Abstract
Background
Hyperphosphatemia has been identified in the past decade as a strong predictor of mortality in advanced chronic kidney disease (CKD). For example, a study of patients in stage CKD 5 (with an annual mortality of about 20%) revealed that 12% of all deaths in this group were attributable to an elevated serum phosphate concentration. Recently, a high-normal serum phosphate concentration has also been found to be an independent predictor of cardiovascular events and mortality in the general population. Therefore, phosphate additives in food are a matter of concern, and their potential impact on health may well have been underappreciated.
Methods
We reviewed pertinent literature retrieved by a selective search of the PubMed and EU databases (www.zusatzstoffe-online.de, www.codexalimentarius.de), with the search terms “phosphate additives” and “hyperphosphatemia.”
Results
There is no need to lower the content of natural phosphate, i.e. organic esters, in food, because this type of phosphate is incompletely absorbed; restricting its intake might even lead to protein malnutrition. On the other hand, inorganic phosphate in food additives is effectively absorbed and can measurably elevate the serum phosphate concentration in patients with advanced CKD. Foods with added phosphate tend to be eaten by persons at the lower end of the socioeconomic scale, who consume more processed and “fast” food. The main pathophysiological effect of phosphate is vascular damage, e.g. endothelial dysfunction and vascular calcification. Aside from the quality of phosphate in the diet (which also requires attention), the quantity of phosphate consumed by patients with advanced renal failure should not exceed 1000 mg per day, according to the guidelines.
Conclusion
Prospective controlled trials are currently unavailable. In view of the high prevalence of CKD and the potential harm caused by phosphate additives to food, the public should be informed that added phosphate is damaging to health. Furthermore, calls for labeling the content of added phosphate in food are appropriate.
The dietary intake of phosphate and the serum phosphate concentration are important matters not just for persons with renal disease, but for the general public as well. It has recently been determined that phosphate additives in food may harm the health of persons with normal renal function (1, e1). This judgment has been made on the basis of large-scale epidemiological studies and is supported by the latest findings of basic research.
It was first recognized in patients with renal disease that a high serum phosphate concentration is a major risk factor for elevated cardiovascular and overall mortality (2, 3). Block et al. studied a cohort of 40 538 hemodialysis patients and determined, after multivariate adjustment, that 12% of the 10 015 deaths occurring over the period of observation were associated with hyperphosphatemia (2). Dietary phosphate restriction has been a standard recommendation for decades for patients with chronic renal failure (4). This approach is also supported by the findings of a prospective five-year observational study of patients receiving chronic hemodialysis, in which a low dietary intake of phosphate was found to confer a significant survival advantage. Patients whose dietary phosphate intake was above the 99th percentile died at a rate 2.37 times higher than those whose dietary phosphate intake was below the first percentile (5). In a controlled interventional trial, persons who were informed about food additives containing phosphates and their presence in different types of food went on to avoid food with phosphate additives and to have lower serum phosphate concentrations.
More recent studies have shown that the association between high phosphate concentrations and higher mortality is not restricted to persons with renal disease; it can also be observed in persons with cardiovascular disease and even in the general population. High-normal serum phosphate concentrations are associated with coronary calcification in young, healthy men (6) and were found to be a predictor of cardiovascular events in the Framingham study (7). Elevated mortality in association with high-normal serum phosphate concentrations was seen mainly among persons with cardiovascular disease who had normal renal function (8) (Figure 1). In the Framingham study, 375 of the 4127 subjects died within 60 months; the adjusted mortality risk was 22% for each 1 mg/dL elevation of the serum phosphate concentration.
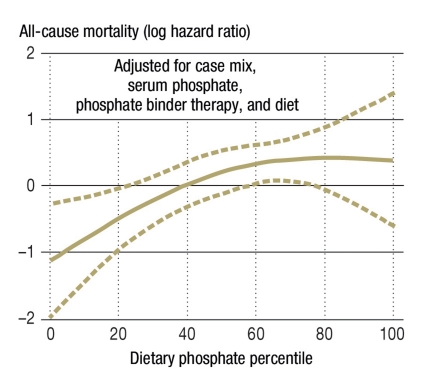
Figure 1.
Percentiles of dietary phosphate intake and overall mortality in 224 hemodialysis patients observed for up to 5 years; the hazard ratio (after full adjustment) in the highest percentile, compared to the lowest percentile, was 2.37
(From: Noori N, et al.: Association of dietary phosphorus intake and phosphorus to protein ratio with mortality in hemodialysis patients. CJASN 2010; 5: 683–92 [5]. Reproduced with the kind permission of the American Society of Nephrology)
Thus, the problem of high serum phosphate concentrations impairing survival is not merely a matter for nephrologists, though admittedly these observational studies do not yet firmly establish a direct cause-and-effect relationship. For the present article, the authors reviewed pertinent publications retrieved by a selective search of the PubMed and EU databases (www.zusatzstoffe-online.de, www.codexalimentarius.de). Much of this literature is of very recent date; in identifying important articles, we benefited from our experience working in international guideline committees (the “Kidney Disease—Improving Global Outcomes” (KDIGO) initiative [MK]) (4) and developing nutrition programs for dialysis patients (the Phosphate Unit Program [Phosphat-Einheiten-Programm, PEP] [MKK]). Our literature search alse revealed the lack of prospective controlled trials on this topic.
The main sources of easily resorbable phosphate
Phosphate occurs naturally in the form of organic esters in many kinds of food, including meat, potatoes, bread, and other farinaceous products; the consumption of such foods cannot be restricted without incurring the risk of lowering protein intake. Naturally occurring phosphate in food is organically bound, and only 40% to 60% of it is absorbed in the gastrointestinal tract (e1).
On the other hand, an avoidable risk to health that has not attracted sufficient attention to date arises from the increased use of phosphate as a food additive and preservative. This “free” (not organically bound) phosphate is very effectively absorbed in the gastrointestinal tract. Typical foods with large amounts of added phosphate are processed meat, ham, sausages, canned fish, baked goods, cola drinks, and other soft drinks. Dietary counseling is all the more difficult because the phosphate content in food—and, in particular, the added phospate content—is not marked on the package.
It used to be thought that the only health risk posed by phosphate lay in the promotion of calcification in blood vessels and bodily organs. Recently, however, important discoveries have been made about the hormonal regulation of phosphate metabolism. It is now known that the serum phosphate concentration is controlled by two newly discovered factors called fibroblast growth factor 23 (FGF23) and klotho; that phosphate causes lasting damage to the cardiovascular system, either by a direct mechanism or by way of these hormonal factors; and that phosphate accelerates aging processes in animal models (e2, e3).
In particular, phosphate added to animal fodder accelerates age-related organ complications such as muscle and skin atrophy, the progression of chronic renal failure, and cardiovascular calcifications (e2). Phosphate added to human food probably has similar effects in man. Inexpensive food containing additives (processed food), and fast food in particular, are extraordinarily rich in phosphate additives. Such foods are consumed in greater amounts by the poor. In the USA, hyperphosphatemia has been found to be twice as common among persons of low income than among persons of high income. In the Chronic Renal Insufficiency Cohort Study, which was carried out in the USA, patients with mildly to moderately impaired renal function were studied over time; the multivariate adjusted risk of hyperphosphatemia (defined as a serum phosphate concentration above 1.45 mmol/L) was higher in persons of the lowest income class than in persons of the highest income class, by a factor of 2.5 to 2.7 (9). As the total phosphate intake among persons in these two groups was roughly the same (1156 versus 1190 mg/day), the finding is presumably attributable to the different types of phosphate that were consumed.
The purpose of this article is to inform physicians of these new aspects of nutritional medicine and, more broadly, to acquaint laypersons interested in health policy with the problem of excessive phosphate intake.
Hormonal phosphate regulation
Until recently, medical students were taught that phosphate was resorbed in the intestines proportionally to the amount consumed in food, and that the resorbed phosphate was then excreted without any further difficulty by the kidneys. Normally, up to 80% of dietary phosphate is resorbed in the intestines, but the transport rate varies depending on the source of phosphate (and on the individual’s vitamin D status). About two-thirds of dietary phosphate is eliminated in the urine, and one-third in the feces (10). It has been discovered only in the last five years that phosphate homeostasis and the renal excretion of phosphate are regulated by a complex endocrine feedback system. The key hormone for phosphate homeostasis is FGF23 (11, e4). In genetically manipulated mice, the absence of FGF23 led to severe hyperphosphatemia and simultaneously to increased renal calcitriol synthesis by way of increased 1-alpha-hydroxylation (12). On the other hand, an elevated concentration of FGF23 leads to increased renal excretion of phosphate and diminishes the activation of vitamin D to calcitriol.
Two hormone systems prevent phosphate accumulation
FGF23 is mainly produced in bone osteocytes (10, 11, e4). Its secretion is stimulated by high intestinal phosphate resorption and a high serum phosphate concentration; it is unclear whether this stimulation occurs directly or by way of as yet unidentified intestinal messenger molecules (10). The FGF23-induced increase in renal phosphate excretion can long delay the development of hyperphosphatemia even in patients with progressive renal failure. Definitely elevated serum phosphate concentrations are not seen until the glomerular filtration rate (GFR) drops below 30 mL/min (CKD stage 4). Normophosphatemia in the early stages of renal failure comes at a price, however: steadily increasing concentrations of FGF23 and parathormone (PTH). A high concentration of FGF23 indirectly stimulates the secretion of parathormone by suppressing renal calcitriol synthesis (secondary hyperparathyroidism). PTH has a phosphaturic effect, as does FGF23. Thus, the body uses two different hormone systems (FGF23, PTH) to stave off hyperphosphatemia as long as possible in the setting of progressive renal failure (13, e5).
FGF23 exerts its effects only in the kidneys and parathyroid glands, which are the only organs that express klotho. Klotho is a beta-glucuronidase located in the cell membrane that serves as a coreceptor for FGF23 (10, 11, e4). The absence of klotho leads to the same changes as a total lack of FGF23, namely, hyperphosphatemia and an elevated concentration of active vitamin D. Moreover, both klotho knockout mice and FGF23-deficient mice express a phenotype that resembles premature aging, with vascular calcification, osteoporosis, skin atrophy, pulmonary emphysema, infertility, and early death (14).
It follows from all of the above that high-normal phosphate concentrations are associated with elevated morbidity and mortality even in persons with normal renal function, particularly if they suffer from cardiovascular disease (6, 7) (Figure 2). Kuro-o, who first described the klotho/FGF23 system, views the serum phosphate concentration as a surrogate marker for the activity of the phosphate/FGF23/klotho endocrine axis. Low klotho activity is associated with a high serum phosphate concentration. Kuro-o calls phosphate the “signal molecule of aging,” but this interpretation is mainly based on data from animal experiments (15).
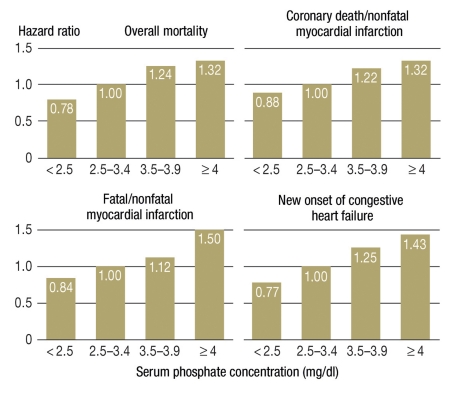
Figure 2.
Post-hoc analysis in the Cholesterol and Recurrent Events (CARE) study (n = 4127): A high-normal phosphate concentration is an independent predictor of mortality and further cardiovascular events in patients who have sustained a myocardial infarction
(From: Tonelli M, et al.: Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 2005; 112: 2627–33 [8]. Reproduced with the kind permission of Wolters Kluwer Health)
Phosphate and blood vessels
Phosphate induces vascular calcification both in vitro and in vivo (16, e6). What occurs is not merely the passive precipitation of calcium × phosphate, but rather an active cellular process in which smooth-muscle cells in blood vessels are reprogrammed to become osteoblast-like cells (“osteogenic transdifferentiation”) (16). This process, originally identified in cell culture and in animal experiments, has since been demonstrated in human arteries as well (17, e7). Moreover, it has recently been shown that increased phosphate intake leads to a marked impairment of endothelial-cell function in the vascular system, both in experimental animals and in man (18). Phosphate-induced vascular changes may be the link connecting elevated serum phosphate concentrations to premature aging and death.
The risks of hypophosphatemia
Lastly, it should be mentioned that the very rare genetic or tumor-associated disturbances of the klotho/FGF23 system lead to severe hypophasphatemia that can be expressed as hypophosphatemic rickets in children and as osteomalacia in adults (10, 11, e4). Aside from these conditions, clinically relevant hypophosphatemia is seen only in extremely malnourished persons, e.g., in septic patients in intensive care, who may have phosphate concentrations of 0.5 mmol/L or lower. In some cases, rhabdomyolysis may ensue as the result of depleted energy stores (intracellular ATP [adenosine triphosphate] deficiency).
Phosphate as a food additive
As mentioned above, organic phosphate esters are found mainly in protein-rich foods such as dairy products, fish, meat, sausages, and eggs. They are slowly hydrolyzed in the gastrointestinal tract and then slowly resorbed from the intestine. About 40% to 60% of the organic phosphate esters consumed in the diet are resorbed (e1, e8).
The phosphates found in grains, nuts, and legumes are mainly in the form of phytic acid (hexaphospho-inositol), which cannot be split in the human intestine because of the lack of the enzyme phytase (19). The bioavailability of vegetable phosphate esters is usually less than 50% (8, 20) and thus much lower than that of the phosphate esters in protein-rich foods. It follows that the phosphate content of food cannot be automatically equated with the phosphate load.
The phosphate content of industrially processed food is much higher than that of natural food, because polyphosphates are commonly used as an additive in industrial food production (Table). In the European Union, sodium phosphate (E 339), potassium phosphate (E 340), calcium phosphate (E 341), and salts of orthophosphoric acid diphosphate (E 450), triphosphate (E 451), and polyphosphate (E 452) can legally be added to food as preservatives, acidifying agents, acidity buffers, and emulsifying agents. Phosphate salts are also added to many foods as stabilizers or taste intensifiers.
Table. The phosphate content of various food groups.
| Portion size | mg of phosphate | Presence of phosphate additives | |
| Meat, sausages, fish, poultry | |||
| Pork, veal, beef, lamb | 150 g | 200–300 | −/+ (frozen products) |
| Cold cuts | 50 g | 50–100 | + (labeling) |
| Sausages (frankfurters, knockwurst, veal sausages, frying sausages, etc.) | 150 g | 200–300 | + (labeling) |
| Fish, seafood | 150 g | 300–400 | −/+ (canned products) |
| Cheese, dairy products, eggs | |||
| Soft cheese (camembert, gorgonzola, mozzarella, butterkäse, etc.) | 50 g | 100–200 | −/+ |
| Hard and sliced cheese (edam, gouda, emmentaler, raclette, etc.) | 50 g | 200–300 | −/+ |
| Processed/parmesan/American cheese | 50 g | 400–500 | ++ |
| Milk, regardless of fat content | 200 mL | 100–200 | − |
| Yoghurt, regardless of fat content | 150 g | 100–200 | −/+ |
| Quark cheese, regardless of fat content | 150 g | 200–300 | − |
| Egg (hen’s) | 60 g | 100–200 | − |
| Vegetable spread for bread | 100 g | 100–200 | + |
| Vegetables, fruit, baked goods, additives for baking | |||
| Potatoes, rice, noodles, semolina | 150 g | 50–100 | − |
| Salad, fruit | 150 g | 0–50 | − |
| White bread | 100 g | 50–100 | −/+ (baking mix) |
| Whole-wheat bread | 100 g | 100–200 | −/+ (baking mix) |
| Peanuts, almonds, pistachios | 100 g | 400–500 | − |
| Chocolate (whole milk) | 50 g | 100–200 | −/+ |
| Baker’s yeast | cube | 200–300 | + |
| Baking powder | packet | 1500 | ++ |
| Beverages | |||
| Cola, mixed drinks containing cola | 200 mL | 50–100 | ++ |
| Beer | 200 mL | 50–100 | −/+ |
| Fruit juice (non-perishable) | 200 mL | 50–100 | + |
| Coffee | 150 mL | 0–100 | −/+ (instant products) |
| Cocoa powder | 20 g | 100–200 | + |
– no added phosphate;
–/+ added phosphate in some products;
+ added phosphate in most products;
++ large quantities of added phosphate in most products
Fast food and ready-to-eat processed foods are the main contributors to today’s rising dietary consumption of phosphate. Because of the increased use of food additives, the estimated daily intake of phosphate-containing food additives has more than doubled since the 1990s, from just under 500 mg/day to 1000 mg/day (21, e9). The findings of another recent study were on the same order of magnitude: In processed meat and poultry products, the phosphate content was nearly twice that of the natural product because of added phosphate (22). Thus, not only patients with impaired renal function need to be put on a low-phosphate diet; patients with cardiovascular diseases should also consume less phosphate, and indeed the general population should as well.
Phosphate additives play an especially important role in the meat industry, where they are used as preservatives. They are also used as a component of melting salts in the production of soft cheese. Phosphates loosen the structure of protein, enabling it to bind more water. Phosphate additives are also found in large quantities in flavored soft drinks and in sterilized, ultra heat treated, thickened, and powdered milk. A further use of phosphates is to prevent the agglomeration of food powders such as powdered coffee and pudding. Cola drinks and flavored soft drinks often contain large quantities of phosphoric acid (E 338) as an acidifying agent. Such agents are given to lower the pH of food and thereby inhibit the growth of yeast, fungi, and bacteria.
Without added phosphate, cola drinks would be black
In particular, the phosphate that is added to cola drinks interrupts a glycation reaction, which, if unhindered, would produce so-called advanced glycation end products (AGE) and color the beverage pitch-black. Thus, cola drinks owe their brown color to phosphate. European regulations allow up to 700 mg/L of phosphate in cola drinks; if this much phosphate were added, one liter of cola would already provide 50% to 75% of the recommended daily allowance of phosphate for adults. The actual amount of phosphate added to each liter of cola is somewhat less, however, about 520 mg.
More than 300 food additives have been approved for use in Europe and given a uniform designation with an E number. According to the guidelines of the European Union, the E numbers of all food additives in packaged foods must be marked on the package. The EU eco-regulation further restricts the use of food additives for organically grown foods; among additives containing phosphate, only calcium phosphate can be used in organic food. The labeling requirement is, unfortunately, only qualitative, and not quantitative. The consumer, or the patient, cannot determine how much phosphate is actually present in each item, as neither the overall phosphate content nor the quantity of added phosphate is indicated.
The need to take action
In view of the known connection between dietary phosphate and organ calcification in patients with renal failure, as well as the growing realization that phosphate can damage health even in persons with normal kidneys, one may ask whether concrete interventions in health policy ought to be taken now, even though such steps cannot yet be supported by any findings from prospective interventional trials.
Public education and food labeling
One important step would be to inform physicians and the public thoroughly about the potential risks to cardiovascular and renal function arising from dietary phosphate consumption. Phosphate has long been known to elevate the cardiovascular risk in dialysis patients, but analogous effects have only recently been shown in persons with moderately impaired renal function (of whom the number is growing) and even in persons with normal renal function (6, 7, 23). The changing age structure of the population, with ever more elderly people, further deepens the implications of this problem for health policy, as does the high prevalence of “diseases of civilization,” such as diabetes mellitus, hypertension, and coronary heart disease, that damage the kidneys and accelerate the age-related decline of renal function. The link between phosphate and progressive renal failure was already suspected and investigated in the early 1980s (24, e10).
To raise public awareness of the health risks of phosphate, we recommend that relevant information should be provided through the mass media. It is important that the subject should be presented in a manner appropriate for laymen, yet without loss of scientific accuracy.
The labeling of phosphate content
Comprehensive labeling of phosphate additives in food—ideally, with a “traffic-light” scheme—would also be desirable, as would a quantitative restriction of phosphate additives. The amount of added phosphate, whether low, medium, or high, should be indicated with a green, yellow, or red sign on the package, as is currently done in Finland and the United Kingdom to indicate sodium chloride content. In order for such measures to be implemented, support should be sought from the food industry, consumer protection organizations, medical societies, and governmental and quasi-governmental entities (the statutory health insurance carriers, the German Federal Ministry of Health, and the European Union). The public is already well informed about the damaging effects of excessive salt consumption as a result of thorough scientific research and the effective public education efforts of medical institutions (25).
It remains to be clarified whether the association of a high serum phosphate concentration with increased morbidity and mortality reflects a direct toxic effect of phosphate or is rather due to pathological concentrations of the phosphate-regulating hormones FGF23 and klotho. Phosphate might thus also be a surrogate parameter for the functioning of this hormone system. We believe that comprehensive public education, with a scientifically well-grounded explanation of the adverse effects of high phosphate intake along with easily understandable labeling of the phosphate content of food, could help considerably to limit the damage done by this newly recognized cardiovascular risk factor.
Key Messages.
Elevated serum phosphate concentrations have recently been found to be correlated with mortality in patients with chronic renal failure, while high-normal serum phosphate concentrations have been found to be correlated with cardiovascular morbidity in the general population.
Unlike naturally occurring phosphate in food, which mainly takes the form of phosphate esters, phosphate in food additives is almost completely resorbed in the gastrointestinal tract.
Phosphate is thought to cause vascular damage and to induce aging processes.
Because of the potential damage to health from excessive phosphate consumption, a labeling requirement should be introduced for foods with added phosphate.
Although extensive pathobiological and epidemiological data are now available on this subject, data from prospective studies are still lacking.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Prof. Ritz has received consultant’s fees from the following companies: Daiichi Sankyo, Abbott, Mitsubishi Tanabe, Rofar, Medice, and Hexal.
Dr. Hahn has received consultant’s fees, lecture honoraria, and reimbursement of travel expenses and continuing medical education participation fees from Genzyme, Shire, and Medice.
Prof. Ketteler has received consultant’s fees and lecture honoraria from FMC, Genzyme, and Shire.
Prof. Kuhlmann has received consultant’s fees from Sanofi-Aventis, Roche, and Fresenius and lecture honoraria from Shire, Amgen, Roche, Fresenius, Braun, and Genzyme.
Prof. Mann states that he has no conflict of interest.
References
- 1.Sullivan C, et al. Effect of food additives on hyperphosphatemia among patients with end-stage renal disease: a randomized controlled trial. JAMA. 2009;301:629–635. doi: 10.1001/jama.2009.96. [DOI] [PubMed] [Google Scholar]
- 2.Block GA, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. JASN. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 3.Kestenbaum B, et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. JASN. 2005;16:520–528. doi: 10.1681/ASN.2004070602. [DOI] [PubMed] [Google Scholar]
- 4.KDIGO. Kidney Int Suppl. 2009. clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) pp. S1–S130. [DOI] [PubMed] [Google Scholar]
- 5.Noori N, et al. Association of dietary phosphorus intake and phosphorus to protein ratio with mortality in hemodialysis patients. CJASN. 2010;5:683–692. doi: 10.2215/CJN.08601209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foley RN, et al. Serum phosphorus levels associate with coronary atherosclerosis in young adults. JASN. 2009;20:397–404. doi: 10.1681/ASN.2008020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhingra R, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879–885. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 8.Tonelli M, et al. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez OM, et al. Low socioeconomic status associates with higher serum phosphate irrespective of race. JASN. 2010;21:1953–1960. doi: 10.1681/ASN.2010020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berndt T, Kumar R. Novel mechanisms in the regulation of phosphorus homeostasis. Physiology (Bethesda) 2009;24:17–25. doi: 10.1152/physiol.00034.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu S, Quarles LD. How fibroblast growth factor 23 works. JASN. 2007;18:1637–1647. doi: 10.1681/ASN.2007010068. [DOI] [PubMed] [Google Scholar]
- 12.Shimada T, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wetmore JB, Quarles LD. Calcimimetics or vitamin D analogs for suppressing parathyroid hormone in end-stage renal disease: time for a paradigm shift? Nat Clin Pract Nephrol. 2009;5:24–33. doi: 10.1038/ncpneph0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuro-o M, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 15.Kuro-o M. A potential link between phosphate and aging—lessons from Klotho-deficient mice. Mech Ageing Dev. 2010;131:270–275. doi: 10.1016/j.mad.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giachelli CM. The emerging role of phosphate in vascular calcification. Kidney Int. 2009;75:890–897. doi: 10.1038/ki.2008.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shroff RC, et al. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. JASN. 2010;21:103–112. doi: 10.1681/ASN.2009060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shuto E, et al. Dietary phosphorus acutely impairs endothelial function. JASN. 2009;20:1504–1512. doi: 10.1681/ASN.2008101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bohn L, Meyer AS, Rasmussen SK. Phytate: impact on environment and human nutrition. A challenge for molecular breeding. J Zhejiang Univ Sci B. 2008;9:165–191. doi: 10.1631/jzus.B0710640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lei XG, Porres JM. Phytase enzymology, applications, and biotechnology. Biotechnol Lett. 2003;25:1787–1794. doi: 10.1023/a:1026224101580. [DOI] [PubMed] [Google Scholar]
- 21.Kalantar-Zadeh K, et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. CJASN. 2010;5:519–530. doi: 10.2215/CJN.06080809. [DOI] [PubMed] [Google Scholar]
- 22.Sherman RA, Mehta O. Phosphorus and potassium content of enhanced meat and poultry products: implications for patients who receive dialysis. CJASN. 2009;4:1370–1373. doi: 10.2215/CJN.02830409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tonelli M, Pannu N, Manns B. Oral phosphate binders in patients with kidney failure. NEJM. 2010;362:1312–1324. doi: 10.1056/NEJMra0912522. [DOI] [PubMed] [Google Scholar]
- 24.Haut LL, et al. Renal toxicity of phosphate in rats. Kidney Int. 1980;17:722–731. doi: 10.1038/ki.1980.85. [DOI] [PubMed] [Google Scholar]
- 25.Klaus D, Hoyer J, Middeke M. Salt restriction for the prevention of cardiovascular disease. Dtsch Arztebl Int. 2010;107(26):457–462. doi: 10.3238/arztebl.2010.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e1.Uribarri J. Phosphorus homeostasis in normal health and in chronic kidney disease patients with special emphasis on dietary phosphorus intake. Semin Dial. 2007;20:295–301. doi: 10.1111/j.1525-139X.2007.00309.x. [DOI] [PubMed] [Google Scholar]
- e2.Ohnishi M, Razzaque MS. Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. FASEB J. 2010;24:3562–3571. doi: 10.1096/fj.09-152488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e3.Dhingra R, et al. Relations of serum phosphorus levels to echocardiographic left ventricular mass and incidence of heart failure in the community. Eur J Heart Fail. 2010;12:812–818. doi: 10.1093/eurjhf/hfq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e4.Prie D, Urena Torres P, Friedlander G. Latest findings in phosphate homeostasis. Kidney Int. 2009;75:882–899. doi: 10.1038/ki.2008.643. [DOI] [PubMed] [Google Scholar]
- e5.Martin KJ, Gonzalez EA. Prevention and control of phosphate retention/hyperphosphatemia in CKD-MBD: what is normal, when to start, and how to treat? Clin J Am Soc Nephrol. 2011;6:440–446. doi: 10.2215/CJN.05130610. [DOI] [PubMed] [Google Scholar]
- e6.Stubbs JR, et al. Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J Am Soc Nephrol. 2007;18:2116–2124. doi: 10.1681/ASN.2006121385. [DOI] [PubMed] [Google Scholar]
- e7.Moe SM, et al. Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney Int. 2002;61:638–647. doi: 10.1046/j.1523-1755.2002.00170.x. [DOI] [PubMed] [Google Scholar]
- e8.Kayne LH, et al. Analysis of segmental phosphate absorption in intact rats. A compartmental analysis approach. J Clin Invest. 1993;91:915–922. doi: 10.1172/JCI116313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e9.Calvo MS, Park YK. Changing phosphorus content of the US. diet: potential for adverse effects on bone. J Nutr. 1996;126(4):1168S–1180S. doi: 10.1093/jn/126.suppl_4.1168S. [DOI] [PubMed] [Google Scholar]
- e10.Alfrey AC. The role of abnormal phosphorus metabolism in the progression of chronic kidney disease and metastatic calcification. Kidney Int Suppl. 2004: S:13–17. doi: 10.1111/j.1523-1755.2004.09003.x. [DOI] [PubMed] [Google Scholar]