Abstract
We previously reported that administration of the synthetic cannabinoid agonist, WIN 55,212-2, causes an increase in norepinephrine (NE) efflux in the frontal cortex (FC). The present study examined the expression levels of α2- and β1-adrenergic receptors (ARs) as well as the norepinephrine transporter (NET) in the FC of rats following exposure to WIN 55,212-2. Rats received systemic injection of WIN 55,212-2 (3 mg/kg) acutely or for seven days. Another group of rats received repeated WIN 55,212-2 treatment followed by a period of abstinence. Control rats received vehicle injections. Rats were euthanized 30 min after the last WIN 55,212-2 injection and the FC was microdissected and protein extracts were probed for α2-AR, β1-AR and NET. Results showed that β1-AR expression was significantly decreased following repeated WIN 55,212-2 treatment but significantly increased following a period of abstinence. α2-AR expression showed no significant change in all groups examined. NET expression was significantly decreased following acute WIN 55,212-2 treatment with no changes following chronic administration or a period of abstinence. Alterations in NET may arise from modulation of cannabinoid receptors (CB1) receptor that are localized to noradrenergic axon terminals as we demonstrate co-localization of CB1 receptor and NET in the same cortical axonal processes. The present findings support significant alterations in adrenergic receptor and NET expression in the FC following WIN 55,212 exposure that may underlie reported changes in attention, cognition and anxiety commonly observed following cannabinoid exposure.
Keywords: α2-adrenergic receptor; β1- adrenergic receptor; norepinephrine transporter; cannabinoid receptor; WIN 55,212
Introduction
A considerable number of studies in humans and animals demonstrate that acute and chronic administration of cannabinoids impairs attention, cognition and vigilance by modulating cannabinoid receptors in many brain regions (D’Souza et al., 2008a; D’Souza et al., 2008b; Egertova and Elphick, 2000; Ranganathan and D’Souza, 2006; Solowij and Michie, 2007; Verrico et al., 2003a). In addition, chronic exposure to cannabinoid agonists is associated with mood disorders including anxiety and depression (Pattij et al., 2008; Schneider et al., 2008). Although an extensive literature supports modulation of glutamate and gamma-amino butyric acid (GABA) transmission by cannabinoids, growing evidence supports an action on catecholamines including norepinephrine (NE), a biogenic amine that has been associated with various central functions that are influenced by cannabinoids (Muntoni et al., 2006; Oropeza et al., 2007; Oropeza et al., 2005; Page et al., 2007; Page et al., 2008).
Systemic administration of WIN 55,212-2, a synthetic cannabinoid agonist, increases indices of noradrenergic activity (Oropeza et al., 2005; Page et al., 2007). For instance, acute administration of WIN 55,212-2 increases NE efflux within the frontal cortex (FC; Oropeza et al., 2005), an area involved in executive functions. The increase in NE efflux in the FC was also observed following a direct local infusion of WIN 55,212-2 into the FC (Page et al., 2008) and this effect was abrogated by pretreatment with the selective CB1 receptor antagonist, SR 141716A (Oropeza et al., 2005; Page et al., 2008) indicating a specific role for CB1 receptors in these effects. Furthermore, chronic WIN 55,212-2 administration increases the extracellular NE efflux in the FC and increases tyrosine hydroxylase (TH) protein expression in the locus coeruleus (LC) with an associated anxiogenic-like response (Page et al., 2007). We also demonstrated the localization of CB1 receptors on noradrenergic axon terminals in the FC (Oropeza et al., 2007) providing evidence for an anatomical and functional link between CB1 receptors and NE output that could impact neurobehavioral changes associated with cannabinoid use.
In the present study, we investigated the effect of cannabinoid agonist exposure on adrenoceptors and NE transporters (NET) using Western blot analysis. Expression levels of α2- and β1-adrenergic receptors (α2-AR and β1-AR) and NET were assessed in the FC of male rats. Cortical protein samples were probed using selective antibodies directed against two adrenoceptors and NET in rats that received systemic WIN 55,212-2 administration either acutely, repeatedly or chronically followed by a period of abstinence.
Materials and Methods
Animals
Twenty seven adult male Sprague-Dawley rats (250–300 g; Harlan Sprague-Dawley, Inc., Indianapolis, IN) were used in the present study. The animal procedures used were approved by the Institutional Animal Care and Use Committee at Thomas Jefferson University and conform with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Rats were housed 2–3 per cage on a 12-h light schedule (lights on at 0700) in a temperature-controlled (20 °C) colony room. They were allowed ad libitum access to standard rat chow and water. Rats were allowed to acclimate to the animal housing facility for several days prior to the onset of the study. All efforts were made to utilize only the minimum number of animals necessary to produce reliable scientific data, and experiments were designed to minimize any animal distress.
Primary antibodies
In the present study, the α2-AR was generated in rabbit using a synthetic peptide (Arg-Ile-Tyr-Gln-Ile-Ala-Lys-Arg-Arg-Thr-Val-Pro-Ser-Arg-Arg-Gly) derived from amino acids 218–235 of human, mouse, rat and pig α2-AR, as the immunogen. This sequence is found within the third intracellular loop of α2-AR subtype. Using Western blotting, the α2-AR antibody recognized a single band of proteins with an approximate molecular weight of 32 kDa. The antibody is purified from rabbit serum by epitope affinity chromatography. The β1-AR antibody was generated in rabbit using a synthetic peptide (His-Gly-Asp-Arg-Pro-Arg-Ala-Gly-Leu-Ala-Arg-Ala-Gly) derived from amino acids 394–408 of mouse and rat β1-AR C-terminal domain, as an immunogen. Using Western blotting the β1-AR antibody recognized single band of proteins with approximate molecular weight 64 kDa. The monoclonal mouse NET antibody was raised against a peptide (amino acids 05–17) coupled to KLH by addition of a C-terminal cysteine. Using Western blotting the NET antibody recognized single band of proteins with approximate molecular weight 62 kDa.
Drug treatment
WIN 55,212-2 (Sigma-Aldrich Inc., St. Louis, MO) was dissolved in 5% dimethyl sulfoxide (DMSO) in 0.9% sodium chloride solution at a concentration of 3 mg/ml. Experimental and control rats were injected intraperitoneally (i.p.) with 0.1 ml/100 g body weight of either WIN 55,212-2 or DMSO, respectively.
Rats were randomly divided into three groups at the beginning of the study. For the acute group, animals were administered WIN 55,212-2 at 3.0 mg/kg once. For the chronic group, animals were administered WIN 55,212-2 at 3.0 mg/kg once daily for seven days. Another group consisted of animals that were administered WIN 55,212-2 for seven days and were subsequently abstinent from WIN 55,212-2 for seven days. For each group examined, the control animals received a solution of DMSO solution i.p. at 0.1 ml/100 g body weight. Thirty minutes following the last WIN 55,212-2 injection, rats were briefly exposed to isoflurane (Abbott Laboratories, North Chicago, IL; 0.5–1.0%, in air) and euthanized by decapitation. Brains were removed for subsequent protein extraction and Western blot analysis.
Protein extraction
Brain tissue was rapidly removed from each animal on ice. Using a trephine, the FC brain region was microdissected from each animal. FC was homogenized with a pestle and extracted in radioimmunoprecipitation assay lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA, USA) on ice for 20 min. Lysates were cleared by centrifugation at 13,000 rpm for 12 min at 41°C. Supernatants or protein extracts were diluted with an equal volume of Novex 2® tris glycine sodium dodecyl sulfate sample buffer (Invitrogen, Carlsbad, CA, USA) containing dithiothreitol (Sigma-Aldrich Inc.). Protein concentrations of the undiluted supernatants were quantified using the bicinchoninic acid protein assay reagent (Pierce, Rockford, IL).
Western blot analysis
Cell lysates containing equal amounts of protein were separated on 4–12% tris-glycine polyacrylamide gels and then electrophoretically transferred to Immobilon-P polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). Membranes were incubated in rabbit anti-α2-AR (1:500; Sigma-Aldrich Inc.), rabbit anti-β1-AR (1:1000; Sigma-Aldrich Inc.) or mouse anti-NET (1:1000, Chemicon International, Temecula, CA) primary antibodies for a minimum of 2 hours and then in alkaline phosphatase-conjugated secondary antibodies for 30 min to probe for the presence of proteins using a Western blotting detection system (Western Breeze Chemiluminescent Kit; Invitrogen). Following incubation in a chemiluminescent substrate (Western Breeze Chemiluminescent Kit), blots were exposed to X-OMAT AR film (Kodak, Rochester, NY, USA) for different lengths of time to optimize exposures. α2-AR, β1-AR or NET was readily detected by immunoblotting in rat FC extracts. α2-AR immunoreactivity was visualized as a single band that migrates at approximately 45kDA, while β1-AR and NET migrate at approximately 64kDA and 62kDA, respectively. Blots were incubated in stripping buffer (Restore Stripping Buffer, Pierce) to disrupt previous antibody-antigen interactions and then re-probed with β-actin (1:5,000, Sigma-Aldrich Inc.) with 1-hour incubation to ensure proper protein loading. The density of each band was quantified using Un-Scan-It blot analysis software (Silk Scientific Inc., Orem, Utah). α2-AR, β1-AR or NET was normalized to β-actin immunoreactivity on each respective blot. Western blot data was analyzed using Student’s t-test (GraphPad Prism 4, GraphPad Software, Inc., San Diego, CA)
Immunofluorescence
Five adult male Sprague-Dawley rats were deeply anesthetized with sodium pentobarbital (80 mg/kg) and transcardially perfused through the ascending aorta with 500 ml of 4% formaldehyde in 0.1 M phosphate buffer (PB; pH 7.4). Brains were then removed and post fixed in 4% formaldehyde overnight at 4°C. Forty micrometer thick coronal sections through the rostrocaudal extent of FC (Paxinos and Watson, 1986) were cut using a Vibratome (Technical Product International, St Louis, MO, USA) and rinsed extensively in 0.1 M PB and 0.1 M tris-buffered saline (TBS; pH 7.6). Sections were placed for 30 min in 1% sodium borohydride in 0.1 M PB to reduce amine-aldehyde compounds. Tissue sections were rinsed in 0.1 M PB and 0.1 M TBS. Sections were then incubated in 0.5% bovine serum albumin (BSA) and 0.25% Triton X-100 in 0.1M TBS for 30 min and rinsed thoroughly in 0.1 M TBS. Following rinses, tissue sections were incubated in a cocktail of mouse anti-NET (1:1000; Chemicon International, Temecula, CA) and rabbit anti-CB1 receptor (1:1,000; kindly provided by Dr. Kenneth Mackie, University of Washington, Seattle, WA) in 0.1% BSA and 0.25% Triton X-100 in 0.1M TBS at room temperature overnight on a rotary shaker. Subsequently, tissue sections were washed in 0.1 M TBS and incubated in a secondary antibody cocktail containing fluorescein isothiocyanate (FITC) donkey-anti-rabbit (1:200; Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) and tetramethyl rhodamine isothiocyanate (TRITC) donkey anti-mouse (1:200; Jackson ImmunoResearch Laboratories Inc.) antibodies prepared in 0.1 % BSA and 0.25% Triton X-100 in 0.1 M TBS for 2 hours in the dark on a rotary shaker.
Following incubation with the secondary antibodies, the tissue sections were washed thoroughly in 0.1 M TBS. The tissue sections were then mounted on slides and allowed to dry in complete darkness. The slides were dehydrated in a series of alcohols, soaked in xylene and coverslipped using DPX (Sigma-Aldrich Inc., St. Louis, MO, USA). Immunofluorescence labeling on tissue sections was visualized using a Zeiss LSM 510 Meta confocal microscope (Carl Zeiss Inc., Thornwood, NY, USA). Digital images of immunofluorescent labeling were captured and imported using the LSM 5 image browser (Carl Zeiss Inc.). Figures were assembled and adjusted for brightness and contrast in Adobe Photoshop.
Results
Specificity of the α2-AR, β1-AR and NET
The specificity of the α2-AR, β1-AR and NET was investigated using immunoperoxidase, immunofluorescence and Western blotting. Recently, we showed specific immunoreactivity of β1-AR in the amygdala using light, immunofluorescence and electron microscopy as well as Western blotting (Rudoy et al., 2008). Preabsorption of α2-AR and β1-AR with the antigenic peptide at 10 μM blocked the α2-AR and β1-AR immunoreactivities in the forebrain. Blots incubated with 10 μg/ml of affinity-purified α2-AR and β1-AR antisera preincubated with antigenic peptide blocked the α2-AR and β1-AR expression in rat FC extracts. Likewise, preabsorption of NET with the antigenic peptide at 0.1 μg/ml and 1.0 μg/ml blocked the NET immunoreactivity in the forebrain. Blots incubated with 1.0 μg/ml and 10 μg/ml of affinity-purified NET antiserum preincubated with antigenic peptide blocked the NET expression in rat FC extracts. Furthermore, some sections were processed in parallel with the rest of the immunohistochemical procedures identical except that one of the primary antisera was omitted. Sections processed in the absence of primary antibody (α2-AR, β1-AR or NET) did not exhibit immunoreactivity (α2-AR-, β1-AR- or NET-immunoreactivity). To evaluate cross-reactivity of labeling of the primary antiserum by secondary antisera, some sections were processed for dual labeling with omission of one of the primary antisera. Omission of the primary antibody abolished any detectable immunoreactivity.
WIN 55,212-2 alters levels of adrenoceptor expression in the frontal cortex
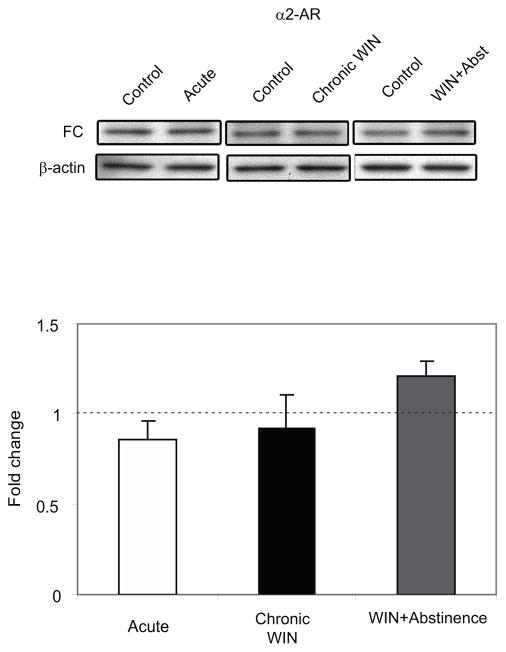
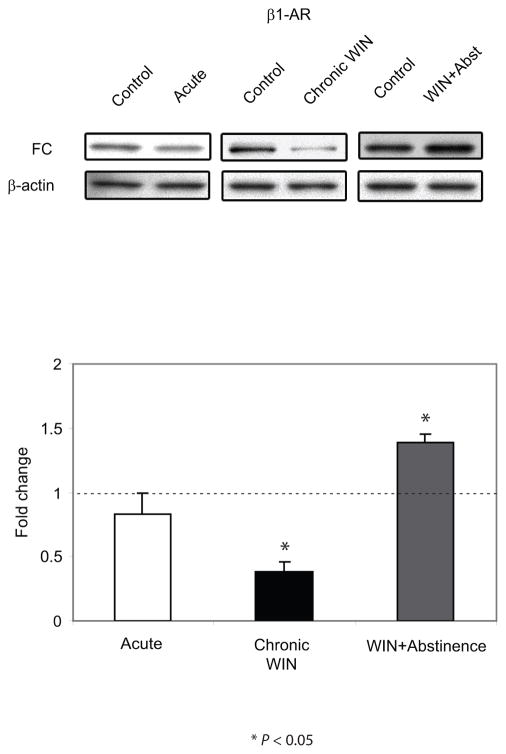
The FC extends from the rostrocaudal segment of the cerebral cortex. Using the Paxinos and Watson rat brain atlas (Paxinos and Watson, 1986), the FC is located at a level 5.2 mm anterior to bregma and extends 3.3 posterior to bregma. It forms the anterior part of the forebrain, and is bounded medially by the median plane dividing the cerebral lobes, ventrally by the medial orbital cortex (5.2 mm anterior to bregma - 3.2 mm anterior to bregma), dorsal peduncular cortex (3.2 mm anterior to bregma - 2.2 mm anterior to bregma) or corpus callosum (1.7 mm anterior to bregma - 3.3 mm posterior from bregma) and caudally by the occipital area (Paxinos and Watson, 1986). In the present study, the FC was microdissected bilaterally (approximately covering the FC area at a level 3.7 mm anterior to bregma extending at a level 1.7 posterior to bregma). α2-AR and β1-AR protein expression was assessed using Western blot analysis. FC extracts from rats that were euthanized following acute and chronic WIN 55,212-2 administration with or without abstinence showed no significant difference in the expression levels of α2-AR compared to their respective DMSO-treated control (Figure 1). Conversely, FC extracts from rats that were euthanized following chronic WIN 55,212-2 administration showed a significant decrease (P < 0.05) in the β1-AR expression levels compared to DMSO-treated control (Figure 2). β1-AR expression following abstinence from chronic WIN 55,212-2 administration was significantly increased (P < 0.05) compared to DMSO-treated control. There was no significant difference found in the expression levels of β1-AR following acute WIN 55,212-2 administration compared to DMSO-treated control.
Figure 1.
Western blot analysis of α2-adrenergic receptor (α2-AR) expression in the frontal cortex (FC) following acute and repeated treatment of WIN 55, 212-2 with or without abstinence. α2-AR expression in the FC of the animals is expressed as a fold change from the control mean when the control equals 1.0 ± SEM. β-actin immunoblotting was used as a control to verify equal protein loading. No significant effect was observed in the α2-AR expression in the FC following WIN 55, 212-2 treatment.
Figure 2.
Western blot analysis of β1-adrenergic receptor (β1-AR) expression in the frontal cortex (FC) following acute and repeated treatment of WIN 55, 212-2 with or without abstinence. β1-AR expression in the FC of the animals is expressed as a fold change from the control mean when the control equals 1.0 ± SEM. β-actin immunoblotting was used as a control to verify equal protein loading. β1-AR was significantly decreased (P < 0.05) following repeated WIN 55, 212-2 treatment compared to the control group, however, a rebound was evident following a period of abstinence compared to the control group. *P < 0.05 vs control group.
WIN 55,212-2 alters levels of norepinephrine transporter expression in the frontal cortex
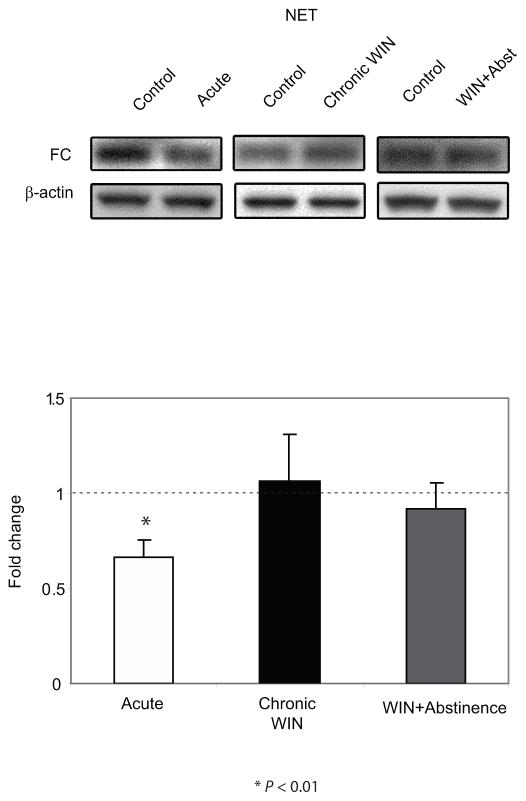
NET expression was also assessed using Western blot analysis. Statistical analysis showed that FC extracts from the rats that were euthanized following acute administration of WIN 55,212-2 exhibited a significant decrease in the expression of NET (P < 0.01) compared to DMSO-treated control (Figure 3). However, FC extracts from rats that underwent a 7-day abstinence following chronic (7 days) WIN 55,212-2 administration did not show significant difference in the expression level of NET compared to DMSO-treated control. Likewise, no significant effects were observed following chronic WIN 55,212-2 administration without abstinence.
Figure 3.
Western blot analysis of norepinephrine transporter (NET) expression in the frontal cortex (FC) following acute and repeated treatment of WIN 55, 212-2 with or without abstinence. NET expression in the FC of the animals is expressed as a fold change from the control mean when the control equals 1.0 ± SEM. β-actin immunoblotting was used as a control to verify equal protein loading. NET was significantly decreased following acute WIN 55, 212-2 treatment compared to the control group, however, no significant change was observed following repeated WIN 55, 212-2 treatment with or without a period of abstinence. *P < 0.01 vs control group.
Norepinephrine transporter-immunoreactive fibers colocalize with CB1 receptor in the frontal cortex
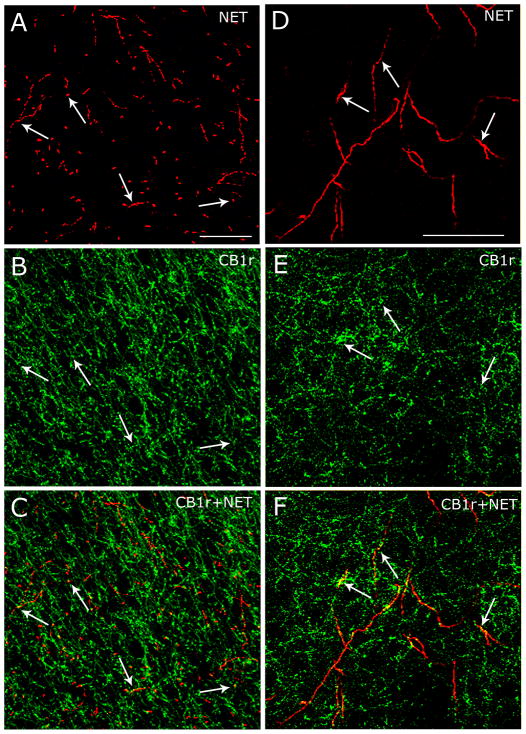
To determine whether NET-immunoreactive fibers contain CB1 receptor, dual immunocytochemical labeling of the CB1 receptor and NET was carried out in the same tissue sections through the FC using immunofluorescence microscopy. Rat brain tissue sections containing FC were labeled for NET using TRITC and CB1 receptor using FITC. NET immunoreactivity was distributed throughout the rostrocaudal extent of the FC (Figure 4A,D) and localized as thick and relatively smooth beaded processes confirming previous anatomical descriptions (Miner et al., 2003). Also, NET-immunoreactive processes resembled fibers labeled for dopamine-β-hydroxylase that we previously described (Oropeza et al., 2007). CB1 receptor immunoreactivity revealed a robust distribution throughout the FC (Figure 4B,E) and was localized to varicose processes. Consistent with our previous report (Oropeza et al., 2007), CB1 receptor was associated prominently with processes that appeared to be presynaptic cellular profiles (Figure 4B,E). Figure 4C and 4F show that NET-immunoreactive processes were also immunoreactive for CB1 receptor indicating that individual processes exhibit NET and CB1 receptor in the FC.
Figure 4.
Norepinephrine transporters (NET) are located on CB1 receptor-containing processes in the frontal cortex (FC). A,D: High magnification confocal fluorescence photomicrographs of two separate coronal sections through the FC labeled for NET using rhodamine isothiocyanate-conjugated secondary antibody (red). Arrows point to individual NET-containing varicose processes. B,E: The same coronal sections shown in panels A and D, respectively, were labeled for CB1 receptor using fluorescein isothiocyanate (green). Arrows point to individual CB1 receptor-containing varicose processes. C,F: Merged images. Arrows point to NET and CB1 receptor dual-labeled varicose processes. Scale bars = 100 μm.
Discussion
The present study extends previous findings from our laboratory by showing alterations in AR and NET expression in the FC following exposure to a synthetic cannabinoid agonist. We also provide neuroanatomical evidence showing that the CB1 receptor is localized to NET-containing processes in the FC. Taken with our previous findings of behavioral changes and alterations in NE neurotransmission in the FC following WIN 55, 212-2 administration (Oropeza et al., 2005; Page et al., 2007; Page et al., 2008), these data demonstrate profound effects of cannabinoids on adrenergic circuitry.
Methodological considerations
Inherent caveats exist with respect to Western blot analysis experiments. These caveats include the accuracy of sampling of the region of interest and the comparison of equal protein quantities across treatment groups. In order to circumvent the variability in tissue excision, a single investigator obtained the brain samples for each experiment. Moreover, to ensure equivalent loading of protein, blots were reprobed with β-actin and results were normalized to this internal standard. β-actin expression was comparable across treatment groups examined.
We addressed the localization of CB1 receptor and NET in the FC using immunofluorescence. The use of Triton X-100 as detergent in the present study most likely enhanced the effectivity of immunolabeling (Ghrebi et al., 2007). This is evident based on the intensity and abundance of CB1 receptor and NET immunoreactivities that could be detected in the FC. Although our analysis was limited to fluorescence microscopy, our data are consistent with our previous study (Oropeza et al., 2007) showing that CB1 receptors are expressed in noradrenergic terminals. Based on this, we did not further investigate the association of CB1 receptor and NET at the ultrastructural level.
Frontal cortex as a target of noradrenergic actions
The FC plays a vital role in a variety of cognitive and behavioral processes including arousal, attention, cognition, motivation, working memory and vigilance (Miller and Cohen, 2001). The release of catecholamines (e.g. NE and dopamine), is related to arousal states that have profound effects on cognitive and behavioral processes involving FC functions (Arnsten 2007; Lapiz and Morilak, 2006). While these modulatory systems engage multiple receptor subtypes including α1-, α2, and β1-(Arnsten, 2007; Lapiz and Morilak, 2006; Ramos et al., 2005), the levels of NE release in FC determines the type of AR engaged in the process (Arnsten, 2007). It has been shown that moderate levels of NE released during nonstressed waking engage high affinity α2-AR, while higher levels of NE released during stress engage lower affinity α1-ARs (Arnsten, 2000).
As the activity of LC neurons is correlated with levels of cortical arousal and influenced by internal and external stimuli (Aston-Jones et al., 1984; Aston-Jones et al., 1999), cannabinoid modulation of coeruleo-cortical pathway may contribute to changes in attention, cognition and vigilance commonly observed following cannabis use (D’Souza et al., 2008a; Ranganathan and D’Souza, 2006; Verrico et al., 2003b). Though it has been demonstrated that NE efflux changes in the FC following acute or chronic cannabinoid exposure (Oropeza et al., 2005; Page et al., 2007; Page et al., 2008), the effect of cannabinoid agonists on adrenoreceptors and NET expression levels on the FC has not been investigated, to date.
Cortical CB1 receptors and norepinephrine transmission
Our present data support our previous published report (Oropeza et al., 2007) and others (Marsicano and Lutz, 1999; Zarate et al., 2008) demonstrating that CB1 receptors are localized to axonal processes in the FC. Anatomical and physiological studies have shown that CB1 modulates release of neurotransmitters (Acquas et al., 2000; Bajo et al., 2008; Hofmann et al., 2008; Malone and Taylor, 1999; Pistis et al., 2002). For example, WIN 55, 212-2 significantly decreases hippocampal excitatory transmission (Bajo et al., 2008; Hofmann et al., 2008). In addition, in vivo microdialysis studies have demonstrated that cannabinoid agonists significantly increase extracellular dopamine and glutamate levels while also significantly increasing GABA and acetylcholine levels in the FC (Acquas et al., 2000; Malone and Taylor, 1999; Pistis et al., 2002).
We have shown that acute and chronic WIN 55,212-2 administration increases extracellular NE efflux in the FC and is accompanied by an anxiety-like response (Oropeza et al., 2005; Page et al., 2007). The enhancement of cortical NE is likely mediated by the CB1 receptors since the increase in NE release is blocked by pretreatment with the cannabinoid receptor antagonist, SR 141716A (Page et al., 2007). Also, we described that CB1 receptors are localized to DBH-containing axon terminals in the FC (Oropeza et al., 2007). Hence, one potential substrate underlying behavioral and neurochemical changes observed following WIN 55, 212-2 is the coeruleo-cortical pathway.
Effects of WIN 55, 212-2 on α-AR, β-AR and norepinephrine transporter expression on frontal cortex
Alterations in noradrenergic transmission following WIN 55, 212-2 exposure may impact certain types of cortical adrenoceptors as selected NE levels can dictate activation of a particular type of adrenoceptor (Arnsten, 2007). In the present study, acute and chronic WIN 55, 212-2 administration significantly affected β1-AR and NET expression levels but not α2-AR suggesting that the presence of NE during acute WIN 55, 212-2 administration impacts NET only, while chronic treatment with or without abstinence influences β1-AR but not α2-AR and NET.
The decrease in cortical NET expression levels following acute WIN 55, 212-2 suggests that WIN 55, 212-2 may impact NET resulting in alterations in NE efflux in the FC since NET is critical for the removal of NE from the extracellular space (Bonisch and Bruss, 1994; Trendelenburg, 1989). Indeed, local application of drugs that block NET into the prefrontal cortex increases extracellular levels of both NE and dopamine (Bymaster et al., 2002; Tanda et al., 1997). This mechanism may contribute to the peak of NE efflux in the FC following acute WIN 55, 212-2 exposure (Oropeza et al., 2005). In addition, NET may be downregulated in response to elevated NE release following acute WIN 55, 212-2 treatment (Oropeza et al., 2005) or a decrease of NET expression may serve as a compensatory mechanism aimed at normalizing NE neurotransmission (Zafar et al., 1997). Previous studies have shown changes in NET expression in different brain regions following a variety of stimuli or treatments. With repeated stressor exposure NET binding sites are significantly decreased (Tejani-Butt et al., 1994). Repeated immobilization stress (Zafar et al., 1997) or desipramine treatment (Szot et al., 1993) significantly up-regulated NET in several brain regions.
We also showed that, in the FC, WIN 55, 212-2 administration does not affect α2-AR expression but decreases β1-AR expression following chronic exposure and increases β1-AR expression following a period of abstinence. A significant decrease in expression levels of β1-AR, a receptor typically coupled to stimulatory G proteins (Wenzel-Seifert et al., 2002), has been reported following chronic administration of antidepressants, desipramine and amitriptyline (Pratt and Bowery, 1993). It remains to be elucidated whether decreases in β1-AR expression observed following WIN 55, 212-2 administration share similar mechanisms as following antidepressant administration (Pratt and Bowery, 1993). It is tempting to speculate that decreases in β1-AR occur in response to altered cortical norepinephrine levels at the synapse (Page et al., 2007). Following a period of abstinence, increased β1-AR expression may result from a rebound in receptor expression similar to that reported following sudden discontinuation of a beta-blocker drug (Lopez-Sendon et al., 2004). Increases in β1-AR expression following abstinence might result from normalization of NE levels. Consistent with this, anxiety-like behavior and NE levels return to control levels following chronic WIN 55, 212-2 exposure followed by a period of drug discontinuation (Page et al., 2007).
In summary, the present study demonstrates alterations in β1-AR and NET expression in the FC following WIN 55, 212-2 administration. Our anatomical data further indicates that alterations in NET expression may influence noradrenergic signaling via CB1 receptors that are localized to the same cortical axonal processes. Taken together, our findings suggest that anxiety-like behaviors observed following cannabinoid exposure may involve β1-AR and NET that are altered following prolonged cannabinoid exposure.
Acknowledgments
We thank Dr. Kenneth Mackie for the CB1 receptor antibody. This project was supported by the National Institutes of Health grant DA #020129 to E.V.B.
References
- Acquas E, Pisanu A, Marrocu P, Di Chiara G. Cannabinoid CB(1) receptor agonists increase rat cortical and hippocampal acetylcholine release in vivo. Eur J Pharmacol. 2000;401:179–185. doi: 10.1016/s0014-2999(00)00403-9. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Through the looking glass: differential noradenergic modulation of prefrontal cortical function. Neural Plast. 2000;7:133–146. doi: 10.1155/NP.2000.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine and second messenger influences on prefrontal cortical networks of “representational knowledge”: a rational bridge between genetics and the symptoms of mental illness. Cereb Cortex. 2007;17(Suppl 1):16–15. doi: 10.1093/cercor/bhm033. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Foote SL, Bloom FE. Anatomy and physiology of locus coeruleus neurons: functional implications. In: Ziegler M, Lake CR, editors. Norepinephrine (Frontiers of Clinical Neuroscience) Baltimore: Williams and Wilkins; 1984. pp. 92–116. [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999;46:1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Bajo M, Roberto M, Schweitzer P. Differential alteration of hippocampal excitatory synaptic transmission by cannabinoid ligands. J Neurosci Res. 2008;87:766–775. doi: 10.1002/jnr.21889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonisch H, Bruss M. The noradrenaline transporter of the neuronal plasma membrane. Ann N Y Acad Sci. 1994;733:193–202. doi: 10.1111/j.1749-6632.1994.tb17269.x. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Braley G, Blaise R, Vendetti M, Oliver S, Pittman B, Ranganathan M, Bhakta S, Zimolo Z, Cooper T, Perry E. Effects of haloperidol on the behavioral, subjective, cognitive, motor, and neuroendocrine effects of Delta-9-tetrahydrocannabinol in humans. Psychopharmacology (Berl) 2008a;198:587–603. doi: 10.1007/s00213-007-1042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T, Perry E, Krystal J. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology. 2008b;33:2505–2516. doi: 10.1038/sj.npp.1301643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egertova M, Elphick MR. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB. J Comp Neurol. 2000;422:159–171. doi: 10.1002/(sici)1096-9861(20000626)422:2<159::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Ghrebi SS, Owen GR, Brunette DM. Triton X-100 pretreatment of LR-white thin sections improves immunofluorescence specificity and intensity. Microsc Res Tech. 2007;70:555–562. doi: 10.1002/jemt.20422. [DOI] [PubMed] [Google Scholar]
- Hofmann ME, Nahir B, Frazier CJ. Excitatory afferents to CA3 pyramidal cells display differential sensitivity to CB1 dependent inhibition of synaptic transmission. Neuropharmacology. 2008;55:1140–1146. doi: 10.1016/j.neuropharm.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapiz MD, Morilak DA. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience. 2006;137:1039–1049. doi: 10.1016/j.neuroscience.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Le Moal M, Simon H. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol Rev. 1991;71:155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- Lopez-Sendon J, Swedberg K, McMurray J, Tamargo J, Maggioni AP, Dargie H, Tendera M, Waagstein F, Kjekshus J, Lechat P, Torp-Pedersen C. Expert consensus document on beta-adrenergic receptor blockers. Eur Heart J. 2004;25:1341–1362. doi: 10.1016/j.ehj.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Malone DT, Taylor DA. Modulation by fluoxetine of striatal dopamine release following Delta9-tetrahydrocannabinol: a microdialysis study in conscious rats. Br J Pharmacol. 1999;128:21–26. doi: 10.1038/sj.bjp.0702753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miner LH, Schroeter S, Blakely RD, Sesack SR. Ultrastructural localization of the norepinephrine transporter in superficial and deep layers of the rat prelimbic prefrontal cortex and its spatial relationship to probable dopamine terminals. J Comp Neurol. 2003;466:478–494. doi: 10.1002/cne.10898. [DOI] [PubMed] [Google Scholar]
- Muntoni AL, Pillolla G, Melis M, Perra S, Gessa GL, Pistis M. Cannabinoids modulate spontaneous neuronal activity and evoked inhibition of locus coeruleus noradrenergic neurons. Eur J Neurosci. 2006;23:2385–2394. doi: 10.1111/j.1460-9568.2006.04759.x. [DOI] [PubMed] [Google Scholar]
- Oropeza VC, Mackie K, Van Bockstaele EJ. Cannabinoid receptors are localized to noradrenergic axon terminals in the rat frontal cortex. Brain Res. 2007;1127:36–44. doi: 10.1016/j.brainres.2006.09.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oropeza VC, Page ME, Van Bockstaele EJ. Systemic administration of WIN 55,212–2 increases norepinephrine release in the rat frontal cortex. Brain Res. 2005;1046:45–54. doi: 10.1016/j.brainres.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Page ME, Oropeza VC, Sparks SE, Qian Y, Menko AS, Van Bockstaele EJ. Repeated cannabinoid administration increases indices of noradrenergic activity in rats. Pharmacol Biochem Behav. 2007;86:162–168. doi: 10.1016/j.pbb.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ME, Oropeza VC, Van Bockstaele EJ. Local administration of a cannabinoid agonist alters norepinephrine efflux in the rat frontal cortex. Neurosci Lett. 2008;431:1–5. doi: 10.1016/j.neulet.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattij T, Wiskerke J, Schoffelmeer AN. Cannabinoid modulation of executive functions. Eur J Pharmacol. 2008;585:458–463. doi: 10.1016/j.ejphar.2008.02.099. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Pistis M, Ferraro L, Pira L, Flore G, Tanganelli S, Gessa GL, Devoto P. Delta(9)-tetrahydrocannabinol decreases extracellular GABA and increases extracellular glutamate and dopamine levels in the rat prefrontal cortex: an in vivo microdialysis study. Brain Res. 2002;948:155–158. doi: 10.1016/s0006-8993(02)03055-x. [DOI] [PubMed] [Google Scholar]
- Pratt GD, Bowery NG. Repeated administration of desipramine and a GABAB receptor antagonist, CGP 36742, discretely up-regulates GABAB receptor binding sites in rat frontal cortex. Br J Pharmacol. 1993;110:724–735. doi: 10.1111/j.1476-5381.1993.tb13872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos BP, Colgan L, Nou E, Ovadia S, Wilson SR, Arnsten AF. The beta-1 adrenergic antagonist, betaxolol, improves working memory performance in rats and monkeys. Biol Psychiatry. 2005;58:894–900. doi: 10.1016/j.biopsych.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, D’Souza DC. The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology (Berl) 2006;188:425–444. doi: 10.1007/s00213-006-0508-y. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Rubio P, Menzaghi F, Merlo-Pich E, Rivier J, Koob GF, Navarro M. Corticotropin-releasing factor (CRF) antagonist [D-Phe12,Nle21,38,C alpha MeLeu37]CRF attenuates the acute actions of the highly potent cannabinoid receptor agonist HU-210 on defensive-withdrawal behavior in rats. J Pharmacol Exp Ther. 1996;276:56–64. [PubMed] [Google Scholar]
- Rudoy CA, Reyes AR, Van Bockstaele EJ. Evidence for beta(1)-Adrenergic Receptor Involvement in Amygdalar Corticotropin-Releasing Factor Gene Expression: Implications for Cocaine Withdrawal. Neuropsychopharmacology. 2008;31:1119–1129. doi: 10.1038/npp.2008.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Schomig E, Leweke FM. Acute and chronic cannabinoid treatment differentially affects recognition memory and social behavior in pubertal and adult rats. Addict Biol. 2008;13:345–357. doi: 10.1111/j.1369-1600.2008.00117.x. [DOI] [PubMed] [Google Scholar]
- Solowij N, Michie PT. Cannabis and cognitive dysfunction: parallels with endophenotypes of schizophrenia? J Psychiatry Neurosci. 2007;32:30–52. [PMC free article] [PubMed] [Google Scholar]
- Szot P, Ashliegh EA, Kohen R, Petrie E, Dorsa DM, Veith R. Norepinephrine transporter mRNA is elevated in the locus coeruleus following short- and long-term desipramine treatment. Brain Res. 1993;618:308–312. doi: 10.1016/0006-8993(93)91281-v. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Frau R, Di Chiara G. Contribution of blockade of the noradrenaline carrier to the increase of extracellular dopamine in the rat prefrontal cortex by amphetamine and cocaine. Eur J Neurosci. 1997;9:2077–2085. doi: 10.1111/j.1460-9568.1997.tb01375.x. [DOI] [PubMed] [Google Scholar]
- Tejani-Butt SM, Pare WP, Yang J. Effect of repeated novel stressors on depressive behavior and brain norepinephrine receptor system in Sprague-Dawley and Wistar Kyoto (WKY) rats. Brain Res. 1994;649:27–35. doi: 10.1016/0006-8993(94)91045-6. [DOI] [PubMed] [Google Scholar]
- Trendelenburg U. The uptake and metabolism of 3H-catecholamines in rat cerebral cortex slices. Naunyn Schmiedebergs Arch Pharmacol. 1989;339:293–297. doi: 10.1007/BF00173580. [DOI] [PubMed] [Google Scholar]
- Verrico CD, Jentsch JD, Dazzi L, Roth RH. Systemic, but not local, administration of cannabinoid CB1 receptor agonists modulate prefrontal cortical acetylcholine efflux in the rat. Synapse. 2003a;48:178–183. doi: 10.1002/syn.10202. [DOI] [PubMed] [Google Scholar]
- Verrico CD, Jentsch JD, Roth RH. Persistent and anatomically selective reduction in prefrontal cortical dopamine metabolism after repeated, intermittent cannabinoid administration to rats. Synapse. 2003b;49(1):61–66. doi: 10.1002/syn.10215. [DOI] [PubMed] [Google Scholar]
- Wenzel-Seifert K, Liu HY, Seifert R. Similarities and differences in the coupling of human beta1- and beta2-adrenoceptors to Gs(alpha) splice variants. Biochem Pharmacol. 2002;64(1):9–20. doi: 10.1016/s0006-2952(02)00924-3. [DOI] [PubMed] [Google Scholar]
- Zafar HM, Pare WP, Tejani-Butt SM. Effect of acute or repeated stress on behavior and brain norepinephrine system in Wistar-Kyoto (WKY) rats. Brain Res Bull. 1997;44:289–295. doi: 10.1016/s0361-9230(97)00140-8. [DOI] [PubMed] [Google Scholar]
- Zarate J, Churruca I, Echevarria E, Casis L, Lopez de Jesus M, Saenz del Burgo L, Salles J. Immunohistochemical localization of CB1 cannabinoid receptors in frontal cortex and related limbic areas in obese Zucker rats: effects of chronic fluoxetine treatment. Brain Res. 2008;1236:57–72. doi: 10.1016/j.brainres.2008.07.100. [DOI] [PubMed] [Google Scholar]