Abstract
Distinct brain regions, reproducible from one person to the next, are specialized for processing different kinds of human expertise, such as face recognition and reading. Here we explore the relationship between age of learning, learning ability, and specialized brain structures. Specifically we ask whether the existence of reproducible cortical domains necessarily means that certain abilities are innate, or innately easily learned, or whether reproducible domains can be formed, or refined, by interactions between genetic programs and common early experience. Functional MRI showed that intensive early, but not late, experience caused the formation of category-selective regions in macaque temporal lobe for stimuli never naturally encountered by monkeys. And behaviorally, early training produced more fluent processing of these stimuli than the same training in adults. One explanation for these results is that in higher cortical areas, as in early sensory areas, experience drives functional clustering and functional clustering determines how that information is processed.
Introduction
In most humans, face processing is localized predominantly to the right posterior ventral temporal lobe (Kanwisher et al., 1997; McCarthy et al., 1997); visual recognition of letters and words is also localized, to about the same part of the temporal lobe, though contralaterally and a bit more lateral and posterior (Cohen and Dehaene, 2004; Cohen et al., 2000). The importance of social interactions in primates could conceivably have driven the generation of a face-specific cortical domain by natural selection, yet it is difficult to imagine how a cortical region specific for written words could have evolved, given that humans have been using written language for only a few thousand years, and literacy has been widespread for at most a few hundred. Thus both reading and face processing are localized to similar parts of the temporal lobe, despite the discrepancy between the apparent innateness of face recognition and the unnaturalness of reading. However, most people do have intensive early experience with both faces and text, raising the possibility that both kinds of domains are not innate, in the sense of being genetically predetermined, but rather emerge as a consequence of experience interacting with development. This prompted us to ask whether intensive early experience could cause monkeys to develop anatomical specializations for processing stimuli they never naturally encounter. We used number and letter symbols, which are simpler than faces and have been honed by human culture to be easily discriminated and remembered. If there is a basis in low-level vision for the particular shapes used in human writing systems and for their ease in processing (Changizi et al., 2006), this basis should be present in macaque monkeys.
Adult and juvenile monkeys learned to associate reward amount with letters and numerals, precisely discriminating 26 symbols. The juvenile monkeys learned the symbols more easily than the adults did, and they responded faster to these symbols than adult learners with comparable training. Furthermore the juveniles, but not the adults, developed regions in their temporal lobes that were more responsive to the learned symbols than to visually similar but unfamiliar shapes. The results suggest that intensive early experience drives the generation, or segregation, of domain-specific modules and that the formation of specialized domains may facilitate the neuronal processing of those clustered categories.
RESULTS
Differences in learning between juveniles and adults
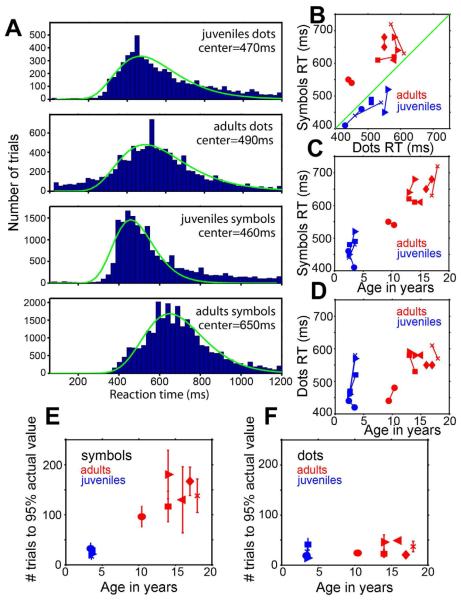
Ten monkeys were intensively trained for 3 years to choose between pairs of dot patterns or pairs of symbols for fluid reward (Figure 1 A,B). Four juvenile (all male) and six adult (two female) Rhesus macaque monkeys learned to use touchscreens in their home cages to choose quite accurately between pairs of stimuli to select a reward amount (Figure 1 C-F). The two stimuli could be arrays of dots inside a circle or two symbols (Arabic numerals or English letters). Reward amounts corresponded to the number of dots in a circle or the assigned value of the symbol--numerals 0 through 9 corresponded to 0 to 9 drops and the letters X Y W C H U T F K L N R M E A J represented 10 through 25 drops. The monkeys were first trained on 0 versus 1, and each new symbol was introduced, in ascending order, only after the monkey’s choice behavior indicated that he or she had learned the value of the preceding symbol. After one year of daily training, during a month-long period while no new symbols were introduced, the monkeys were tested on alternate days with symbol pairs or dot pairs exclusively, with values between 0 and the maximum learned symbol (21 for the juveniles and various lower values for each of the adults). Reaction-time histograms (Figure 2A) for adults and juveniles were similar when they chose between dot arrays (peak of a log Gaussian fitted to the distribution = 470ms for the juveniles; 490ms for the adults), and reaction times for juveniles were about the same regardless of whether they chose between symbols (peak=460ms) or between dots (470ms). Adults only, however, were slower specifically when choosing between symbols (peak=650ms).
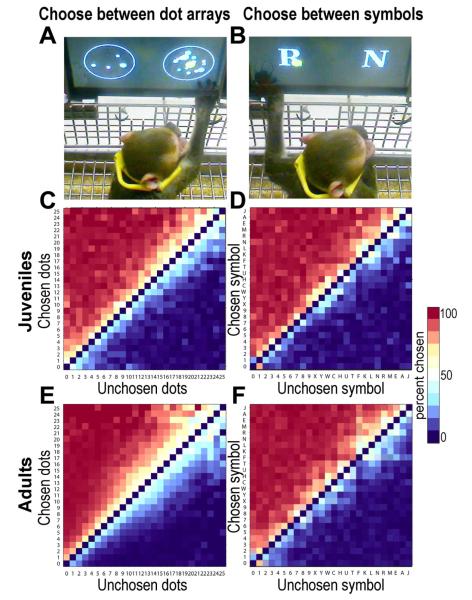
Figure 1.
(A) Juvenile monkey using a touchscreen to choose 14 dots in preference to 4 dots (the large yellow dot shows where he first touched the screen). The dots vary in color, size, and position randomly (the colors are more distinct from each other than they appear on this video image). The monkey’s mouth is on the juice tube. (B) Monkey choosing the symbol R (worth 21 drops) in preference to N (20 drops). (C) Average performance choosing between pairs of dot patterns for 4 juvenile monkeys over a 1 month period (~7000 trials per monkey). The color of each square in the matrix indicates the percentage of trials in which the monkey chose the number corresponding to the square’s vertical position over the number corresponding to its horizontal position. Thus all the squares above the diagonal represent trials when the monkey chose the larger number of dots over the smaller, and the squares below the diagonal show trials when the monkey chose the smaller numerosity. (D) Average performance for choosing between two symbols for 4 juvenile monkeys over a one month period (~7000 trials per monkey). (E) Average performance choosing between two dot patterns for 5 adult monkeys over a 2 month period (~15,000 trials per monkey). (F) Average performance for choosing between two symbols for 5 adult monkeys over a one month period (~7000 trials per monkey). Note that both adult and juvenile monkeys showed an increasing number of ‘errors’ for proportionately smaller differences between dot choices(Livingstone et al., 2010). Note also that both adults and juveniles were more accurate in their value choices when those values were represented by symbols than by dot arrays (Livingstone et al., 2010).
Figure 2.
Reaction times and learning rates for monkeys choosing between pairs of dot arrays or between symbol pairs. (A) Reaction time histograms for 4 juveniles compared to 6 adults after one year each of training; histograms were clipped at 1.2 seconds but extended to 10 seconds. Histograms were fit with a log Gaussian (green curve) using least squares. (B) Reaction times (peak of the log Gaussian fitted to the histogram for each monkey), measured during two 1-month periods separated by 1 year; the two data points for each monkey are linked by a line; each symbol represents a different monkey. The scanned monkeys were: blue x=Juvenile1; blue square=Juvenile2; blue circle=Juvenile3; red circle=Adult1; red right triangle=Adult2. (C) Symbol reaction times versus monkey age. (D) Dots reaction times versus monkey age. (E) Number of trials+sem, averaged over all novel symbols above 5 for each monkey, required for the monkey to learn to choose each novel symbol at a behavioral choice value of 95% of its actual value. The highest symbol value learned by all 4 juveniles was 21 at the first testing and 25 at the second, which was the highest we tested, and the adults had progressed to 21, 19, 17, 17, 15, and 16 (youngest to oldest) by the first testing and to 25, 25, 24, 25, and 23 by the second testing. (F) Number of trials+sem required for each monkey to attain behavioral choice values of 95% of the actual value for novel dot numerosities, averaged over all novel numerosities > 5.
One year later, after learning more symbols (up to value 25 for the juveniles and various lower values for each of the adults), the reaction times of all the monkeys were measured again during another month-long period while no new symbols were introduced. The peak of the fitted reaction time distribution was 490 ms for juveniles using dots, 510ms for adults using dots, 450ms for juveniles using symbols, and 630ms for adults using symbols. Thus the average reaction times were stable, and adults choosing between symbols were slower than adults choosing between dots or juveniles choosing between either symbols or dots. Figure 2B compares the peaks of the fitted reaction time distributions between dots and symbols for each monkey, over the two testing periods. Reaction times were not significantly different between the two testing periods (t(19)=−1.894, p=0.08, two-tailed t-test) so the reaction time distributions from the two test periods were combined to obtain a single peak time for each monkey for statistical comparisons between adults and juveniles and between dots and symbols. The juveniles responded slightly faster to symbols than to dots, but the difference was not significant (t(6)=−0.99, p=0.36, two-tailed t-test), while the adults showed slower reaction times for symbols than for dots (t(10)=2.66, p=0.04, one-tailed t-test, corrected for multiple comparisons). Furthermore the adults’ symbol reaction times were significantly slower than the juveniles’ symbol reaction times (t(8)=−6.06. p=0.0005, one-tailed t-test, corrected for multiple comparisons). The symbol reaction times increased with increasing age (linear regression, r2=0.94, p<0.001), so the youngest adult was about as fast as the slowest juvenile; nevertheless this adult was slower responding to symbols than to dots, and none of the juveniles were (Figure 2C&D). In contrast, the dots reaction times for the adults and the juveniles were not significantly different (t(8)=−2.13, p=0.07, one-tailed t-test). Thus the adults responded slower to symbols than the juveniles did, but this difference cannot be explained by the adults being less motivated or having slower reaction times in general, since they were as fast as the juveniles in the non-symbolic dots task.
Once the touchscreen task had been mastered, and after symbols 0 through 5 had been learned, it became clear that the juvenile monkeys learned new symbols faster than the adult monkeys. Figure 2E shows the number of trials required, averaged over each new symbol above 5, for each monkey to respond to novel symbols at a choice value of 95% of the novel symbol’s actual value, calculated as the point of subjective equality between the novel symbol and all other symbols. New symbols were introduced in ascending order, so a new symbol always represented a reward one drop larger than the last learned symbol. Choice patterns for novel symbols indicated that juvenile monkeys learned the value represented by novel symbols faster than the adults did (Figure 2E); the number of trials required to reach criterion was significantly larger for adults learning symbols than for juveniles learning symbols (t(8)=−6.2, p=0.005, one-tailed t-test, corrected for multiple comparisons). In contrast to the symbol learning behavior, both adults and juveniles quickly learned the optimum rule for dot arrays (Figure 2F) (no significant difference between trials to criterion between juveniles learning dots and adults learning dots, t(8)=−1.03, p=0.33, two-tailed t-test). Both adults and juveniles tended to choose the larger number of dots even when one or both numerosities were novel, consistent with previous reports that monkeys can learn rules for making choices based on numerosity (Cantlon and Brannon, 2007). Thus the adults learned novel symbols slower than the juveniles and responded to the symbols more slowly, even though they were just as facile at learning and responding to dot numerosities.
Differences in functional anatomy between adult and juvenile symbol learners
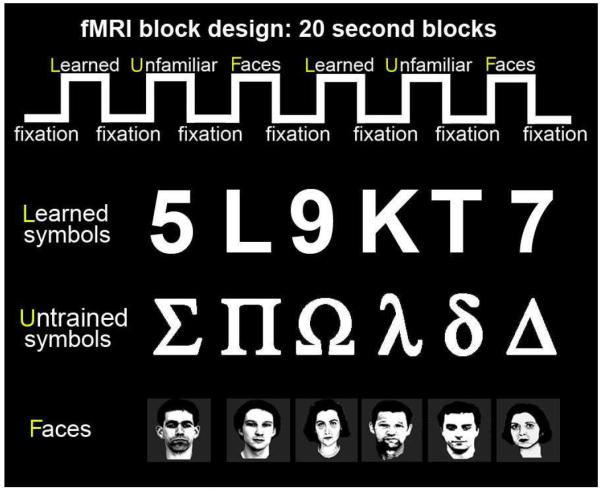
To find out what parts of the monkeys’ brains were involved in recognizing symbols after this prolonged intensive training, we performed functional MRI on 6 monkeys: 2 adults and 3 juveniles that had learned the symbol/value associations, and one adult who had not been trained in this task. For various technical reasons we could not scan any more of the trained animals (see methods). Each of the trained monkeys had had at least 2 years daily exposure to the symbols at the time of scanning. We scanned alert monkeys while they passively viewed 20-second blocks of Learned symbols, Untrained shapes (other human symbols differing in shape from the Learned symbols), and Faces, alternating with 20-second blocks of a small fixation spot (Figure 3).
Figure 3.
Functional MRI stimulus block design. Each scan lasted 260 seconds, and consisted of alternating 20 second blocks of visual stimuli and a fixation spot. The visual categories were Learned symbols, Untrained shapes, and Faces. All the image categories had the same number of white pixels on average.
We first calculated maximum likelihood maps of responsiveness to each stimulus category (Learned symbols, Untrained shapes, Faces) using general linear model methods (Boynton et al., 1996), wherein a hemodynamic impulse response function was convolved with the stimulus paradigm. We defined three category contrasts by performing t-tests between responses to different pairs of stimulus categories: Learned symbols versus Faces (LvsF); Learned symbols versus Untrained shapes (LvsU); and Faces Vs Untrained shapes (FvsU). Then we defined three category selectivity maps using a conjunction analysis (Bell et al., 2009; Price et al., 1997) on the three contrast conditions, using odd-numbered scans: Face selective voxels were defined as being more responsive to both F>U AND F>L, both contrasts p<0.001 (corrected for multiple comparisons, see methods), Shape selective regions satisfied both L>F AND U>F at p<0.001, and Learned symbol selective regions satisfied both L>U AND L>F at p<0.001. The maps in Figures 4 & 5 show these category selective regions, projected onto semi-inflated anatomical maps for each monkey.
Figure 4.
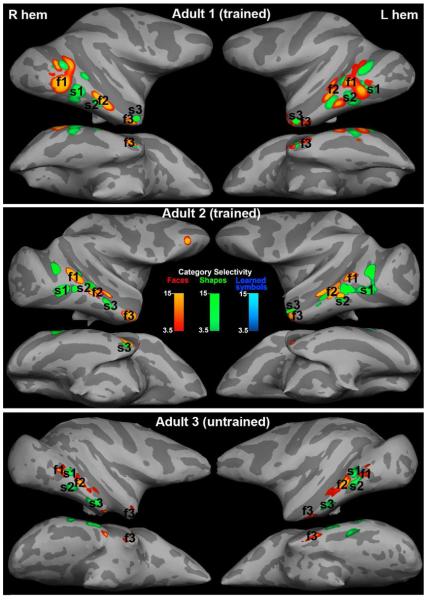
Category selectivity maps of two adult symbol learners, and one adult who was not trained in the symbol task, for Learned symbols, Untrained shapes or Faces; color scale indicates t-score. Voxels that responded significantly more to Faces than to Shapes (conjunction of Faces>Learned symbols AND Faces>Untrained shapes) are indicated in red; the three largest and most consistent face-selective regions are labeled f1, f2 & f3, and are the loci used to calculate the corresponding percent activations in Figure 7 and the time-courses in Figure 8. Voxels that responded significantly more to Shapes than to Faces (conjunction of Learned symbols> Faces AND Untrained shapes> Faces) are indicated in green; the three largest and most consistent shape-selective regions are labeled s1, s2 & s3. No regions in any of the adults showed more activation to Learned symbols compared to Untrained shapes (blue).
Figure 5.
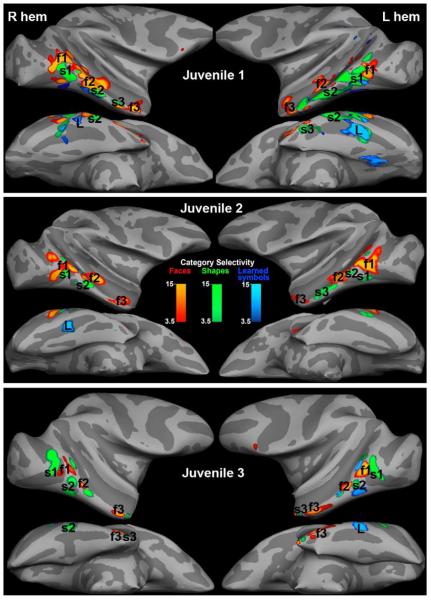
Category selectivity maps of three juvenile symbol learners. Conventions as in Figure 4. All 3 juvenile monkeys showed regions that were activated more by Learned symbols compared to Untrained shapes (L>U & L>F), in addition to the pattern observed in adults of alternating regions relatively selective for Faces or for Shapes.
In all six monkeys several bilateral regions of the inferior temporal lobe were more active to Faces than to either shape category (F>U AND F>L), consistent with previous reports of face selective regions in the temporal lobe (Tsao et al., 2003). These Face selective regions showed > 90 % overlap between the left and right hemispheres for all 6 monkeys (Supplemental Table S1); therefore we averaged together the left and right Face-selective activations. We identified the three largest Face patches in each monkey as f1, f2, and f3 (posterior to anterior). We projected the Face selective patches from each individual monkey onto a common semi-inflated left hemisphere (Figure 6A, red patches); the patches were roughly overlapping in this projection, indicating some consistency in location from monkey to monkey, except for the most anterior Face region, which could comprise two patches or may simply be less reproducible in location from monkey to monkey. The location of the maximally selective voxels in each of the Face-selective patches in each monkey are given in Supplemental Table S1. The most posterior Face patch (f1) was located in posterior Area TEO, sometimes extending into anterior V4, on the ventral bank of the STS near the anterior tip of IOS, with the region of maximum overlap between monkeys at A1. The middle Face patch (f2) was mostly in Area TEa with the region of maximum overlap at A8. The most anterior Face patch (f3) was more variable in location and was located in TEm on the ventral bank of the STS or on the inferotemporal gyrus centered at A18.
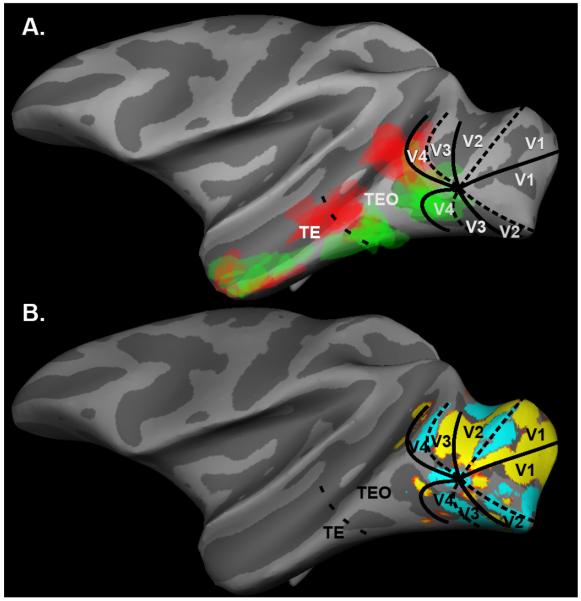
Figure 6.
A. Overlay of all Shape (green) and Face (red) selective patches (p<0.001) from both hemispheres of all 6 monkeys aligned to the left hemisphere of Juvenile 2, with estimated areal borders. B. Areal borders of Juvenile 2. To locate borders of retinotopic visual areas, the monkey viewed blocks of vertical wedges of flashing checks alternating with horizontal wedges. Retinotopic areal boundaries were drawn by hand according to the alternating pattern of vertical (dotted lines) and horizontal (solid lines) meridian activations (Fize et al., 2003; Sereno et al., 1995). The TE/TEO border was drawn by hand through the anterior tip of the posterior middle temporal sulcus (PMTS) (Boussaoud et al., 1991).
In addition, in all six monkeys several regions were reproducibly more active to Shapes (both Learned symbols and Untrained shapes) than to Faces (conjunction of L > F & U > F contrast maps) (Figures 4-6, green patches). Three Shape selective regions (s1, s2, s3, posterior to anterior) were consistent between the two hemispheres for each monkey so we again averaged the two hemispheres together to project each monkey’s Shape selectivity maps onto a common hemisphere (Figure 6, green patches). Again, by inspection of Figure 6, several regions are commonly Shape selective. The maximally selective voxels in each of the three largest Shape selective regions for each monkey are listed in Supplemental Table S1. The posterior-most Shape patch (s1) was consistently localized ventral and slightly posterior to Face patch f1 in posterior area TEO or in anterior V4, at the anterior tip of IOS, with maximal overlap at A2. The middle Shape patch (s2) extended from the bank of the STS near the anterior tip of PMTS out onto the inferotermporal gyrus, maximal overlap at A4 mostly within Area TEpd or area TEO. The anterior most Shape patch (s3) was less consistent between monkeys; it was located in TEa/TEm, varying in position from A12 to A16. Shape selective regions that are distinct from Face selective patches have also been previously described (Denys et al., 2004; Sawamura et al., 2005).
In all six monkeys the relative category selective regions formed 3 pairs of regions more responsive to Faces than to Symbols (Learned and Untrained), or the reverse, distributed along the inferotemporal gyrus (Figure 6A). The locations of the two posterior pairs of patches roughly correspond to the borders between the major subdivisions of the ventral temporal lobe (Boussaoud et al., 1991; Desimone and Underleider, 1989; Saleem and Logothetis, 2007)--V4/TEO and TEO/TE (Figure 6A). The anterior patches may be located at the TE/TG border, but their position was too variable to really say. Because our stimuli covered only the central visual field, the patches may correspond to foveal confluences between areas (Kolster et al., 2009). Alternating face, body, and object selective regions have been described previously in macaque temporal lobe (Bell et al., 2009; Denys et al., 2004; Op de Beeck et al., 2008) and have been proposed to represent alternating regions selective for animate versus inanimate categories (Bell et al., 2009; Op de Beeck et al., 2008). Our results are consistent with this hypothesis, and in one of our monkeys we confirmed that the regions activated by Shapes>Faces were also selectively activated by images of inanimate objects (data not shown).
In the three juveniles the pairs of Shape and Face selective regions distributed along inferotemporal cortex were similar to those in adults, but in addition, in all three juvenile learners, we saw selective responsiveness to Learned symbols compared to Untrained shapes between A0 and A4 in or on the lateral bank of the occipitotemporal sulcus (OTS) (Figure 5, Supplemental Table S1), bilaterally in Juvenile 1, and unilaterally in the other two juveniles. This Learned symbol selective region (labeled L in Figure 5) was close to the same location as what we identify as the middle shape patch (s2), but we cannot say whether it is a distinct patch or part of s2, since this region was also Shape selective in that it responded slightly better to Untrained shapes than to Faces (see Figures 7&8). Only in the three juveniles did any regions pass our threshold/clustering criterion for the Learned symbol category selectivity (L>U AND L>F p<0.001; see Methods). This novel Learned symbol selective region is anterior to early retinotopic visual areas (Figure 6B), defined by vertical and horizontal meridian mapping (Fize et al., 2003).
Figure 7.
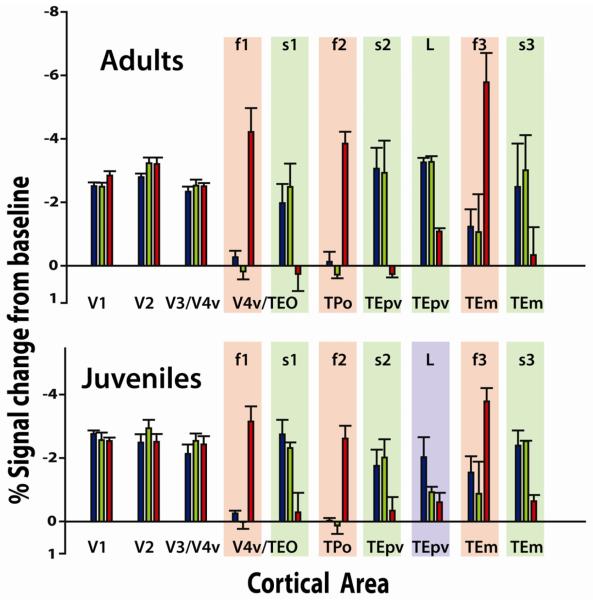
Average percentage signal change from baseline for adults vs juveniles in response to each category in early visual areas and category selective regions, in caudo-rostral order. Regions showing less than 2% change in all monkeys are not shown. Red bars represent the percent signal change for the 20 second period in which Face stimuli were displayed; Blue bars for Learned symbols; Green bars for Untrained shapes. Red shading indicates regions with statistically significant relative selectivity for Faces (F>U & F>L); Blue shading for Learned symbols (L>U & L>F), and green for Shape stimuli (L>F & U>F). The activation labeled L for the adults was taken from the same average location as the Learned symbol selective regions in the juveniles; it comprises part of the region s2.
Figure 8.
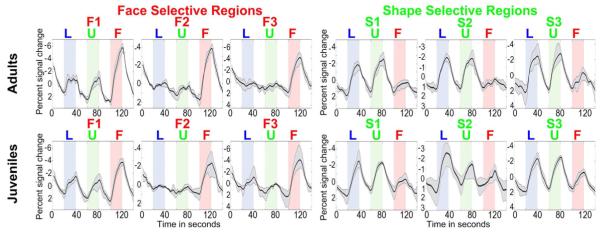
Signal time-courses in category-selective ROIs. The graphs show the MRI signal time-course for 40 voxel ROIs centered on each category-selective region indicated by corresponding labels in Figures 4&5, averaged over 60 scans for each monkey. The timecourse labeled L for the adults was taken from the same average location as the Learned symbol selective regions in the juveniles. The thick line shows the average signal, and the thinner lines show the maximum and minimum values for each time-point. Blue, green, and red blocks indicate presentation of Learned symbols, Untrained shapes, and Faces respectively, alternating with fixation blocks (white). Increased neuronal activity produces a decrease in signal because an iron blood-pool contrast agent was used, so peaks are negative from baseline=0, and the graphs are inverted for clarity.
We identified an ROI for each category selective region in each monkey using odd-numbered scans, taking 40 maximally selective contiguous voxels for each patch. Since the adults did not show a Learned symbol selective region that passed the clustering criterion (see methods), we defined for the adults a Learned symbol ROI as 40 voxels centered on the average of the coordinates of the three Learned symbol patches for the juveniles (coordinates: 21.5 mm lateral of the midline, 2.0 mm anterior and 9.5mm above ear-bar zero). We calculated the signal change, adjusted for hemodynamic delay, in response to presentation of each stimulus type, for each category selective patch, using even-numbered scans. Figure 7 compares the percentage signal change from baseline in response to each stimulus category (Learned, Untrained and Faces) in each category-selective region, averaged across adults (top) and juveniles (bottom). Because all three categories consisted of high contrast images that alternated with a small fixation spot, we observed strong visual activations in early visual areas that were about equal for all three categories, but the category selective patches showed differential responses to Faces, Learned symbols and Untrained shapes. The time courses of activations for each ROI, averaged within each subgroup (adults vs juveniles) are shown in Figure 8.
A three way between groups ANOVA was performed on the percentage change within each stimulus block for all category selective ROIs in each monkey. The Face-selective and Shape-selective patches revealed no effect of age or subject but did show a main effect of stimulus category across all monkeys (face selective regions- f1: F(2,17) = 357.76, p < 0.005; f2: F(2,17) = 45.43; p < 0.05; f3: F(2,17) = 33.37; p <0.05; shape selective regions- s1: F(2,17) = 23.63, p < 0.05; s2: F(2,17) = 56.59, p < 0.05; s3: F(2,14) = 11.37, p < 0.05). The Learned symbol selective region (L), in addition to a main effect of category also showed a main effect of age and a significant 2-way interaction between age and category (Age effect: F(4,14)=75.01,p<0.001; Category effect: F(2,17) = 175.27, p < 0.05; Interaction between age and Category: F(2,17) = 212.04, p < 0.05).
Pairwise comparisons of L>F, L>U and U>F were done on the hemodynamic responses in each category selective ROI to each stimulus (Supplemental Table S2). Face selective regions showed a statistically higher percent signal change to Face stimuli than to Learned or Untrained shapes, and all the Shape selective regions showed significantly higher signal change to Learned symbols and Untrained shapes compared to Face stimuli. The Learned symbol region showed significantly higher signal change to Learned symbols compared to Untrained shapes and Faces, in juveniles but not in adults.
To explore the difference between juveniles and adults in the responsiveness of the Learned symbol region we first defined an Average Learned symbol ROI by combining scans from all 3 juvenile monkeys and aligning them to a standard monkey template (McLaren et al., 2009). The Average Learned symbol selective ROI comprised 114 contiguous voxels that were preferentially more active in the combined juveniles data set to Learned symbols than to Untrained shapes or to Faces (p<0.001 for both contrasts). We counted the voxels in all 6 individual monkeys within the Average Learned symbol ROI that were selectively responsive to Learned symbols in each monkey (Supplemental Table S3). This average ROI contained significantly more voxels selectively responsive to Learned symbols in juveniles (mean = 28) compared to adults (mean = 4); (t(10)=--3.17, p=0.011, two-tailed t-test).
The fact that fMRI showed a Learned symbol selective region in juveniles but not in adults could reflect the better performance of the juveniles compared to the adults, rather than a qualitative difference between the two groups. Therefore to ask whether the Learned symbol region was exclusively present in juveniles, and not simply less active, or in a different place, in adult monkeys, we further calculated, in each whole brain, the number of voxels that were significantly selective for Learned symbols, at three different thresholds (Supplemental Table S4), without smoothing or clustering. Juvenile monkeys showed significantly more voxels selective for Learned symbols than adults did, irrespective of the threshold used, indicating that the juveniles showed qualitatively different responses to the Learned symbols (p<0.01 at all thresholds tested).
The novel functional specialization in juveniles for Learned symbols is probably not due to low-level differences between Learned symbols and Untrained shapes, such as degree of curvature or retinotopic representation, or to attentional differences, because we did not see any Learned symbol specialization in either of the adult-trained monkeys or in the naïve adult. Furthermore we scanned two of the juveniles with a different Learned symbol font, with the image blocks in a different order (U, L, F), with each image position randomly jittered by 1 degree from the center, and using 1.5 fold larger images. We saw the same regions selectively activated by Faces, Shapes, and Learned symbols irrespective of stimulus size, order, font, or position (Supplemental Figure S2). Because of their age we could not scan the juveniles before we commenced Symbol training, so we cannot rule out the unlikely possibility that the four juvenile monkeys might have exhibited Learned symbol selective cortical domains without training, though the absence of a Learned symbol selective region in any of the adults makes this unlikely.
Discussion
Four juvenile monkeys learned to recognize symbols faster than six sexually mature adults and showed faster reaction times than the adults in choosing between symbols, even though the reaction times and learning rates of the adults were comparable to the juveniles when choosing between dot arrays. Functional MRI on the juvenile monkeys showed novel domains that were more active when the monkeys viewed the Learned symbols, compared to visually similar but Untrained shapes, and Faces. The same location in the adults responded as strongly to Untrained shapes as to Learned symbols. The anatomical results indicate that intensive early, but not late, experience can cause the formation of a novel specialized cortical domain, or cause an existing domain to become specialized for the trained shapes. The association of a specialized domain with faster learning and responding suggests that having a specialized domain bestows a behavioral advantage. These results raise two important questions:
How could intensive early experience cause the formation of a novel functional domain?
Why would a novel functional domain correlate with a behavioral advantage?
The relationship between intensive early experience and novel domain formation
Our results are completely consistent with the possibility that early symbol learning modifies the tuning properties of cells in an innately specialized domain (Dehaene and Cohen, 2007). We would like, however, to propose an alternative hypothesis: The emergence, only in the juvenile-trained monkeys, of a domain selective for an artificial object category raises the possibility that early experience plays a causal role in the formation or specialization of functional domains. The functional domains for faces and shapes were not in precisely the same location in each monkey, but the paired pattern of face and shape domains within each of the major subdivisions along inferotemporal cortex was similar in all the monkeys, and was similar to what has been previously reported (Bell et al., 2009; Denys et al., 2004). The experience-dependence of the novel functional domain, coupled with the pattern of one pair of face and shape functional domains within each major cortical area, suggests a self-organizing Hebbian mechanism. Stereotyped patterns of modular organization within a cortical area without precise localization of any particular domain are observed in ocular dominance and orientation columns in V1 (Hubel and Wiesel, 1977), cytochrome oxidase domains in V2 (Hubel and Livingstone, 1987), and rodent barrel fields (Van der Loos and Woolsey, 1973), which are all thought to form by activity dependent sorting mechanisms (Hebb, 1949; Wiesel, 1982). Several groups have documented shape learning in individual neurons in temporal cortex and proposed that such changes could occur as a consequence of competitive segregation of those neurons’ inputs by Hebbian mechanisms (Fukushima et al., 1988; Kourtzi and DiCarlo, 2006; Rolls and Tovee, 1995; Sohal and Hasselmo, 2000). Polk and Farah (1995) explicitly proposed that activity-dependent Hebbian mechanisms could drive the coarser segregation of neurons responsive to learned stimulus categories, like letters and words, from neurons responsive to other shapes. Here we hypothesize that self-organizing segregation within cortical areas could underlie the formation of functional domains in the temporal lobe.
In the same way that differential activity in the two eyes drives the segregation of ocular dominance columns within V1, or tactile experience with differential whisker activity drives the organization of whisker barrels within each somatosensory cortical area, we propose that differential early experience with face parts being experienced conjunctively with other face parts, but disjunctively with other objects, and vice versa, could drive the segregation of category selective domains within cortical areas in inferotemporal cortex. We propose that intensive early experience with symbols drives the segregation of a domain selective for those learned symbols, and by extension, we propose that intensive early experience with faces and other objects drives the segregation of face and shape domains. Figure 6 indicates that this segregation occurs independently several times along inferotemporal cortex, suggesting an underlying organizational principle of modular segregation within each cortical area. This general organizational principle probably further involves interconnectivity between functionally related modules: modules in V1 are selectively interconnected with functionally related modules in V2 (Livingstone and Hubel, 1984), and face selective modules in different parts of IT are selectively interconnected (Moeller et al., 2008). By inspection of figure 6, there is another peculiar similarity between the face/shape modular architecture in IT and other modules in the visual system, namely that the modular divisions within each area tend to run perpendicular to the areal border: Ocular dominance columns in old world monkeys, orientation columns in new world monkeys, and functional domains (cytochrome oxidase stripes) in V2 are all oriented perpendicular to the V1/V2 border (Blasdel and Campbell, 2001; Hubel and Freeman, 1977; Tootell et al., 1983). This similarity is noteworthy because it is consistent with our hypothesis of a common rule-based organization.
The reproducible location of face-selective domains in humans and macaques, the fact that newborn humans and macaques selectively track faces (Goren et al., 1975; Johnson et al., 1991), the fact that monkeys preferentially look at faces even when they have never seen them before (Sugita, 2008), and the effects of early brain damage all argue that some aspects of face processing must be innate (Farah et al., 2000). However our results, and the selective responsiveness to written words in the human Visual Word Form area, indicate that experience must also be important in the formation or refinement of category-selective domains in the temporal lobe (Baker et al., 2007; Cohen and Dehaene, 2004; Cohen et al., 2000; Glezer et al., 2009). These two lines of evidence may not be contradictory, but may instead address different things--individual neuronal response selectivity versus the spatial clustering of neurons with similar selectivity. Behavioral responsiveness to faces at birth necessitates that some face-selective neurons be present in newborns; cortical domains involve the spatial organization of such response selectivity. In earlier parts of the visual system selective response properties emerge in the absence of visual experience (Wiesel and Hubel, 1974), yet visual experience exerts profound effects on the spatial organization and clustering of these cells within visual cortex (Wiesel, 1982) and in other sensory systems (Hensch, 2004). Therefore we suggest that neuronal selectivity to faces and shapes may be innate, but segregation into category selective domains could be driven by extensive visual experience of these categories. Indeed, Dehaene et al. (2010) recently reported that in illiterate adult humans the part of the brain corresponding to the Visual Word Form Area responds preferentially to faces; this intriguing result is consistent with face and symbol-selective regions being segregated by activity-dependent competition.
Behavioral consequences of functional domains in the temporal lobe
We found a behavioral juvenile advantage that correlated with differences in cortical organization, suggesting that the acquisition of a novel domain in our juvenile learners is the basis for their enhanced fluency. Tsao and Livingstone (2008) proposed that the clustering of cells responsive to faces could explain the fine distinctions characteristic of face processing, because such proximity would favor interactions between cells with similar response selectivity. Clustering not only makes interconnectivity more likely, but it also facilitates opponency, or comparisons, between cells with similar response properties because of the local nature of cortical inhibition. Proximity thus facilitates fine, within-category comparisons. Therefore, expert processing could emerge simply as a consequence of clustering.
Cortical modules in the temporal lobe could exist because the biological importance of certain categories drives the evolution of specialized circuitry for processing these categories in optimal ways. We suggest a different, mechanistically plausible, hypothesis: That natural selection evolved a mechanism for adapting to the environment by which intensive early experience drives the segregation within a cortical area of inputs by frequency of co-activiation, causing clustering of category selective responsiveness, and that the resultant clustering permits finer discrimination within these categories than for less frequently experienced shapes whose neuronal selectivity is dispersed among neurons selective for other kinds of objects. Hebbian competition, in which inputs with temporally correlated firing patterns coalesce, is thought to be the means by which immature, expansive neuronal projections are refined into precise retinotopic, tonotopic, or somatotopic maps. We propose that in temporal cortex developmental Hebbian mechanisms segregate and refine maps for object category, and we further suggest a novel consequence of category maps, namely expert processing of those clustered categories. Although adults can learn, children are better than adults at learning some things, and differences between adult and juvenile learning abilities may correlate with critical periods for the location or scale of potential neuronal plasticity (Castro-Caldas et al., 2009; Dehaene et al., 2010; Hensch, 2004; Van der Loos and Woolsey, 1973; Wiesel, 1982).
Faces and symbols are both kinds of learned expertise, and we propose that the localized domains for such categories are both a consequence of intensive experience and the basis for the resultant expertise. This hypothesis is a compromise between the idea that the FFA is a domain innately specialized to process faces (Farah, 1996; Yovel and Kanwisher, 2004) and the idea that it processes objects of expertise (Gauthier et al., 1999; Gauthier et al., 2000). Our ideas are not inconsistent with the contention that the unique, holistic, characteristics of face (Farah et al., 1998; Kanwisher et al., 1998; Tanaka and Farah, 1993; Yin, 1969) and word processing (Anstis, 2005) imply that these processes must be carried out by a specialized type of cortical circuitry because clustering is a kind of specialized wiring, but a kind of specialization that can be understood mechanistically and has precedents in the field.
Experimental Procedures
Behavior
Four juvenile male macaque monkeys, starting at 1 year of age, and six sexually mature adults (2 females, 4 males) participated in the behavioral experiments, beginning training 3 years ago (Livingstone et al., 2010). The youngest adult male was 9 years old at the beginning of training, and the ages of the other adults were estimated from their weight at time of acquisition: the two females were both ~12 years old at the beginning of training, and the other the adult males were between 14 and 16 years old. One of the adult males died accidentally during routine TB testing, and therefore participated in only the first part of the experiment. The 4 adult males had been trained previously to sit quietly in a primate chair and fixate on a screen for a reward, and the juvenile males and the two females were trained concurrently with this study to sit quietly in a primate chair and fixate on a screen. However, none of the monkeys had been trained in any operant tasks previously, and none had used touchscreens before.
In a compartment of the monkeys’ home cages a touchscreen monitor (Elo TouchSystems, Menlo Park, CA) was mounted at eye level with a stainless steel juice delivery tube positioned so the monkey could comfortably reach the screen and drink at the same time. Pairs of stimuli were presented simultaneously, one on the left and one on the right side of the monitor. Each stimulus value was randomly chosen from a set of values from 0 to a maximum that could go as high as 25; the two stimuli were never the same value. Dots varied randomly in color, size, and position, and were constrained so that whenever two dots in an array overlapped, the smaller dot was drawn in front and differed in color from the other dot. For each dot pair presentation, the dot patterns were freshly generated with a random number generator off-screen before presentation, and presented instantaneously on the screen.
Animals chose one of the two stimuli by touching it. Monkeys were rewarded with the same number of drops as the assigned value of the chosen stimulus. Solenoid openings were longer (200-300ms) early in training, when both options were small, but as the average reward value increased solenoid openings were reduced to 25ms, resulting in one drop per opening. Drops were delivered at 4Hz, and each drop was accompanied by a beep sound. Each stimulus pair was presented for 10 seconds or until the animal responded by touching either stimulus. A new stimulus was presented 3 seconds after the end of the preceding trial. Monkeys were allowed to work alone to satiety for at least 3 hours per day, and they usually stopped working after 2 hours, or 300-600 trials. The monkeys’ average daily fluid intake was always more than 30 ml/kg. They had ad lib food.
Reaction time histograms for each monkey individually or for the adults or juveniles as a population were fit by least squares with a log Gaussian:
where A is the amplitude of the Gaussian, c is the center, and σ is the width; we took c, the center of the log Gaussian as the reaction time for each distribution.
To find out how long it took each monkey to learn novel symbols, we calculated the behavioral value of each novel symbol, as each symbol was introduced. To do this, we extracted from the entire data set all trials in which the novel symbol was one of the options and took bins of 10 trials (per monkey), and for each bin calculated the fraction of larger (novel symbol) choices as a function of the value of the other choice. For each bin we took the behavioral value of the novel symbol as the point of subjective equality as a function of the other choice values from the best fitting sigmoid (cumulative normal distribution) for those points. We then used the exponential:
where y is the calculated behavioral value of the novel stimulus, as a function of x (10-trial bin number), and V is the actual value of the novel symbol (# of drops delivered); we used least squares to estimate the λ that gave the best fit to the data points for each novel symbol. For each novel symbol we calculated the number of trials needed for the behavioral value to reach 95% of the actual value by finding the bin number where the fitted exponential curve crossed 95% of the actual value.
As an alternative method of calculating how long it took each monkey to learn each novel symbol, we calculated a running average of the frequency of larger choices for each novel symbol by averaging across a moving window of ±10 consecutive trials; using only trials in which the novel symbol was one of the options. We calculated how many trials it took each monkey to attain 90% correct (larger) choices for each novel symbol. Results were almost identical to the equivalent value calculations described above, in that the adults took more trials to learn each novel symbol than the juveniles did.
We tested all the monkeys behaviorally with 1.5 and 2 fold larger and smaller fonts, for which they maintained the same accuracy as with the original size. We tested the monkeys behaviorally using a serif font (Utopia), instead of the sans serif font (Helvetica) they first learned, and they all recognized this font accurately after a few days.
Functional MRI
Six monkeys were scanned to look for localization of Learned symbol responsiveness: two adults (one male and one female) who had learned symbols, three juveniles who had learned symbols, and one adult male who had not been exposed to the symbol task. These 6 animals represent the maximum number of our trained monkeys who could be scanned; the fourth trained juvenile and the other adult female were not willing to sit still enough in the scanner for fMRI, and the other trained adult males are too large to scan. The monkeys were scanned using techniques similar to those pioneered by Vanduffel and colleagues (Vanduffel et al., 2001). The monkeys lay comfortably in a horizontal primate chair in a ‘sphynx’ position, free to move limbs, but with the head restrained. The heads of four of the monkeys (the adult female and the three juveniles) were held stationary during scanning using a non-invasive vacuum helmet restraint (Srihasam et al., 2010), and the two adult males were held still using previously implanted delrin headposts (Tsao et al., 2006; Vanduffel et al., 2001). Each monkey was trained to sit in the chair and habituated to the sounds of MR scanning in a “mock” MR bore. The monkeys were water scheduled during the period of testing, and behavioral control was achieved using operant conditioning techniques. They were trained on a fixation task, and eye position was monitored using a pupil-corneal reflection tracking system (RK-726PCI, ISCAN, Cambridge, MA). Monkeys were rewarded for maintaining fixation within a 2° square fixation window. The i nterval between rewards was decreased systematically (from 2000 to 500 msec) as the monkey maintained fixation within the window during the trials. After fixation performance reached an asymptote (20-50 training sessions), the monkeys were scanned in a 3-T horizontal GE scanner (Signa) or in a 3T Siemens Tim Trio with an AC88 gradient insert. Similar results were obtained using both scanners, though at higher resolution in the Siemens scanner.
We used custom-made 4 channel coil arrays (made by Azma Maryam at the Martinos Imaging Center or by Resonance Innovations, Omaha NE) that fit closely over the monkeys’ heads. In order to enhance contrast, before each scanning session, the monkey was injected with 10mg/kg of a Monocrystalline Iron Oxide Nanoparticle contrast agent (Feraheme, AMAG Pharmaceuticals, Cambridge, MA). Each session consisted of 10-30 functional scans, each lasting 260 seconds (2-D Gradient-Echo Planar Imaging (GE-EPI); Repetition Time (TR) = 2 Sec, Echo Time (TE) = 14 msec). In the GE scanner: 64 × 64 matrix; 1.2 × 1.2 × 1.2 mm voxels, 35 contiguous horizontal slices. In the Siemens scanner: 96 × 84 matrix; 1 × 1 × 1 mm voxels, 50 contiguous horizontal slices]. Slices were positioned to cover the entire brain. In a separate session, a high resolution anatomical scan (0.35 × 0.35 × 0.45 mm) was obtained for each monkey in the Siemens scanner using a surface coil while the monkey was anesthetized.
Visual stimuli were projected onto a screen at the end of the bore 57 cm from the animal’s eyes. Each image subtended 3° x 3°. The st imuli consisted of symbols the monkeys had learned to associate with reward amount: 5 6 7 8 9 X Y W C H U T F K L N R M E A J, untrained shapes: @ β d Δ D $ Λ Ξ γ Φ Π Θ Σ Φ Γ # h Ω P % V, and 21 high-contrast faces. The Learned symbol blocks never contained symbols the monkey being scanned had not yet learned, and the number of possible images for each category was always the same There was always a fixation spot at the center of the screen. Each scan lasted 260 seconds, consisting of 20-second blocks of 20 images (1 second presentation of each image) from one category, Learned symbols (L), Untrained shapes (U), or Faces (F). Visual blocks were separated by 20-second blocks of the fixation spot alone. Stimuli were randomly selected from the appropriate category, with the constraint that consecutive stimuli not be identical.
Data Analysis
Data were analyzed using AFNI (Cox, 1996) and Freesurfer (Dale et al., 1999; Fischl et al., 1999). Only scans in which the monkey fixated within the 2° x 2° fixation window for >90% of the duration were used for statistical analysis. Prior to data analysis, all functional data were aligned to each monkey’s anatomical template individually using JIP software (http://www.nitrc.org/projects/jip) to remove distortions of the functional images, due to field variations induced by body position and movement between scans.
For four monkeys (all Juveniles and Adult 1), we used a total of 120 scans from 5 separate sessions each for our analysis. For monkeys Adult 2 and Adult 3, we used 60 scans from two separate sessions each. To identify category selective regions for each monkey we analyzed the odd and even scans separately, using the odd scans to identify regions selectively activated by each category (Figures 4&5) and the even scans to calculate response time courses (Figure 8), the percentage signal change (Figure 7) and significance (Supplemental Table S2) at each locus. Data were motion-corrected, quadratically detrended and smoothed after flattening with a Gaussian kernel of 2mm full-width-at-half-magnitude (fwhm). To calculate the maximum likelihood maps of responses to each stimulus category we used a modified gamma-variate function approximating monkey hemodynamic changes in cerebral blood volume with Monocrystalline Iron Oxide Nanoparticle contrast agent (Leite et al., 2002; Mandeville et al., 1999). We ran Monte Carlo simulations to get the clustering criterion needed to eliminate false positives arising from multiple comparisons (threshold/clustering criterion: p<0.001, minimum cluster size 15 voxels).
For each monkey we defined three category contrasts by performing t-tests between pairs of stimulus categories: Learned symbols versus Faces (LvsF); Learned symbols versus Untrained shapes (LvsU); and Faces Vs Untrained shapes (FvsU). Three stimulus category selective maps (Learned L; Shapes S and Faces F) were defined by conjunction analyses (Bell et al., 2009; Price et al., 1997).
We used odd-numbered scans to define regions of interest (ROIs) for each category selective patch as the best 40 contiguous voxels centered on the maximally active voxel, to alleviate any adverse effects on results due to differences in cluster sizes. We then used even-numbered scans to calculate time-courses for each category selective ROI. This gave us 130 measurements for blood flow changes, adjusted for hemodynamic delay, during three stimulus conditions. We averaged two stimulus cycles to get 70 measurements for each ROI .i.e. 10 values for the average blood flow during the 20 seconds display interval for each stimulus condition. Baseline was calculated by averaging the activity during the fixation periods, and was used to calculate the percentage signal change in Figure 7. Three way ANOVA was performed on the average signal change for each stimulus block to test for effects of stimulus category, subject, and age. Wilcoxon rank sum test was done on the stimulus block data for statistical significance of the patches (Supplemental Table S2). The percentage signal change for the early visual areas (V1, V2, V3/V4) were calculated using 40 contiguous voxels in the central visual field part of each area, identified using retinotopic mapping (Srihasam et al., 2010) and a macaque atlas (Saleem and Logothetis, 2007).
To identify an average ROI for the Learned symbol selective region, we randomly took 30 odd numbered scans from each of the three juvenile monkeys (90 scans in total). These functional volumes were then warped into a standard template (McLaren et al., 2009) to compensate for the individual differences using a non-rigid mapping software, JIP. Data were analyzed as described above for individual monkeys. Two ROIs were then defined in left and right TEpv between A0 and A4 in or on the lateral bank of the OTS, by taking all the voxels selectively more active for Learned symbols than for Untrained shapes and Faces within this sub-region (114 voxels).
Software for stimulus presentation and reward delivery was developed in-house and was written in C++. All experiments were done in accordance with procedures approved by the Harvard Medical School Standing Committee on Animals.
Supplementary Material
Highlights.
Juvenile monkeys learned symbols faster and more fluently than adults did
Juvenile learners developed symbol-selective regions in their temporal lobe
There is a regular organization of category-selective modules along IT
This study links intensive early experience, expertise, and modular organization
Acknowledgements
This work was supported by NIH grant EY16187. We thank Wim Vanduffel for much help developing scanning technology, Tristram Savage for monkey training, and Winrich Freiwald, Doris Tsao, and Heather Sternshein for advice and comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anstis S. Last but not least. Perception. 2005;34:237–240. doi: 10.1068/p5412. [DOI] [PubMed] [Google Scholar]
- Baker CI, Liu J, Wald LL, Kwong KK, Benner T, Kanwisher N. Visual word processing and experiential origins of functional selectivity in human extrastriate cortex. Proc Natl Acad Sci U S A. 2007;104:9087–9092. doi: 10.1073/pnas.0703300104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AH, Hadj-Bouziane F, Frihauf JB, Tootell RB, Ungerleider LG. Object representations in the temporal cortex of monkeys and humans as revealed by functional magnetic resonance imaging. J Neurophysiol. 2009;101:688–700. doi: 10.1152/jn.90657.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasdel G, Campbell D. Functional retinotopy of monkey visual cortex. J Neurosci. 2001;21:8286–8301. doi: 10.1523/JNEUROSCI.21-20-08286.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussaoud D, Desimone R, Ungerleider LG. Visual topography of area TEO in the macaque. J Comp Neurol. 1991;306:554–575. doi: 10.1002/cne.903060403. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantlon JF, Brannon EM. How much does number matter to a monkey (Macaca mulatta)? J Exp Psychol Anim Behav Process. 2007;33:32–41. doi: 10.1037/0097-7403.33.1.32. [DOI] [PubMed] [Google Scholar]
- Castro-Caldas A, Nunes MV, Maestu F, Ortiz T, Simoes R, Fernandes R, de La Guia E, Garcia E, Goncalves M. Learning orthography in adulthood: a magnetoencephalographic study. J Neuropsychol. 2009;3:17–30. doi: 10.1348/174866408X289953. [DOI] [PubMed] [Google Scholar]
- Changizi MA, Zhang Q, Ye H, Shimojo S. The structures of letters and symbols throughout human history are selected to match those found in objects in natural scenes. Am Nat. 2006;167:E117–139. doi: 10.1086/502806. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S. Specialization within the ventral stream: the case for the visual word form area. Neuroimage. 2004;22:466–476. doi: 10.1016/j.neuroimage.2003.12.049. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff MA, Michel F. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123(Pt 2):291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Cox R. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L. Cultural recycling of cortical maps. Neuron. 2007;56:384–398. doi: 10.1016/j.neuron.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Pegado F, Braga LW, Ventura P, Nunes Filho G, Jobert A, Dehaene-Lambertz G, Kolinsky R, Morais J, Cohen L. How learning to read changes the cortical networks for vision and language. Science. 2010;330:1359–1364. doi: 10.1126/science.1194140. [DOI] [PubMed] [Google Scholar]
- Denys K, Vanduffel W, Fize D, Nelissen K, Peuskens H, Van Essen D, Orban GA. The processing of visual shape in the cerebral cortex of human and nonhuman primates: a functional magnetic resonance imaging study. J Neurosci. 2004;24:2551–2565. doi: 10.1523/JNEUROSCI.3569-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Underleider LG. Neural mechanism of visual processing in monkeys. In: Boller E, Grafman BV, editors. Handbook of Neurophychology. Elsivier Science Publishers; 1989. [Google Scholar]
- Farah MJ. Is face recognition “special”? Evidence from neuropsychology. Behavioural Brain Research. 1996;76:181–189. doi: 10.1016/0166-4328(95)00198-0. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Rabinowitz C, Quinn GE, Liu GT. Early commitment of neural substrates for face recognition. Cognitive Neuropsychology. 2000;17:117–123. doi: 10.1080/026432900380526. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Wilson KD, Drain M, Tanaka JN. What is “special” about face perception? Psychol Rev. 1998;105:482–498. doi: 10.1037/0033-295x.105.3.482. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno M, Dale A. Cortical surface-based analysis II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fize D, Vanduffel W, Nelissen K, Denys K, Chef d’Hotel C, Faugeras O, Orban GA. The retinotopic organization of primate dorsal V4 and surrounding areas: A functional magnetic resonance imaging study in awake monkeys. J Neurosci. 2003;23:7395–7406. doi: 10.1523/JNEUROSCI.23-19-07395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima K, Miyake S, Ito T. Computer Society Press Technology Series Neural Networks. IEEE Computer Society Press Los Alamitos; CA, USA: 1988. Neocognitron: a neural network model for a mechanism of visual pattern recognition; pp. 136–144. [Google Scholar]
- Gauthier I, Behrmann M, Tarr MJ. Can face recognition really be dissociated from object recognition? J Cogn Neurosci. 1999;11:349–370. doi: 10.1162/089892999563472. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Skudlarski P, Gore JC, Anderson AW. Expertise for cars and birds recruits brain areas involved in face recognition. Nat Neurosci. 2000;3:191–197. doi: 10.1038/72140. [DOI] [PubMed] [Google Scholar]
- Glezer LS, Jiang X, Riesenhuber M. Evidence for highly selective neuronal tuning to whole words in the “visual word form area”. Neuron. 2009;62:199–204. doi: 10.1016/j.neuron.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren CC, Sarty M, Wu PY. Visual following and pattern discrimination of face-like stimuli by newborn infants. Pediatrics. 1975;56:544–549. [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior; a neuropsychological theory. Wiley; New York: 1949. [Google Scholar]
- Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Freeman DC. Projection into the visual field of ocular dominance columns in macaque monkey. Brain Res. 1977;122:336–343. doi: 10.1016/0006-8993(77)90299-2. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Livingstone MS. Segregation of form, color, and stereopsis in primate area 18. J Neurosci. 1987;7:3378–3415. doi: 10.1523/JNEUROSCI.07-11-03378.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Ferrier lecture. Functional architecture of macaque monkey visual cortex. Proc R Soc Lond B Biol Sci. 1977;198:1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Dziurawiec S, Ellis H, Morton J. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40:1–19. doi: 10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Tong F, Nakayama K. The effect of face inversion on the human fusiform face area. Cognition. 1998;68:B1–11. doi: 10.1016/s0010-0277(98)00035-3. [DOI] [PubMed] [Google Scholar]
- Kanwisher NG, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolster H, Mandeville JB, Arsenault JT, Ekstrom LB, Wald LL, Vanduffel W. Visual field map clusters in macaque extrastriate visual cortex. J Neurosci. 2009;29:7031–7039. doi: 10.1523/JNEUROSCI.0518-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtzi Z, DiCarlo JJ. Learning and neural plasticity in visual object recognition. Curr Opin Neurobiol. 2006;16:152–158. doi: 10.1016/j.conb.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Leite FP, Tsao D, Vanduffel W, Fize D, Sasaki Y, Wald LL, Dale AM, Kwong KK, Orban GA, Rosen BR, et al. Repeated fMRI using iron oxide contrast agent in awake, behaving macaques at 3 Tesla. Neuroimage. 2002;16:283–294. doi: 10.1006/nimg.2002.1110. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. Anatomy and physiology of a color system in the primate visual cortex. J Neurosci. 1984;4:309–356. doi: 10.1523/JNEUROSCI.04-01-00309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS, Srihasam K, Morocz IA. The benefit of symbols: monkeys show linear, human-like, accuracy when using symbols to represent scalar value. Anim Cogn. 2010;13:711–719. doi: 10.1007/s10071-010-0321-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJ, Ayata C, Zaharchuk G, Moskowitz MA, Rosen BR, Weisskoff RM. Evidence of a cerebrovascular postarteriole windkessel with delayed compliance. J Cereb Blood Flow Metab. 1999;19:679–689. doi: 10.1097/00004647-199906000-00012. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Gore JC, Allison T. Face-specific processing in the human fusiform gyrus. Journal of Cognitive Neuroscience. 1997;9:605–610. doi: 10.1162/jocn.1997.9.5.605. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Kosmatka KJ, Oakes TR, Kroenke CD, Kohama SG, Matochik JA, Ingram DK, Johnson SC. A population-average MRI-based atlas collection of the rhesus macaque. Neuroimage. 2009;45:52–59. doi: 10.1016/j.neuroimage.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller S, Freiwald WA, Tsao DY. Patches with links: a unified system for processing faces in the macaque temporal lobe. Science. 2008;320:1355–1359. doi: 10.1126/science.1157436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op de Beeck HP, Deutsch JA, Vanduffel W, Kanwisher NG, DiCarlo JJ. A stable topography of selectivity for unfamiliar shape classes in monkey inferior temporal cortex. Cereb Cortex. 2008;18:1676–1694. doi: 10.1093/cercor/bhm196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk TA, Farah MJ. Brain localization for arbitrary stimulus categories: a simple account based on Hebbian learning. Proc Natl Acad Sci U S A. 1995;92:12370–12373. doi: 10.1073/pnas.92.26.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Friston KJ. Subtractions, conjunctions, and interactions in experimental design of activation studies. Hum Brain Mapp. 1997;5:264–272. doi: 10.1002/(SICI)1097-0193(1997)5:4<264::AID-HBM11>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Tovee MJ. The responses of single neurons in the temporal visual cortical areas of the macaque when more than one stimulus is present in the receptive field. Exp Brain Res. 1995;103:409–420. doi: 10.1007/BF00241500. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Logothetis N. A combined MRI and histology atlas of the rhesus monkey brain in stereotaxic coordinates. Burlington, MA, Academic; London: 2007. [Google Scholar]
- Sawamura H, Georgieva S, Vogels R, Vanduffel W, Orban GA. Using functional magnetic resonance imaging to assess adaptation and size invariance of shape processing by humans and monkeys. J Neurosci. 2005;25:4294–4306. doi: 10.1523/JNEUROSCI.0377-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RB. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Hasselmo ME. A model for experience-dependent changes in the responses of inferotemporal neurons. Network. 2000;11:169–190. [PubMed] [Google Scholar]
- Srihasam K, Sullivan K, Savage T, Livingstone MS. Noninvasive functional MRI in alert monkeys. Neuroimage. 2010;51:267–273. doi: 10.1016/j.neuroimage.2010.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita Y. Face perception in monkeys reared with no exposure to faces. Proc Natl Acad Sci U S A. 2008;105:394–398. doi: 10.1073/pnas.0706079105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka JW, Farah MJ. Parts and wholes in face recognition. Q J Exp Psychol A. 1993;46:225–245. doi: 10.1080/14640749308401045. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Silverman MS, De Valois RL, Jacobs GH. Functional organization of the second cortical visual area in primates. Science. 1983;220:737–739. doi: 10.1126/science.6301017. [DOI] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Knutsen TA, Mandeville JB, Tootell RB. Faces and objects in macaque cerebral cortex. Nat Neurosci. 2003;6:989–995. doi: 10.1038/nn1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Tootell RB, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311:670–674. doi: 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Livingstone MS. Mechanisms of face perception. Annu Rev Neurosci. 2008;31:411–437. doi: 10.1146/annurev.neuro.30.051606.094238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Loos H, Woolsey TA. Somatosensory cortex: structural alterations following early injury to sense organs. Science. 1973;179:395–398. doi: 10.1126/science.179.4071.395. [DOI] [PubMed] [Google Scholar]
- Vanduffel W, Fize D, Mandeville JB, Nelissen K, Van Hecke P, Rosen BR, Tootell RB, Orban GA. Visual motion processing investigated using contrast agent-enhanced fMRI in awake behaving monkeys. Neuron. 2001;32:565–577. doi: 10.1016/s0896-6273(01)00502-5. [DOI] [PubMed] [Google Scholar]
- Wiesel TN. Postnatal development of the visual cortex and the influence of environment. Nature. 1982;299:583–591. doi: 10.1038/299583a0. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Ordered arrangement of orientation columns in monkeys lacking visual experience. J Comp Neurol. 1974;158:307–318. doi: 10.1002/cne.901580306. [DOI] [PubMed] [Google Scholar]
- Yin R. Looking at upside-down faces. J Exp Psychol. 1969;81:141–145. [Google Scholar]
- Yovel G, Kanwisher N. Face perception: domain specific, not process specific. Neuron. 2004;44:889–898. doi: 10.1016/j.neuron.2004.11.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.