Abstract
The rationale for in utero repair of myelomeningocele (MMC) in the context of pathologic observations, animal models, and outcomes from the initial experience with human fetal myelomeningocele repair is presented. This has now culminated in a randomized trial, Management of Myelomeningocele Study (the MOMS Trial), the findings of which are listed. The story is focused on the milestone contributions of members of the Center for Fetal Diagnosis and Treatment at the Children's Hospital of Philadelphia (CHOP) on the road to successful fetal surgery for spina bifida. This is now performed in selected patients and presents an additional therapeutic alternative for expectant mothers carrying a fetus with MMC.
Keywords: Myelomeningocele, spina bifida, hydrocephalus, hindbrain herniation, prenatal diagnosis, fetal surgery, Management of Myelomeningocele Study (MOMS)
Introduction
It is an honor and a privilege for me to give the Isabella Forshall lecture at the British Association of Paediatric Surgeons meeting. The BAPS is the oldest and most distinguished international pediatric surgical society in the world, and one of its founding members its second President, Isabella Forshall, was a pediatric surgery pioneer. Described by her peers as a superb technical surgeon and having a formidable presence, she served as a creative force to enhance the surgical care of children.
Open spina bifida or myelomeningocele (MMC) is caused by primary failure of neural tube closure during the embryologic period, and it is by far the most common and devastating form of spina bifida.[1] Advances in prenatal diagnosis now permit diagnosis of spina bifida as early as the first trimester, and extensive research into the etiology of neural tube defects has elucidated both genetic and micronutrient causes.[2] Folic acid fortification of foodstuffs has had a significant impact on the prevention of MMC, but this salutary effect has now leveled off.[3,4] Currently, one in every 3000 live-births in the USA is an infant with MMC which translates to about 1500 infants born each year.[5-7] The incidence is even higher in the UK and Ireland. This excludes an estimated 25-50% of MMC pregnancies in which the fetus is aborted. [8,9]
Myelomeningocele is a devastating congenital defect of the central nervous system for which there is no cure and is characterized by protrusion of the meninges and spinal cord through open vertebral arches leading to lifelong paralysis. The natural history includes a constellation of findings which correlate with the proximal anatomic extent of the defect. These findings include hydrocephalus, hindbrain herniation, motor and cognitive impairments, bladder and bowel incontinence, social and emotional challenges, and lifelong quality of life issues.
Postnatal treatment of MMC consists of surgical closure of the spinal canal at birth and lifelong supportive care. For prenatally diagnosed cases, cesarean section at term is performed to prevent birth trauma to the exposed spinal cord.[10] About 85% of MMC infants require placement of a ventriculoperitoneal shunt (VP), and 45% of those will undergo shunt revision within their first year due to complications such as occlusion and infection.[11] About 15% of MMC infants do not survive beyond 5 years, and this rises to about 35% in those with symptoms of brainstem dysfunction secondary to the hindbrain herniation feature of the Arnold-Chiari malformation.[12] Clinical presentation of this malformation depends on the age of the child, but typically it includes dysfunction of the cerebellum, medullary respiratory center, and cranial nerves IX and X as well as hydrocephalus. Surgical management for symptomatic hindbrain herniation is beneficial in only selected patients and consists of laminectomy and decompression of the cranio-cervical junction.[13] Ongoing treatment of orthopedic, urologic, bowel and shunt complications is common. While 70% of patients have an IQ >80; only half are able to live independently as adults, even with adapted accommodations.[14] The emotional and financial impact on the family and community are, of course, enormous.[15]
In Utero Intervention Rationale and Animal Studies
The rationale for in utero repair of MMC is based on the “two-hit hypothesis”: the first “hit” is a failure of neural tube closure, followed by the second “hit” in which a relatively normal spinal cord becomes secondarily damaged by amniotic fluid exposure, direct trauma, hydrodynamic pressure, or a combination of these factors. In theory, it is this secondary damage which may be ameliorated by early gestation fetal surgical repair.
The husband and wife team of Martin Meuli and Claudia Meuli-Simmen came to the University of California San Francisco (UCSF) as research fellows in the early 1990s to work with me on fetal therapy. Martin Meuli had read an abstract by Grover Hutchins, a pathologist at Johns Hopkins University, who had examined the spinal cords of eight human fetuses with MMC and carefully described the relationships of the spinal cord, meninges, and dermal-epidermal junction. Hutchins showed varying degrees of neural tissue loss at the defect site, but normal appearing dorsal and ventral horns were present at the most proximal aspect of the lesion.[16] This group were the first to suggest the two-hit pathophysiology, since they attributed these alterations to injuries occurring subsequent to primary neural tube formation.[17] The traditional thinking about spina bifida embryology had been turned on its head.
Additional support for the two-hit hypothesis came from sonographic observations of human fetuses with MMC [18,19], and analysis of some of the less severe variants of spinal dysraphism which are interesting “experiments of nature”.[20-22] Over the past 25 years, animal models of MMC were developed to test the hypothesis that in utero intervention can prevent further spinal cord damage and the consequent neurologic deficits.[23-27] While these studies support the principle of improved neurologic function with in utero coverage of the spinal cord, a large animal model with prolonged periods of time in utero after surgical manipulation was needed before extrapolation of these findings to humans.
Beginning in 1993, we performed a series of experiments which demonstrated the similarities between a surgically created large animal model and human MMC, and documented neurologic improvement following in utero repair.[28,29] We enlisted the expertise of Grover Hutchins for the pathological analysis. A sheep model was created in fetal lambs at 75 days gestation (term 145 days) by excision of skin, paraspinal musculature, vertebral arches of lumbar vertebrae (L1-4), and the exposed dorsal dura mater. The pregnancy was then continued to near term, and cesarean section performed at 140 days gestation. The lambs developed lumbar cystic sacs with abnormal spinal cord tissue on the dorsal aspect. Histology revealed loss of neural tissue, disruption of neural bundles, and areas of cord necrosis in the exposed segments, strikingly similar to that seen in human MMC. The spinal cord and its coverings proximal to the lesion appeared normal. Clinically, the lambs demonstrated incontinence of urine and stool, flaccid paraplegia, as well as lack of sensation in the hindlimbs which was confirmed by somatosensory evoked potentials.
We then performed in utero closure of the spine using this same fetal lamb model.[29, 30] Spina bifida-type lesions were created at 75 days, followed by a second procedure at 100 days gestation. A reversed latissimus dorsi flap technique developed by plastic surgeon Claudia Meuli-Simmen was used to cover the exposed spinal cord placode, and the animals were then delivered by cesarean section just prior to term.[31] The repaired group showed near normal motor function, apparent continence of stool and urine, and intact sensation by clinical evaluation and somatosensory evoked potentials. These animals, compared to normal sheep, had some neurologic delay and hindlimb weakness, but they were able to stand, walk, and climb stairs. Histologically, the spinal cord, nerve roots, and spinal ganglia had well preserved cytoarchitecture in all specimens, with only flattening and mild dilation of the central canal. The paper summarizing these findings was published in Nature Medicine in 1995.[30]
This was the first large animal experiment that demonstrated a spinal cord lesion could be created in utero and repaired at a later time point with preservation of neurologic function. Unlike the previous animal models, this sheep model more closely resembled that of human MMC in duration of exposure of the cord to the environment, clinical examination, and histology, as we presented at the BAPS meeting in St. Helier, Jersey, Channel Islands in 1996.[32] Subsequent sheep studies by Rusty Jennings at UCSF and Sarah Bouchard at the Children's Hospital of Philadelphia (CHOP) showed that this model, when combined with a lumbar myelotomy to enhance CSF drainage out through the lumbar laminectomy, leads to hindbrain herniation, and that in utero closure resulted in reversal of hindbrain herniation.[33,34] Reversal of hindbrain herniation after fetal MMC surgery in these animals supports the hypothesis that the MMC permits excessive drainage of cerebrospinal fluid (CSF) through the open defect, leading to loss of hydrostatic pressure and consequent downward herniation and caudal displacement of the cerebellar vermis and brainstem into the cervical spinal canal. By closing the MMC early in fetal life and thereby sealing the CSF leak, the hydrostatic back pressure is again established in the posterior fossa, which disimpacts the brain from the spinal canal and reestablishes a more normal CSF drainage pathway (Figure 1 a & b).
Figure 1.
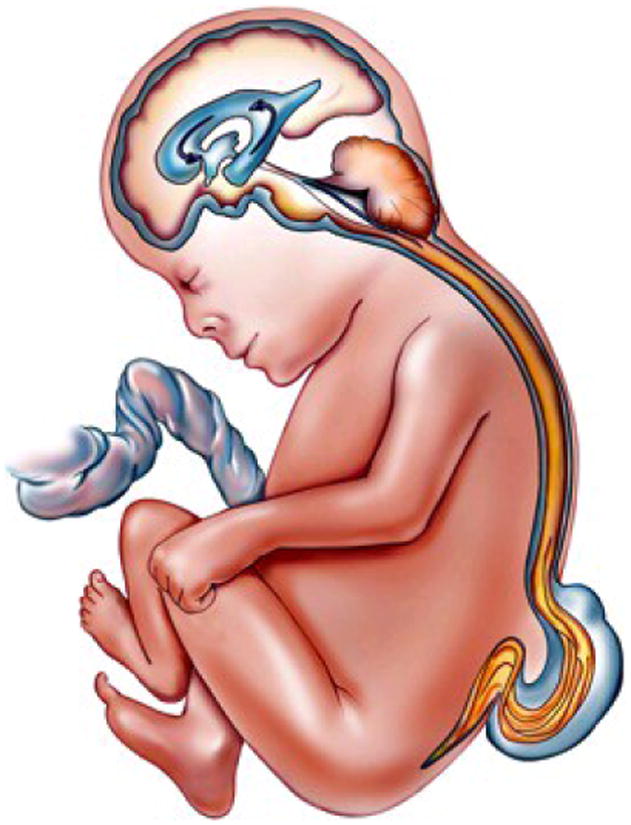

The pathophysiology of hindbrain herniation in myelomeningocele (MMC).
Fig. 1a The cerebrospinal fluid (CSF) leaks out through the fetal MMC defect leading to loss of hydrostatic pressure and descent of the hindbrain through the foramen magnum into the cervical spinal canal.
Fig. 1b: After fetal MMC closure, the CSF leak is sealed, the hydrostatic pressure column is restored, the hindbrain ascends into the posterior fossa, and a more normal CSF drainage pathway is established.
One of the criticisms of surgical models of MMC is that the lesion is artificially created at mid- gestation and is therefore unable to replicate the primary defect in neurulation, limiting their experimental relevance to the “secondary” injuries of mechanical or chemical trauma. To address this criticism, Enrico Danzer and Alan Flake at CHOP developed a novel short-gestation animal model of isolated MMC in fetal rats by maternal administration of all-trans retinoic acid (RA).[35] Prenatal administration of RA induces a primary defect during neural tube formation so that the RA-induced MMC model enables investigation of the evolution of abnormalities in the development of MMC from the point of defective neurulation forward. Fetal rats exposed to RA develop MMC lesions that are confined to the lumbo-sacral area and the histopathological features of the lesion are quite similar to human MMC. Fetal rats with MMC develop clubfoot deformity, abnormal bladder function, and features of the Arnold-Chiari II malformation identified on postnatal MR imaging. Using this animal model we were able to provide clear evidence that most MMC rat fetuses have normal neurological function early in gestation despite the absence of normal primary neurulation.[35-37] Loss of this function is associated with neurodegeneration that is acquired later in gestation. The degree of neurological dysfunction correlates with the concentration of glial fibrillary acidic protein (GFAP) levels in the amniotic fluid as gestation proceeds, so GFAP levels may serve as a biomarker of neurologic damage.[38] The fetal rat is large enough for surgical manipulation and has minimal propensity for preterm labor after fetal surgery, so we are now using this model to study various approaches for prenatal coverage of MMC and the subsequent effect on postnatal neurologic outcome.
The CHOP Clinical Experience Prior to the MOMS Trial
Before 1995, we considered only fetuses with life-threatening anomalies and very poor predicted outcomes to be candidates for fetal surgery.[39] However, the promising results of animal research, and the development of diagnostic fetal magnetic resonance imaging (MRI) techniques at CHOP led to increased consideration of prenatal intervention. Beginning in Philadelphia in 1995, expectant mothers considering in utero therapy for myelomeningocele underwent extensive prenatal evaluation to include obstetrical evaluation, genetic screening, ultrasonography, and ultrafast MRI. Although most cases of MMC are isolated abnormalities, genetic screening permits identification of some of the genetic and chromosomal syndromes associated with spinal dysraphism. Ultrasonography assesses lower extremity function, identifies club foot anomalies, and determines the spinal level of the defect by localizing vertebral arch defects. As a rule, fetuses with thoracolumbar defects have the worst functional outcomes, while those with progressively lower lesions tend to do better.[40] Using ultrafast MR sequencing techniques developed by Anne Hubbard and her colleagues at CHOP, we were able to define the presence or absence of the Arnold-Chiari malformation, hydrocephalus, and any other brain abnormalities.[41] We were able to enhance prenatal counseling of parents and planning for possible fetal surgery by careful correlation of imaging results with known clinical outcomes.
Fetal surgery has significant risks; so it was initially offered only to those mothers in which the fetus had a large thoracolumbar defect, the Arnold-Chiari malformation, mild or moderate ventriculomegaly, normal leg movements, no apparent clubbing of the feet, normal karyotype, and absence of other anomalies. Encouraging results with the first few patients led to surgical repair of smaller spinal defects provided the other criteria were met. It is mandatory that fetal surgery candidates exhibit hindbrain herniation, since those fetuses are most likely to suffer from hydrocephalus or life-threatening brainstem symptoms, both of which require frequent postnatal surgery.
We speculated that the surgical procedure was ideally performed between 19 and 25 weeks gestation based upon our experience with other fetal surgical interventions and our animal models. Repair at this age minimizes the length of time during which neuronal damage to the exposed cord may occur as before this age fetal tissues are quite gelatinous making the procedure technically difficult. We also believed early repair might limit progression of hydrocephalus, since increasing ventricular size over the course of gestation is characteristic of fetal MMC.[42]
The first successful open fetal MMC surgery in an early gestation human fetus was performed at CHOP and reported in The Lancet in 1998.[43] A 23-week gestation fetus with a T11-S1 lesion, hindbrain herniation, and normal lower extremity movement underwent open surgical coverage of the dysraphic defect. Fetal surgery and recovery were uneventful, and the fetus was subsequently delivered by cesarean section at 30 weeks of gestation after the onset of preterm labor. The baby had a right clubfoot deformity, and neuromotor function at the L4 level on the right and L5 level on the left. Whereas hindbrain herniation was found preoperatively, postnatal MRI documented hindbrain herniation reversal and absence of ventriculomegaly, so shunting has never been required. Unfortunately, this first patient developed severe tethering of the spinal cord at the repair site after 6 months of age leading to loss of lower extremity function and requiring operative release. In this first case, the MMC defect had been covered only with skin flaps, and the neural placode became adherent to the overlying skin. This late decline in function due to tethering motivated us to investigate better repair techniques and coverage materials for fetal MMC repair. Subsequent studies reported by neurosurgeons Leslie Sutton from CHOP and Noel Tulipan from Vanderbilt University showed that infants treated prenatally had improvement in hindbrain herniation after fetal MMC surgery and had a diminished need for shunting relative to infants that underwent standard postnatal neurosurgical repair.[44,45] Compared to historical controls it was estimated that fetal MMC surgery reduced the need for VP shunt placement from 80-90% to 40%.[46]
The intraoperative and postoperative management algorithm for fetal MMC surgery has been extensively described in the recent MOMS trial publication in the New England Journal of Medicine.[47] After maternal laparotomy followed by hysterotomy using a uterine stapling device, the fetus is positioned with the MMC lesion visible through the uterine incision. We have shown that intraoperative fetal echocardiographic monitoring is imperative.[48] The cystic membrane of the MMC is excised and the attachments of the meninges to the skin and soft tissues are detached. If possible, native dura is closed over the spinal cord as a first layer, followed by closure of paraspinal myofascial flaps, and then the skin surrounding the lesion is mobilized and closed to complete the repair (Figure 2 a & b). When the skin cannot be closed primarily, an acellular human dermis graft is used to complete the closure.
Figure 2.
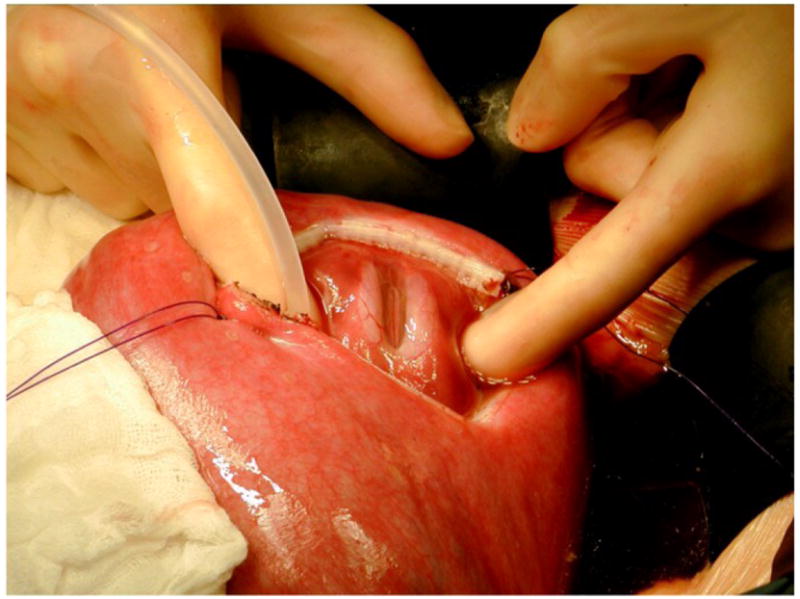
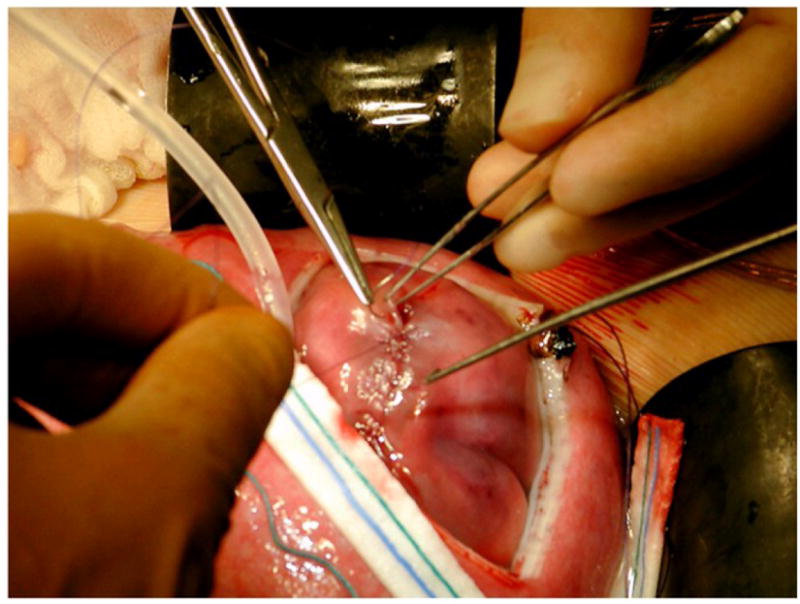
Fig. 2a. Exposure of 22 week gestation fetus through hysterotomy showing the MMC.
Fig. 2b. After dural closure and myofascial flap closure, the skin is closed.
Mark Johnson reported our experience at CHOP with 58 patients treated with fetal surgery from 1998-2003 prior to the beginning of the MOMS Trial.[49] There were 4 deaths due to preterm delivery, and the average age at delivery was 34 weeks, 4 days. Comprehensive follow-up examinations were performed at 1, 2, 3 and 5 years of age. There was resolution of hindbrain herniation in nearly all patients treated in utero, and the ascent of hindbrain structures could be shown within 3 weeks of the fetal closure using serial MRI. Hindbrain herniation reversal was significant in three ways for improved outcomes. First, the VP shunt rate was 46%, which is much lower that the predicted overall shunt rate of 84% shown by CHOP neonatologist Natalie Rintoul based upon 297 historical controls followed at the CHOP Spina Bifida Clinic between 1983 and 2000.[50] Second, the overall head size is very small in MMC fetuses, but it increased towards normal after fetal surgery due to the restoration of extra-axial CSF spaces and increase in cortical indices.[51] Third, the vast majority of children demonstrated no or minimal brainstem dysfunction symptoms at follow-up.[52]
In assessing motor skills, fetal surgery usually resulted in better than predicted lower extremity function at birth: 57% had lower extremity neuromotor function that was better than predicted by a median of 2 functional levels, 24% had neuromotor function as predicted, while 19% had worse than predicted function by a median of 1 functional level. Sixty-nine percent of children who underwent fetal MMC repair at CHOP were independent walkers at a mean follow-up age of 66 months.[53] Despite these promising findings, a few of the patients developed clinically symptomatic spinal cord tethering in association with dermoid inclusion cysts at the fetal closure site and required repeat surgery. A possible late decline in neurological function due to tethering ± dermoid inclusion cysts underscores the importance of careful long-term neurologic surveillance of these children.[54]
Neurodevelopmental evaluation was performed at 2 years and 5 years of age in studies lead by Mark Johnson, Marsha Gerdes, and Enrico Danzer at CHOP.[55,56] By 5 years of age, the majority (83%) of children had overall cognitive functioning in the average to high range. There was a pattern of consistently higher scores in verbal areas compared to scores for visual-motor or non-verbal reasoning, which suggests the possibility of later learning difficulties.
These preliminary observations and outcomes are significant. After fetal MMC repair, ascent of the hindbrain and improved CSF hydrodynamics reduces hydrocephalus and averts the need and morbidity of ventricular shunts. With a more normal anatomic location of the hindbrain, the symptomatic sequelae of the Arnold-Chiari malformation and need for subsequent surgery should be reduced. In the case of lower lumbar and sacral lesions where less impairment in lower extremity function may be predicted, normalizing hindbrain position and minimizing the need for postnatal VP shunt placement may be the primary indication for surgery. Persistence of improved lower extremity function, especially in patients with lesions at higher spinal levels, should permit greater independence and potentially improved quality of life. A reduction risk of club feet and other orthopedic anomalies should limit the need for surgical intervention and enhance the possibility of future ambulation. Two follow-up studies of women who underwent open fetal surgery at CHOP demonstrated no impairment of future reproductive capacity, and the hysterotomy risks were comparable to those of a classic cesarean section.[57,58] The latter finding mandates cesarean delivery for the fetal surgery pregnancy and all subsequent pregnancies.
Although fetoscopic techniques that involve making multiple puncture wounds in the uterus are theoretically appealing to potentially mitigate maternal morbidity, clinical reports on their use are limited and the results have been disappointing, primarily because of uterine membrane problems leading to premature birth 3 to 6 weeks after the procedure and delivery before 30 weeks gestation. The first cases of fetal MMC surgery using an endoscopic approach were reported in 1997 at Vanderbilt University. This technique proved disastrous (two of four fetuses died) and was abandoned.[59] In 2003, Farmer and colleagues from UCSF reported three patients that underwent fetoscopic MMC surgery.[60] Fetoscopic coverage was successfully completed in one, but the patch partially detached after fetal intervention and the infant required standard repair and shunt placement postnatally. Due to technical difficulties, the MMC defect in the second fetus was never completely covered and the fetus was delivered prematurely at 31 weeks of gestation. Postnatally the newborn required neurosurgical repair of the lesion and VP shunt placement and subsequently died of urosepsis at one month of age. The third fetus required conversion to an open approach secondary to an anterior placenta and difficulties in appropriately positioning the fetus. Fetoscopic patch coverage has also been tried in Europe in a small series of patients, and has also proven very problematic.[61, 62] Complete coverage of the defect was only achieved in 11 of 16 (69%) fetuses. In four fetuses the surgery was terminated prior to completion of the procedure secondary to bleeding at the trocar sites. Mean age at delivery was 28 weeks which is considerably earlier than the reported mean gestational age at delivery of 34-35 weeks for the open approach.[47,49] Oligohydramnios developed in 9(56%) pregnancies. Overall survival was only 81% (the 3 deaths were due to severe prematurity, intraoperative demise, and termination of pregnancy after fetal surgery). As compared with the open fetal surgery technique, fetoscopic repair of MMC has resulted in higher rates of fetal death, premature rupture of the membranes, chorioamnionitis, premature delivery, and persistent hindbrain herniation. If the problems of membrane rupture associated with multiple-port fetoscopy can be solved, this minimally invasive approach to repairing MMC before birth should be tested clinically.
Management of Myelomeningocele Study (MOMS): A Randomized, Prospective Clinical Trial
Due to the lack of a control group of children with MMC who had not undergone prenatal surgery, the initial clinical results of fetal MMC surgery have been compared to previously published cohorts. Infants treated prenatally represent a highly-selected subset of affected individuals. Comparison between MMC patients who were treated prenatally and previously reported controls are subject to bias. For these reasons the National Institutes of Health (NIH) sponsored a multicenter, prospective, randomized clinical trial comparing outcome after prenatal and postnatal surgery for MMC beginning in 2003.[47] Enrollment was stopped by the Data Safety and Monitoring Board in December 2010 because of the efficacy of fetal surgery after recruitment and randomization of 183 of a planned sample size of 200 patients. The study was performed by three fetal surgery units including CHOP (led by Scott Adzick), Vanderbilt University (led in succession by Joseph Bruner, Edmund Yang, and John Brock), and UCSF (led by Michael Harrison followed by Diana Farmer); the Data Study and Coordinating Center at George Washington University (led by Elizabeth Thom); and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (led by Catherine Spong). Prior to the beginning of the trial, all other US centers voluntarily agreed not to offer fetal surgery for MMC outside of the trial, essentially closing a “back door” to the intervention until the trial was completed.
Potential patients were referred to the closest center based on geographic criteria. Patients willing to accept either procedure were randomized after consent to either prenatal surgery or postnatal surgery at that center. All prenatal and postnatal patient care protocols were standardized among the three centers. Patient inclusion and exclusion criteria for the MOMS trial are shown in Table 1.
Table 1. Our current inclusion and exclusion selection criteria are the same as for the MOMS trial.
| Inclusion criteria |
|---|
|
| Exclusion criteria |
|
The objective of the trial was to evaluate if intrauterine repair of MMC between 19 to 25 weeks gestation improves outcome compared with standard neurosurgical repair. One primary outcome was a composite of fetal or neonatal death or the need for VP shunt placement by the age of 12 months. A second primary outcome was the assessment of mental development and motor function at 30 months. A variety of secondary neonatal and maternal outcome measures were also examined. The long-term psychological and reproductive consequences in mothers who undergo intrauterine repair of MMC are currently being compared to those in the postnatal repair group. During the study, the investigators were blinded to the results, since the follow-up evaluation of the children and mothers was performed by an independent medical team.
Similar to the earlier, non-randomized results of patients who underwent fetal MMC repair, the MOMS trial has shown a significant reduction of VP shunt placement at one year of age following fetal MMC surgery (prenatal group: 40% vs. postnatal group: 82%, P<0.001). The trial also demonstrated a substantial improvement in the overall neuromotor function at 30 months of age by a variety of measures including the finding that 42% in the fetal surgery group were walking independently compared to only 21% in the postnatal surgery group (P<0.01). Finally, hindbrain herniation was significantly reversed in the fetal surgery group compared to the postnatal surgery group (no hindbrain herniation in 36% and 4% of the infants, respectively, and severe herniation in 6% and 22%, respectively, P<0.001).
Despite these promising results, the MOMS trial also revealed that fetal MMC surgery increases the risks for spontaneous rupture of membranes (prenatal surgery: 46% vs. postnatal surgery: 8%, P<0.001), oligohydramnios (21% vs. 4%, P=0.001), and preterm delivery (79% vs. 15%, P<0.001) including 13% of fetal surgery group that were born before 30 weeks of gestation. The average gestational age at delivery in the fetal surgery group was 34.1 weeks gestation compared to 37.3 weeks in the postnatal surgery group. At the time of delivery, approximately one-quarter of mothers in the fetal surgery group showed evidence of thinning of the uterine wound, and 10% showed variable degrees of dehiscence at the hysterotomy site, but none had a hysterotomy rupture.
The MOMS Trial elucidated the benefits and risks of fetal MMC repair. The mother carrying a fetus with MMC at less than 24 weeks gestation now has three choices: termination of the pregnancy, continuation of the pregnancy with near-term cesarean section and postnatal repair, or prenatal surgery. At CHOP, prenatal surgery for MMC is a new standard of care option for these families if the mother and fetus meet inclusion and exclusion criteria (Table 1), and if the family chooses fetal surgery.
Future Studies
Future improvements in fetal MMC surgery will depend on a number of factors. First, the results of the non-randomized and randomized studies regarding prenatal therapy for MMC are less than perfect, and it is clear that prenatal surgery is not a cure for MMC. Despite fetal closure, 40% still required shunting, and not all had improved neuromotor function or complete reversal of hindbrain herniation. Because the trial was closed early due to the efficacy of fetal surgery, complete follow-up of the entire 183 patient MOMS trial cohort at 12 and 30 months of age is important, and prenatal anatomic predictors of outcome need to be delineated. Completion of the MOMS Trial dataset should help answer many questions. Does fetal ventricular size >20 mm increase the likelihood of a postnatal shunt even after prenatal surgery? What impact does prenatally diagnosed bilateral or unilateral talipes have on postnatal motor function at 30 months? How accurate is prenatal ultrasound compared to postnatal X-ray or MRI in predicting the anatomic level of the MMC? What are the urologic findings in the two groups? What is the effect of prenatal surgery compared to postnatal surgery on health care costs and on maternal morbidity, future reproductive capacity, and psychology? Long-term follow-up is crucial to assess the durability of the initial benefits, and the NIH has funded a followup study of the MOMS trial patients at 5-9 years of age.
Second, the results of our studies cannot be generalized to patients that either undergo fetal MMC surgery at less experienced centers or have fetal surgery outside the eligibility criteria set forth by the MOMS Trial (Table 1). Outcomes may be less favorable than those in the trial, and maternal and fetal complications may be greater as part of the recognized “learning curve” at new centers. For patient safety and optimal outcome, fetal MMC surgery should be limited to high-volume fetal surgery centers with a committed multidisciplinary team of experts following a standardized patient care protocol.
Finally, the timing and technique of fetal MMC surgery needs to be optimized. The development of minimally invasive approaches for fetal MMC surgery may not only minimize preterm labor and delivery, but may also permit prenatal coverage of the lesion much earlier in gestation. We evaluated gelatin-hydrogel based scaffolds embedded with growth factors for early gestation prenatal coverage of MMC in fetal rats with RA-induced MMC and showed that these scaffolds adhere to the MMC and subsequently promote tissue coverage over the defect.[63] This study supports the therapeutic potential of a tissue engineering approach for prenatal MMC coverage, perhaps by introducing these tissue engineered components through a fetoscope or through an amniocentesis needle under ultrasound guidance. Such coverage must be completely “water tight” to prevent the leakage of CSF through the MMC defect that leads to hindbrain herniation, and to prevent amniotic fluid exposure which damages the neural tissues in the MMC defect. Rigorous experimental testing and comparisons with open fetal MMC surgery techniques will be required in an effort to decrease the risks to the mother and fetus and to improve outcomes.
Acknowledgments
The Management of Myelomeningocele Study (MOMS) is supported by a National Institutes of Health U10 grant. Studies at CHOP are supported in part by the Michael and Katherine Mulligan Research Fund. I would like to acknowledge the members of our group in Philadelphia: Lori Howell, Mark Johnson, Leslie Sutton, Alan Flake, Holly Hedrick, Michael Bebbington, Julie Moldenhauer, Nahla Khalek, Jack Rychik, Natalie Rintoul, Michael Carr, Enrico Danzer, David Cohen, Kha Tran, Lynne Maxwell, Jeffrey Feldman, Beverly Coleman, Steven Horii, Ann Johnson, Teresa Victoria, Patrick Pasquariello, Larissa Bilaniuk, Deborah Zarnow, Jamie Koh, Stefanie Kasperski, Susan Spinner, Sue Miesnik, Jane Wright, Susan Scully, Martha Hudson and Elizabeth Rozovsky.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mitchell LE, Adzick NS, Melchionne J, et al. Spina bifida. Lancet. 2004;364:1885–1895. doi: 10.1016/S0140-6736(04)17445-X. [DOI] [PubMed] [Google Scholar]
- 2.Botto LD, Moore CA, Khoury MJ, et al. Neural-tube defects. N Engl J Med. 1999;341:1509–1519. doi: 10.1056/NEJM199911113412006. [DOI] [PubMed] [Google Scholar]
- 3.Medical Research Council Vitamin Research Study Group. Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- 4.CDC. Knowledge and use of folic acid by women of childbearing age-United States, 1995 and 1998. MMWR Morb Mortal Wkly Rep. 1999;48:325–327. [PubMed] [Google Scholar]
- 5.Edmonds LD, James LM. Temporal trends in the prevalence of congenital malformations at birth based on the birth defects monitoring program, United States, 1979-1987. MMWR Morb Mortal Wkly Rep CDC Surveill Summ. 1990;39:19–23. [PubMed] [Google Scholar]
- 6.Lary JM, Edmonds LD. Prevalence of spina bifida at birth--United States, 1983-1990: a comparison of two surveillance systems. MMWR Morb Mortal Wkly Rep CDC Surveil Summ. 1996;45:15–26. [PubMed] [Google Scholar]
- 7.Shaw GM, Jensvold NG, Wasserman CR, et al. Epidemiologic characteristics of phenotypically distinct neural tube defects among 0.7 million California births, 1983-1987. Teratology. 1994;49:143–149. doi: 10.1002/tera.1420490210. [DOI] [PubMed] [Google Scholar]
- 8.Roberts HE, Moore CA, Cragan JD, et al. Impact of prenatal diagnosis on the birth prevalence of neural tube defects , Atlanta, 1990-1991. Pediatrics. 1995;96:880–883. [PubMed] [Google Scholar]
- 9.Velie EM, Shaw GM. Impact of prenatal diagnosis and elective termination on prevalence and risk estimates of neural tube defects in California, 1989-1991. Am J Epidemiol. 1996;144:473–479. doi: 10.1093/oxfordjournals.aje.a008953. [DOI] [PubMed] [Google Scholar]
- 10.Luthy DA, Wardinsky T, Shurtleff DB, et al. Cesarean section before the onset of labor and subsequent motor function in infants with myelomeningocele diagnosed antenatally. N Engl J Med. 1991;324:662–666. doi: 10.1056/NEJM199103073241004. [DOI] [PubMed] [Google Scholar]
- 11.Caldarelli M, DiRocco C, LaMarca F. Shunt complications in the first postoperative year in children with meningomyelocele. Childs Nerv Syst. 1996;12:748–754. doi: 10.1007/BF00261592. [DOI] [PubMed] [Google Scholar]
- 12.Oakeshott P, Hunt GM. Long-term outcome in open spina bifida. Br J Gen Pract. 2003;53:632–636. [PMC free article] [PubMed] [Google Scholar]
- 13.Oaks W, Gaskill S. Symptomatic Chiari malformations in childhood. In: Park T, editor. Spinal Dysraphism. Boston: Blackwell Scientific Publications, Inc; 1992. pp. 104–125. [Google Scholar]
- 14.Hunt GM. Open spina bifida: outcome for a complete cohort treated unselectively and followed into adulthood. Devel Med Child Neurol. 1990;32:108–188. doi: 10.1111/j.1469-8749.1990.tb16910.x. [DOI] [PubMed] [Google Scholar]
- 15.Waitzman NJ, Romano PS, Scheffler RM. Estimates of the economic costs of birth defects. Inquiry. 1994;31:188–205. [PubMed] [Google Scholar]
- 16.Hutchins GM, McGowan KD, Blakemore KJ. Spinal dysraphia: Not a neural tube defect? Am J Hum Genet. 1992;51:A319. [Google Scholar]
- 17.Hutchins GM, Meuli M, Meuli-Simmen C, et al. Acquired spinal cord injury in human fetuses with myelomeningocele. Pediatr Pathol Lab Med. 1996;16:701–712. [PubMed] [Google Scholar]
- 18.Korenromp MJ, Van Good JD, Bruinese HW, et al. Early fetal movements in myelomeningocele. Lancet. 1986;1:917–918. doi: 10.1016/s0140-6736(86)91022-6. [DOI] [PubMed] [Google Scholar]
- 19.Sival DA, Begeer JH, Staal-Schreinemachers AL, et al. Perinatal motor behaviour and neurological outcome in spina bifida aperta. Early Hum Develop. 1997;50:27–37. doi: 10.1016/s0378-3782(97)00090-x. [DOI] [PubMed] [Google Scholar]
- 20.Pang D, Dias MS. Cervical myelomeningoceles. Neurosurgery. 1993;33:363–372. doi: 10.1227/00006123-199309000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Sutton LN. Lipomyelomeningocele. Neurosurg Clin North Am. 1995;6:325–338. [PubMed] [Google Scholar]
- 22.Duckworth T, Sharrard WJ, Lister J, et al. Hemimyelocele. Dev Med Child Neurol. 1968;10:69–75. doi: 10.1111/j.1469-8749.1968.tb04849.x. [DOI] [PubMed] [Google Scholar]
- 23.Michejda M. Intrauterine treatment of spina bifida. Primate model. Z Kinderchir. 1984;39:259–261. doi: 10.1055/s-2008-1044221. [DOI] [PubMed] [Google Scholar]
- 24.Heffez DS, Aryanpur J, Rotellini NA, et al. Intrauterine repair of experimental surgically created spina bifida. Neurosurg. 1993;32:1005–1010. doi: 10.1227/00006123-199306000-00021. [DOI] [PubMed] [Google Scholar]
- 25.Heffez DS, Aryanpur J, Hutchins GM, et al. The paralysis associated with myelomeningocele: clinical and experimental data implicating a preventable spinal cord injury. Neurosurg. 1990;26:987–992. [PubMed] [Google Scholar]
- 26.Steifel D, Copp AJ, Meuli M. Fetal spina bifida in a mouse model: loss of neural function in utero. J Neurosurg. 2007;106:213–221. doi: 10.3171/ped.2007.106.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stiefel D, Meuli M. Scanning electron microscopy of fetal murine myelomeningocele reveals growth and development of the spinal cord in early gestation and neural tissue destruction around birth. J Pediatr Surg. 2007;42:1561–1565. doi: 10.1016/j.jpedsurg.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 28.Meuli M, Meuli-Simmen C, Yingling CD, et al. Creation of myelomeningocele in utero: a model of functional damage from spinal cord exposure in fetal sheep. J Pediatr Surg. 1995;30:1028–1032. doi: 10.1016/0022-3468(95)90335-6. [DOI] [PubMed] [Google Scholar]
- 29.Meuli M, Meuli-Simmen C, Yingling CD, et al. In utero repair of experimental myelomeningocele spares neurologic function at birth. J Pediatr Surg. 1996;31:397–340. doi: 10.1016/s0022-3468(96)90746-0. [DOI] [PubMed] [Google Scholar]
- 30.Meuli M, Meuli-Simmen C, Hutchins GM, et al. In utero surgery rescues neurologic function at birth in sheep with spina bifida. Nature Medicine. 1995;1:342–347. doi: 10.1038/nm0495-342. [DOI] [PubMed] [Google Scholar]
- 31.Meuli-Simmen C, Meuli M, Hutchins GM, et al. Fetal reconstructive surgery: Experimental use of latissimus dorsi flap to correct myelomeningocele in utero. Plast Reconstr Surg. 1995;96:1007–1011. [PubMed] [Google Scholar]
- 32.Meuli M, Meuli-Simmen C, Hutchins GM, et al. The spinal cord lesion in human fetuses with myelomeningocele: Implications for fetal surgery. J Pediatr Surg. 1997;32:448–452. doi: 10.1016/s0022-3468(97)90603-5. [DOI] [PubMed] [Google Scholar]
- 33.Paek BW, Farmer DL, Wilkinson CC, et al. Hindbrain herniation develops in surgically created myelomeningocele but is absent after repair in fetal lambs. Am J Obstet Gynecol. 2000;183:1119–1123. doi: 10.1067/mob.2000.108867. [DOI] [PubMed] [Google Scholar]
- 34.Bouchard S, Davey MG, Rintoul NE, et al. Correction of hindbrain herniation and anatomy of the vermis after in utero repair of myelomeningocele in sheep. J Pediatr Surg. 2003;38:451–458. doi: 10.1053/jpsu.2003.50078. [DOI] [PubMed] [Google Scholar]
- 35.Danzer E, Schwarz U, Wehrli S, et al. Retinoic acid induced myelomeningocele in fetal rats: characterization by histopathological analysis and magnetic resonance imaging. Exp Neurol. 2005;194:467–475. doi: 10.1016/j.expneurol.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 36.Danzer E, Kiddoo DA, Redden RA, et al. Structural and functional characterization of bladder smooth muscle in fetal rats with retinoic acid-induced myelomeningocele. Am J Physiol Renal Physiol. 2007;292:F197–206. doi: 10.1152/ajprenal.00001.2006. [DOI] [PubMed] [Google Scholar]
- 37.Danzer E, Radu A, Robinson LE, et al. Morphologic analysis of the neuromuscular development of the anorectal unit in fetal rats with retinoic acid induced myelomeningocele. Neurosci Lett. 2008;430:157–162. doi: 10.1016/j.neulet.2007.10.048. [DOI] [PubMed] [Google Scholar]
- 38.Danzer E, Zhang L, Radu A, et al. Amniotic fluid levels of glial fibrillary acidic protein in fetal rats with retinoic acid induced myelomeningocele: a potential marker for spinal cord injury. Am J Obstet Gynecol. 2011;204:e1–11. doi: 10.1016/j.ajog.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 39.Adzick NS, Harrison MR. Fetal surgical therapy. Lancet. 1994;343:897–902. doi: 10.1016/s0140-6736(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 40.Cochrane DD, Wilson RD, Steinbok P, et al. Prenatal spinal evaluation and functional outcome of patients born with myelomeningocele: information for improved prenatal counselling and outcome prediction. Fetal Diag Ther. 1996;11:159–168. doi: 10.1159/000264297. [DOI] [PubMed] [Google Scholar]
- 41.Quinn TM, Hubbard AM, Adzick NS. Prenatal magnetic resonance imaging enhances fetal diagnosis. J Pediatr Surg. 1998;33:553–558. doi: 10.1016/s0022-3468(98)90315-3. [DOI] [PubMed] [Google Scholar]
- 42.Babcook CJ, Goldstein RB, Barth RA, et al. Prevalence of ventriculomegaly in association with myelomeningocele: Correlation with gestational age and severity of posterior fossa deformity. Radiol. 1994;190:703–707. doi: 10.1148/radiology.190.3.8115615. [DOI] [PubMed] [Google Scholar]
- 43.Adzick NS, Sutton LN, Crombleholme TM, Flake AW. Successful fetal surgery for spina bifida. Lancet. 1998;352:1675–1676. doi: 10.1016/S0140-6736(98)00070-1. [DOI] [PubMed] [Google Scholar]
- 44.Sutton LN, Adzick NS, Bilaniuk LT, et al. Improvement in hindbrain herniation by serial fetal MRI following fetal surgery for myelomeningocele. JAMA. 1999;282:1826–1831. doi: 10.1001/jama.282.19.1826. [DOI] [PubMed] [Google Scholar]
- 45.Bruner JP, Tulipan N, Paschall RL, et al. Intrauterine repair of myelomeningocele, ‘hindbrain restoration’ and the incidence of shunt-dependent hydrocephalus. JAMA. 1999;282:1819–1825. doi: 10.1001/jama.282.19.1819. [DOI] [PubMed] [Google Scholar]
- 46.Tulipan N, Sutton LN, Bruner JP, et al. The effect of intrauterine myelomeningocele repair on the incidence of shunt-dependent hydrocephalus. Pediatr Neurosurg. 2003;38:27–33. doi: 10.1159/000067560. [DOI] [PubMed] [Google Scholar]
- 47.Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364:993–1004. doi: 10.1056/NEJMoa1014379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rychik J, Tian Z, Cohen MS, et al. Acute cardiovascular effects of fetal surgery in the human. Circulation. 2004;21:1549–1556. doi: 10.1161/01.CIR.0000142294.95388.C4. [DOI] [PubMed] [Google Scholar]
- 49.Johnson MP, Adzick NS, Rintoul N, et al. Fetal myelomeningocele repair: Short-term clinical outcomes. Am J Ob Gyn. 2003;189:482–487. doi: 10.1067/s0002-9378(03)00295-3. [DOI] [PubMed] [Google Scholar]
- 50.Rintoul NE, Sutton LN, Hubbard AM, et al. A new look at myelomeningoceles: Functional level, vertebral level, shunting, and the implications for fetal intervention. Pediatrics. 2002;109:409–413. doi: 10.1542/peds.109.3.409. [DOI] [PubMed] [Google Scholar]
- 51.Danzer E, Johnson MP, Bebbington M, et al. Fetal head biometry assessed by fetal magnetic resonance imaging following in utero myelomeningocele repair. Fetal Diagn Ther. 2007;22:1–6. doi: 10.1159/000095833. [DOI] [PubMed] [Google Scholar]
- 52.Danzer E, Finkel RS, Rintoul NE, et al. Reversal of hindbrain herniation after fetal surgery for myelomeningocele subsequently reduces the incidence and severity of brain-stem dysfunction and cranial nerve compression. Neuropediatrics. 2010;41:140–143. [Google Scholar]
- 53.Danzer E, Gerdes M, Bebbington M, et al. Lower extremity neuromotor function and short-term ambulatory potential following in utero myelomeningocele surgery. Fetal Diagn Ther. 2009;25:47–53. doi: 10.1159/000197359. [DOI] [PubMed] [Google Scholar]
- 54.Danzer E, Adzick NS, Rintoul NE, et al. Intraductal inclusion cysts following in utero closure of myelomeningocele: Clinical implications and followup findings. J Neurosurg Pediatrics. 2008;6:406–413. doi: 10.3171/PED.2008.2.12.406. [DOI] [PubMed] [Google Scholar]
- 55.Johnson MP, Gerdes M, Rintoul NE, et al. Fetal surgery for myelomeningocele: Neurodevelopmental outcomes at 2 years of age. Am J Ob Gyn. 2006;194:1145–1150. doi: 10.1016/j.ajog.2006.01.072. [DOI] [PubMed] [Google Scholar]
- 56.Danzer E, Gerdes M, Zarnow DM, et al. Preschool neurodevelopmental outcome of children following fetal myelomeningocele closure. Am J Ob Gyn. 2010;202:e1–9. doi: 10.1016/j.ajog.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 57.Wilson RD, Johnson MP, Flake AW, et al. Maternal reproductive outcomes in pregnancies following an open fetal surgery pregnancy. Am J Ob Gyn. 2004;191:1430–1436. doi: 10.1016/j.ajog.2004.05.054. [DOI] [PubMed] [Google Scholar]
- 58.Wilson RD, Kamerand K, Johnson MP, et al. Reproductive outcomes in subsequent pregnancies after a pregnancy complicated by maternal-fetal surgery. Am J Ob Gyn. 2010;203:e1–6. doi: 10.1016/j.ajog.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 59.Bruner JP, Tulipan NB, Richards WO. Endoscopic coverage of fetal open myelomeningocele in utero. Am J Obstet Gynecol. 1997;176:256–257. doi: 10.1016/s0002-9378(97)80050-6. [DOI] [PubMed] [Google Scholar]
- 60.Farmer DL, von Koch CS, Peacock WJ, et al. In utero repair of myelomeningocele: Experimental pathophysiology, initial clinical experience, and outcomes. Arch Surg. 2003;138:872–878. doi: 10.1001/archsurg.138.8.872. [DOI] [PubMed] [Google Scholar]
- 61.Kohl T, Gembruch U. Current status and prospects of fetoscopic surgery for spina bifida in human fetuses. Fetal Diagn Ther. 2008;24:318–320. doi: 10.1159/000158549. [DOI] [PubMed] [Google Scholar]
- 62.Verbeek R, Heep A, Maurits N, et al. Does fetal endoscopic closure of the myelomeningocele prevent loss of neurologic function in spina bifida aperta? Cerebrospinal Fluid Res. 2010;7(Suppl 1):S18. [Google Scholar]
- 63.Watanabe M, Jo J, Radu A, et al. A tissue engineering approach for prenatal closure of myelomeningocele with gelatin sponges incorporating basic fibroblast growth factor. Tissue Eng Part A. 2010;16:1645–1646. doi: 10.1089/ten.TEA.2009.0532. [DOI] [PubMed] [Google Scholar]


