Summary
Adipose tissue expansion involves the enlargement of existing adipocytes, the formation of new cells from committed preadipocytes, and the coordinated development of the tissue vascular network. Here we find that murine endothelial cells (EC) of classic white and brown fat depots share ultrastructural characteristics with pericytes, which are pluripotent and can potentially give rise to preadipocytes. Lineage tracing experiments using the VE-cadherin promoter reveal localization of reporter genes in EC, and also in preadipocytes and adipocytes of white and brown fat depots. Furthermore, capillary sprouts from human adipose tissue, which have predominantly EC characteristics, are found to express Zfp423, a recently identified marker of preadipocyte determination. In response to PPARγ activation, endothelial characteristics of sprouting cells are progressively lost, and cells form structurally and biochemically defined adipocytes. Together these data support an endothelial origin of murine and human adipocytes, suggesting a model for how adipogenesis and angiogenesis are coordinated during adipose tissue expansion.
Keywords: adipose stem cells, preadipocytes, adipogenesis, white adipose tissue, electron microscopy, in vivo lineage tracing, adipose tissue explants
Introduction
The obesity epidemic associated with increased risk of type 2 diabetes have underscored the need to understand the relationship between excess caloric intake, white adipose tissue (WAT) development, and metabolic disease. Mammals, including humans, also have metabolically active brown adipose tissue (BAT) (Cypess et al., 2009; van Marken Lichtenbelt et al., 2009; Virtanen et al., 2009), and BAT precursors can be found in adipose organ of adult humans (Zingaretti et al., 2009). BAT has anti-obesity properties, therefore the mechanisms by which brown adipocytes emerge under different physiological and pharmacological conditions is under intensive investigation.
Adipose tissue growth is mediated by adipocyte hypertrophy, but in obesity adipose tissue may reach 60–70% of body weight, requiring hyperplasic growth (Prins and O'Rahilly, 1997; Hausman et al., 2001). Therefore, defining the identity of adipocyte precursors is an area of intense interest. Markers of precursor cells giving rise to committed preadipocytes are being identified (Gupta et al., 2010), and a population of early adipocyte progenitor cells expressing stem cell markers has been characterized in mouse WAT (Rodeheffer et al., 2008). Furthermore, PPARγ lineage tracing studies indicate that the vascular wall of adipose tissue capillaries represents the niche of adipocyte precursors (Tang et al., 2008).
Adipose tissue growth requires concomitant expansion of its capillary network (Hausman and Richardson, 2004; Christiaens and Lijnen, 2010), but how adipocyte formation and capillary expansion are coordinated is unclear. The stromal-vascular fraction (SVF) cells of adipose tissue differentiate in vitro into either an adipogenic or a perivascular phenotype (Cinti et al., 1984; Gregoire, 2001), but the identity of these progenitor cells in vivo is unknown (Gesta et al., 2007). Since cells morphological features are closely related to their function, a careful ultrastructural analysis during development could provide information on the origin and anatomical localization of adipose tissue precursors.
VE-cadherin is required for the formation of vasculature, and is expressed specifically in endothelial cells (EC) of fetal and adult mice. VE-cadherin-Cre-dependent LacZ and eGFP reporter strains show localization of the reporters in EC of many tissues (Alva et al., 2006; Monvoisin et al., 2006; Zovein et al., 2008; Speck and Iruela-Arispe, 2009). However, VE-cadherin is also expressed in sub-populations of hematopoietic cells before E11.5, and thus their descendants are potentially labelled into adulthood (Alva et al., 2006; Monvoisin et al., 2006; Zovein et al., 2008). To circumvent this problem, mice in which VE-cadherin-driven Cre is induced during adulthood have also been created, resulting in negligible excision (lower than 0.4%) in the hematopoietic lineage (Monvoisin et al., 2006). Lineage tracing with constitutive and inducible VE-cadherin driven Cre can provide information on the relationship between vascular and adipose cell development by allowing the identification of cells that express this EC gene at any point during embryonic and postnatal periods.
An additional tool to define the relationships between newly formed vasculature and the genesis of new adipocytes is the use of mouse and human adipose tissue explants cultured ex vivo (Greenway et al., 2007; Gealekman et al., 2011). In this manuscript, we have used these morphological, genetic and functional approaches, and obtained evidence that EC of capillaries in developing WAT and BAT depots can give rise to mature adipocytes. Our findings will enable further studies of the biochemical and physiological cues controlling adipose tissue growth.
Results and Discussion
Ultrastructural identification of endothelial-pericytic cells as possible intermediate between EC and preadipocytes
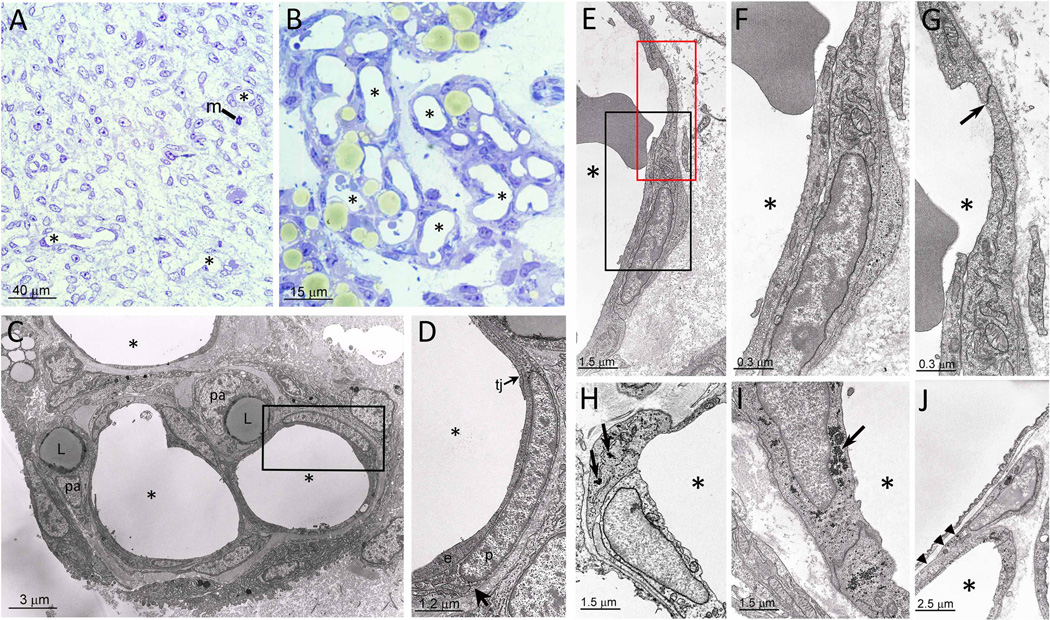
Before birth, rat (Figure 1A) and mouse (not illustrated) epididymal fat (eWAT) show the typical features of a poorly differentiated mesenchymal tissue, consisting of a homogeneous population of fibroblast-like cells, often in mitosis (Figure 1A, m), in a loose connective matrix with small and sparse capillaries (Figure 1A, asterisks). In contrast, at postnatal day 6–8 (P6–8) (Figure 1B), well-circumscribed areas with majority of cells identifiable as adipocytes due to the predominantly unilocular cytoplasmic lipid droplets (Figure 1B, yellowish colour) are observed. These areas are delimited by fibroblast-like cells, and contain numerous large capillaries (Figure 1B, asterisks). These are about 3-fold larger than capillaries found in eWAT of adult animals, suggesting they have functions additional to oxygen and nutrient exchange. Adipogenesis appears to be restricted into these vasculo-adipocytic islets. Electron microscopy of these islets reveals dense collagen fibrils in the interstitial matrix, and thick capillary walls due to the presence of abundant pericytes (Figure 1C and D). Most of the cells located in the peri-capillary position of the vasculo-adipocytic islets, correspond to the cells described above by EM, and show nuclear staining for transcription factors widely considered as markers of adipogenic conversion, such as PPARγ, C/EBPα and C/EBPβ (Supplemental Figure 1A–C). These results are consistent with studies suggesting that pericytes are precursors of preadipocytes (Iyama et al., 1979; Tang et al., 2008). Some EC are also positive for C/EBPβ (Supplemental Figure1D, arrowhead), which is located upstream of PPARγ and C/EBPα in transcriptional control of adipogenesis (Farmer, 2006).
Figure 1. Murine embryonic and postnatal eWAT morphology.
A. eWAT depot at E18 composed of poorly differentiated mesenchymal cells (m = mitosis; some capillaries indicated with asterisk). B. eWAT at P7, where adipocytes appear yellowish in areas with abundant large capillaries (asterisks). C,D. EM micrograph of a vasculo-adipocytic islet showing EC (e; elongated cells), tight junctions (tj), pericytes (p; poorly differentiated cells with glycogen granules and surrounded by a distinct basal membrane), preadipocytes (pa; cells with small lipid droplets, (L)), and glycogen granules in pericyte (arrow). E. EC and pericytes of a capillary wall (asterisk indicates the capillary lumen). F. Enlargement of the black squared area in E revealing EC in endothelial-pericytic position. G. Enlargement of the red squared area in E highlighting typical oblique tight junction (arrow) joining cell in endothelial-pericytic position to adjacent EC. H. Example of cell in endothelial-pericytic position containing abundant glycogen (arrows). I. Example of a "pure" EC containing abundant glycogen (arrow). J. Example of a cell partially associated with the capillary wall (arrowheads) and partially abutting into the interstitial space.
In about 1–3% of vasculo-adipocytic islet capillaries, EC exhibited novel, unusual features by being exposed to the capillary lumen, but also extended over a vicinal EC to adopt a pericytic position (Figure 1E–G, endothelial-pericytic cells). Importantly, the junction of these cells with adjacent EC was composed of typical oblique tight junctions (Figure 1G, arrow), confirming the endothelial nature of these cells. EC and pericytes were sometimes joined by tight junctions between a protrusion of the EC crossing the basal membrane, and the complementary indentation in the pericyte (Supplemental Figure 1E). Some of the EC and endothelial-pericytic cells contain glycogen granules (Figure 1H–J, arrows), a characteristic feature of adipocyte progenitors (Tavassoli, 1976). Almost all pericytes (Figure 1H), some EC (Figure 1I, arrow), and cells that are partially associated with the capillary wall and also abut into the interstitial space (Figure 1J) also contain glycogen particles. These data reveal a complex relationship between cells in the vasculo-adipocytic islets where extensive adipogenesis is ongoing, and suggest that endothelial-pericytic cells represent an intermediate between endothelial and preadipocyte stages (drawing in Supplemental Figure 1F).
Lineage tracing studies show an endothelial origin of adipocytes
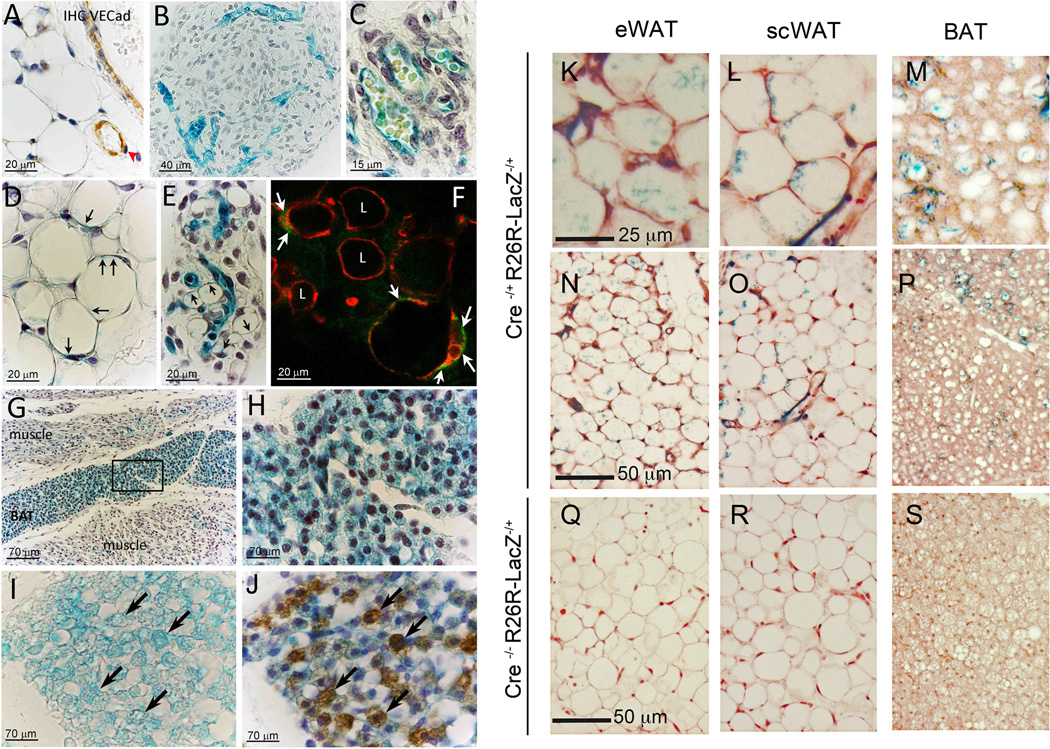
Data shown above suggest that EC may be amongst the cells that give rise to new adipocytes. To address this hypothesis, we performed VE-cadherin promoter-driven lineage tracing experiments. VE-cadherin was expressed only in EC, and not in pericytes (red arrowhead) or adipocytes of subcutaneous (scWAT) (Figure 2A) and eWAT (data not shown) of adult animals. Similarly, prior to adipocyte development in fetal and early postnatal eWAT (Figure 2B, C) and scWAT (not shown) of VE-cadherin-Cre/R26R-LacZ mice, only EC were X-gal positive. In contrast, from P6–8 (Figure 2D, E) to adult (Supplemental Figure 2A), adipocytes at different stages of lipid accumulation in eWAT and scWAT were also X-gal positive, indicating that VE-cadherin promoter was expressed at some point during adipocyte development. Control animals were always X-gal negative (Supplemental Figure 2B). To verify that the reporter was localized to the adipocyte cytoplasm, we used EM and observed a precise localisation of X-gal crystals in EC, pericytes, adipocytes (Supplemental Figure 2C), and preadipocytes (Supplemental Figure 2D). Similar results were found using eGFP as a reporter; in developing eWAT adipocytes of VE-cadherin-Cre/R26R-eGFP mice, perilipin and eGFP colocalized in adipocytes (Figure 2F). Internal positive control (vessels), and other eGFP positive adipocytes are shown in Supplemental Figure 2E. These results further support the hypothesis that adipocytes can develop in vivo from cells of endothelial origin.
Figure 2. VE-Cadherin lineage tracing in WAT and BAT.
A. Immunohistochemical analysis on scWAT from VE-cadherin-Cre/R26Rmice showing the specific expression of VE-cadherin only in EC (brown); to note the negative pericytes indicated by red arrowhead. B, C. In early neonatal eWAT only vasculature is X-gal positive. D, E. scWAT (D) and eWAT (E) from P7 mice revealing X-gal staining (arrows) in developing and mature adipocytes. F. Confocal microscopy of eWAT from VE-cadherin-Cre/eGFPRmice showing a single optical plane of adipocytes (arrows), containing eGFP (green) and perilipin (red). Some eGFP-negative/perilipin-positive adipocytes are also visible (L). G. X-gal positive staining in developing BAT, and in muscle capillaries. H. Enlargement of the squared area in G. I, J. X-gal(I) and UCP-1 (J) colocalization to brown adipocytes (arrows). K-P. eWAT (K, N), scWAT (L, O), and BAT (M, P) adipose tissue from VE-cadherin-CreERT2−/+/R26R−/+ mice showing X-gal positive staining in adipocytes and EC. Q–S, Adipose tissues from VE-cadherin-CreERT2−/−/R26R−/+ control mice.
These data prompted us to investigate whether brown adipocytes might also have an endothelial origin. Immunohistochemistry for VE-cadherin confirmed its presence in EC and its absence in BAT pericytes and adipocytes (not illustrated). However, the interscapular region of E17–19 VE-cadherin-Cre/R26R-LacZ mice showed strong X-gal staining in both EC and adipocytes (Figure 2G, H), while in the surrounding muscles only EC were stained (Figure 2G). The X-gal staining colocalized with the classical brown adipocyte marker UCP-1 (Compare Figure 2I and J). Moreover, in the developing interscapular brown fat depot (Supplemental Figure 3A–B), some EC display characteristic markers (Supplemental Figure 3C–E) and structural features (glycogen and mitochondria) of UCP-1- positive brown adipocyte precursors (Supplemental Figure 3F–I). Taken together, these data strongly suggest that EC of developing WAT and BAT capillaries are a source of adipocyte precursors. This is consistent with the finding by Gupta et al. (Cell Metabolism, in press) that pericytes and some EC of mouse adipose tissue express GFP driven by the promoter for Zfp423, which marks cells determined to form preadipocytes (Gupta et al., 2010). Moreover, PPARγ excision with the use of the Tie-2 promoter driven Cre-recombinase, which is expressed in a mosaic pattern in capillary endothelium (Anghelina et al., 2005), results in a decrease in adiposity and adipocyte size in response to rosiglitazone treatment in a manner dependent on endothelial, but not bone-marrow, PPARγ expression (Kanda et al., 2009). These findings constitute further evidence supporting the possibility that certain EC populations can give rise to adipocytes.
A hematopoietic origin of adipocytes has been suggested (Crossno et al., 2006; Koh et al., 2007; Tomiyama et al., 2008). To exclude the possibility that X-gal positive adipocytes originate from hematopoietic lineage, we complemented our studies with a tamoxifen-inducible transgenic VE-cadherin-CreERT2 model in which cells derived from hematopoietic precursors are not labeled (Monvoisin et al., 2006). VE-cadherin-CreERT2/R26R-LacZ mice at eight weeks of age were induced by tamoxifen injection for five consecutive days. Three weeks later, X-gal staining was observed in capillaries of adipose tissue, but also in numerous adipocytes distributed among the eWAT (Figure 2K, N), inguinal (L, O), and brown (M, P) depots. Number of X-gal positive adipocytes was lower, as compared to the results described above, possibly due to a lower number of adipocytes being formed during this postnatal expansion period, but still clearly significantly above the background, observed in negative controls (Figure 2Q–S). PPARγ agonists affect murine adipose tissue by increasing multilocularization of existing adipocytes and by stimulating pre-adipocyte differentiation into mature adipocytes (Koh et al., 2009; Tang et al., 2011). Mice treated with rosiglitazone for three weeks following induction displayed numerous multilocular adipocytes, many of which were X-gal positive (Supplemental Figure 3J–L). Thus, under both normal and stimulated adipogenesis, adipocytes arise from VE-cadherin expressing progenitors that are not of hematopoietic origin.
A vascular origin for white and brown adipocytes may seem to be in contradiction with the work of Seale et al., in which a common origin of BAT and skeletal muscle cells (Seale et al., 2008) was proposed. In this work, the authors found expression of YFP in skeletal muscle and BAT from Myf5-Cre/R26R3-YFP mice, consistent with both these tissues being derived from a myogenic, Myf5 expressing progenitor. Interestingly, De Angelis et al. have shown the existence of progenitor cells from embryonic dorsal aorta that express both myogenic and endothelial markers including Myf5 and VE-cadherin (De Angelis et al., 1999). Thus, one explanation for the results of Seale et al., and ours is that BAT is derived from a population of VE-cadherin positive and Myf5 positive cells, while muscle can be derived from Myf5 only positive cells. Alternatively, as the Myf5-Cre mouse model labels multiple cell lineages from somite origin (Jinno et al., 2010), somite-derived cell progenitors that subsequently express VE-cadherin could in theory give rise to BAT. If the brown adipocyte and muscle cell fates were determined before the expression of VE-cadherin, lineage tracing experiments using the VE-cadherin-Cre would not label myocytes.
Human adipose tissue endothelial sprouts give rise to adipocytes
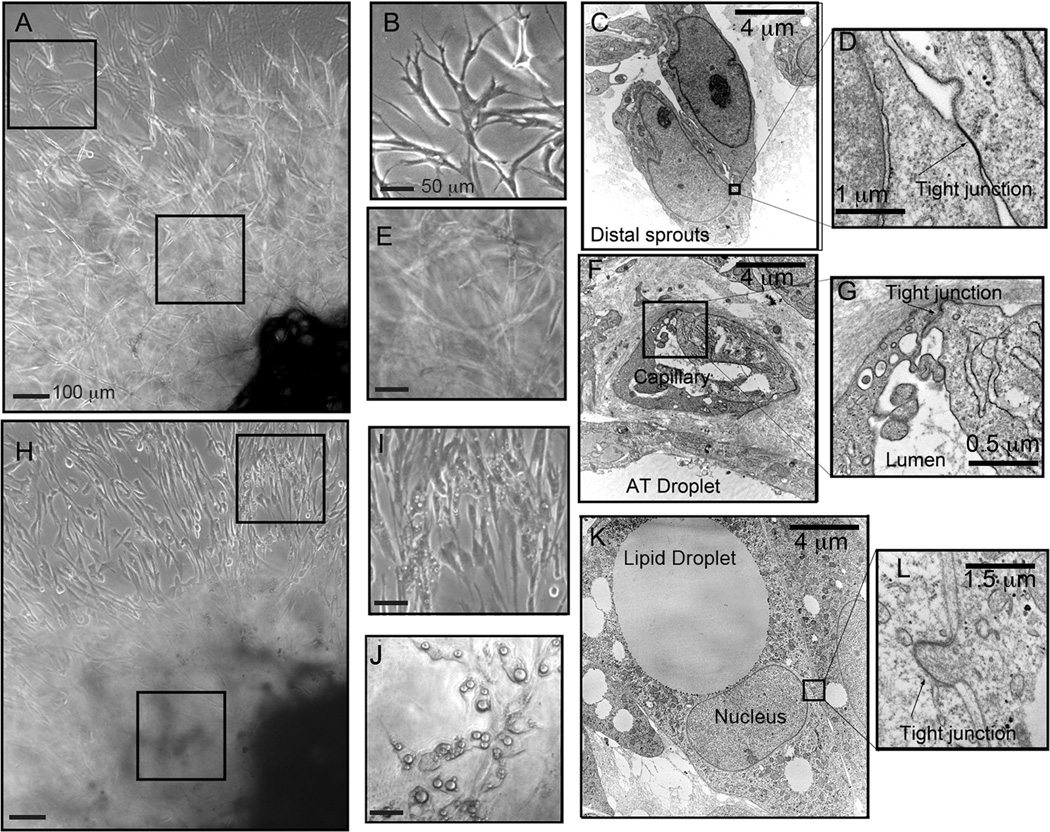
The finding that murine cells with endothelial characteristics can give rise to adipocytes prompted us to investigate whether human adipocytes share this origin. Fragments of human adipose tissue give rise to capillary sprouts when embedded in Matrigel, and cultured in pro-angiogenic media (Gealekman et al., 2011). Classical EC ultrastructural features, including tight junctions, irregular nuclei and pinocytic vesicles were seen in cells comprising these sprouts (Figure 3A–D). In regions more proximal to the tissue explant, capillary lumens could be observed (Figure 3E–G), indicating that angiogenic development is recapitulated ex vivo. Upon exposure to the PPARγ agonist rosiglitazone, many cells within the emerging capillary sprouts produced lipid droplets (Figure 3H–J). These cells displayed canonical ultrastructural features of white adipocytes, including a large lipid droplet, displaced nucleus, small and elongated mitochondria and glycogen particles distributed in the cytoplasm (Figure 3K). Interestingly, these adipocytes showed tight junctions identical to those found in sprouting EC, and between EC and pericytes in vivo (Figure 3L, compare with Supplemental Figure 1). These results suggest that the processes of capillary expansion and adipocyte formation seen in explants ex vivo reflect those occurring in the intact organism.
Figure 3. Effect of rosiglitazone on angiogenic sprouts originating from human adipose tissue.
A. Capillary outgrown after 15 days of culture in the absence of rosiglitazone, indicating areas distal and proximal to the embedded explants. B. Enlargement of area distal to the explants. C. Electron microscopy of area similar to that shown in B, where tight junctions can be seen to connect cells. D. Enlargement of tight junction found between two EC. E. Enlargement of area proximal to the explant. F. Electron microscopy of area similar to that shown in E, revealing lumenized capillaries formed by EC joined by tight junctions. G. Enlargement of area shown in F. H. Capillary outgrowth after 15 days in the presence of rosiglitazone. I. Enlargement of area distal to the explant, revealing lipid droplets in cells interspersed among the capillary sprouts. J. Area proximal to the explant containing cells harboring larger lipid droplets. K. Electron microscopy of lipid-laden cells revealing features of classical white adipocytes, and of EC such as the tight junction in the squared area. L. Enlargement of area outlined in K.
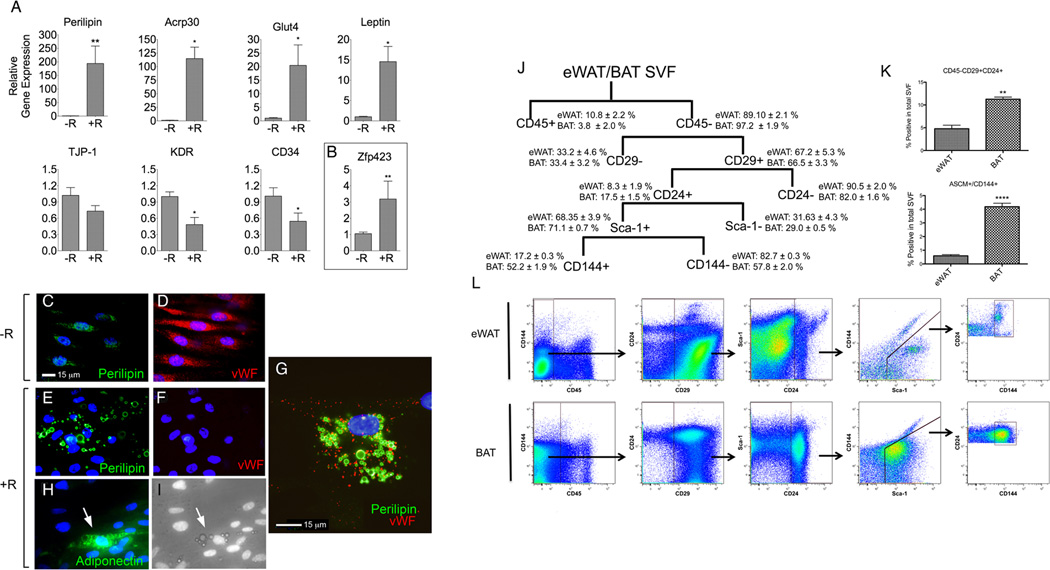
We then asked whether these morphologically defined adipocytes express genes that confer adipocyte function. Induction of mRNAs for Glut4, adiponectin, and leptin, and an >100-fold increase in the expression of perilipin mRNA were observed (Figure 4A, top row) in parallel with decreased expression of EC markers; tight junction protein (TJP-1), CD34, and KDR (Figure 4A, bottom row). In addition, the transcription factor Zfp423, considered a marker of adipocyte lineage pre-determination (Gupta et al., 2010), was detected and increased significantly in response to rosiglitazone (Figure 4B). The presence of this pre-determination marker, and the strong induction of genes that define adipocyte function, is consistent with the possibility that cells with endothelial characteristics can give rise to adipocytes in human adipose tissue.
Figure 4. Co-expression of endothelial and adipose cell genes in human and mouse systems.
A. Relative mRNA levels of canonical adipocyte (top row) or EC (bottom row) genes in capillary outgrowth from explants grown for 14 days in the absence (−R) or presence (+R) of rosiglitazone. B. Relative mRNA levels of the adipocyte pre-determination marker Zfp423. Plotted are the means and SEM of 6–8 independent experiments. Statistical significance was assessed by non-paired, two tailed student t-test *=p<0.05; **=p< 0.001; C, D. In absence of rosiglitazone cells growing from human adipose explants are mostlyperilipin-negative (green) and vWF-positive (red). E–G. In the presence of rosiglitazone, perilipin coating around lipid droplets is present (E), with majority of cells becoming vWF-negative (F), and about 5% of cells co-expressing perilipin (green) and vWF (red) (G). H, I. Cells growing from adipose explants, that accumulate lipid droplets in the presence of rosiglitazone (I) are also adiponectin-positive (H). J. FACS analysis scheme of cells from SVF of eWAT and BAT. K. Comparison of CD45-CD29+CD24+Sca1+ population and CD45-CD29+CD24+Sca1+CD144+ (ASCM+) between depots. L. Gating strategy for experiments. Plotted in graphs (K) are the means and SEM of 3–4 independent experiments. Data were analyzed using two-tailed student t-tests, **p< 0.005 and *p < 0.00005. Fluorescence-minus-one antibody controls are in Supplemental Figure S4.
To further examine the relationship between endothelial and adipocyte markers at a single cell level, we performed immunofluorescence analysis. In the absence of rosiglitazone, most cells contained low or undetectable levels of perilipin (Figure 4C), and high levels of von-Willebrand Factor (vWF) (Figure 4D). In the presence of rosiglitazone, majority of cells became vWF-negative (Figure 4F), but exhibited typical morphological feature of adipocytes, a perilipin-coated lipid droplets (Figure 4E). Nevertheless, approximately 5% of cells containing perilipin-coated lipid droplets also contained vWF (Figure 4G). Similar cells containing lipid droplets were also found to express adiponectin (Figure 4H and I), another specific feature of mature adipocytes. These results are consistent with the hypothesis that adipocytes arise from EC, or precursors expressing EC features. In line with these observations, recent findings indicate that EC can be converted into mesenchymal stem cells, which can differentiate into adipocytes, chondrocytes and osteoblasts (Medici et al., 2010). Furthermore, others have found that adipocytes have the potential to rapidly acquire an endothelial phenotype in vitro (Planat-Benard et al., 2004), raising the possibility that adipocytes and EC are plastic enough to undergo inter-conversion to maintain a homeostatic equilibrium during adipose tissue expansion and reduction.
Expression of VE-cadherin in adipocyte progenitors characterized by stem-cell markers
To further examine the possibility that adipocytes and EC derived from each other or share a common precursor, we asked whether cells expressing adipocyte stem cell markers (Rodeheffer et al., 2008), were also positive for VE-cadherin (CD144). CD45- cells from the SVF were successively gated for the presence of CD29, CD24 and Sca1 (Figure 4H). Approximately 5% and 10% of the cells in the SVF of eWAT and BAT, respectively, were CD45-CD29+CDC24+, and this difference was statistically significant (Figure 4I, upper panel). Approximately 17% and 52% of the CD45-CD29+CDC24+Sca-1+ population in eWAT and BAT, respectively, were also positive for VE-cadherin (CD144) (Figure 4H), comprising 0.5% and 4% of the cells in the SVF of eWAT and BAT, respectively (Figure 4I, lower panel). The percentages of adipogenic stem cells expressing VE-cadherin is in line with the amount of endothelial-pericytic cells found in WAT by EM studies in vivo and the much larger proportion of VE-cadherin positive cells in BAT is consistent with its denser vascular network. These data support the hypothesis of an endothelial origin of adipocyte populations in these depots.
In summary, we present morphological and genetic evidence that adipocytes in white and brown depots originate from cells that display endothelial characteristics. Further research to identify the physiological signals that determine adipocyte and/or EC fates will lead to a better understanding of the mechanisms responsible for coordinating the formation of new adipocytes with angiogenic expansion during adipose tissue growth.
Experimental Procedures
Animals
Male Sprague Dawley rats and B6 Mice (Harlan, Udine, Italy) were studied at different developmental stages. At least 5 animals per group were used for morphological studies performed by light, confocal, electron microscopy and immunohistochemistry. Homozygous VE-cadherin (VE-cadherin-Cre-recombinase) transgenic mice were crossed to homozygous ROSA26R (R26R) reporter and ROSA-eGFPR lines (Jackson Labs., Bar Harbor, ME; stock n. 6137, 3474 and 4077 respectively) to reveal the activity of the Cre-recombinase by detection of LacZ and eGFP reporter genes. The VE-cadherin-R26R and VE-cadherin-eGFPR mice were studied at developmental stage E18 (± 12h) and at P6–8. Heterozygous VE-cadherin tamoxifen-inducible Cre-recombinase transgenic mice (VE-cadherin-CreERT2) were crossed to homozygous R26R reporter animals. VE-cad-CreERT2/R26R mice (8 weeks old) were IP injected with tamoxifen (2mg/day) for five consecutive days (Monvoisin et al., 2006). After injections, mice were fed normal diet with or without rosiglitazone (10mg/kg/day) for three weeks. Mice were then sacrificed, and BAT and WAT from epididymal and inguinal depots was harvested for X-gal staining. Animal care and handling were in accordance with Italian Institutional Guidelines and the Animal Care and Use Committee at UMass Medical School.
Light microscopy and immunohistochemistry
Mouse and rat embryos were collected after mother perfusion with 4% paraformaldehyde in 0.1M PB pH 7.4 and fixed by overnight immersion in the same solution. Each embryo was then dehydrated, paraffin embedded and oriented to be cross-sectioned through the interscapular region (head-neck). Newborn rats and mice were perfused intracardially using the same fixative; bilateral testis with peri-epididymal fat were dissected and embedded in paraffin. Light microscopy, immunohistochemistry and confocal microscopy were performed using standard methods described in the Supplemental Experimental Procedures.
Electron microscopy and immuno-gold staining
After perfusion, small fragment of iBAT of fetal and postnatal eWAT were fixed in 2% glutaraldehyde – 2% paraformaldehyde in 0.1M PB pH 7.4 for at least 4 h, post-fixed in a solution of 1% osmium-tetroxide and - 1% potassium hexacyanoferrate (II), dehydrated in acetone and finally epoxy-resin embedded. For immune-gold staining, iBAT fragments fixed in 4% paraformaldehyde were embedded in LR White Resin (London Resin Company, Reading, UK). Sectioning, mounting and examination are further described in the Supplemental Experimental Procedures.
X-gal staining for β-galactosidase tissue localization
Male E18–19 and P6–8 and adult VE-cadherin-Cre/R26R mice were fixed in 2% paraformaldehyde, 0.25% glutaraldehyde in PBS pH 7.3 for 1 h and washed in PBS. Thick (500µm) cross-sections through the interscapular region of iBAT and eWAT were stained for β-galactosidase with the chromogenic substrate X-gal, sectioned, counterstained and imaged as described in the Supplemental Experimental Procedures.
Human adipose tissue explants
Human scWAT was obtained from discarded tissue of patients undergoing panniculectromy at UMASS Memorial Hospital. Pieces of 1mm3 were prepared and embedded in Growth Factor Reduced Matrigel (BD Biosciences) on 35 mm glass-bottom culture dishes (MatTek Corporation) as described previously (Gealekman et al., 2011), in the absence or presence of 1 µM rosiglitazone maleate. After 14 days in culture immunofluorescence was performed as described previously (Gealekman et al., 2011). Primary antibodies used are stated in the Supplemental Experimental Procedures. For RT-qPCR, the adipose tissue fragment was mechanically excised and the endothelial sprouts remaining in the Matrigel were isolated using Dispase II (Roche, 2.4 U/mL), centrifuged, and RNA extracted from the pellet using an Ambion RNA extraction kit. Probes used are specified in the Supplemental Experimental Procedures.
Fluorescence Activated Cell Sorting
SVF from eWAT or inter-scapular BAT from 8 week old mice were isolated as described (Fitzgibbons et al., 2011), and stained with blue stain (Invitrogen) at 4°C for 20 min. Cells were incubated with anti-mouse CD16/CD32 (BD Biosciences) for 15 min and then with respective FACS antibody for 2h at room temperature. Antibodies used are stated in the Supplemental Experimental Procedures.
Research Highlights.
Progression of phenotypes from endothelial to pre-adipose seen in developing adipose tissues.
VE-cadherin promoter driven tracers are found in adipocytes from white and brown adipose tissues.
Endothelial cells growing from human adipose tissue explants can develop mature adipocyte features.
A population of adipocyte stem cells from white and brown adipose tissue expresses VE-cadherin
Supplementary Material
Acknowledgements
The research described in this study was supported by grants from Università Politecnica delle Marche and Cariverona Foundation to Saverio Cinti and NIH grant DK089101to Silvia Corvera. The FACS analysis was funded in part by the NIDDK Diabetes and Endocrinology Research Center DK52530.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicting interests statement. The authors declare that they have no competing financial interests
References
- Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, Iruela-Arispe ML. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235:759–767. doi: 10.1002/dvdy.20643. [DOI] [PubMed] [Google Scholar]
- Anghelina M, Moldovan L, Moldovan NI. Preferential activity of Tie2 promoter in arteriolar endothelium. J Cell Mol Med. 2005;9:113–121. doi: 10.1111/j.1582-4934.2005.tb00341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiaens V, Lijnen HR. Angiogenesis and development of adipose tissue. Mol Cell Endocrinol. 2010;318:2–9. doi: 10.1016/j.mce.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Cinti S, Cigolini M, Bosello O, Bjorntorp P. A morphological study of the adipocyte precursor. J Submicrosc Cytol. 1984;16:243–251. [PubMed] [Google Scholar]
- Crossno JT, Jr, Majka SM, Grazia T, Gill RG, Klemm DJ. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J Clin Invest. 2006;116:3220–3228. doi: 10.1172/JCI28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis L, Berghella L, Coletta M, Lattanzi L, Zanchi M, Cusella-De Angelis MG, Ponzetto C, Cossu G. Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration. J Cell Biol. 1999;147:869–878. doi: 10.1083/jcb.147.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, Czech MP. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. American journal of physiology Heart and circulatory physiology. 2011;301:H1425–H1437. doi: 10.1152/ajpheart.00376.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gealekman O, Guseva N, Hartigan C, Apotheker S, Gorgoglione M, Gurav K, Tran KV, Straubhaar J, Nicoloro S, Czech MP, et al. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation. 2011;123:186–194. doi: 10.1161/CIRCULATIONAHA.110.970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Greenway FL, Liu Z, Yu Y, Caruso MK, Roberts AT, Lyons J, Schwimer JE, Gupta AK, Bellanger DE, Guillot TS, et al. An assay to measure angiogenesis in human fat tissue. Obes Surg. 2007;17:510–515. doi: 10.1007/s11695-007-9089-z. [DOI] [PubMed] [Google Scholar]
- Gregoire FM. Adipocyte differentiation: from fibroblast to endocrine cell. Exp Biol Med (Maywood) 2001;226:997–1002. doi: 10.1177/153537020122601106. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Arany Z, Seale P, Mepani RJ, Ye L, Conroe HM, Roby YA, Kulaga H, Reed RR, Spiegelman BM. Transcriptional control of preadipocyte determination by Zfp423. Nature. 2010;464:619–623. doi: 10.1038/nature08816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev. 2001;2:239–254. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- Hausman GJ, Richardson RL. Adipose tissue angiogenesis. J Anim Sci. 2004;82:925–934. doi: 10.2527/2004.823925x. [DOI] [PubMed] [Google Scholar]
- Iyama K, Ohzono K, Usuku G. Electron microscopical studies on the genesis of white adipocytes: differentiation of immature pericytes into adipocytes in transplanted preadipose tissue. Virchows Arch B Cell Pathol Incl Mol Pathol. 1979;31:143–155. doi: 10.1007/BF02889932. [DOI] [PubMed] [Google Scholar]
- Jinno H, Morozova O, Jones KL, Biernaskie JA, Paris M, Hosokawa R, Rudnicki MA, Chai Y, Rossi F, Marra MA, et al. Convergent genesis of an adult neural crest-like dermal stem cell from distinct developmental origins. Stem Cells. 2010;28:2027–2040. doi: 10.1002/stem.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda T, Brown JD, Orasanu G, Vogel S, Gonzalez FJ, Sartoretto J, Michel T, Plutzky J. PPARgamma in the endothelium regulates metabolic responses to high-fat diet in mice. The Journal of clinical investigation. 2009;119:110–124. doi: 10.1172/JCI36233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh YJ, Kang S, Lee HJ, Choi TS, Lee HS, Cho CH, Koh GY. Bone marrow-derived circulating progenitor cells fail to transdifferentiate into adipocytes in adult adipose tissues in mice. The Journal of clinical investigation. 2007;117:3684–3695. doi: 10.1172/JCI32504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh YJ, Park BH, Park JH, Han J, Lee IK, Park JW, Koh GY. Activation of PPAR gamma induces profound multilocularization of adipocytes in adult mouse white adipose tissues. Exp Mol Med. 2009;41:880–895. doi: 10.3858/emm.2009.41.12.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16:1400–1406. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monvoisin A, Alva JA, Hofmann JJ, Zovein AC, Lane TF, Iruela-Arispe ML. VE-cadherin-CreERT2 transgenic mouse: a model for inducible recombination in the endothelium. Dev Dyn. 2006;235:3413–3422. doi: 10.1002/dvdy.20982. [DOI] [PubMed] [Google Scholar]
- Planat-Benard V, Silvestre JS, Cousin B, André M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Pénicaud L, Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation 10. 2004;109(5):656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- Prins JB, O'Rahilly S. Regulation of adipose cell number in man. Clin Sci (Lond) 1997;92:3–11. doi: 10.1042/cs0920003. [DOI] [PubMed] [Google Scholar]
- Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck NA, Iruela-Arispe ML. Conditional Cre/LoxP strategies for the study of hematopoietic stem cell formation. Blood Cells Mol Dis. 2009;43:6–11. doi: 10.1016/j.bcmd.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Zeve D, Seo J, Jo AY, Graff JM. Thiazolidinediones regulate adipose lineage dynamics. Cell metabolism. 2011;14:116–122. doi: 10.1016/j.cmet.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavassoli M. Ultrastructural development of bone marrow adipose cell. Acta Anat (Basel) 1976;94:65–77. doi: 10.1159/000144545. [DOI] [PubMed] [Google Scholar]
- Tomiyama K, Murase N, Stolz DB, Toyokawa H, O'Donnell DR, Smith DM, Dudas JR, Rubin JP, Marra KG. Characterization of transplanted green fluorescent protein+ bone marrow cells into adipose tissue. Stem cells. 2008;26:330–338. doi: 10.1634/stemcells.2007-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, Nedergaard J, Cinti S. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. Faseb J. 2009;23:3113–3120. doi: 10.1096/fj.09-133546. [DOI] [PubMed] [Google Scholar]
- Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, Becker MS, Zanetta L, Dejana E, Gasson JC, et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008;3:625–636. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.