Abstract
Mutations in the pattern of CpG methylation imprinting of the human genome have been correlated with a number of diseases including cancer. In particular, aberrant imprinting of tumor suppressor genes by gain of CpG methylation has been observed in many cancers and thus represents an important alternative pathway to gene mutation and tumor progression. Inhibitors of DNA methylation display therapeutic effects in the treatment of certain cancers and it has been assumed that these effects are due to the reversal of mutant gene imprinting. However, significant reactivation of imprinted tumor suppressor genes is rarely observed in vivo following treatment with DNA methylation inhibitors. A recent study revealed an unexpected requirement for CpG methylation in the synthesis and assembly of the ribosome, an essential function for cell growth and proliferation. As such, the data provide an unforeseen explanation of the action of DNA methylation inhibitors in restricting cancer cell growth.
Key words: DNA methylation, meCpG, DNA methyltransferase-inhibition, DNMT1-/-, DNMT3b-/-, aza-deoxycytidine, gene silencing, ribosome biogenesis, cancer therapy
Introduction
Epigenetic modification of nuclear chromatin is responsible for the transcriptional silencing of a large part of the human genome. Establishment of this silencing is an essential aspect of human development and, as such, must be inherited over many cell generations. Epigenetic silencing is established by a combination of the post-translational modification of the histone proteins of nuclear chromatin and the direct methylation of the underlying genomic DNA at CpG dinucleotides. However, it is predominantly DNA methylation that establishes the heritable imprint of genome silencing.1 “Mutations” in this imprint have been correlated with a number of diseases including cancer2 and both loss and gain of CpG methylation have been observed in human cancers.3–5 As tumor cells increase their invasive properties, they display a tendency to lose CpG methylation. This leads to the reactivation of imprinted genes and to genomic instability, both factors known to contribute to tumor progression. However, the gain of CpG methylation at certain tumor suppressor genes, such as CDKN2A, SFRPs and GATA4 and 5, is also observed in many cancers and provides an important alternative pathway to cell transformation by aberrantly silencing these genes.4,5 These latter findings have stimulated the search for drugs capable of correcting such epigenetic mutations. Inhibitors of DNA methylation such as 5-azacytidine and 5-aza-2′-deoxycytidine have been found to reactivate imprinted genes when used in vitro and to display promising therapeutic effects in the treatment of certain human cancers.3–6 Thus, it has been generally assumed that the therapeutic effects of these drugs are due to the demethylation and reactivation of epigenetically imprinted tumor suppressor genes. However, recent data question this assumption since these drugs do not appear to induce significant demethylation and reactivation of imprinted tumor suppressor genes in vivo.5,7–10 Gagnon-Kugler et al.11 recently revealed an unexpected requirement for CpG methylation in the synthesis of the ribosome or ribosome biogenesis, an essential function for cell and tumor growth. The data provide a significant insight into the functioning of the ribosomal RNA (rRNA) genes and present a compelling alternative explanation for the ability of DNA methylation inhibitors to restrict tumor growth.
Establishment of Epigenetic Imprinting
How the stable patterns of CpG methylation are established in mammals is still somewhat unclear. At least in part they are determined by local modification of the four core histones of nuclear chromatin. In particular, methylation of lysine 4 of histone H3 (H3K4me) correlates with gene activation, while lysine 9 methylation (H3K9me) correlates with inactivation.1 Maintenance of the H3K9me modification leads to methylation of the underlying DNA sequences at CpG dinucleotides and determines stable, heritable gene silencing. In mammals, CpG dinucleotides are statistically underrepresented in the genome and most are found methylated on the C5 of the cytosine ring. The major exceptions to this are the “CpG islands,” short relatively CpG-rich DNA segments associated with active and potentially active genes.12 The initial genome-wide pattern of DNA methylation is established during early development, but de novo methylation continues throughout the life span of an individual and may be associated with aging.13–15 Once established, CpG methylation patterns are maintained during DNA replication by a copying mechanism and, in this way, are able to provide a long-term memory of the epigenetic program. Methylation of single copy DNA is largely erased in the germline, presumably to re-establish the pluripotent state.16–19 However, repetitive DNA sequences such as satellite DNAs, centromeric and telomeric sequences and the many hundreds of rRNA genes exhibit significant levels of DNA methylation even in the germline. 16,20 An inability to maintain the methylation of these repetitive DNAs is correlated with genome instability and disease in humans.16
The rRNA Genes and Their Expression
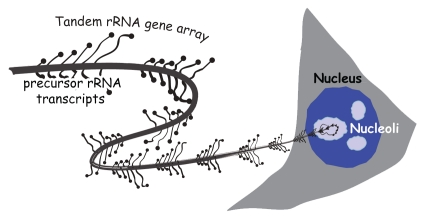
The rRNA genes, globally referred to as the rDNA (Fig. 1), present the special case of a repetitive DNA that is subject both to saturation levels of transcription and to stringent epigenetic silencing. This apparent contradiction is possible since these genes are transcribed exclusively by RNA polymerase I (RPI or PolI) and their transcription by the other two nuclear RNA polymerases (RPII/PolII and RPIII/PolIII) is stringently suppressed. Each rRNA gene encodes the 18S, 5.8S and 28S catalytic RNAs of the ribosome as part of a single precursor rRNA that is cleaved and assembled with over 80 ribosomal proteins into the two subunits of the ribosome.21 Ribosome biogenesis engages around 50% of the total transcriptional capacity of a proliferating human cell. Around 35% of this transcription is dedicated to producing the rRNAs and the rest to the synthesis of the mRNAs encoding the several hundred proteins required to assemble the ribosome. As such, the activity of the rRNA genes is essential for cell growth and proliferation and is strictly regulated by nutrients, growth factors and by tumor suppressors.21,22 The rRNA genes of eukaryotes from yeast to human exist within the genome as one or more tandem repeats. For example, yeast has a single tandem array of 150 rRNA genes that occupies the majority of the long arm of chromosome 12, the amphibian Xenopus laevis has a single array of ∼500 genes laying at the secondary constriction on the short arm of chromosome 3,23 while closely related Xenopus species have several rRNA gene arrays lying on multiple chromosomes. The mouse and human haploid genomes each contain around 200 rRNA genes laying in tandem arrays at the secondary constrictions, the so-called nucleolar organizers, or nors, of five different chromosomes.24,25 In all species, the rRNA genes are subject to silencing at two distinct levels.21,26 The first is the complete or near-complete inhibition of transcription by RPII (PolII); I will call this “RPII exclusion.” The second silencing level limits the number of rRNA genes that are available for productive rRNA synthesis by RPI (PolI); this will be referred to as “transcriptional silencing.”
Figure 1.
Schematic representation of an actively transcribed tandem array of rRNA genes spooling out from the nucleolus of a cell. Each gene is shown with a number of characteristic lateral transcripts.
Transcriptional Silencing of the rRNA Genes
How eukaryotes determine the number of rRNA genes that remain available for transcription has been the subject of extensive study.27,28 In the absence of DNA methylation, transcriptional silencing of the rRNA genes of yeast is in large part defined by histone modification. In mammals, amphibians and plants, DNA methylation has also been shown to enforce silencing as well as to determine nucleolar dominance, e.g., the choice of the rRNA gene arrays expressed in plant hybrids (reviewed in refs. 21 and 26). The biologically relevant question, why eukaryotes silence a large fraction of their rRNA genes, has attracted much less attention. In general, it has been tacitly assumed that regulation of the number of actively transcribed rRNA genes is a mechanism to control rRNA synthesis. However, no natural circumstance, whether in mammals, plants or yeast, has yet been described in which all, or even most, of the rRNA genes are actively transcribed.21,29 Engineered laboratory strains of yeast carrying as few as 20 of their normal 150 rRNA genes display no growth rate limitation even in rich medium30 (Griesenbeck J, personal communication) and viable laboratory mice strains may lack two of their five wild-type rRNA arrays.14 Further, significant growth factor upregulation of rRNA gene transcription does not require de novo gene activation.29 Thus, it is extremely unlikely that the excessively large numbers of rRNA genes present within eukaryotic genomes are required for rRNA synthesis. Rather, the high copy number of rRNA genes is probably necessary to fulfill some other function. In support of this conclusion, the maintenance of a large number of transcriptionally silent rRNA genes in yeast was recently shown to be essential for sister chromatid cohesion, recombinational repair and genome stability.30
Two Modes of rRNA Gene Silencing
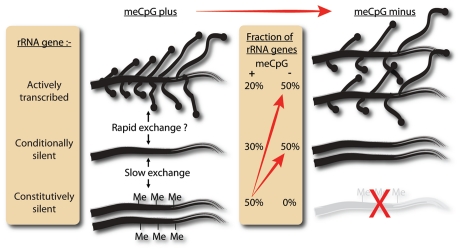
In human, as in most other mammals, a fraction of the rRNA genes are epigenetically imprinted by hypermethylation at CpGs and this hypermethylation has been shown to enforce transcriptional silencing.27 Since hypermethylation of the rRNA genes, as for other repetitive DNAs, is stably inherited, it clearly could not play any dynamic role in regulating the number of active rRNA genes. Thus, why are some of the mammalian and, in particular, the human rRNA genes silenced by hypermethylation? In an attempt to answer this question, Gagnon-Kugler et al.11 sought a robust system in which the effects of the loss of this hypermethylation could be studied. The use of antisense reagents against DNA methyltransferases (DNMTs), the enzymes responsible for CpG methylation, has been inconclusive,31,32 and small molecule inhibitors such as aza-deoxycytidine and related compounds display poor target specificity and high cytotoxicity.5,33 Luckily, the laboratory of B. Vogelstein had undertaken the difficult work of establishing human HCT116 colorectal carcinoma cell lines lacking both DNA methyltransferases DNMT1 and 3b.34,35 Analysis of CpG methylation in DNMT1-/-, DNMT3b-/- (DKO) cells showed that all 58 CpG dinucleotides across the rRNA gene promoter were unmethylated.11 In contrast, parent HCT116 cells revealed two distinct rRNA gene categories, one displaying near full methylation at all 58 sites and the other no methylation at any of these sites (Fig. 2).
Figure 2.
The distribution of the ∼200 human rRNA genes of HCT116 cells among actively transcribed, conditionally silent (unmethylated) and constitutively silent (methylated) categories. Left: data for the meCpG positive parent HCT116 cells. Right: data for the DNMT1-/-, DNMT3b-/- (DKO) cells. The gene diagrams indicate (1) the relative number of transcription complexes per actively transcribed gene [=RPI (PolI) loadings] and (2) the relative number of genes in each category. The probable rates of exchange between the different gene categories is also indicated. “Slow exchange” indicates that exchange could take place only after several cell generations, while “fast exchange” might occur within minutes. Whether such exchange actually happens is still unknown.
Not unexpectedly, the lack of rRNA gene methylation in DKO cells corresponded with a significant increase in the number of actively transcribed rRNA genes. However, half of the genes still remained transcriptionally silent (Fig. 2). Clearly, human rRNA genes are transcriptionally silenced by at least two distinct mechanisms: one dependent on CpG methylation and the other independent of it. The situation of rRNA gene silencing in DKO cells thus resembles that in yeast, where a large fraction of rRNA genes also maintain transcriptional silence in the absence of DNA methylation.
Loss of DNA Methylation Suppresses rRNA Synthesis and Processing and Enhances Recombination
Despite the persistence of rRNA gene silencing, Gagnon-Kugler et al.11 found that the DKO cells actively transcribe more than twice as many genes as the parent HCT cells (Fig. 2). Hence, these cells might be expected to display increased rRNA synthesis and grow more rapidly. In fact, the contrary is true: DKO cells grow much more slowly than the parent cells.35 Consistent with this, rRNA synthesis rates in DKO cells are strongly suppressed,11 demonstrating again a lack of correlation between the number of active genes and rRNA transcription rate. The reduced rate of rRNA transcription in DKO cells was later found to be due to a slowing of the transcription elongation rate, a previously recognized major mode of rRNA gene regulation in human, mouse and yeast.36,37
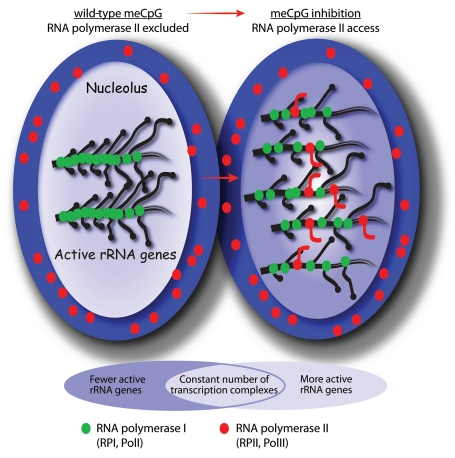
In addition to displaying reduced rRNA gene transcription, DKO cells display an unexpected defect in rRNA processing.11 These cells aberrantly accumulate precursor rRNAs that are either not processed or are processed extremely slowly. Why loss of DNA methylation should affect rRNA processing was unclear until it was found that the rRNA genes of DKO cells are also aberrantly transcribed by RPII. The RPII exclusion mode of silencing is then clearly defective in DKO cells. In these cells RPII transcripts derive from both strands of the rRNA genes and from coding and non-coding regions alike (Fig. 3). These RPII transcripts are the direct cause of the processing defect, since exogenous expression of similar transcripts in parent HCT116 cells reproduced the defect in processing of the precursor rRNA. Why this aberrant RPII transcription affects rRNA processing could have several explanations. However, as a very early step in processing, several hundred bases of the precursor rRNA are methylated or converted to pseudo-uridine, and this modification process requires the help of hundreds of small nucleolar guide RNAs (snoRNAs).38 The precursor rRNAs accumulating in DKO cells appear to be unmethylated,11 suggesting that their processing is blocked upstream of this modification step. It is then possible that aberrant RPII transcripts produced in the DKO cells prevent rRNA modification by acting as antisense competitors for the snoRNA guides. Alternatively, RPII transcripts might interact non-productively with ribosome assembly proteins. RPII transcription of the yeast rRNA gene locus is also known to enhance recombination, and hence cause genomic instability and cellular senescence.39 A similar enhancement in the level of rRNA gene recombination was noted in DKO cells. Thus, loss of DNA methylation not only suppresses rRNA synthesis and processing, but may also destabilize the human genome.
Figure 3.
A summary of the changes occurring in the cell nucleus when CpG methylation is prevented or inhibited. On the left the meCpG positive cells maintain a stringent compartmentalization of RPI and RPII transcription, the former in the nucleolus (light blue) on the rRNA genes, the latter in the nucleoplasm (dark blue). Loss of CpG methylation increases the number of active rRNA genes, but not the number of RPI (green) transcription complexes, and allows the incursion of RPII (red) and the production of aberrant RNA transcripts. The rRNA genes are normally densely occupied by RPI transcription complexes and this acts to exclude RPII, while the increase in the number of active rRNA genes after loss of DNA methylation reduces the density of RPI complexes and allows access to RPII with consequent effects on rRNA processing and ribosome biogenesis.
Defects in Ribosome Biogenesis May Explain the DKO Slow Growth Phenotype
The slow growth phenotype of DKO cells is believed to be due to the reactivation of the CDKN2A tumor suppressor gene, the single copy of which is epigenetically repressed by DNA methylation in the HCT116 colorectal carcinoma cells.35 However, data of Gagnon-Kugler et al.11 point to an alternative explanation, that of a defect in the ability to produce ribosomes. The proliferation rate of a cell displays a second order relationship with ribosome biogenesis, a two-fold increase in proliferation requiring a four-fold increase in the production of ribosomes.40 Thus, the defects in ribosome biogenesis are very probably an important cause of the slow growth phenotype of DKO cells.
DNMT Inhibitors Also Target Ribosome Biogenesis
The data of Gagnon-Kugler et al.11 clearly show that the elimination of CpG methylation has unexpected effects on ribosome biogenesis and genome stability, and this likely limits growth and proliferation. Analogs of aza-deoxycytidine induce untargeted DNA demethylation and would therefore also be expected to affect ribosome biogenesis. Consistent with this, treatment of HCT116 cells with aza-deoxycytidine closely simulated the effects of loss of DNA methylation. Aza-deoxycytidine reactivated the methylated fraction of the silent rRNA genes. This reactivation was associated with a suppression of rRNA synthesis and with defective rRNA processing.11
Ribosome Biogenesis: A Therapeutic Target of DNA Methylation Inhibitors?
As mentioned above, recent data from clinical evaluations of DNA methyltransferase inhibitors such as aza-deoxycytidine questions the assumption that they reactivate imprinted tumor suppressor genes (reviewed in ref. 5). The data of Gagnon-Kugler et al.11 suggest that in fact the therapeutic effects of inhibitors of DNA methylation in general, and of aza-deoxycytidine in particular, may in large part be mediated by an inhibition of ribosome biogenesis rather than by the reactivation of tumor suppressor genes. Consistent with this, aza-cytidine has long been known to block precursor rRNA processing and ribosome biogenesis.41 Incorporation of aza-cytidine into rRNA would, of course, prevent its methylation, explaining the arrest of rRNA processing. Consistent with this direct targeting of ribosome biogenesis, aza-cytidine is far more toxic than its 2′-deoxy analog, aza-deoxycytidine.5 Thus, both aza-deoxycytidine and azacytidine inhibit ribosome biogenesis in similar ways, albeit via different mechanistic routes.
Conclusion
The loss of CpG methylation, whether by genetic or pharmaceutical means, is likely to have a major effect on cell and tumor growth as the result of the suppression of rRNA processing and ribosome biogenesis. Given the interest in modified nucleosides and other DNMT inhibitors in the treatment of human cancers, the recognition that these drugs may target a completely different cellular function than previously thought should help guide drug design and the development of combined drug therapies. In fact, several unrelated drugs used in chemotherapy, such as cisplatin,42 rapamycin analogs43 and others,44 may all share ribosome biogenesis as the key target. As such, the hundreds of proteins necessary for the assembly and function of the ribosome may represent a rich new source of anticancer drug targets.
Acknowledgements
The author thanks Drs. V. Stefanovsky and J.Y. Masson and F. Lessard for their thoughtful opinions on the manuscript. This work was funded by operating grant MOP12205 from the Canadian Institutes of Health Research (CIHR). The Research Centre of the CHUQ, in which the Cancer Research Centre is housed, is also supported by the FRSQ (Québec).
Abbreviations
- rRNA
ribosomal RNA
- rDNA
ribosomal DNA (the DNA of the rRNA genes)
- DNMT
DNA methyltransferase
- CpG
deoxycytidine-deoxyguanosine dinucleotide
- meCpG
5 methyl-CpG
References
- 1.Cedar H, Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet. 2009;10:295–304. doi: 10.1038/nrg2540. [DOI] [PubMed] [Google Scholar]
- 2.Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6:597–610. doi: 10.1038/nrg1655. [DOI] [PubMed] [Google Scholar]
- 3.Cihak A. Biological effects of 5-azacytidine in eukaryotes. Oncology. 1974;30:405–422. doi: 10.1159/000224981. [DOI] [PubMed] [Google Scholar]
- 4.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kristensen LS, Nielsen HM, Hansen LL. Epigenetics and cancer treatment. Eur J Pharmacol. 2009;625:131–142. doi: 10.1016/j.ejphar.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Christman JK. 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene. 2002;21:5483–5495. doi: 10.1038/sj.onc.1205699. [DOI] [PubMed] [Google Scholar]
- 7.Zhu WG, Dai Z, Ding H, Srinivasan K, Hall J, Duan W, et al. Increased expression of unmethylated CDKN2D by 5-aza-2′-deoxycytidine in human lung cancer cells. Oncogene. 2001;20:7787–7796. doi: 10.1038/sj.onc.1204970. [DOI] [PubMed] [Google Scholar]
- 8.Soengas MS, Capodieci P, Polsky D, Mora J, Esteller M, Opitz-Araya X, et al. Inactivation of the apoptosis effector Apaf-1 in malignant melanoma. Nature. 2001;409:207–211. doi: 10.1038/35051606. [DOI] [PubMed] [Google Scholar]
- 9.Gius D, Cui H, Bradbury CM, Cook J, Smart DK, Zhao S, et al. Distinct effects on gene expression of chemical and genetic manipulation of the cancer epigenome revealed by a multimodality approach. Cancer Cell. 2004;6:361–371. doi: 10.1016/j.ccr.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 10.Flotho C, Claus R, Batz C, Schneider M, Sandrock I, Ihde S, et al. The DNA methyltransferase inhibitors azacitidine, decitabine and zebularine exert differential effects on cancer gene expression in acute myeloid leukemia cells. Leukemia. 2009;23:1019–1028. doi: 10.1038/leu.2008.397. [DOI] [PubMed] [Google Scholar]
- 11.Gagnon-Kugler T, Langlois F, Stefanovsky V, Lessard F, Moss T. Loss of human ribosomal gene CpG methylation enhances cryptic RNA polymerase II transcription and disrupts ribosomal RNA processing. Mol Cell. 2009;35:414–425. doi: 10.1016/j.molcel.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Illingworth RS, Bird AP. CpG islands—‘a rough guide’. FEBS Lett. 2009;583:1713–1720. doi: 10.1016/j.febslet.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Ahuja N, Li Q, Mohan AL, Baylin SB, Issa JP. Aging and DNA methylation in colorectal mucosa and cancer. Cancer Res. 1998;58:5489–5494. [PubMed] [Google Scholar]
- 14.Swisshelm K, Disteche CM, Thorvaldsen J, Nelson A, Salk D. Age-related increase in methylation of ribosomal genes and inactivation of chromosome-specific rRNA gene clusters in mouse. Mutat Res. 1990;237:131–146. doi: 10.1016/0921-8734(90)90019-n. [DOI] [PubMed] [Google Scholar]
- 15.Oakes CC, Smiraglia DJ, Plass C, Trasler JM, Robaire B. Aging results in hypermethylation of ribosomal DNA in sperm and liver of male rats. Proc Natl Acad Sci USA. 2003;100:1775–1780. doi: 10.1073/pnas.0437971100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lees-Murdock DJ, Walsh CP. DNA methylation reprogramming in the germ line. Epigenetics. 2008;3:5–13. doi: 10.4161/epi.3.1.5553. [DOI] [PubMed] [Google Scholar]
- 17.Weaver JR, Susiarjo M, Bartolomei MS. Imprinting and epigenetic changes in the early embryo. Mamm Genome. 2009;20:532–543. doi: 10.1007/s00335-009-9225-2. [DOI] [PubMed] [Google Scholar]
- 18.Feil R. Epigenetic asymmetry in the zygote and mammalian development. Int J Dev Biol. 2009;53:191–201. doi: 10.1387/ijdb.082654rf. [DOI] [PubMed] [Google Scholar]
- 19.Corry GN, Tanasijevic B, Barry ER, Krueger W, Rasmussen TP. Epigenetic regulatory mechanisms during preimplantation development. Birth Defects Res C Embryo Today. 2009;87:297–313. doi: 10.1002/bdrc.20165. [DOI] [PubMed] [Google Scholar]
- 20.Thurston A, Lucas ES, Allegrucci C, Steele W, Young LE. Region-specific DNA methylation in the preimplantation embryo as a target for genomic plasticity. Theriogenology. 2007;68:98–106. doi: 10.1016/j.theriogenology.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Moss T, Langlois F, Gagnon-Kugler T, Stefanovsky V. A housekeeper with power of attorney: the rRNA genes in ribosome biogenesis. Cell Mol Life Sci. 2007;64:29–49. doi: 10.1007/s00018-006-6278-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lessard F, Morin F, Ivanchuk S, Langlois F, Stefanovsky V, Rutka J, et al. The ARF tumor suppressor controls ribosome biogenesis by regulating the RNA polymerase I transcription factor TTF-I. Mol Cell. 2010;38:539–550. doi: 10.1016/j.molcel.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Callan HG, Gall JG, Murphy C. The distribution of oocyte 5S, somatic 5S and 18S + 28S rRNA sequences in the lampbrush chromosomes of Xenopus laevis. Chromosoma. 1988;97:43–54. [Google Scholar]
- 24.Rowe LB, Janaswami PM, Barter ME, Birkenmeier EH. Genetic mapping of 18S ribosomal RNA-related loci to mouse chromosomes 5, 6, 9, 12, 17, 18, 19 and X. Mamm Genome. 1996;7:886–889. doi: 10.1007/s003359900262. [DOI] [PubMed] [Google Scholar]
- 25.Henderson AS, Warburton D, Atwood KC. Location of ribosomal DNA in the human chromosome complement. Proc Natl Acad Sci USA. 1972;69:3394–3398. doi: 10.1073/pnas.69.11.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moss T. At the crossroads of growth control; making ribosomal RNA. Curr Opin Gen Dev. 2004;14:210–217. doi: 10.1016/j.gde.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 27.McStay B, Grummt I. The epigenetics of rRNA genes: from molecular to chromosome biology. Annu Rev Cell Dev Biol. 2008;24:131–157. doi: 10.1146/annurev.cellbio.24.110707.175259. [DOI] [PubMed] [Google Scholar]
- 28.Tucker S, Vitins A, Pikaard CS. Nucleolar dominance and ribosomal RNA gene silencing. Curr Opin Cell Biol. 2010;22:351–356. doi: 10.1016/j.ceb.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stefanovsky VY, Moss T. Regulation of rRNA synthesis in human and mouse cells is not determined by changes in active gene count. Cell Cycle. 2006;5:735–739. doi: 10.4161/cc.5.7.2633. [DOI] [PubMed] [Google Scholar]
- 30.Ide S, Miyazaki T, Maki H, Kobayashi T. Abundance of ribosomal RNA gene copies maintains genome integrity. Science. 2010;327:693–696. doi: 10.1126/science.1179044. [DOI] [PubMed] [Google Scholar]
- 31.Ting AH, Jair KW, Suzuki H, Yen RW, Baylin SB, Schuebel KE. CpG island hypermethylation is maintained in human colorectal cancer cells after RNAi-mediated depletion of DNMT1. Nat Genet. 2004;36:582–584. doi: 10.1038/ng1365. [DOI] [PubMed] [Google Scholar]
- 32.Robert MF, Morin S, Beaulieu N, Gauthier F, Chute IC, Barsalou A, et al. DNMT1 is required to maintain CpG methylation and aberrant gene silencing in human cancer cells. Nat Genet. 2003;33:61–65. doi: 10.1038/ng1068. [DOI] [PubMed] [Google Scholar]
- 33.Fandy TE. Development of DNA methyltransferase inhibitors for the treatment of neoplastic diseases. Curr Med Chem. 2009;16:2075–2085. doi: 10.2174/092986709788612738. [DOI] [PubMed] [Google Scholar]
- 34.Rhee I, Jair KW, Yen RW, Lengauer C, Herman JG, Kinzler KW, et al. CpG methylation is maintained in human cancer cells lacking DNMT1. Nature. 2000;404:1003–1007. doi: 10.1038/35010000. [DOI] [PubMed] [Google Scholar]
- 35.Rhee I, Bachman KE, Park BH, Jair KW, Yen RW, Schuebel KE, et al. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature. 2002;416:552–556. doi: 10.1038/416552a. [DOI] [PubMed] [Google Scholar]
- 36.Stefanovsky VY, Langlois F, Gagnon-Kugler T, Rothblum LI, Moss T. Growth factor signaling regulates elongation of RNA polymerase I transcription in mammals via UBF phosphorylation and rchromatin remodeling. Mol Cell. 2006;21:629–639. doi: 10.1016/j.molcel.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, SmithD A, 4th, Renfrow MB, Schneider DA. The RNA polymerase-associated factor 1 complex (Paf1C) directly increases the elongation rate of RNA polymerase I and is required for efficient regulation of rRNA synthesis. J Biol Chem. 2010;285:14152–14159. doi: 10.1074/jbc.M110.115220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bachellerie JP, Cavaille J, Huttenhofer A. The expanding snoRNA world. Biochimie. 2002;84:775–790. doi: 10.1016/s0300-9084(02)01402-5. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi T, Ganley AR. Recombination regulation by transcription-induced cohesin dissociation in rDNA repeats. Science. 2005;309:1581–1584. doi: 10.1126/science.1116102. [DOI] [PubMed] [Google Scholar]
- 40.Maaloe O, Kjeldgaard NO. In: Control of macromolecular synthesis: A study of DNA, RNA and protein synthesis in bacteria. Benjamin WA, editor. New York: 1966. [Google Scholar]
- 41.Cihak A, Weiss JW, Pitot HC. Characterization of polyribosomes and maturation of ribosomal RNA in hepatoma cells treated with 5-azacytidine. Cancer Res. 1974;34:3003–3009. [PubMed] [Google Scholar]
- 42.Zhai X, Beckmann H, Jantzen HM, Essigmann JM. Cisplatin-DNA adducts inhibit ribosomal RNA synthesis by hijacking the transcription factor human upstream binding factor. Biochemistry. 1998;37:16307–16315. doi: 10.1021/bi981708h. [DOI] [PubMed] [Google Scholar]
- 43.Gibbons JJ, Abraham RT, Yu K. Mammalian target of rapamycin: discovery of rapamycin reveals a signaling pathway important for normal and cancer cell growth. Semin Oncol. 2009;36:3–17. doi: 10.1053/j.seminoncol.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Rubbi CP, Milner J. Disruption of the nucleolus mediates stabilization of p53 in response to DNA damage and other stresses. EMBO J. 2003;22:6068–6077. doi: 10.1093/emboj/cdg579. [DOI] [PMC free article] [PubMed] [Google Scholar]