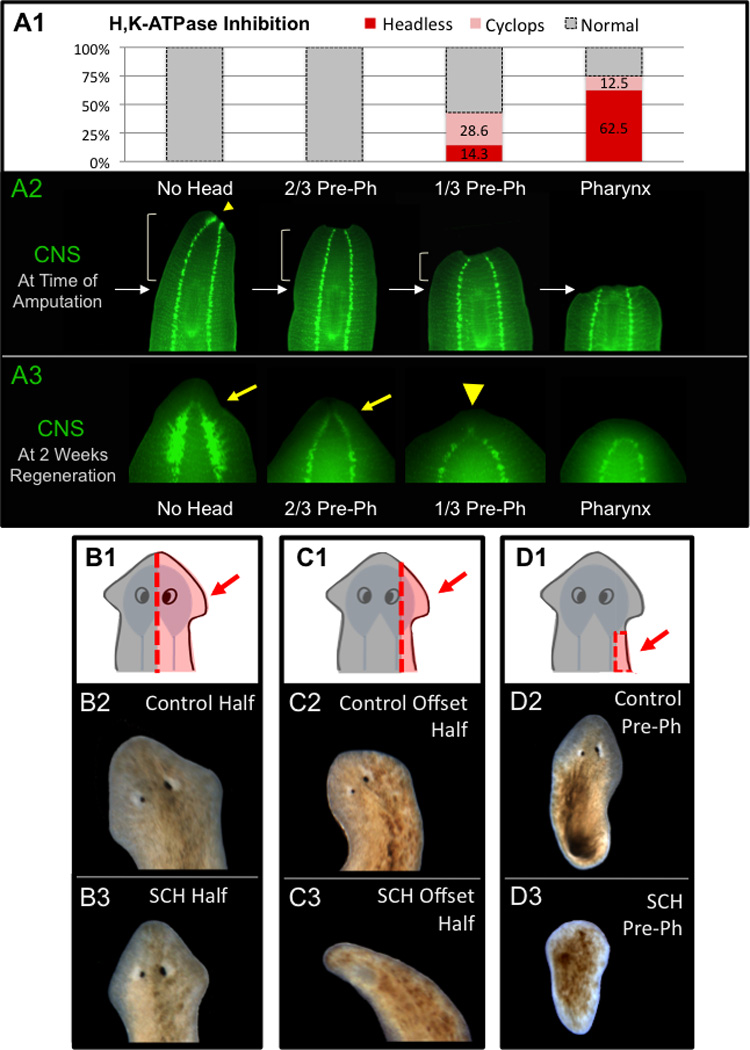
(A) Anterior amputations of SCH-treated worms. (A1) Worms amputated between the head and pharynx, scored at 2 weeks. 95% CI for 1/3-Pre-pharynx fragments: headless (CI=0.18), cyclops (CI=0.24); for Pharynx fragments: headless (CI=0.24), cyclops (CI=0.19). (A2) CNS remaining at time of amputation. Arrowhead=posterior brain remnant. Arrows=anterior tip of pharynx. Brackets=amount of pre-pharyngeal region remaining. (A3) CNS at 2 weeks regeneration. Arrows=brain regeneration. Arrowhead=cyclopic brain. CNS marker: Synapsin. (B–C) Sagittal (longitudinal) cuts including (B, even halves) or excluding (C, offset halves) CNS. (B2–C2) Control and (B3–C3) H,K-ATPase-inhibited fragments. (B3, 0% headless, n=42). Excluding anterior CNS allows headless regenerates (C3, 26% headless, n=43). (D) Small pre-pharynx fragments cut to exclude CNS (ventral nerve cords). Excluding anterior CNS allows headless regenerates in SCH-resistant pre-pharynx fragments (D3, 11.8% headless, n=34). Red dashed lines=amputation plane. Arrows & red shaded areas=fragments shown below. Anterior is up.
To determine if the anterior CNS specifically is involved, whole worms were cut sagittally (longitudinally) either through the midline (anterior CNS left in both halves, Figure 4B), or offset to one side where most anterior CNS was left in the larger “half” and little-to-none in the smaller “half” (Figure 4C). The results reveal that when brain tissue is present, head formation occurs even with H,K-ATPase inhibition (Figure 4B3). However when the CNS is excluded, H,K-ATPase inhibition is sufficient to block head regeneration, resulting in headless worms (Figure 4C3). In fact, although pre-pharyngeal transverse fragments are SCH resistant (Figure 1D), pre-pharyngeal fragments that exclude the ventral nerve cords (CNS) become susceptible to H,K-ATPase-inhibition (Figure 4D). This suggests it is not merely anterior tissue, but anterior CNS specifically, that is capable of rescuing H,K-ATPase inhibition.
These results suggest the presence of anterior signaling is sufficient to drive head regeneration independent of H,K-ATPase activity. If correct, re-initiating regeneration, which would re-supply such signaling, should also rescue head formation. Therefore, headless regenerates (at 2–3 weeks of regeneration) were wounded at the “anterior” end without further SCH exposure and assayed for head regeneration (Figure 5). The data revealed that wound healing alone was not sufficient to restore head structures (Figure 5A). However, the presence of an unpigmented blastema did rescue head structures (Figure 5B,C). Interestingly, smaller wounds that induced a tiny blastema (Figure 5B3) developed cyclopia (Figure 5B4), while large blastemas (Figure 5C3) regenerated full heads (Figure 5C4). This perhaps reflects the difference between a small, localized spot of anterior gene expression and the broader signaling provided by a larger blastema. We conclude that wound healing alone is not sufficient to initiate anterior morphogenesis, but that anterior signals are provided by the new tissue (blastema). This suggests a functional difference between wound healing (driven by tissue expansion) and true regeneration (where blastema formation initiates extensive patterning and tissue remodeling).
Figure 5. Regeneration rescues H,K-ATPase-inhibited worms.
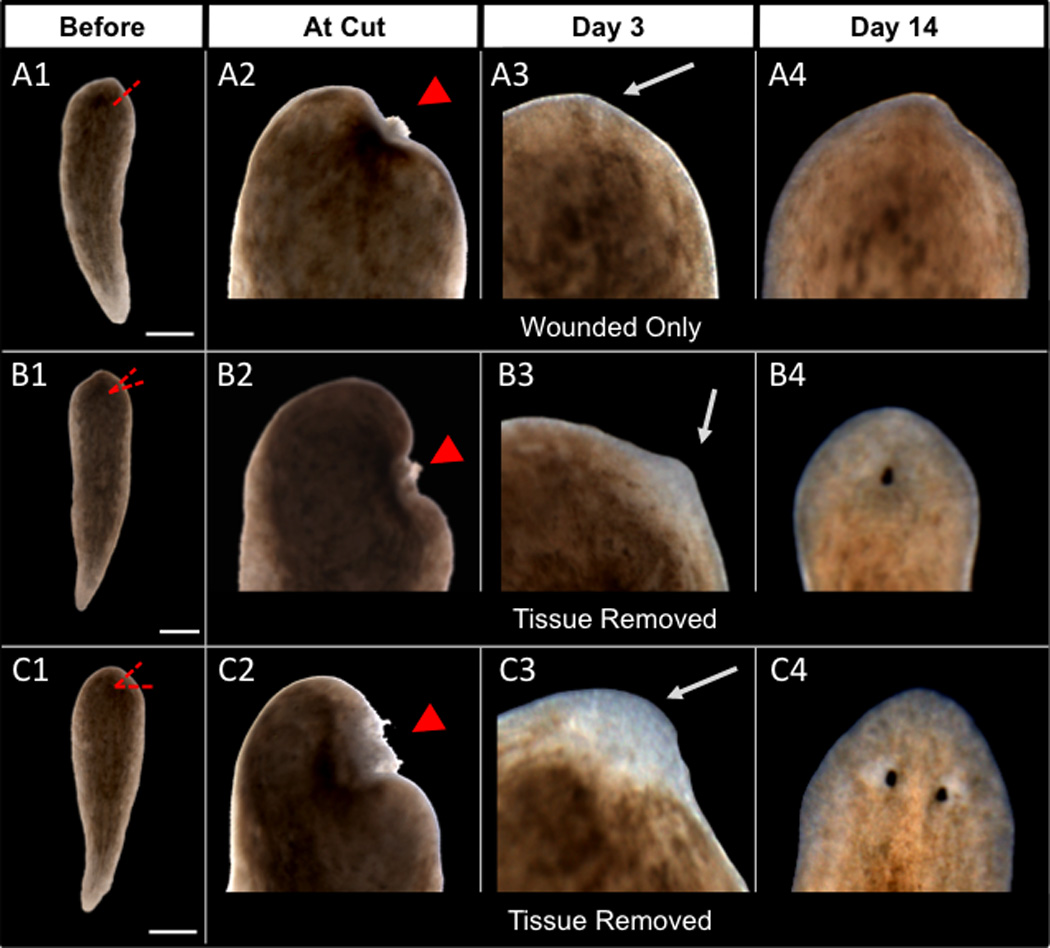
Headless regenerates wounded without drug at the “anterior” end (determined by pharynx direction). (A) Wound with one amputation plane and no blastema (A3) fails to rescue head (A4). 100% headless (n=16). (B) Wound with two amputation planes and small bit of tissue removed produces a small blastema (B3) and cyclopia (B4). 75% cyclopic (n=4). (C) Wound with more tissue removed produces a large blastema (C3) and full anterior rescue (C4). 75% head rescue (n=24). Panels show same worms before re-cutting (A1–C1), ½ hour after cutting (A2–C2), at 3 days (A3–C3) and 14 days (A4–C4) regeneration. Dashed red lines=amputation planes. Arrowheads=wound. Arrows=blastema (unpigmented regions in B3–C3) or wound site (for A3). Anterior is up. Scale bars=500µm.
As expected, the ability to restore head structures appears to be from regeneration-specific signaling, since in wounded headless worms the first eye invariably arose within the blastema. This rescue is not caused merely by indiscriminate regeneration, as blastemas formed by wounds in the tail never promoted head regeneration (100%, n=11). Only blastema formation in what should be the anterior end of headless worms resulted in head regeneration. The rescue of H,K-ATPase inhibition by inducing regeneration in the headless region shows that “non-head” (non-posterior, non-anterior) tissue is still competent to form anterior structures, provided the correct signals are made available. This demonstrates that H,K-ATPase activity is not involved in the initial determination of anterior polarity, as blastemas in the headless region still know to form head structures. Instead, the sufficiency of anterior signals to rescue head regeneration is consistent with data showing that H,K-ATPase activity is required for anterior gene expression upstream of anterior morphogenetic patterning, thus supporting our conclusion that patterning is downstream of H,K-ATPase-dependent signaling.
H,K-ATPase Activity Regulates Head Regeneration Through Membrane Voltage
In light of the mechanisms by which the electroneutral H,K-ATPase controls left-right polarity (in concert with a partnered K+ channel), we examined membrane voltage (Vmem) regulation during regeneration. We used the DiBAC voltage-reporting dye (Adams et al., 2007; Oviedo et al., 2008c) to assay for relative changes in steady-state Vmem in 24-hour pharynx fragments with one end (either anterior or posterior) cut at a slant to mark the original A/P axis (Figure 6). After 24 hours of regeneration, the anterior blastema in controls was depolarized (Figure 6B2). Control fragments displayed a head-to-tail membrane voltage gradient, with the anterior blastema significantly (p<0.001) more depolarized and the tail blastema just marginally more hyperpolarized than the original tissue. With H,K-ATPase-inhibition this anterior blastema depolarization was lost (Figure 6B1), and regenerates were significantly more hyperpolarized than controls (p<<0.0001), even after only 24 hours of inhibition (Figure 6C). When allowed to regenerate fully, these hyperpolarized fragments displayed the headless phenotype (Figure 6B4).
Figure 6. H,K-ATPase activity depolarizes the anterior blastema membrane potential.
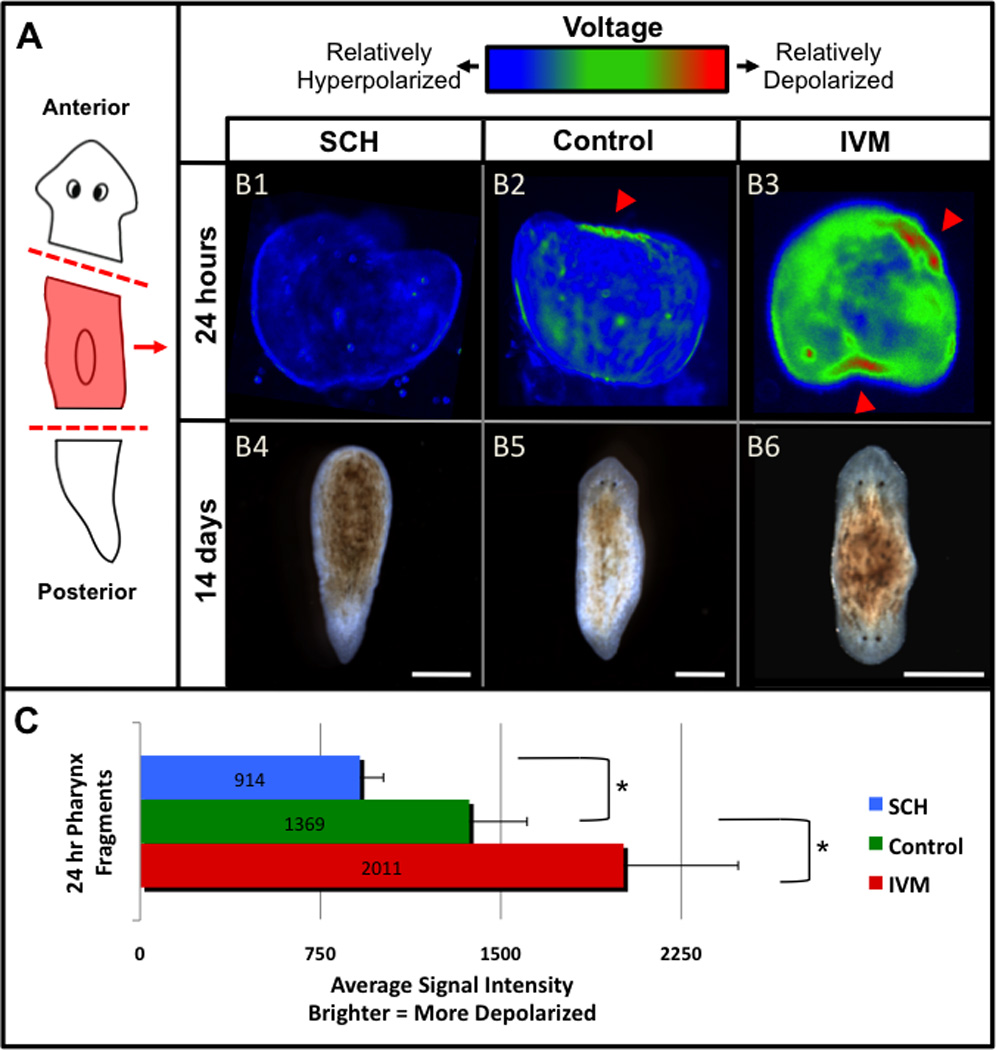
Membrane voltage assay using DiBAC. (A) Diagram of cuts. Pharynx fragments cut with slanted anterior ends to mark original A/P axis. (B) Regenerates assayed at 24 hours post amputation (B1–B3), and phenotypes scored at 14 days (B4–B6). Images are pseudocolored blue-green-red. Brighter pixels (green to red) indicate relatively depolarized cells (inside more positive with respect to outside), while darker pixels (blue) indicate relatively hyperpolarized cells (inside more negative). (B1, B4) SCH-treated, (B2, B5) control, and (B3, B6) ivermectin (IVM)-treated fragments. Arrowheads=depolarized blastemas. Anterior is up. Scale bars=500µm. (C) Whole fragment comparisons of 24-hour pharynx regenerates. SCH-treated (n=13), control (n=19), and IVM-treated (n=11). Asterisks=p<<0.0001. Error bars=standard deviation. See also Figure S3.
Hyperpolarization correlated closely with inhibited head regeneration: 62.5% of SCH-treated fragments had relative Vmem values lower (more polarized) than the lowest (least polarized) control fragment, consistent with the 67.4% of SCH-treated pharynx regenerates that are headless. To ensure inhibited head regeneration was truly due to membrane voltage changes and not some unknown effect of SCH, we manipulated a different regulator of polarization, external potassium. Alone, increased K+ concentrations in the media (worm water) had no effect (100%, n=30). However, increased K+ in the media rescued H,K-ATPase inhibition (5.6% headless, compared to 52.6% with SCH alone). This suggests that in planarians, the H,K-ATPase works in tandem with a partner K+ channel to modulate Vmem, keeping K+ flux in balance and maintaining the proper polarization needed to drive anterior morphogenesis. Most crucially, the data confirm that the critical H,K-ATPase activity during head morphogenesis is regulating membrane voltage by establishing the required K+ gradient.
Blastema Depolarization is Sufficient to Drive Anterior Polarity
The hyperpolarization associated with loss of head structures suggests depolarization may be required for head regeneration. To directly test a role for membrane voltage and to further rule out off-target effects of SCH, voltage-independent H,K-ATPase functions, or potassium-specific mechanisms, we developed a method of controlling membrane potential independently of the H,K-ATPase. Ivermectin is a well-characterized, irreversible, highly specific activator of the invertebrate glutamate-gated chloride (GluCl) channel (Shan et al., 2001). It is known to depolarize tissues in C. elegans (Pemberton et al., 2001) and offers a convenient and powerful tool with which to drive changes in membrane voltage. Once the GluCl channel is opened by ivermectin, modulation of external chloride levels can cause Cl− influx (hyperpolarizing cells) or efflux (depolarizing cells) based on the direction of the Cl− gradient.
We treated planarian pharynx fragments with 1µM ivermectin for the first 72 hours of regeneration, and remarkably a second head formed at the posterior wound in 91.7% of regenerates (Figure 6B6). When assayed for relative Vmem at 24 hours of regeneration, ivermectin-treated regenerates had two regions of high depolarization, one at each blastema (Figure 6B3); indeed, ivermectin treatment significantly depolarized regenerates compared to controls (Figure 6C, p<0.0001). While previous results indicated that H,K-ATPase activity is necessary for regenerating head structures, these data demonstrate that depolarization alone is sufficient to drive anterior regeneration even in posterior wounds. This suggests membrane voltage is an early, instructive step for anterior fate specification in newly regenerating tissues. To confirm that Vmem changes after GluCl activation resulted from directed changes in membrane potential and not other effects of ivermectin, we raised external chloride concentrations. Increased Cl− concentrations in the media rescued ivermectin treatment, inhibiting ectopic head formation (36.6% double-headed, compared to 91.7% for ivermectin alone). This further supports our conclusion that hyperpolarization of the anterior blastema is sufficient to block anterior morphogenesis.
Interestingly, in intact worms the head region was significantly depolarized compared to the tail (Figure S3, n=6, p<0.0001), supporting a role for membrane voltage in regulating anterior genes. However, treatment of uncut worms with either ivermectin or SCH had no effect, despite confirmation that these worms were indeed depolarized or hyperpolarized, respectively (DiBAC analysis, Figure S3), suggesting Vmem regulation is essential for regeneration but not for tissue homeostasis.
Membrane Voltage Acts Through Calcium Signaling to Drive Anterior
Our data demonstrate that membrane voltage regulates anterior gene expression in blastema cells. One mechanism for transducing changes in membrane voltage into transcriptional activity is through modulating the activity of voltage-gated calcium channels (Cav), such as in the calcium-activated CaM-Kinase/CREB pathway (Weick et al., 2003). A recent planarian study showed that Cav activation by praziquantel treatment results in two-headed worms (consistent with our ivermectin data), and is rescued by RNAi to Cavβ subunits (Nogi et al., 2009). Therefore, we investigated the possibility that membrane depolarization of the anterior blastema controls patterning via calcium dynamics.
We used Fura-2-AM, a ratiometric intracellular Ca2+ reporting dye, to examine calcium levels in 24-hour pharynx fragments where one end was cut at a slant to mark the original A/P axis (Figure 7A). Control regenerates exhibited a significant upregulation of calcium in the anterior blastema, which was not seen in posterior blastemas (Figure 7A1–A2, n=8, p<<0.0001). H,K-ATPase inhibition eliminated this increase in intracellular calcium, consistent with the headless phenotype (Figure 7A3–A4, controls n=8 and SCH-treated n=9, p<0.001). No significant difference was found in calcium levels in either the original tissue (p=0.22) or the posterior blastema (p=0.72), suggesting H,K-ATPase-mediated calcium changes are restricted to the anterior blastema.
Figure 7. Membrane voltage regulates blastema polarity.
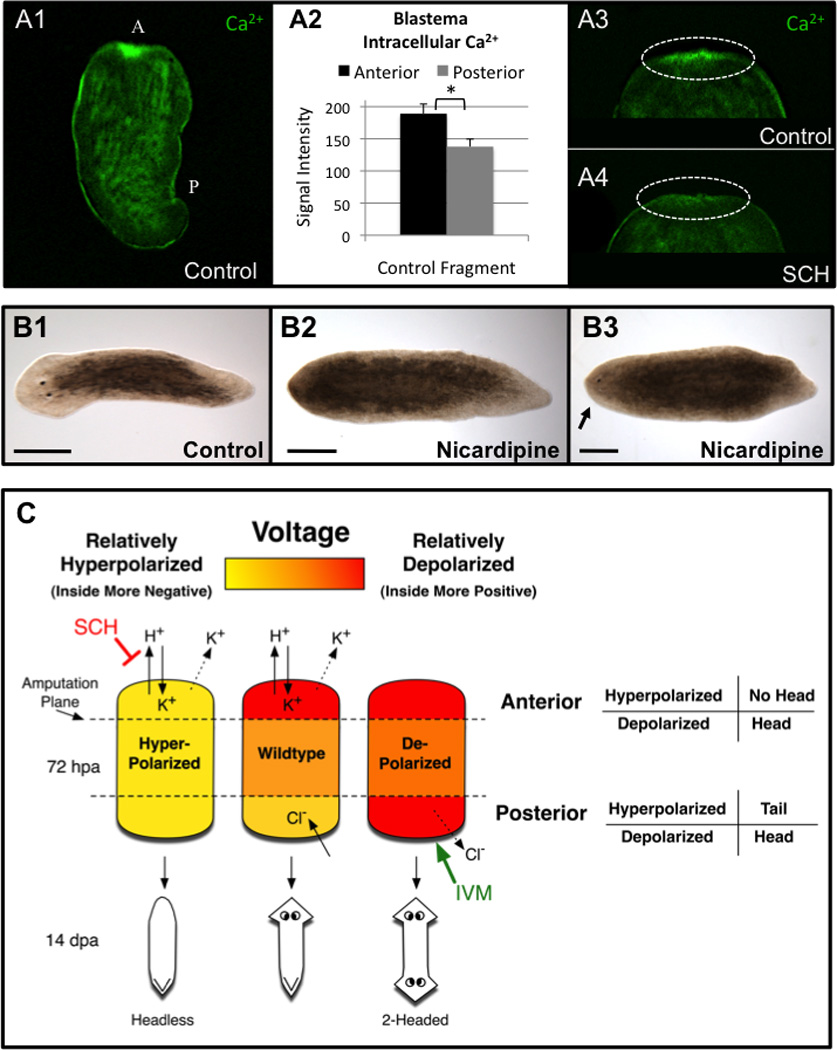
(A) Intracellular calcium assay using Fura-2-AM. 24 hour pharynx regenerates. (A1–A2) Calcium is significantly upregulated in control anterior blastemas (letter A) compared to posterior blastemas (P). Asterisk=p<<0.0001. (A3–A4) H,K-ATPase inhibition eliminates this upregulation (p<0.001). (B) Inhibition of voltage-gated calcium channels using nicardipine. Pharynx regenerates at 14 days regeneration. (B1) Control. (B2-3) Nicardipine regenerates have reduced anterior: (B2) headless (8.2%) and (B3) failure to form both eyes (34.4%, arrow). Scale bars=500µm. (C) Model. Wildtype fragments have a membrane potential gradient with the head blastema most depolarized and the tail blastema least depolarized. SCH-mediated H,K-ATPase inhibition results in relative hyperpolarization and ivermectin treatment in relative depolarization. Hyperpolarized blastemas become either tail or headless, while depolarized blastemas always result in head formation. IVM=ivermectin, hpa=hours post amputation, dpa=days post amputation.
To directly test the involvement of Cav channels, we treated regenerating fragments with nicardipine (Figure 7B), an inhibitor of L-type voltage-gated calcium channels (Elmslie, 2004). Following a 4-hour delay to prevent interference with wound closure (Shabir and Southgate, 2008), pharynx fragments were exposed to nicardipine from 4–72 hours of regeneration. At 2 weeks, 42.6% of nicardipine-treated regenerates had reduced anterior regeneration (n=61), including headless worms (Figure 7B2, 8.2%) and a failure to fully form both eyes (Figure 7B3, 34.4%). These data suggest calcium signaling as a mechanism for membrane voltage-mediated changes in anterior gene expression, specifically implicating a role for Cav in anterior head regeneration. Together, the data highlight ion transport regulation as a particularly convenient tool for controlling polarity, morphogenesis and gene expression downstream of adult stem cell proliferation and blastema formation.
DISCUSSION
Here we demonstrate a powerful chemical genetics approach for investigating the regeneration of axial polarity, uncovering a novel role for ion transport and membrane voltage in initiating anterior gene expression and morphogenesis downstream of stem cell proliferation. The use of pharmacological reagents has several advantages over molecular tools such as RNAi. Animals do not always recover from RNAi, unlike many chemical compounds which are reversible, such as the H,K-ATPase inhibitor SCH-28080. This allows for time-course studies and the investigation of late events without inhibiting early ones. Additionally, with RNAi only a few genes are usually targeted at one time. In contrast, SCH inhibits all H,K-ATPase genes expressed (while being highly specific to H,K-ATPases and not other P2C class family members). This feature allows for the identification of phenotypes that would be masked by redundancy when targeting single gene products.
A Membrane Voltage Model for Regulating Polarity During Planarian Regeneration
The data suggest a model for membrane voltage and its role in the regeneration of axial polarity (Figure 7). Endogenous H,K-ATPase activity depolarizes the anterior blastema, probably in tandem with a partnered K+ channel. Hyperpolarizing K+ ions flow outward through this K+ channel, while the H,K-ATPase pumps K+ ions back inward—the mechanism by which the H,K-ATPase keeps the membrane depolarized. When SCH prevents H,K-ATPase activity, the H,K-ATPase-driven K+ influx is blocked, but the partnering K+ channel’s efflux is unaffected. This results in a net outward flow of K+ ions that hyperpolarizes the blastema (preventing head regeneration). When SCH treatment was combined with increased K+ in the media, this outward efflux of K+ ions is stopped by the new gradient, restoring the ion balance and allowing head formation. This implicates the presence of strong endogenous biological buffers, which sequester increasing intracellular H+ levels when the H,K-ATPase is blocked. Excess H+ is buffered in the cell, so protons do not contribute to overall membrane voltage. This is supported by the fact that A/P polarity in regenerating planaria was not affected by altering the pH of the media.
The model focuses on membrane voltage as a transducer of morphogenetic signals. As absolute calibration of reporter dyes in vivo is difficult to achieve, our model is framed in terms of relative values (most depolarized versus least depolarized). Additionally, while it is not known whether chloride channels are an endogenous component of polarity regulation in planarians, they were a convenient target for functional perturbations. We modulated Cl− flux as an H,K-ATPase-independent method to determine if membrane voltage alone regulates blastema polarity. The drug ivermectin was used to keep glutamate-gated chloride channels open, allowing Cl− to flow along its gradient (Shan et al., 2001), producing ectopic heads. Since increased Cl− concentrations in the media (causing the gradient to flow inward) rescued the double-headed ivermectin phenotype, this suggests the natural gradient for Cl− is outwards—hyperpolarizing the posterior blastema and keeping anterior genes turned off. Together the data confirmed that membrane depolarization alone is sufficient to drive anterior polarity.
The data suggest membrane voltage influences gene expression by altering intracellular calcium levels in the anterior blastema, in part through regulation of membrane voltage-gated calcium channels. L-type calcium channels (inhibited by nicardipine) are activated by depolarization (Lipscombe et al., 2004), and can drive gene expression through CREB activation (Weick et al., 2003). Membrane voltage may have additional effects on the permeability of gap junctions, resulting in changes in second messenger movements, including calcium (Rackauskas et al., 2010). This is consistent with data showing that gap junctions are also required for correct A/P polarity in regenerating planaria (Nogi and Levin, 2005; Oviedo et al., 2010). Together, the evidence suggests calcium signaling is the functional target of anterior blastema membrane depolarization and head regeneration.
Integrating Ion Transport with Anterior-Posterior Wnt Signaling Pathways
As in vertebrates, planarian Wnt signaling plays a major role in establishing the primary axis (Kobayashi et al., 2007; Petersen and Reddien, 2009). Specifically, β-catenin signaling drives posterior fates (Gurley et al., 2008; Iglesias et al., 2008; Yazawa et al., 2009), while Hedgehog signaling in turn regulates β-catenin (Rink et al., 2009; Yazawa et al., 2009). β-catenin-RNAi injected animals regenerate ectopic heads at posterior wounds (Petersen and Reddien, 2008; Oviedo et al., 2010). Very early work demonstrated that polarity could also be reversed at posterior wounds by direct application of an exogenous electrical field (Marsh and Beams, 1952). However, the molecular basis for this effect, and the role of membrane potential in blastema cells, has not previously been investigated.
H,K-ATPase inhibition was able to block ectopic head formation caused by β-catenin-RNAi. This suggests H,K-ATPase activity and membrane depolarization are required for the ectopic initiation of anterior morphogenesis in posterior tissues. However, H,K-ATPase-inhibited worms with hyperpolarized anterior blastemas failed to form ectopic tails. This suggests the posterior signals required to drive tail regeneration are not present in anterior tissues. Temporally, H,K-ATPase activity is required for the first 72 hours of regeneration, consistent with a role upstream of tissue differentiation. In the planaria S. mediterranea, wound healing is finished by 6 hours (Salo et al., 2009); followed by stem cell proliferation and blastema formation; and at 24–48 hours stem cell differentiation begins to occur (Eisenhoffer et al., 2008). Although evidence suggests the D. japonica used here regenerate somewhat faster (Inoue et al., 2004; Cebria et al., 2007), the data are consistent with the placement of membrane voltage as an early requirement for anterior polarity, upstream of morphogenesis and anterior gene expression.
Ivermectin-mediated depolarization of the posterior blastema is sufficient to reverse endogenous polarity and produce ectopic heads. However, endogenous H,K-ATPase activity, while necessary for head regeneration, is not the initial determinate of anterior polarity. When H,K-ATPase-inhibited headless regenerates are re-cut (without inhibitor), the new blastema is able to determine it should have anterior polarity and thus regenerate a head. Although this could be due to timing issues (reflecting an initial incomplete block of anterior gene expression before the inhibitor saturates blastemal cells), it is most likely that there is a temporal requirement for membrane depolarization downstream of early differentiation (which specifies the blastema as “anterior”) but upstream of late differentiation (which leads to tissue-specific gene expression).
The Role of Ion Transport in Development and Disease
The development of molecular-genetic tools for characterizing and manipulating ion flows in vivo (Adams and Levin, 2006b; Smith et al., 2007; Adams, 2008; Tsutsui et al., 2008) has revealed that bioelectric signals regulate a variety of developmental processes not typically associated with ion flux. Membrane voltage-mediated signaling (downstream of individual ion transporters) is an important regulator of regenerative outgrowth and proliferation (Adams et al., 2006). For instance, in Xenopus tail regeneration, a very early H+ flux repolarizes blastema cells, acting as a cue for downstream gene expression and axonal outgrowth (Adams et al., 2007). Proton transport and regulation of steady-state membrane voltage (over hours and days) are also required for embryonic left-right polarity in a wide variety of organisms (Levin et al., 2002; Hibino et al., 2006; Raya and Izpisua Belmonte, 2006; Shimeld and Levin, 2006).
In planarians, we showed that interfering with H,K-ATPase-mediated ion transport results in complete loss of head structures. It is not uncommon for seemingly minor alterations in ion flux to produce significant defects at the organismal level; ion transport disruptions produce so many distinct diseases (e.g. cystic fibrosis, epilepsy, deafness) that the term channelopathies has entered our disease lexicon (Hubner and Jentsch, 2002). The disregulation of membrane voltage gradients and ion transport is also associated with patterning defects in cancer (Mycielska and Djamgoz, 2004), as well as the induction of neoplastic phenotypes in embryonic stem cells (Morokuma et al., 2008b). New methods for controlling the runaway growth and de-differentiation of cancer are being sought amongst ion channel-targeting compounds (Arcangeli et al., 2009). Moreover, pharmacological modulation of endogenous ion transporters are being used to augment epithelial wound healing upstream of cellular signaling pathways (Zhao et al., 2006). Since cancer progression and tissue regeneration share a number of fundamental underlying mechanisms (Oviedo and Beane, 2009), developing techniques for modulating the ion flux in specific groups of cells as illustrated here is likely to contribute to progress in several important areas of biomedicine. Both ivermectin and SCH are approved for human use and offer the potential for versatile therapeutic control of membrane potentials (ivermectin by modulating chloride levels, and SCH through regulation of potassium flux). We show that both compounds predictably alter the patterning of regenerative tissues, respectively activating or inhibiting associated signaling, to control the axial identity of the new growth. Perfecting techniques to control bioelectrical tissue properties will be essential if we are to realize the promise of regenerative therapies.
SIGNIFICANCE
This work identifies a physiological property, membrane voltage, as a novel regulator of polarity during regeneration in adult planarians. This exciting discovery reveals an entirely novel and biomedically useful method for controlling morphogenesis in adult stem cell-derived tissues, including brain. Current research efforts in regenerative medicine have strongly focused on stem cell maintenance and proliferation, as the first vital hurdle for regenerative therapies is to induce regeneration in non-regenerative tissues. However, any new tissues generated must be correctly patterned in the context of the whole organism. As demonstrated here, chemical modulation of ion transport using known drugs (which have already gone through clinical testing and are approved for human use) could be a powerful tool to induce the regrowth of lost tissues (organs and limbs) that are correctly patterned and thus functional. Crucially, we showed that pharmacologically-induced changes in membrane voltage are sufficient to initiate an entire morphogenetic program (head regeneration) downstream of stem cell proliferation, serving as a master regulator of a highly orchestrated patterning cascade that would be undesirable to micromanage directly. Chemical genetic approaches, such as the ones described here, circumvent the need to regulate each signaling pathway and epigenetic mechanism individually. Development of such techniques for the regulation of ion flow, direction, and timing of instructive Vmem changes will one day significantly augment our ability to induce medically-relevant regenerative responses.
EXPERIMENTAL PROCEDURES
Colony Care
An asexual clonal strain (GI) of Dugesia japonica (GI) was kept and maintained as in (Oviedo et al., 2008a). Worms 4–7 mm long were used, except for Figure 4A&D where ~1 cm were used. All worms were starved for ≥1 week prior to use.
Pharmacology and Amputations
For each treatment, SCH-28080 (Sigma S4443) was suspended to 0.18 M in newly opened DMSO (ATTC #4-X), used at 18 µM on day 1, and refreshed on days 2 & 3 with aliquots stored at −80°C. Ivermectin (Sigma I8898) was kept as a 10 mM stock in DMSO, from which a 100 µM dilution in water was made. Worms were pre-soaked in 1 µM IVM for 3 days prior to cuts, when IVM was refreshed. External K+ was increased with 2.8 mM potassium-D-gluconate (Sigma). External Cl− was increased with 11.9 mM N-methyl-D-glucamine-HCl (TCI). Nicardipine HCl (Sigma) in DMSO was used at 3–5 µM. DMSO-treated controls had no effect. Amputations were performed as in (Nogi et al., 2005).
Immunofluorescence and Immunohistochemistry
Worms were fixed as in (Nogi et al., 2005). For immunofluorescence, worms were processed as in (Reddien et al., 2005). 10 antibodies: α-phosphorylated histone H3, 1:250 (Upstate); α-arrestin, 1:10,000 (kind gift from K. Watanabe); and synapsin, 1:50 (Developmental Studies Hybridoma Bank). 20 antibodies: goat anti-mouse Alexa488, 1:400 (Sigma); or for H3P an HRP-conjugated anti-Rabbit with TSA-Alexa568 anti-HRP (Molecular Probes). In situ hybridizations were performed as in (Nogi and Levin, 2005). Probes: Hox9 (Abd-ba, Nogi and Watanabe, 2001); Innexin7 (Nogi and Levin, 2005); 0251_HH and 0821_HN (Nakazawa et al., 2003) were a kind gift from T. Gojobori.
Behavioral Analyses
Movements were quantified with a custom-made computer vision system (Hicks et al., 2006). Worms were kept in the dark for 10 minute trials (data recorded at 5 hertz). Coordinates were analyzed in Microsoft Excel and heat maps generated with J-Specimen (Ebiotics).
Gene Knockdown with RNAi
dsRNA was generated and injected as in (Oviedo et al., 2008b). β-catenin-RNAi (Oviedo et al., 2010) was injected on days 1–3, and worms cut on day 7. Control worms were injected on days 1–3 with either water or PC2-RNAi and cut on day 14.
Membrane Voltage and Calcium Reporter Assays
Voltage: DiBAC4(3) (bis-[1,3-dibarbituric acid]-trimethine oxanol) (Invitrogen) was used on PC2-RNAi immbolized worms as in (Adams et al., 2007; Oviedo et al., 2008c). Stock (1.9 mM in DMSO) was used at 0.475 µM in worm water, with ≥30 minute incubation before imaging. Calicum: 10mM Fura-2-AM (Sigma) in DMSO was kept in aliquots at −20°C, then used at 10 µM in worm water. PC2-RNAi regenerates were incubated for 1 hour, then washed 3X prior to imaging. Images were produced using an Olympus BX61 compound microscope (FITC filter) with a Hamamatsu Orca AG CCD camera and IP Labs imaging software (intensity measured with “Quantify Segment”).
Image Collection and Processing
All other images were collected using a Nikon SMZ1500 microscope with a Retiga 2000R camera (Q-Imaging) and Q-Capture imaging software. Adobe Photoshop was used to orient and scale images and improve clarity (not for Vmem or pH images). Data were neither added nor subtracted; original images available upon request.
Statistical Analyses
Error bars for % phenotypes were calculated using 95% Confidence Intervals (CI). Error bars for average pixel intensity are ± 1 standard deviation. Microsoft Excel was used to calculate Student's t-Test (2-tailed distribution, two independent samples, unequal variance).
HIGHLIGHTS.
H,K-ATPase is required for head regeneration and anterior blastema depolarization
H,K-ATPase-independent depolarization is able to drive ectopic head regeneration
Membrane voltage acts through calcium signaling to drive gene expression
Control of Vmem is a novel method to regulate stem cell-mediated patterning
Supplementary Material
ACKNOWLEDGEMENTS
We thank Kelly Tseng and other lab members for discussion and suggestions, and K. Watanabe and T. Gojobori for reagents. WSB also thanks JLB for continued support and NJ Oviedo for planarian instruction. WSB was supported by NIH Kirschstein-NRSA grant F32 GM08354. M.L. gratefully acknowledges support by NSF grant IBN# 0347295, NHTSA grant DTNH22-06-G-00001, and NIH grant HD055850. DSA was supported by NIH K22-DE016633. This paper is dedicated to G. Marsh and G. W. Beams, whose pioneering work inspired this project.
REFERENCES
- Adams DS. A new tool for tissue engineers: ions as regulators of morphogenesis during development and regeneration. Tissue Eng Part A. 2008;14:1461–1468. doi: 10.1089/ten.tea.2008.0080. [DOI] [PubMed] [Google Scholar]
- Adams DS, Levin M. Inverse drug screens: a rapid and inexpensive method for implicating molecular targets. Genesis. 2006a;44:530–540. doi: 10.1002/dvg.20246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DS, Levin M. Strategies and Techniques for Investigation of Biophysical Signals in Patterning. In: Whitman M, Sater AK, editors. Analysis of Growth Factor Signaling in Embryos. CRC Press; 2006b. pp. 177–262. [Google Scholar]
- Adams DS, Masi A, Levin M. H+ pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development. 2007;134:1323–1335. doi: 10.1242/dev.02812. [DOI] [PubMed] [Google Scholar]
- Adams DS, Robinson KR, Fukumoto T, Yuan S, Albertson RC, Yelick P, Kuo L, McSweeney M, Levin M. Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development. 2006;133:1657–1671. doi: 10.1242/dev.02341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcangeli A, Crociani O, Lastraioli E, Masi A, Pillozzi S, Becchetti A. Targeting ion channels in cancer: a novel frontier in antineoplastic therapy. Curr Med Chem. 2009;16:66–93. doi: 10.2174/092986709787002835. [DOI] [PubMed] [Google Scholar]
- Aw S, Adams DS, Qiu D, Levin M. H,K-ATPase protein localization and Kir4.1 function reveal concordance of three axes during early determination of left-right asymmetry. Mech Dev. 2008;125:353–372. doi: 10.1016/j.mod.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston DJ, McLaughlin KA, Levin M. Bioelectric controls of cell proliferation: ion channels, membrane voltage and the cell cycle. Cell Cycle. 2009;8:3519–3528. doi: 10.4161/cc.8.21.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebria F, Guo T, Jopek J, Newmark PA. Regeneration and maintenance of the planarian midline is regulated by a slit orthologue. Dev Biol. 2007;307:394–406. doi: 10.1016/j.ydbio.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducibella T, Schultz RM, Ozil JP. Role of calcium signals in early development. Semin Cell Dev Biol. 2006;17:324–332. doi: 10.1016/j.semcdb.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Eisenhoffer GT, Kang H, Sanchez Alvarado A. Molecular analysis of stem cells and their descendants during cell turnover and regeneration in the planarian Schmidtea mediterranea. Cell Stem Cell. 2008;3:327–339. doi: 10.1016/j.stem.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmslie KS. Calcium channel blockers in the treatment of disease. J Neurosci Res. 2004;75:733–741. doi: 10.1002/jnr.10872. [DOI] [PubMed] [Google Scholar]
- Forrester JV, Lois N, Zhao M, McCaig C. The spark of life: the role of electric fields in regulating cell behaviour using the eye as a model system. Ophthalmic Res. 2007;39:4–16. doi: 10.1159/000097901. [DOI] [PubMed] [Google Scholar]
- Forsthoefel DJ, Newmark PA. Emerging patterns in planarian regeneration. Curr Opin Genet Dev. 2009;19:412–420. doi: 10.1016/j.gde.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita A, Horio Y, Higashi K, Mouri T, Hata F, Takeguchi N, Kurachi Y. Specific localization of an inwardly rectifying K(+) channel, Kir4.1, at the apical membrane of rat gastric parietal cells; its possible involvement in K(+) recycling for the H(+)-K(+)-pump. J Physiol. 2002;540:85–92. doi: 10.1113/jphysiol.2001.013439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley KA, Rink JC, Sanchez Alvarado A. Beta-catenin defines head versus tail identity during planarian regeneration and homeostasis. Science. 2008;319:323–327. doi: 10.1126/science.1150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino T, Ishii Y, Levin M, Nishino A. Ion flow regulates left-right asymmetry in sea urchin development. Dev Genes Evol. 2006;216:265–276. doi: 10.1007/s00427-005-0051-6. [DOI] [PubMed] [Google Scholar]
- Hicks C, Sorocco D, Levin M. Automated analysis of behavior: a computer-controlled system for drug screening and the investigation of learning. J Neurobiol. 2006;66:977–990. doi: 10.1002/neu.20290. [DOI] [PubMed] [Google Scholar]
- Hubner CA, Jentsch TJ. Ion channel diseases. Hum Mol Genet. 2002;11:2435–2445. doi: 10.1093/hmg/11.20.2435. [DOI] [PubMed] [Google Scholar]
- Iglesias M, Gomez-Skarmeta JL, Salo E, Adell T. Silencing of Smed-betacatenin1 generates radial-like hypercephalized planarians. Development. 2008;135:1215–1221. doi: 10.1242/dev.020289. [DOI] [PubMed] [Google Scholar]
- Inoue T, Kumamoto H, Okamoto K, Umesono Y, Sakai M, Sanchez Alvarado A, Agata K. Morphological and functional recovery of the planarian photosensing system during head regeneration. Zoolog Sci. 2004;21:275–283. doi: 10.2108/zsj.21.275. [DOI] [PubMed] [Google Scholar]
- Jaisser F, Beggah AT. The nongastric H+-K+-ATPases: molecular and functional properties. Am J Physiol. 1999;276:F812–F824. doi: 10.1152/ajprenal.1999.276.6.F812. [DOI] [PubMed] [Google Scholar]
- Kaufhold MA, Krabbenhoft A, Song P, Engelhardt R, Riederer B, Fahrmann M, Klocker N, Beil W, Manns M, Hagen SJ, Seidler U. Localization, trafficking, and significance for acid secretion of parietal cell Kir4.1 and KCNQ1 K+ channels. Gastroenterology. 2008;134:1058–1069. doi: 10.1053/j.gastro.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Klaassen CH, Fransen JA, Swarts HG, De Pont JJ. Glycosylation is essential for biosynthesis of functional gastric H+,K+-ATPase in insect cells. Biochem J. 1997;321(Pt 2):419–424. doi: 10.1042/bj3210419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi C, Saito Y, Ogawa K, Agata K. Wnt signaling is required for antero-posterior patterning of the planarian brain. Dev Biol. 2007;306:714–724. doi: 10.1016/j.ydbio.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Lambrecht NW, Yakubov I, Scott D, Sachs G. Identification of the K efflux channel coupled to the gastric H-K-ATPase during acid secretion. Physiol Genomics. 2005;21:81–91. doi: 10.1152/physiolgenomics.00212.2004. [DOI] [PubMed] [Google Scholar]
- Levin M. Large-scale biophysics: ion flows and regeneration. Trends Cell Biol. 2007;17:261–270. doi: 10.1016/j.tcb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Levin M. Bioelectric mechanisms in regeneration: Unique aspects and future perspectives. Semin Cell Dev Biol. 2009;20:543–556. doi: 10.1016/j.semcdb.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M, Thorlin T, Robinson KR, Nogi T, Mercola M. Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell. 2002;111:77–89. doi: 10.1016/s0092-8674(02)00939-x. [DOI] [PubMed] [Google Scholar]
- Lipscombe D, Helton TD, Xu W. L-type calcium channels: the low down. J Neurophysiol. 2004;92:2633–2641. doi: 10.1152/jn.00486.2004. [DOI] [PubMed] [Google Scholar]
- Lyu RM, Farley RA. Amino acids Val115-Ile126 of rat gastric H(+)-K(+)-ATPase confer high affinity for Sch-28080 to Na(+)-K(+)-ATPase. Am J Physiol. 1997;272:C1717–C1725. doi: 10.1152/ajpcell.1997.272.5.C1717. [DOI] [PubMed] [Google Scholar]
- Marsh G, Beams HW. Electrical control of morphogenesis in regenerating Dugesia tigrina. I. Relation of axial polarity to field strength. J Cell Physiol. 1952;39:191–213. doi: 10.1002/jcp.1030390203. [DOI] [PubMed] [Google Scholar]
- McCaig CD, Song B, Rajnicek AM. Electrical dimensions in cell science. J Cell Sci. 2009;122:4267–4276. doi: 10.1242/jcs.023564. [DOI] [PubMed] [Google Scholar]
- Morokuma J, Blackiston D, Levin M. KCNQ1 and KCNE1 K+ channel components are involved in early left-right patterning in Xenopus laevis embryos. Cell Physiol Biochem. 2008a;21:357–372. doi: 10.1159/000129628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morokuma J, Blackiston D, Adams DS, Seebohm G, Trimmer B, Levin M. Modulation of potassium channel function confers a hyperproliferative invasive phenotype on embryonic stem cells. Proc Natl Acad Sci U S A. 2008b;105:16608–16613. doi: 10.1073/pnas.0808328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee T, Mandal D, Bhaduri A. Leishmania plasma membrane Mg2+-ATPase is a H+/K+-antiporter involved in glucose symport. Studies with sealed ghosts and vesicles of opposite polarity. J Biol Chem. 2001;276:5563–5569. doi: 10.1074/jbc.M008469200. [DOI] [PubMed] [Google Scholar]
- Mycielska ME, Djamgoz MB. Cellular mechanisms of direct-current electric field effects: galvanotaxis and metastatic disease. J Cell Sci. 2004;117:1631–1639. doi: 10.1242/jcs.01125. [DOI] [PubMed] [Google Scholar]
- Nakazawa M, Cebria F, Mineta K, Ikeo K, Agata K, Gojobori T. Search for the evolutionary origin of a brain: planarian brain characterized by microarray. Mol Biol Evol. 2003;20:784–791. doi: 10.1093/molbev/msg086. [DOI] [PubMed] [Google Scholar]
- Nogi T, Watanabe K. Position-specific and non-colinear expression of the planarian posterior (Abdominal-B-like) gene. Dev Growth Differ. 2001;43:177–184. doi: 10.1046/j.1440-169x.2001.00564.x. [DOI] [PubMed] [Google Scholar]
- Nogi T, Levin M. Characterization of innexin gene expression and functional roles of gap-junctional communication in planarian regeneration. Dev Biol. 2005;287:314–335. doi: 10.1016/j.ydbio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Nogi T, Zhang D, Chan JD, Marchant JS. A novel biological activity of praziquantel requiring voltage-operated Ca2+ channel beta subunits: subversion of flatworm regenerative polarity. PLoS Negl Trop Dis. 2009;3:e464. doi: 10.1371/journal.pntd.0000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogi T, Yuan YE, Sorocco D, Perez-Tomas R, Levin M. Eye regeneration assay reveals an invariant functional left-right asymmetry in the early bilaterian, Dugesia japonica. Laterality. 2005;10:193–205. doi: 10.1080/1357650054200001440. [DOI] [PubMed] [Google Scholar]
- Oviedo N, Levin M. The planarian regeneration model as a context for the study of drug effects and mechanisms. In: Raffa R, Rawls S, editors. Planaria: A Model for Drug Action and Abuse. Austin: RG Landes Co.; 2008. pp. 95–104. [Google Scholar]
- Oviedo NJ, Beane WS. Regeneration: The origin of cancer or a possible cure? Semin Cell Dev Biol. 2009;20:557–564. doi: 10.1016/j.semcdb.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo NJ, Nicolas CL, Adams DS, Levin M. Establishing and Maintaining a Colony of Planarians. Cold Spring Harb. Protoc. 2008a doi: 10.1101/pdb.prot5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo NJ, Nicolas CL, Adams DS, Levin M. Gene Knockdown in Planarians Using RNA Interference. Cold Spring Harb. Protoc. 2008b doi: 10.1101/pdb.prot5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo NJ, Nicolas CL, Adams DS, Levin M. Live Imaging of Planarian Membrane Potential Using DiBAC4(3) Cold Spring Harb. Protoc. 2008c doi: 10.1101/pdb.prot5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo NJ, Morokuma J, Walentek P, Kema IP, Gu MB, Ahn JM, Hwang JS, Gojobori T, Levin M. Long-range neural and gap junction protein-mediated cues control polarity during planarian regeneration. Dev Biol. 2010;339:188–199. doi: 10.1016/j.ydbio.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagan OR, Rowlands AL, Azam M, Urban KR, Bidja AH, Roy DM, Feeney RB, Afshari LK. Reversal of cocaine-induced planarian behavior by parthenolide and related sesquiterpene lactones. Pharmacol Biochem Behav. 2008;89:160–170. doi: 10.1016/j.pbb.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Pemberton DJ, Franks CJ, Walker RJ, Holden-Dye L. Characterization of glutamate-gated chloride channels in the pharynx of wild-type and mutant Caenorhabditis elegans delineates the role of the subunit GluCl-alpha2 in the function of the native receptor. Mol Pharmacol. 2001;59:1037–1043. doi: 10.1124/mol.59.5.1037. [DOI] [PubMed] [Google Scholar]
- Petersen CP, Reddien PW. Smed-betacatenin-1 is required for anteroposterior blastema polarity in planarian regeneration. Science. 2008;319:327–330. doi: 10.1126/science.1149943. [DOI] [PubMed] [Google Scholar]
- Petersen CP, Reddien PW. A wound-induced Wnt expression program controls planarian regeneration polarity. Proc Natl Acad Sci U S A. 2009;106:17061–17066. doi: 10.1073/pnas.0906823106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackauskas M, Neverauskas V, Skeberdis VA. Diversity and properties of connexin gap junction channels. Medicina (Kaunas) 2010;46:1–12. [PubMed] [Google Scholar]
- Raya A, Izpisua Belmonte JC. Left-right asymmetry in the vertebrate embryo: from early information to higher-level integration. Nat Rev Genet. 2006;7:283–293. doi: 10.1038/nrg1830. [DOI] [PubMed] [Google Scholar]
- Reddien PW, Sanchez Alvarado A. Fundamentals of planarian regeneration. Annu Rev Cell Dev Biol. 2004;20:725–757. doi: 10.1146/annurev.cellbio.20.010403.095114. [DOI] [PubMed] [Google Scholar]
- Reddien PW, Bermange AL, Murfitt KJ, Jennings JR, Sanchez Alvarado A. Identification of genes needed for regeneration, stem cell function, and tissue homeostasis by systematic gene perturbation in planaria. Dev Cell. 2005;8:635–649. doi: 10.1016/j.devcel.2005.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink JC, Gurley KA, Elliott SA, Sanchez Alvarado A. Planarian Hh signaling regulates regeneration polarity and links Hh pathway evolution to cilia. Science. 2009;326:1406–1410. doi: 10.1126/science.1178712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb SM, Ross E, Sanchez Alvarado A. SmedGD: the Schmidtea mediterranea genome database. Nucleic Acids Res. 2008;36:D599–D606. doi: 10.1093/nar/gkm684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo E, Abril JF, Adell T, Cebria F, Eckelt K, Fernandez-Taboada E, Handberg-Thorsager M, Iglesias M, Molina MD, Rodriguez-Esteban G. Planarian regeneration: achievements and future directions after 20 years of research. Int J Dev Biol. 2009;53:1317–1327. doi: 10.1387/ijdb.072414es. [DOI] [PubMed] [Google Scholar]
- Sanchez Alvarado A, Newmark PA, Robb SM, Juste R. The Schmidtea mediterranea database as a molecular resource for studying platyhelminthes, stem cells and regeneration. Development. 2002;129:5659–5665. doi: 10.1242/dev.00167. [DOI] [PubMed] [Google Scholar]
- Sarnat HB, Netsky MG. The brain of the planarian as the ancestor of the human brain. Can J Neurol Sci. 1985;12:296–302. doi: 10.1017/s031716710003537x. [DOI] [PubMed] [Google Scholar]
- Shabir S, Southgate J. Calcium signalling in wound-responsive normal human urothelial cell monolayers. Cell Calcium. 2008;44:453–464. doi: 10.1016/j.ceca.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Shan Q, Haddrill JL, Lynch JW. Ivermectin, an unconventional agonist of the glycine receptor chloride channel. J Biol Chem. 2001;276:12556–12564. doi: 10.1074/jbc.M011264200. [DOI] [PubMed] [Google Scholar]
- Sheiman IM, Zubina EV, Kreshchenko ND. Regulation of appetitive behavior in planaria Dugesia (Girardia) tigrina. Zh Evol Biokhim Fiziol. 2002;38:322–325. [PubMed] [Google Scholar]
- Shibata T, Hibino H, Doi K, Suzuki T, Hisa Y, Kurachi Y. Gastric type H+,K+-ATPase in the cochlear lateral wall is critically involved in formation of the endocochlear potential. Am J Physiol Cell Physiol. 2006;291:C1038–C1048. doi: 10.1152/ajpcell.00266.2006. [DOI] [PubMed] [Google Scholar]
- Shimeld SM, Levin M. Evidence for the regulation of left-right asymmetry in Ciona intestinalis by ion flux. Dev Dyn. 2006;235:1543–1553. doi: 10.1002/dvdy.20792. [DOI] [PubMed] [Google Scholar]
- Shin JM, Munson K, Vagin O, Sachs G. The gastric HK-ATPase: structure, function, and inhibition. Pflugers Arch. 2009;457:609–622. doi: 10.1007/s00424-008-0495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PJS, Sanger RH, Messerli MA. Principles, Development and Applications of Self-Referencing Electrochemical Microelectrodes to the Determination of Fluxes at Cell Membranes. In: Michael AC, Borland LM, editors. Electrochemical Methods for Neuroscience. Boca Raton: CRC Press; 2007. pp. 373–405. [PubMed] [Google Scholar]
- Smukste I, Stockwell BR. Advances in chemical genetics. Annu Rev Genomics Hum Genet. 2005;6:261–286. doi: 10.1146/annurev.genom.6.080604.162136. [DOI] [PubMed] [Google Scholar]
- Tsutsui H, Karasawa S, Okamura Y, Miyawaki A. Improving membrane voltage measurements using FRET with new fluorescent proteins. Nat Methods. 2008;5:683–685. doi: 10.1038/nmeth.1235. [DOI] [PubMed] [Google Scholar]
- Vagin O, Denevich S, Munson K, Sachs G. SCH28080, a K+-competitive inhibitor of the gastric H,K-ATPase, binds near the M5-6 luminal loop, preventing K+ access to the ion binding domain. Biochemistry. 2002;41:12755–12762. doi: 10.1021/bi025921w. [DOI] [PubMed] [Google Scholar]
- Weick JP, Groth RD, Isaksen AL, Mermelstein PG. Interactions with PDZ proteins are required for L-type calcium channels to activate cAMP response element-binding protein-dependent gene expression. J Neurosci. 2003;23:3446–3456. doi: 10.1523/JNEUROSCI.23-08-03446.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler GN, Brandli AW. Simple vertebrate models for chemical genetics and drug discovery screens: lessons from zebrafish and Xenopus. Dev Dyn. 2009;238:1287–1308. doi: 10.1002/dvdy.21967. [DOI] [PubMed] [Google Scholar]
- Yazawa S, Umesono Y, Hayashi T, Tarui H, Agata K. Planarian Hedgehog/Patched establishes anterior-posterior polarity by regulating Wnt signaling. Proc Natl Acad Sci U S A. 2009;106:22329–22334. doi: 10.1073/pnas.0907464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh JR, Crews CM. Chemical genetics: adding to the developmental biology toolbox. Dev Cell. 2003;5:11–19. doi: 10.1016/s1534-5807(03)00200-4. [DOI] [PubMed] [Google Scholar]
- Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, Sasaki T, Suzuki A, Forrester JV, Bourne HR, Devreotes PN, McCaig CD, Penninger JM. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457–460. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


