Abstract
Background:
This open-label study evaluated the efficacy and safety of a new leuprolide acetate 45 mg 6-month depot formulation in 151 men with prostate cancer who received 2 intramuscular injections administered 24 weeks apart.
Methods:
The primary efficacy measurement was the proportion of patients achieving suppression of serum testosterone to ⩽50 ng dl−1 from week 4 through week 48. Adverse events (AEs) and hormonal and safety laboratory values were monitored.
Results:
The primary efficacy end point was achieved in 93.4% of subjects (95% confidence interval (89.2%, 97.6%)). There were nine escapes from testosterone suppression during the study, none of which were accompanied by a rise in PSA. By week 4, mean testosterone concentration was suppressed below castrate levels to 15.9 ng dl−1; suppression was maintained for the entire 24-week duration of each depot injection. No mean increase in testosterone was observed after the second injection. Mean PSA levels were maintained below 3 ng ml−1 from week 14 through the 48-week treatment period. The most frequent AE was flushing (58.3%). Injection site reactions were reported in 24.5% of patients.
Conclusions:
Leuprolide acetate 45 mg 6-month depot demonstrated rapid and sustained testosterone suppression through 12 months and was well tolerated. This 6-month leuprolide acetate depot will decrease the number of annual injections in the treatment of prostate cancer.
Keywords: leuprolide acetate, gonadotropin-releasing hormone analog, testosterone, PSA, LH-RH
Introduction
Prostate cancer is the second most frequently diagnosed cancer among men in the United States, and estimates predict that approximately 217 730 new cases were diagnosed in 2010.1 For patients with metastatic prostate cancer and locally advanced disease, androgen deprivation therapy is the mainstay of treatment, and can be achieved through the use of gonadotropin-releasing hormone agonists (GnRHa) or bilateral orchiectomy.2, 3, 4
Leuprolide acetate is a GnRHa, which is widely used for the treatment of hormone-dependent conditions in men and women. A chronic administration of GnRHa leads to downregulation of GnRH receptors in the pituitary gland, which results in a complete suppression of luteinizing hormone (LH), follicle-stimulating hormone and gonadal steroids after an initial stimulatory phase (hormonal flare). Usually, within 4 weeks of initiation of leuprolide acetate therapy, serum testosterone is reduced to near surgically castrated levels.5, 6, 7 Leuprolide acetate 3- and 4-month depot formulations are frequently used for the treatment of advanced prostate cancer. This new 6-month depot formulation of leuprolide acetate was developed to provide an additional treatment option for prostate cancer patients, which reduces the number of injections needed to maintain effective testosterone suppression to two per year.
This study evaluated the efficacy and safety of two formulations (A and B) of leuprolide acetate 6-month depot 45 mg in men with prostate cancer using a sequential study design. The primary efficacy end point was the proportion of patients who achieved suppression of serum testosterone to ⩽50 ng dl−1 from week 4 through week 48.
Only formulation A met prespecified criteria for efficacy and the results of this part of the study are reported herein. Formulation B demonstrated a higher mean initial plasma drug concentration with a lower plasma concentration at the end of the injection period. More subjects escaped from testosterone suppression, and this part of the study was prematurely terminated. The pharmacokinetic data from this study will be reported separately.
Materials and methods
Study design
This open-label clinical trial was conducted from February 2008 to June 2009 at 39 sites in the United States. The study consisted of a screening period (up to 28 days), a 12-month (48-week) treatment period and a 30-day follow-up period. Subjects received two intramuscular injections of leuprolide acetate 6-month depot administered 6 months (24 weeks) apart. Study drug was supplied in a prefilled, dual chamber syringe that contained sterile lyophilized powder and diluent, which were mixed just before injection.
Subjects
Male subjects, 18 years or older, with histologically confirmed prostate cancer (National Cancer Institute (NCI) stages 2–4), or rising PSA following either radical prostatectomy (⩾0.2 ng ml−1 increase from previous test on two consecutive assessments) or prostate irradiation (⩾2.0 ng ml−1 increase above the nadir) were included. Subjects had a prestudy serum testosterone level of >150 ng dl, an Eastern Cooperative Oncology Group performance score of ⩽2, a life expectancy of at least 18 months, and at least 32 weeks had elapsed since prior hormone therapy. Patients were discontinued from the study if serum testosterone was not maintained at therapeutic levels. Institutional review board approval was obtained at each site and written informed consent was provided by each subject before screening or any study-related procedures.
Efficacy end points
The primary efficacy end point was the suppression of serum testosterone to ⩽50 ng dl−1 from week 4 through week 48. The prespecified secondary efficacy end points were the change from baseline in PSA concentrations at study visits, the mean testosterone concentration at each treatment visit and ‘acute-on-chronic' changes in testosterone and LH concentrations from just before the second (week 24) injection through the visit 14 days after the second injection. A post hoc analysis examined the incidence of PSA recurrence defined as a PSA ⩾5 ng ml−1 and an increase in PSA of 50% compared with nadir.
Hormonal assays
Efficacy measurements during treatment included the assessment of LH and testosterone at visits on days 1, 2 and 8 and at weeks 2, 4, 8, 14, 20 and 24 and again after the second injection on day 169 and at days 170, 171 and 176 and at weeks 26, 30, 34, 40, 46 and 48. PSA was measured at days 1 and 8 and weeks 14, 24, 25, 30, 40 and 48. Serum testosterone was measured using liquid chromatography with mass spectrometry detection after extraction (lower limit of quantitation=2.5–3 ng dl−1) by Esoterix (Calabasas Hills, CA, USA) and Abbott Bioanalysis (Abbott Park, IL, USA). Serum LH was measured by immunochemiluminometric assay (lower limit of quantitation=0.02 mIU ml−1) by Esoterix. PSA was measured by a chemiluminescent immunoassay (Access Hybritech PSA; lower limit of quantitation=0.01 ng ml−1) by Esoterix.
Safety
Safety assessments included evaluation of adverse events (AEs) and vital signs at all visits, and clinical laboratory tests at most visits. The injection site was monitored closely for signs of reaction by the clinical investigator for up to 2 weeks following each depot injection.
Statistics
All efficacy analyses were performed on the intent-to-treat population, which included those subjects who received at least 1 dose of study drug and who did not have anti-androgen usage during the first 32 days of study drug. Based on a regulatory request, the primary efficacy analysis was adjusted to censor those four subjects who received an anti-androgen after day 32 at the last testosterone value before use of the anti-androgen. One additional subject was censored, due to a laboratory error, at the last measurement before the error. Both the results from the adjusted and prespecified results are reported. Both of these primary efficacy analyses did not include prematurely discontinued subjects whose final testosterone value was measured before day 19 and was not suppressed, as week 4 suppression could not be determined. The percentage and 90% 2-sided confidence interval (CI) of subjects suppressed for the primary efficacy end point were calculated using a Kaplan–Meier method for right-censored observations, as were the percentages and CI for the subgroups. The prespecified requirement for efficacy was that the lower limit of the 2-sided 90% CI for suppression had to be ⩾87%. Tests of pairwise comparisons between subgroups were based on Z statistics using the Kaplan–Meier estimates and their standard errors.
Summary statistics were generated for the serum testosterone and PSA concentrations at each treatment visit, and ‘acute-on-chronic' changes in testosterone and LH concentrations. For subjects with PSA >4 ng ml−1 at baseline, the number and percentage who achieved a PSA value ⩽4 ng ml−1 were calculated. The percentage of subjects with ⩾50% increase in PSA from nadir and PSA ⩾5 ng ml−1 was also calculated.
AEs were summarized by the percentage of subjects with events during treatment coded by the MedDRA dictionary (version 11.1; http://www.ich.org/products/meddra.html). Injection site reactions that were assessed by the investigator to be AEs were reviewed via an accumulation of all associated terms. Change from baseline in safety laboratory and the percentages of subjects with laboratory values meeting predefined potentially clinically significant thresholds were summarized.
Results
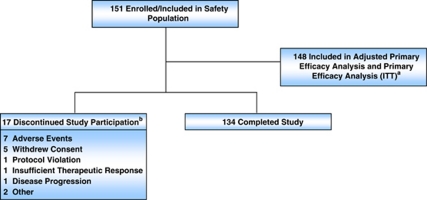
A total of 151 subjects enrolled and received at least 1 dose of study drug and 134 subjects (88.7%) completed the study (Figure 1), and 148 were included for both the adjusted and prespecified primary efficacy end point analyses. Subject demographics and baseline characteristics are presented in Table 1.
Figure 1.
Subject disposition. Flow of subjects through the trial. aThree patients had no testosterone value for week 4 and were excluded fron the intent-to-treat (ITT) population for the primary efficacy endpoint, 5 subjects were censored in the adjusted primary endpoint; bsubject accountability determined by investigator.
Table 1. Subject demographics and baseline characteristics.
| Characteristic | ITT population for primary efficacy end point (N=148) |
|---|---|
| Race, n (%) | |
| White | 116 (78.4) |
| Black or African-American | 30 (20.3) |
| Asian | 1 (0.7) |
| Multi race | 1 (0.7) |
| Ethnicity | |
| Not Hispanic or Latino | 141 (95.3) |
| Age, n (%) | |
| ⩾65 years | 131 (88.5) |
| Age, years | |
| Mean±s.d. | 74.9±8.42 |
| Median (range) | 76.0 (48.0–92.0) |
| Weight, kg | |
| Mean±s.d. | 85.1±13.95 |
| Median (range) | 84.0 (49.0–129.4) |
| Height, cm | |
| Mean±s.d. | 175.3±7.24 |
| Median (range) | 175.3 (149.9–193.0) |
| BMI, kg m−2 | |
| Mean±s.d. | 27.7±4.20 |
| Median (range) | 27.3 (18.0–41.5) |
| Time since first histological diagnosis of prostate cancer (N=147) | |
| Mean±s.d. | 4.6±4.7 |
| Range | 0.0–19.6 |
| Prostate cancer staging (N=142), n (%) | |
| II | 101 (71.1) |
| III | 20 (14.1) |
| IV | 21 (14.8) |
| Testosterone, ng dl−1 | |
| Mean±s.d. | 433.0±175.12 |
| Median (range) | 401.0 (114.0–1060.0) |
| LH, mIU ml−1 | |
| Mean±s.d. | 7.2±6.54 |
| Median (range) | 5.6 (0.1–45.0) |
| PSA (N=146), ng ml−1 | |
| Mean±s.d. | 35.7±138.22 |
| Median (range) | 10.0 (0.2–1517.3) |
| Gleason score category (N=142), n (%) | |
| 4 | 3 (2.1) |
| 5 | 1 (0.7) |
| 6 | 45 (31.7) |
| 7 | 53 (37.3) |
| 8 | 28 (19.7) |
| 9 | 12 (8.5) |
Abbreviations: BMI, body mass index; ITT, intent-to-treat; LH, luteinizing hormone.
Serum testosterone
For the adjusted primary end point, serum testosterone was suppressed to ⩽50 ng dl−1 from week 4 through week 48 in 93.4% of subjects (95% CI (89.2%, 97.6%)). The 90% CI was (89.9%, 96.9%) so the lower limit of the 2-sided 90% CI exceeded the prespecified minimum (87%) for efficacy (Table 2). For the prespecified end point, serum testosterone was suppressed to ⩽50 ng dl−1 from week 4 through week 48 in 93.6% of subjects (90% CI (90.2%, 97.0%)). For the nine subjects who did not have successful suppression of testosterone by each primary end point analysis, one subject failed to achieve suppression by day 32, three subjects escaped suppression just before the second injection and five subjects escaped suppression after the second injection. Of the five subjects who escaped suppression after the second injection, three subjects experienced a transient increase in testosterone within 2 weeks of the second injection (‘acute-on-chronic' reaction), one subject escaped on study day 211 and the remaining subject escaped at the end of the second treatment cycle. Notably, most (5/8) testosterone escapes were transient, and none were associated with increases in PSA. Of the eight escapes, four occurred in patients who were African-American.
Table 2. Suppression of serum testosterone from week 4 through week 48a.
| N | Percent suppressedb | s.e. | Two-sided 90% CI |
|---|---|---|---|
| 148 | 93.4% | 2.13% | 89.9%, 96.9% |
Abbreviation: CI, confidence interval.
Adjusted primary end point.
Suppression of testosterone occurred by study day 32 and with no escape through week 48.
Subgroup analyses of testosterone suppression by NCI prostate cancer stage based on tumor node metastasis, body mass index and race were performed and the results are presented in Table 3. The suppression rate was numerically higher for Caucasian subjects than for African-American subjects, but the difference was not statistically significant (P=0.179). The only significant difference was between those subjects with stage IV prostate cancer vs those subjects with stage II prostate cancer, with the subjects with more advanced prostate cancer (stage IV) having a higher rate of response to treatment (100 vs 91.8%, P=0.003).
Table 3. Suppression of serum testosterone by subgroups of prostate cancer stage, BMI and race.
| Subgroup | N | Percent suppresseda (%) | s.e. (%) | Two-sided 90% CI |
|---|---|---|---|---|
| NCI stage | ||||
| Stage II | 101 | 91.8 | 2.8 | 87.2%, 96.4% |
| Stage III | 20 | 94.1 | 5.7 | 84.7%, 100.0% |
| Stage IV | 21 | 100.0 | 0.0 | 100.0%, 100.0% |
| BMI, kg m−2 | ||||
| <25 | 44 | 95.0 | 3.5 | 89.3%, 100.0% |
| ⩾25 to <30 | 67 | 92.3 | 3.3 | 86.9%, 97.8% |
| ⩾30 | 37 | 94.4 | 3.8 | 88.2%, 100.0% |
| Race | ||||
| Caucasian | 116 | 95.4 | 2.0 | 92.1%, 98.7% |
| African-American | 30 | 86.5 | 6.3 | 76.2%, 96.8% |
| Other | 2 | 100.0 | 0.0 | 100.0%, 100.0% |
Abbreviations: BMI, body mass index; CI, confidence interval; NCI, National Cancer Institute.
Suppression of testosterone from week 4 through week 48 based on prespecified analysis.
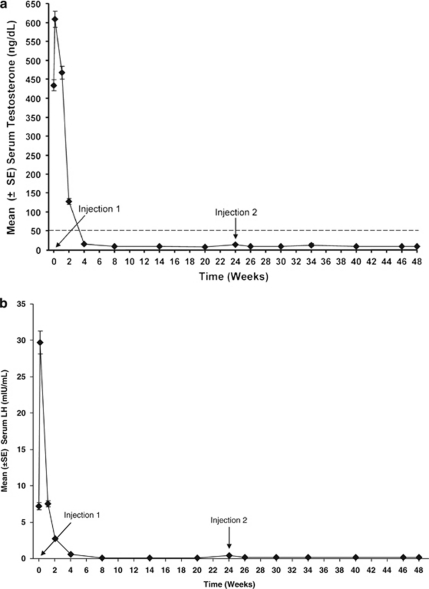
After a transient hormonal flare reaction after the first injection, treatment with leuprolide acetate suppressed mean serum testosterone to below castrate levels for the entire 24-week duration of each depot injection (Figure 2a). At baseline, mean serum testosterone concentration was 434.6 ng dl−1, but decreased to suppression levels as early as week 4 (15.9 ng dl−1) and remained ⩽11 ng dl−1 at the end of each treatment cycle. Similarly, after initial stimulation after the first injection, LH was suppressed for the 24-week interval following each depot injection (Figure 2b).
Figure 2.
Mean testosterone levels (a) and mean luteinizing hormone (LH) concentrations (b) during the treatment period. The dashed line in (a) represents castrate levels for serum testosterone (50 ng dl−1). Time points for administration of leuprolide acetate 6-month depot injections are indicated in both panels.
‘Acute-on-chronic' response
Small, but statistically significant, mean increases in serum LH levels (0.1–0.2 mIU ml−1) were observed from just before the second injection on week 24 to serial time points within 2 weeks after the second injection (‘acute-on-chronic' reaction). In contrast, small mean decreases (0.0–2.0 ng dl−1) in testosterone levels were observed even though three subjects experienced transient testosterone escapes during the ‘acute-on-chronic' phase.
Serum PSA
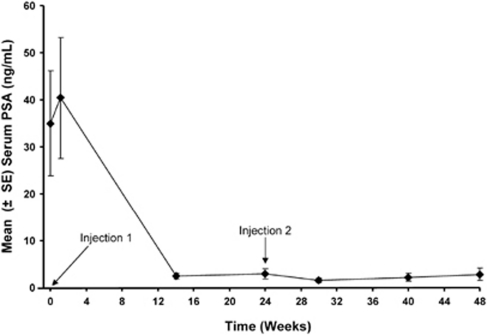
Mean serum PSA concentrations through the 48-week treatment period are shown in Figure 3. At baseline, 75% of subjects (112/149) had serum PSA concentrations that were >4 ng ml−1. Similar to testosterone, there was a transient rise in mean serum PSA concentrations after the first injection. Within 14 weeks of treatment, 87.4% (97/111) of those subjects with elevated PSA levels at baseline achieved a PSA ⩽4 ng ml−1. At all subsequent visits, at least 86% of subjects with a baseline PSA >4 ng ml−1 had PSA values ⩽4 ng ml−1. The incidence of PSA recurrence was low, castration-resistant prostate cancer occurred in only 4.6% of subjects (7/151).
Figure 3.
Mean PSA levels during the treatment period. Time points for administration of leuprolide acetate 6-month depot injections are indicated.
Safety
Treatment-emergent AEs were reported for 94.7% of subjects during the treatment period; the majority of AEs were mild to moderate in severity (Table 4). AEs of injection site reaction were reported in 37 subjects (24.5%), including injection site pain in 27 subjects (17.9%), injection site discomfort and injection site erythema each in 3 subjects (2.0%) and injection site hematoma and injection site swelling in 2 subjects each (1.3%). All were mild or moderate in severity, and none led to discontinuation of study drug.
Table 4. Incidence and severity of treatment-emergent AEs (⩾5% of subjects).
| Adverse eventa |
Leuprolide acetate 45 mg 6-month depot (N=151) |
|||
|---|---|---|---|---|
| All events, n (%) |
Severityb |
|||
| Mild | Moderate | Severe | ||
| Hot flush | 88 (58.3) | 58 | 23 | 7 |
| Injection site pain | 27 (17.9) | 22 | 5 | 0 |
| Fatigue | 18 (11.9) | 15 | 2 | 1 |
| Constipation | 15 (9.9) | 9 | 6 | 0 |
| Arthralgia | 14 (9.3) | 8 | 6 | 0 |
| Insomnia | 13 (8.6) | 12 | 1 | 0 |
| Headache | 11 (7.3) | 6 | 4 | 1 |
| Cough | 10 (6.6) | 7 | 3 | 0 |
| Hematuria | 10 (6.6) | 7 | 3 | 0 |
| Nasopharyngitis | 10 (6.6) | 6 | 4 | 0 |
| Dysuria | 9 (6.0) | 7 | 2 | 0 |
| Hypertension | 9 (6.0) | 3 | 6 | 0 |
| Rash | 9 (6.0) | 8 | 1 | 0 |
| Anemia | 8 (5.3) | 6 | 1 | 1 |
| Back pain | 8 (5.3) | 4 | 4 | 0 |
| Chronic obstructive pulmonary disease | 8 (5.3) | 3 | 2 | 3 |
| Dizziness | 8 (5.3) | 6 | 2 | 0 |
| Musculoskeletal pain | 8 (5.3) | 7 | 1 | 0 |
| Nocturia | 8 (5.3) | 5 | 3 | 0 |
| Upper respiratory tract infection | 8 (5.3) | 4 | 4 | 0 |
| Urinary tract infection | 8 (5.3) | 4 | 3 | 1 |
Abbreviation: AEs, adverse events.
Coded using MedDRA 12.0 Preferred Term.
Subjects were counted for most severe event, as assessed by the investigator, for each preferred term.
In addition to injection site pain, AEs of anemia, bone fracture, diabetes and cardiac events were of interest because of their possible association with androgen deprivation therapy. Ten subjects had AEs of anemia or decreased hemoglobin. Two of these AEs were considered serious, and the subjects received blood transfusions, but these events were considered not related to study drug by the investigator (alternative etiologies of suspected subacute upper gastrointestinal bleeding and metastatic cancer). Only 2 of the 10 AEs of anemia or decreased hemoglobin were considered treatment related. No subjects prematurely discontinued due to an AE of anemia. Five subjects experienced bone fractures as a result of falls or other trauma, but only one event was considered possibly related to study drug, and no subjects discontinued from the study due to an AE related to bone fractures. A total of eight subjects experienced AEs that were related to diabetes mellitus (n=2), increases in serum glucose (n=5) or hypoglycemia (n=1), which included one report of non-related new onset diabetes and only one possibly related event (increase in serum glucose).
Overall, a high proportion of subjects (88.1%) had a history of cardiovascular disease. One or more cardiac events were reported for 18 subjects. Ten subjects experienced serious cardiac AEs, all of whom had medical histories with significant risk factors (for example, hypertension, coronary artery disease and hypercholesterolemia). One subject prematurely discontinued the study due to worsening coronary artery disease, weakness and hyperkalemia. A total of four treatment-related AEs were reported in three subjects: angina pectoris, tachycardia and mitral valve incompetence and tricuspid valve incompetence.
Overall, the incidence of sexual dysfunction during the study, reported as AEs, was low. One patient each reported reduced libido or loss of libido, and two patients reported erectile dysfunction.
Serious AEs were reported for 31 subjects (20.5%), including one death that was not considered related to study drug by the investigator. The subject was 92-years old, had a history of chronic obstructive pulmonary disease, and died as the result of a treatment-emergent AE of aspiration pneumonia that began 11 days after he completed the study. Two subjects had serious AEs that were considered possibly related to study drug: colonic pseudo-obstruction in one and angina pectoris in one. Both serious AEs resolved, and the subjects completed the study. Two subjects had serious AEs that resulted in discontinuation from the study: coronary artery disease, asthenia and hyperkalemia in one and non-Hodgkins lymphoma stage IV in one.
Glucose
At baseline, the mean fasting blood glucose concentration was above normal (5.8 mmol l−1) and further increased by 0.4±1.8 mmol l−1 at the final treatment visit. However, only one subject, who had a history of diabetes, had a glucose value that was considered potentially clinically significant (⩾16.6 mmol l−1) during the treatment period.
Hemoglobin
Mean hemoglobin values decreased through the first 14 weeks of treatment and then were stable through the end of the treatment period, with a mean decrease of 1.05±1.01 g dl−1 at the final treatment visit. All other laboratory changes were unremarkable and consistent with the study population.
Discussion
This study evaluated the efficacy and safety of a new 6-month depot formulation of leuprolide acetate and demonstrated that testosterone suppression to and below castration levels (⩽50 ng dl−1) was rapid and sustained throughout the 12-month (48-week) treatment period; 93.4% of subjects had castrate levels of serum testosterone from week 4 through week 48. None of the escapes from testosterone suppression were associated with increases in PSA.
The suppression of testosterone achieved with this leuprolide acetate 6-month depot formulation is consistent with that achieved with the leuprolide acetate 3- and 4-month depot formulations.6, 7 A cross-study comparison, using the simple percentage of subjects who did not achieve testosterone suppression to ⩽50 ng dl−1 by week 4 or escaped from suppression on or before week 48, indicated that the leuprolide acetate 6-month depot formulation is at least as efficacious as the marketed 3- and 4-month depot formulations (failure rates of 6.0, 10.3 and 10.9% for 6-month, 3-month and 4-month formulations, respectively).6, 7
Overall, subgroup analyses indicate that leuprolide acetate 6-month depot was effective for testosterone suppression regardless of prostate cancer stage, body mass index or race. In the total population, treatment with leuprolide acetate 6-month depot elicited a statistically significantly higher rate of response in the patients with the most advanced disease (P=0.003, stage IV vs stage II). However, while not statistically significant, there was a numerical difference in the percentage of African-American men with testosterone suppression (86.5%) compared with Caucasian men (95.4%). The reasons for this difference are unclear.
With leuprolide acetate 6-month depot treatment, mean testosterone levels after week 4 were suppressed below 15 ng dl−1 for the remainder of the 48-week treatment period and were ⩽11 ng dl−1 at the end of each treatment cycle. In this study, the use of anti-androgens was excluded per protocol because use of these drugs would interfere with assessment of hormonal response (such as the primary end point) and potentially with the effects of leuprolide acetate on testosterone suppression during the initial phase of treatment. In our study, mean testosterone increases were not observed with the second leuprolide acetate injection. Small mean decreases in testosterone were observed from before the second injection to serial time points after the injection, and only three individual subjects experienced increases above 50 ng dl−1, indicating that suppression of testosterone remained stable even after repeated exposure to leuprolide acetate 6-month depot in the vast majority of patients. In addition, PSA levels, which are used as a surrogate marker for prostate cancer progression, paralleled the decreases in testosterone concentrations, and only 4.6% of subjects experienced PSA recurrence, which occurred without an increase in serum testosterone.
Leuprolide acetate 6-month depot was well tolerated, and the type and incidence of AEs and laboratory findings reported were consistent with the safety profiles of the marketed leuprolide acetate intramuscular 3- and 4-month depot formulations6, 7 and a marketed subcutaneous 6-month depot formulation,8 as expected for this patient population.7, 8, 9 Overall, the incidence of injection site reactions was 24.5%, which is higher than reported in other studies with 6-month depot formulations of GnRHa.8, 10, 11 The rigorous monitoring for injection site reactions may have increased the frequency of adverse reaction reporting in our study population. The relatively low incidence of sexual side effects, which were collected as AEs, might be due to under reporting of these events in this study.
The mean changes in glucose and hemoglobin observed in this study were consistent with the mean changes observed for the marketed leuprolide acetate 3- and 4-month depot formulations.7, 9 Androgen deprivation therapy has been shown to increase insulin resistance and increase the risk of new onset diabetes.12, 13, 14, 15 Notably, there was only one potentially significant increase in glucose in a subject with established diabetes and only one report of new onset diabetes during this study. Reductions in hemoglobin are known to occur after orchiectomy and GnRHa therapy as a result of loss of androgen stimulation of the hematopoietic system.16, 17, 18
This study demonstrates the efficacy and safety of the leuprolide acetate 6-month depot formulation for sustained reduction of serum testosterone to castrate levels in men with locally advanced and advanced prostate cancer. The availability of a leuprolide acetate 6-month depot formulation will limit the number of injections needed per year to two, and better align with the treatment schedules of prostate cancer patients, which may improve patient compliance and outcomes.
Acknowledgments
Financial support for this study was provided by Abbott Laboratories. Medical writing support was provided by Amanda J Fein and Theresa J Peterson on behalf of Abbott. We would like to thank all of the study investigators who participated in this clinical trial. Clinicaltrials.gov identifier: NCT00626431.
AS has received honoraria for speaking on behalf of Watson, Pfizer, Solvay and GlaxoSmithKline. JY was a consultant for Abbott. LL, CMG, JD and KC are all employed by Abbott and own Abbott stock.
References
- American Cancer Society Cancer Facts and Figures 2010Available at http://www.cancer.org/acs/groups/content/@nho/ documents/document/acspc-024113.pdf . Accessed 12 August 2010.
- Cassileth BR, Soloway MS, Vogelzang NJ, Schellhammer PS, Seidmon EJ, Hait HI, et al. Patients' choice of treatment in Stage D prostate cancer. Urology. 1989;33 (5 Suppl:57–62. doi: 10.1016/0090-4295(89)90108-8. [DOI] [PubMed] [Google Scholar]
- Klotz L, Boccon-Gibod L, Shore ND, Andreou C, Persson BE, Cantor P, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102:1531–1538. doi: 10.1111/j.1464-410X.2008.08183.x. [DOI] [PubMed] [Google Scholar]
- McLeod DG. Hormonal therapy: historical perspective to future directions. Urology. 2003;61 (2 Suppl 1:3–7. doi: 10.1016/s0090-4295(02)02393-2. [DOI] [PubMed] [Google Scholar]
- Berges R, Bello U. Effect of a new leuprorelin formulation on testosterone levels in patients with advanced prostate cancer. Curr Med Res Opin. 2006;22:649–655. doi: 10.1185/030079906X96425. [DOI] [PubMed] [Google Scholar]
- Chu FM, Jayson M, Dineen MK, Perez R, Harkaway R, Tyler RC. A clinical study of 22.5 mg La-2550: a new subcutaneous depot delivery system for leuprolide acetate for the treatment of prostate cancer. J Urol. 2002;168:1199–1203. doi: 10.1016/S0022-5347(05)64625-3. [DOI] [PubMed] [Google Scholar]
- Sartor O, Dineen MK, Perez-Marreno R, Chu FM, Carron GJ, Tyler RC. An eight-month clinical study of LA-2575 30.0 mg: a new 4-month, subcutaneous delivery system for leuprolide acetate in the treatment of prostate cancer. Urology. 2003;62:319–323. doi: 10.1016/s0090-4295(03)00330-3. [DOI] [PubMed] [Google Scholar]
- Crawford ED, Sartor O, Chu F, Perez R, Karlin G, Garrett JS. A 12-month clinical study of LA-2585 (45.0 mg): a new 6-month subcutaneous delivery system for leuprolide acetate for the treatment of prostate cancer. J Urol. 2006;175:533–536. doi: 10.1016/S0022-5347(05)00161-8. [DOI] [PubMed] [Google Scholar]
- Tunn UW, Bargelloni U, Cosciani S, Fiaccavento G, Guazzieri S, Pagano F.Comparison of LH-RH analogue 1-month depot and 3-month depot by their hormone levels and pharmacokinetic profile in patients with advanced prostate cancer Urol Int 199860(Suppl 19–16.discussion 16–17. [DOI] [PubMed] [Google Scholar]
- Lundstrom EA, Rencken RK, van Wyk JH, Coetzee LJ, Bahlmann JC, Reif S, et al. Triptorelin 6-month formulation in the management of patients with locally advanced and metastatic prostate cancer: an open-label, non-comparative, multicentre, phase III study. Clin Drug Investig. 2009;29:757–765. doi: 10.2165/11319690-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Tunn UW, Wiedey K. Safety and clinical efficacy of a new 6-month depot formulation of leuprorelin acetate in patients with prostate cancer in Europe. Prostate Cancer Prostatic Dis. 2009;12:83–87. doi: 10.1038/pcan.2008.52. [DOI] [PubMed] [Google Scholar]
- Alibhai SM, Duong-Hua M, Sutradhar R, Fleshner NE, Warde P, Cheung AM, et al. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol. 2009;27:3452–3458. doi: 10.1200/JCO.2008.20.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- Nobes JP, Langley SE, Laing RW. Metabolic syndrome and prostate cancer: a review. Clin Oncol (R Coll Radiol) 2009;21:183–191. doi: 10.1016/j.clon.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Smith MR, Lee H, McGovern F, Fallon MA, Goode M, Zietman AL, et al. Metabolic changes during gonadotropin-releasing hormone agonist therapy for prostate cancer: differences from the classic metabolic syndrome. Cancer. 2008;112:2188–2194. doi: 10.1002/cncr.23440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asbell SO, Leon SA, Tester WJ, Brereton HD, Ago CT, Rotman M. Development of anemia and recovery in prostate cancer patients treated with combined androgen blockade and radiotherapy. Prostate. 1996;29:243–248. doi: 10.1002/(SICI)1097-0045(199610)29:4<243::AID-PROS5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Fonseca R, Rajkumar SV, White WL, Tefferi A, Hoagland HC. Anemia after orchiectomy. Am J Hematol. 1998;59:230–233. doi: 10.1002/(sici)1096-8652(199811)59:3<230::aid-ajh8>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Shahani S, Braga-Basaria M, Maggio M, Basaria S. Androgens and erythropoiesis: past and present. J Endocrinol Invest. 2009;32:704–716. doi: 10.1007/BF03345745. [DOI] [PubMed] [Google Scholar]