Abstract
Objectives
To assess the prognostic value of global magnetic resonance (MR) myocardial perfusion imaging (MPI) in women with suspected myocardial ischemia and no obstructive (stenosis <50%) coronary artery disease (CAD).
Background
The prognostic value of global MR-MPI in women without obstructive CAD remains unknown.
Methods
Women (n=100, mean age 57±11 years, range 31–76), with symptoms of myocardial ischemia and with no obstructive CAD as assessed by coronary angiography, underwent MR-MPI and standard functional assessment. During follow-up (34±16 months), time to first adverse event (death, myocardial infarction or hospitalization for worsening anginal symptoms) was analyzed using global MPI and left ventricular ejection fraction (EF) data.
Results
Adverse events occurred in 23 (23%). By univariable Cox proportional hazards regression modeling, variables found to be predictive of adverse events were global MR-MPI average uptake slope (p<0.05), the ratio of MR-MPI peak signal amplitude to uptake slope (p<0.05), and ejection fraction (EF) (p<0.05). Two multivariable Cox models were formed, one using variables that are performance-site dependent: ratio of MR-MPI peak amplitude to uptake slope together with EF (Chi-squared 13, p<0.005), and a model using variables that are performance-site independent: MR-MPI slope and EF (Chi-squared 12, p<0.005). Each of the two multivariable models remained predictive of adverse events after adjustment for age, disease history and Framingham risk score. For each of the Cox models, patients were categorized as high-risk if they were in the upper quartile of the model and not high-risk otherwise. Kaplan-Meier analysis of time to event was performed for high-risk vs. not high-risk for site-dependent (log rank 15.2, p<0.001) and site-independent (log rank 13.0, p<001) models.
Conclusions
Among women with suspected myocardial ischemia and no obstructive CAD, MR-MPI determined global measurements of normalized uptake slope and peak signal uptake, together with global functional assessment of EF appear to predict prognosis.
Keywords: Prognosis, Perfusion, Magnetic Resonance Imaging, Women
Introduction
In the United States, cardiovascular disease is the leading cause of mortality in women (1). However, women are less likely than men to have either classical symptoms or obstructive coronary artery disease (CAD) but paradoxically have a worse prognosis (2,3). This may be an expression of the failure to identify factors contributing to a woman’s risk (4–6). Conventional non-invasive testing in this population is particularly challenging due to the relatively low prevalence of obstructive CAD, coupled with the observations that first cardiovascular events are often fatal in women (7), and that stenoses <70% are associated with the majority of acute ischemic cardiac events (8). Thus, there is a need to develop risk assessment approaches for women to better assess risk of adverse events as opposed to being at high-risk for significant or obstructive CAD (9). The Women’s Ischemia Syndrome Evaluation (WISE) study has established that women who are symptomatic of ischemia but without obstructive CAD experience more adverse events than non-symptomatic controls (10).
Magnetic resonance myocardial perfusion imaging is an evolving high-resolution tomographic modality with no ionizing radiation that allows detection of regional hypoperfusion. We and others reported that obstructive CAD can be detected with a sensitivity and specificity comparable to conventional radionuclide imaging (11,12). A prior report suggested that MR-MPI can detect non-segmental, subendocardial abnormalities suggestive of myocardial ischemia in patients without obstructive CAD (13), however the relationship between this observation and risk has not been evaluated. We hypothesized that in a cohort of women with suspected ischemia but no obstructive epicardial CAD, global MR-MPI measures may predict prognosis.
Methods
Study Population
Symptomatic women with stable angina and suspected myocardial ischemia were prospectively enrolled in the NIH-NHLBI sponsored WISE study and underwent quantitative coronary angiography (QCA). From this enrolled population, 100 consecutive women with coronary artery stenoses <50% were selected for this sub-study and underwent first pass contrast MR-MPI within one week of enrollment. This prospective sub-study was performed at a single WISE site, the University of Alabama at Birmingham (UAB) between the dates of November 1993 and October 1998, and included WISE participants with no contraindications for MR examination. All subjects provided informed consent using forms and procedures approved by the Institutional Review Board at UAB.
Baseline Evaluation
The WISE methodology for acquisition of MR-MPI and QCA data has been described previously (14,15). In brief, demographic data, risk factors for ischemic heart disease, medical and reproductive history, functional capacity, and blood samples were acquired and evaluated. Coronary artery status was assessed qualitatively and quantitatively using cine angiographic films evaluated at the WISE angiographic core laboratory (Rhode Island Hospital, Providence, RI) (15).
MR-MPI Acquisition
Magnetic resonance first-pass MPI data were acquired using an optimized Philips ACS 1.5T scanner (Philips Medical System, Best, The Netherlands) using previously described methods (12). In brief, imaging was performed in the short-axis orientation during the passage of a bolus (0.1 mm/kg) of gadolinium contrast agent (ProHance, Berlex, New Jersey) using a power injector (Spectris® MR Injection System, Medrad Inc., Pittsburgh, PA). Imaging parameters included a field of view of 250–450 mm (depending on patient dimensions) and two slices imaged with slice selective saturation pulse applied 150 ms prior to imaging using a key-hole approach (TR/TE/flip 7/3.5/20°) to sample 32 lines of k-space inserted into a 1282 matrix. Data were acquired for each heartbeat and signal reception was accomplished using a body-coil. Stress hyperemia was induced by intravenous administration of dipyridamole (0.14 mg/kg/min or 0.56 mg/kg over 4 minutes). To maximize the hyperemic response, patients were instructed to abstain from methylxanthine (e.g. caffeinated and theobromine) containing foods such as coffee, tea and chocolate and from use of medications containing nitrates for at least 12 hours prior to the study. Myocardial perfusion status was analyzed at rest and under stress hyperemic conditions to extract myocardial perfusion variables.
MR-MPI Analysis
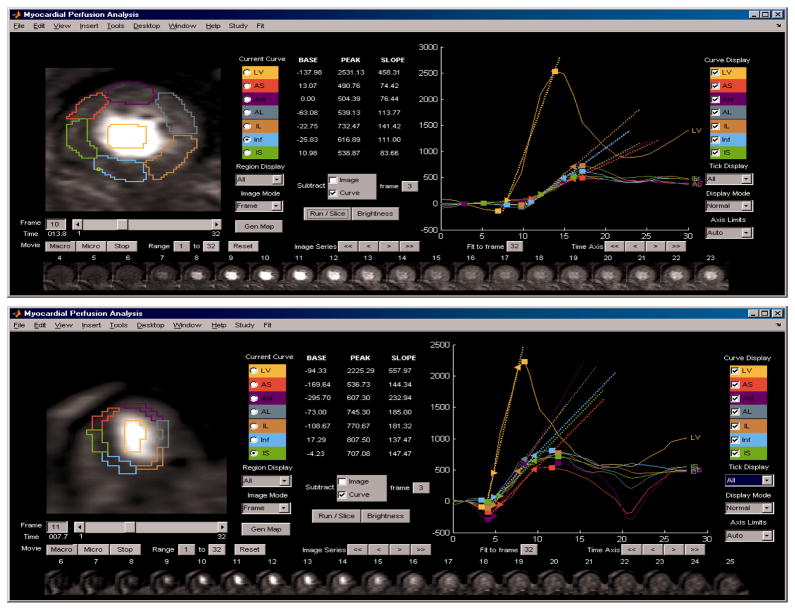
Global MPI indices, including average uptake slope and peak signal amplitude, were measured in the time-resolved MR data using custom-designed software developed at UAB (Figure 1). To compensate for respiratory motion, each image frame was displaced such that the left ventricle was registered over the time series. The uptake slopes were low-pass filtered to remove noise (body coil reception resulted in relatively high noise levels). Perfusion variables were extracted from six manually drawn, circumferentially defined, regions in each of two short-axis slices covering the mid and apical left ventricular (LV) sections. Intensity-time curves for each myocardial region were extracted by semi-automatically identifying the inflection point of signal increase and the peak signal amplitude achieved during the first pass. Using the uptake data between these two points, a linear line was curve-fitted and the slope of signal intensity per unit time was extracted. The uptake slope for each myocardial region was normalized by dividing by the slope of the LV blood pool signal (the dominant curve seen in Fig 1) and the normalized value scaled by multiplication by 1000 to make the values comparable to average peak signal intensity (arbitrary units). The peak signal intensity was calculated as the difference between mean baseline signal prior to contrast arrival and the peak signal intensity. Each parameter was evaluated separately at rest and at stress. All perfusion data were obtained and recorded prior to unmasking clinical and follow-up results.
Figure 1.
Magnetic resonance (MR) myocardial perfusion image (MPI) analysis, for, top panel, a patient with a low average MPI level and, lower panel, for a patient with a high average MPI level. Six myocardial regions and a left ventricular blood pool region are drawn on one frame (lower inset). Extracted and parameterized intensity-time curves are shown in the right-hand display section for the left ventricular blood pool (highest peak) and each of six myocardial sections (closely grouped uptake curves). In these patients no perfusion defects or obstructive stenoses were present, while the patient with low MPI subsequently died and the patient with high MPI survived event free.
MR Cardiac Function Acquisition
During the MRI scan, short axis cine views were acquired using a gradient-recalled echo (TR/TE/flip, 7/4/30) in multiple contiguous slices covering the base to the apex and oriented parallel to the base. The endocardial boundaries were planimetered using a commercial analysis package (Medis, The Netherlands) to extract end-diastolic and end-systolic volumes, and the ejection fraction (EF) calculated.
Follow-up Procedures
For follow-up, a scripted telephone interview was performed by experienced research coordinators at 6-weeks after enrollment and yearly thereafter. The events of interest included all-cause mortality, first incidence of myocardial infarction (MI) or hospitalization for worsening anginal symptoms. Median follow-up was 34±16 months. In the event of death, a death certificate and/or hospital record was obtained when available.
Statistical Analysis
Continuous values were presented as mean ± S.D. and categorical variables as percent frequency. Continuous clinical and demographic characteristics were compared between groups using the independent samples t-test; the chi-square test was used for categorical comparisons. The predictors of adverse events (death, myocardial infarction, hospitalization for worsening angina) were identified by univariable Cox regression analyses. Two multivariable Cox proportional hazard regression models (Cox) were formed for variables shown to be predictive in univariable analysis at the level p<0.05, one model incorporating performance-site-dependent variables, and one model incorporating performance-site-independent variables. Patients in the upper quartile of each of the two multivariable Cox models were designated high-risk, with not high-risk status assigned to all others. The proportional hazards assumption of invariant hazard ratios was found to be met for the high-risk and not-high risk predictors in the model. Kaplan-Meier analysis was performed to assess the effect of risk stratification on survival and tested using the log rank statistic. All p values are two-tailed. A p value <0.05 was considered to be statistically significant. Statistical analyses were performed using SPSS 14.0 (SPSS Inc., Chicago, Illinois).
Results
Population Characteristics
The mean age was 57±11 years (range 31–76); 30% were ethnic minorities, primarily African-Americans, and the average maximal stenosis level was 19%±19 (median= 22.0, range 0–49%). Demographic data are summarized in Table 1. Adverse events occurred in 23 women (23%) consisting of 19 hospitalizations for worsening anginal symptoms, 2 deaths, and 2 MIs. All deaths were deemed cardiovascular and were confirmed by the National Death Index (NDI). Also shown in Table 1 are the demographic values for event and event-free groups.
Table 1.
Population Characteristics of Women
| Variable | Total (n = 100) | Event-Free (n=77) | Adverse Event (n=23) |
|---|---|---|---|
| Black or Hispanic (%) | 30 | 30 | 30 |
| Age (years) (mean±SD)† | 57±11 | 57±10 | 58±12 |
| Hypertension (%) | 66 | 63 | 74 |
| Dyslipidemia (%) | 50 | 49 | 73 |
| History of Smoking (%) | 47 | 43 | 61 |
| Obesity (Body Mass Index ≥30) (%) | 42 | 39 | 52 |
| Diabetes Mellitus (%) | 20 | 19 | 22 |
| Typical Angina Presentation (%) | 34 | 41§ | 13§ |
| Family History of Premature CAD* (%) | 76 | 75 | 78 |
| Coronary artery stenosis (%)(mean±SD)† | 19±19 | 18±19 | 21±21 |
| Left Ventricular Ejection Fraction (%)(mean±SD)† | 62±9 | 64±7§ | 59±12§ |
| Left Ventricular Ejection Fraction less than 55% (%) | 15 | 12 | 26 |
| Resting Heart Rate (BPM)‡ (mean±SD)† | 68±13 | 67±12 | 71±16 |
| Hyperemic Heart Rate (BPM)‡ (mean±SD)† | 84±13 | 83±12 | 85±14 |
| Systolic Blood Pressure (mean±SD)† | 137±22 | 137±22 | 138±22 |
| Diastolic Blood Pressure (mean±SD)† | 75±11 | 75±11 | 76±10 |
| ATP III 10 year risk of MI** | 4.5±4.2 | 4.4±4.4 | 4.9±3.9 |
CAD=coronary artery disease,
SD = standard deviation,
Beats per minute,
Adult Treatment Panel,
indicates a p values less than 0.05 between event-free and adverse event groups
Site-Dependent MR-MPI and Cardiac Function Predictors of Adverse Events
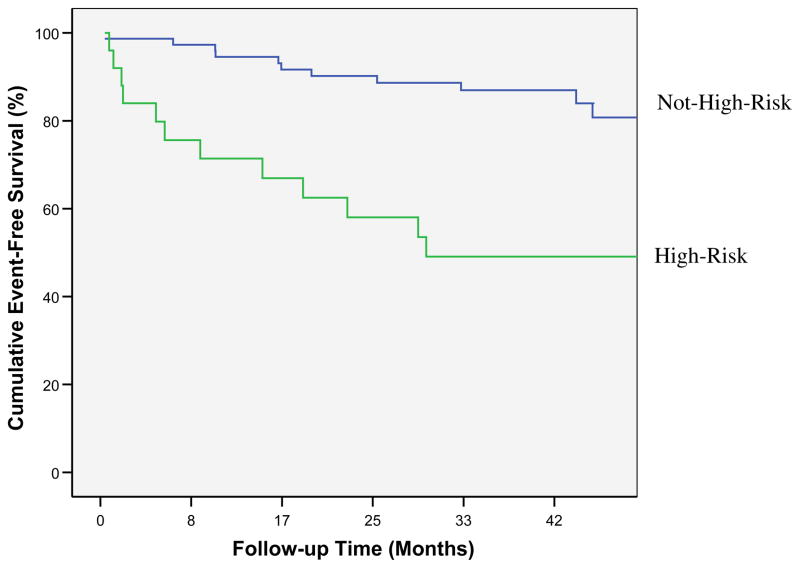
Imaging variables predictive of events by univariable Cox regression modeling were: the global MR-MPI ratio of average peak signal to normalized uptake slope at stress (p<0.05) and EF (p<0.05) (Table 2). Since the peak signal amplitude is measured in arbitrary units, it is a performance-site dependant variable. Using the forward selection method the variables EF and the ratio of average peak signal to normalized uptake slope were entered to form a multivariable Cox model (chi-square13.2, p<0.001). The multivariable Cox model was divided into quartiles and those in the highest risk quartile were categorized as high-risk, with all others categorized as not high-risk. Table 3 compares demographic values between high-risk and not high-risk patients. Kaplan-Meier survival curves indicated that there was a significant difference in time to adverse event between risk groups, Figure 2 (log rank = 15, p<0.001). Annualized event rates were 12% for those in the high-risk group compared with 4% for patients in the not high-risk group. In the high-risk group there were two deaths and 10 hospitalizations for worsening angina, while in the low-risk group, there were two myocardial infarctions and 9 hospitalizations for worsening angina.
Table 2.
Cox Model Parameters
| PREDICTOR | HAZARD RATIO | 95% LOWER CI | 95% UPPER CI |
|---|---|---|---|
| EF | 0.949 | 0.911 | 0.990 |
| Amp/slope *1000 | 0.516 | 0.314 | 0.848 |
| Slope *1000 | 1.005 | 1.001 | 1.009 |
CI= confidence interval
Table 3.
Population Characteristics of Women in High-Risk vs. Not High-Risk Groups
| Variable | Not High-Risk (n=75) | High-Risk (n=25) |
|---|---|---|
| Black or Hispanic (%) | 25 | 44 |
| Age (years) (mean±SD)† | 58±11 | 55±11 |
| Hypertension (%) | 67 | 64 |
| Dyslipidemia (%) | 53 | 60 |
| History of Smoking (%) | 40§ | 68§ |
| Obesity (Body Mass Index ≥30) (%) | 36§ | 60§ |
| Diabetes Mellitus (%) | 17 | 28 |
| Typical Angina Presentation (%) | 36 | 28 |
| Family History of Premature CAD* (%) | 75 | 79 |
| Coronary artery stenosis (%)(mean±SD)† | 19±20 | 20±20 |
| Left Ventricular Ejection Fraction (%)(mean±SD)† | 65±7# | 57±102# |
| Left Ventricular Ejection Fraction less than 55% (%) | 9# | 32# |
| Resting Heart Rate (BPM)‡ (mean±SD)† | 66±12§ | 72±16§ |
| Hyperemic Heart Rate (BPM)‡ (mean±SD)† | 82±13§ | 90±13§ |
| Systolic Blood Pressure (mean±SD)† | 135±21 | 144±25 |
| Diastolic Blood Pressure (mean±SD)† | 73±11§ | 79±8§ |
| ATP III 10 year risk of MI** | 4.7±4.5 | 3.8±3.4 |
CAD=coronary artery disease,
SD = standard deviation,
Beats per minute,
Adult Treatment Panel,
indicates a p values less than 0.05 and
indicates a p value less than 0.001 between not high-risk and high-risk groups
Figure 2.
Kaplan-Meier cumulative event-free survival curves for adverse events (p<0.005) in high-risk and not high-risk patients stratified by highest quartile of Cox model incorporating the ratio of MR-MPI average amplitude to normalized uptake slope and ejection fraction.
Site-Independent MR-MPI and Cardiac Function Predictors of Adverse Events
Since the MR-MPI variable of normalized uptake slope is a ratio, it can be measured in a site-independent manner. The normalized uptake slope at stress was entered into a univariable Cox model, and was predictive of events (p<0.05), Table 2. We formed a multivariable Cox regression model by conditionally entering normalized uptake slope and EF (chi-square12.0, p<0.001). The multivariable Cox model was divided into quartiles, and those in the highest quartile were categorized as high-risk, with all others categorized as not high-risk. Kaplan-Meier survival analysis showed significant difference in time to adverse event between high-risk vs. not high-risk groups (log rank = 13.0, p<0.001). Annualized event rates were 12% for those in the high-risk group compared with 4% for those in the not high-risk group.
Discussion
Ours is the first study to show that global MR-MPI variables are predictive of adverse events among women with suspected ischemia but no obstructive CAD. This is an important observation given the controversy in the current literature concerning the existence of MR-MPI detected perfusion defects in similar populations of patients. One recent study demonstrated a strong correlation between perfusion defects by MR-MPI and impaired coronary microvascular function (16), while another study failed to demonstrate these correlations (17). In a recent editorial, Pennell addressed the divergent opinions by noting the importance of correctly categorizing patient populations (18). The current study represents a well-studied and categorized population of women with symptoms of ischemia and objective evidence of no obstructive CAD, assessed using variables measured in core laboratories and using standardized collection and data analyses methods.
We showed that when considering MPI parameters at stress, averaged over all regions, possessing a low uptake signal with a steep normalized uptake slope was associated with increased events (i.e. a sharp uptake of perfusion, but only reaching a low level). These conditions correspond to low perfusion levels, possibly representative of abnormal subendocardial perfusion secondary to microvascular coronary dysfunction (19). These globally low perfusion conditions would normally not be detected by standard comparative analyses aimed at identifying segmental perfusion abnormalities. Thus, when using the MPI data to evaluate patients with suspected ischemia, two separate analyses should be performed, one to detect low perfusion regions consistent with obstructive CAD and one to detect globally low myocardial perfusion conditions which are independently associated with adverse events.
In addition to globally low perfusion conditions, the highest risk patients had a low EF value. From Table 3 it is apparent that high-risk patients tended to be obese, with a history of smoking, and higher resting and stress heart rates. High-risk patients were those with MPI and EF parameters consistent with the presence of microvascular coronary dysfunction. Importantly, a comprehensive evaluation of regional and global perfusion conditions, along with functional status, can be performed non-invasively during a single MRI examination.
Pathophysiologic mechanisms to explain the relatively high prevalence of adverse events associated with abnormal global MR-MPI measures and poor cardiac function include primary myocyte and/or microvascular coronary dysfunction even in the absence of obstructive CAD. Other WISE data suggest that patients with symptoms of ischemia but no obstructive CAD likely have microvascular coronary dysfunction (20), which is associated with an adverse prognosis (21–22). More work is needed to characterize both the pathophysiology and noninvasive diagnostic tools such as global MR-MPI.
Current diagnostic modalities used to detect myocardial ischemia include radionuclide perfusion and echocardiography imaging. The diagnostic accuracy of these tests in women is limited by the optimization of these techniques for the detection of segmental abnormalities most correlative to obstructive CAD, as well as the confounding effects in radionuclide imaging tissue attenuation (23). Cardiac MR imaging has excellent soft tissue characterization and contrast, three-dimensionality and overall good temporal and spatial resolution. Prior studies document its utility for detection of segmental perfusion defects (12), and the current results suggest that cardiac MR may uniquely be positioned to detect global perfusion abnormalities that are of prognostic significance.
Clinical Relevance
Women with signs and symptoms suggestive of myocardial ischemia, but no obstructive CAD, present a major challenge because current management is largely based on the degree of obstructive CAD visualized by traditional coronary angiography. Recent study demonstrates that there is a sizable subset of patients, often female, with evidence of ischemia but no obstructive CAD that have an adverse prognosis. We showed that global, non-traditional myocardial perfusion variables predicted events. Further validation of these results, with application in patients with suspected ischemia but no obstructive CAD, could enhance diagnosis and prognosis abilities over current modalities.
Limitations
The sample size and number of events are relatively small and current results are underpowered to detect the relationship between specific MR-MPI measures and serious adverse events. Due to the small sample size and low number of events, it was not feasible to consider covariates in the Cox models. Improvements in MR-MPI resolution and extent of myocardium covered have occurred since these data were collected that now allow analyses to be performed separately for endocardial, mid and epicardial layers, and should provide improved evaluation and understanding of myocardial perfusion. The relatively low resolution of our perfusion images in the current study precluded performing separate analyses for subendocardial versus other myocardial layers. No late gadolinium enhancement was performed to detect nonviable myocardium. Since a body coil was used for acquisition, perfusion uptake signal required filtering to remove excessive noise. Cut points for not high-risk versus high-risk patients for adverse events by global MR-MPI and functional variables were defined using clinical outcomes data for adverse events. This methodology was then used to retrospectively predict outcome and is likely to over-estimate the strength of the model. Even lesions less than 50% could progress during the follow-up time and be explanatory of events.
Conclusions
Among women with suspected ischemia but no obstructive CAD, global MR-MPI perfusion abnormalities predict adverse events. These data add to the literature and suggest that global MR-MPI abnormalities may be indicative of global myocardial ischemia possibly secondary to microvascular coronary dysfunction.
Acknowledgments
This work was supported by contracts from the National Heart, Lung and Blood Institutes, nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, RO1-HL-073412-01, grants U0164829, U01 HL649141, U01 HL649241, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, New Jersey, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, California, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, Pennsylvania, and QMED, Inc., Laurence Harbor, New Jersey, and the Edythe L. Broad Endowment, Cedars-Sinai Medical Center, Los Angeles, California, and the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles.
ABBREVIATIONS
- au
Arbitrary Units
- CI
Confidence Intervals
- CAD
Coronary Artery Disease
- MI
Myocardial Infarction
- MPI
Myocardial Perfusion Imaging
- MR
Magnetic Resonance
- QCA
Quantitative Coronary Artery Angiography
- SD
Standard Deviation
- UAB
University of Alabama at Birmingham
- WISE
Women’s Ischemia Syndrome Evaluation
Footnotes
Conflict of Interest Disclosures: We are grateful to Bracco, Princeton, NJ, for providing the ProHance contrast agent.
References
- 1.Mieres JH, Shaw LJ, Arai A, Budoff MJ, et al. Cardiac Imaging Committee. Role of Noninvasive Testing in the Clinical Evaluation of Women With Suspected Coronary Artery Disease Consensus Statement From the Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Circulation. 2005;111:682–696. doi: 10.1161/01.CIR.0000155233.67287.60. [DOI] [PubMed] [Google Scholar]
- 2.Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. New England J Med. 1999;341(4):217–25. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Smith SC, Jr, Cooper RS, Hill MN, Luepker RV. Task force #1--magnitude of the prevention problem: opportunities and challenges. 33rd Bethesda Conference. J Am Coll Cardiol. 2002;40(4):588–603. doi: 10.1016/s0735-1097(02)02082-x. [DOI] [PubMed] [Google Scholar]
- 4.Gordon EE. Coronary artery disease in women: the role of diagnostic imaging. Echocardiography. 1993;10(3):321–30. doi: 10.1111/j.1540-8175.1993.tb00043.x. [DOI] [PubMed] [Google Scholar]
- 5.Shaw LJ, Hachamovitch R, Redberg RF. Current evidence on diagnostic testing in women with suspected coronary artery disease: choosing the appropriate test. Cardiol in Rev. 2000;8(1):65–74. doi: 10.1097/00045415-200008010-00011. [DOI] [PubMed] [Google Scholar]
- 6.Mieres JH, Shaw LJ, Hendel RC, et al. American Society of Nuclear Cardiology Task Force on Women and Heart Disease Writing Group on Perfusion Imaging in Women. A report of the American Society of Nuclear Cardiology Task Force on Women and Heart Disease. J Nucl Cardiol. 2003;10(1):95–101. doi: 10.1067/mnc.2003.130362. [DOI] [PubMed] [Google Scholar]
- 7.Mosca L, Grundy SM, Judelson D, et al. Guide to preventive cardiology for women: AHA/ACC Scientific Statement: consensus panel statement. Circulation. 1999;99:2480–2484. doi: 10.1161/01.cir.99.18.2480. [DOI] [PubMed] [Google Scholar]
- 8.Fishbein MC, Siegel RJ. How Big Are Coronary Atherosclerotic Plaques That Rupture? Circulation. 1996;94:2662–2666. doi: 10.1161/01.cir.94.10.2662. [DOI] [PubMed] [Google Scholar]
- 9.Shaw LJ, Peterson ED, Mark DB. Clinical Recognition: Risk Assessment Screening. In: Wilansky S, Willerson JT, editors. Heart Disease in Women. Churchill Livingstone; New York: 2002. [Google Scholar]
- 10.Gulati M, Cooper-DeHoff RM, McClure C, et al. Adverse Cardiovascular Outcomes in Women With Nonobstructive Coronary Artery Disease. Arch Intern Med. 2009;169(9):843–850. doi: 10.1001/archinternmed.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagel E, Klein C, Paetsch I, et al. Magnetic Resonance Perfusion Measurements for the Noninvasive Detection of Coronary Artery Disease. Circulation. 2003;108:432–437. doi: 10.1161/01.CIR.0000080915.35024.A9. [DOI] [PubMed] [Google Scholar]
- 12.Doyle M, Fuisz A, Kortright E, et al. for the WISE Study Group. The Impact of Myocardial Flow Reserve on the Detection of Coronary Artery Disease by Perfusion Imaging Methods: An NHLBI WISE Study. J Card Magn Reson. 2003;5(3):475–485. doi: 10.1081/jcmr-120022263. [DOI] [PubMed] [Google Scholar]
- 13.Panting JR, Gatehouse PD, Yang GZ, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. NEJM. 2002;346:1948–1953. doi: 10.1056/NEJMoa012369. [DOI] [PubMed] [Google Scholar]
- 14.Bairey Merz CN, Kelsey SF, Pepine CJ, et al. The Women’s ischemia syndrome evaluation (WISE) study: protocol design, methodology and feasibility report. J Am Coll Cardiol. 1999;33:1453–1461. doi: 10.1016/s0735-1097(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 15.Sharaf BL, Pepine CJ, Kerensky RA, et al. Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation [WISE] Study Angiographic Core Laboratory) Am J Cardiol. 2001;15,87(8):937–41. doi: 10.1016/s0002-9149(01)01424-2. [DOI] [PubMed] [Google Scholar]
- 16.Lanza Gaetano A, Buffon Antonino, Sestito Alfonso, et al. Relation Between Stress-Induced Myocardial Perfusion Defects on Cardiovascular Magnetic Resonance and Coronary Microvascular Dysfunction in Patients With Cardiac Syndrome X. J Am Coll Cardiol. 2008;51:466–472. doi: 10.1016/j.jacc.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 17.Vermeltfoort Ilse AC, Bondarenko Olga, Raijmakers Pieter GHM, et al. Is subendocardial ischaemia present in patients with chest pain and normal coronary angiograms? A cardiovascular MR study. European Heart Journal. 2007;28(13):1554–1558. doi: 10.1093/eurheartj/ehm088. [DOI] [PubMed] [Google Scholar]
- 18.Pennell Dudley J. Perfusion Abnormality, Normal Coronaries, and Chest Pain. J Am Coll Cardiol. 2008;51:473–475. doi: 10.1016/j.jacc.2007.09.049. [DOI] [PubMed] [Google Scholar]
- 19.Pepine CJ, Kerensky RA, Lambert CR, et al. Some thoughts on the vasculopathy of women with ischemic heart disease. J Am Coll Cardiol. 2006;47:S30–5. doi: 10.1016/j.jacc.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 20.Reis SE, Holubkov R, Lee JS, et al. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary artery disease. JACC. 1999;33:1469–75. doi: 10.1016/s0735-1097(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 21.Johnson BD, Shaw LJ, Buchthal SD, et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circ. 2004;109:2993–9. doi: 10.1161/01.CIR.0000130642.79868.B2. [DOI] [PubMed] [Google Scholar]
- 22.von Mering GO, Arant CB, Wessel TR, et al. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004 Feb 17;109(6):722–725. doi: 10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- 23.Shaw LJ, Bairey Merz CN, Pepine CJ, et al. Insights from the NHLBI-sponsored women’s ischemia syndrome evaluation (WISE) study. JACC. 2006;47:4S–20S. [Google Scholar]