Abstract
Mucin 1 (MUC1) is a heterodimeric protein that is aberrantly overexpressed in diverse human carcinomas and certain hematologic malignancies. The transmembrane MUC1-C subunit confers tumorigenicity and is a target for anti-cancer drug development. In this regard, the MUC1-C cytoplasmic domain interacts with multiple effectors that have been linked to transformation. Here we report on the generation of a mouse monoclonal antibody (MAb) against the human MUC1-C cytoplasmic domain (MUC1-CD). This IgG1 MAb, designated anti-MUC1-CD, reacts with the NYGQLDIFP epitope. We show that anti-MUC1-CD is useful in immunoblotting and immunoprecipitation experiments. In addition, anti-MUC1-CD can be used to detect expression of the MUC1-C subunit in formalin-fixed, paraffin-embedded tissues. The MUC1-C inhibitor has entered Phase I evaluation for patients with refractory solid tumors. The present results indicate that the anti-MUC1-CD antibody could be useful as a biomarker to identify patients with tumors that may be responsive to MUC1-C inhibitors.
Introduction
Mucin 1 (MUC1) is a heterodimeric protein that is aberrantly overexpressed in diverse types of human carcinomas and certain hematologic malignancies.(1) Estimates indicate that, of the 1.4 million cancers diagnosed annually in the United States, about 900,000 have increased MUC1 levels. With regard to the development of antibodies against MUC1, it is important to emphasize that MUC1 consists of two subunits.(2) MUC1 is translated as a single polypeptide that undergoes autocleavage, resulting in N-terminal (MUC1-N) and C-terminal (MUC1-C) fragments, which in turn form a complex at the cell surface.(3) MUC1-N contains glycosylated tandem repeats that are found in mucin family members. The MUC1-C subunit contains a 58 amino acid (aa) extracellular domain, a 28 aa region that spans the cell membrane, and a 72 aa cytoplasmic domain.(3) The MUC1-N and MUC1-C subunits are thus unrelated structurally and are distinct from genetic α and β isoforms.(3,4) The MUC1-N tandem repeats are highly immunogenic in mice and thus have been the target of multiple anti-MUC1 antibodies.(1,5) By contrast, few antibodies against the MUC1-C subunit, particularly the cytoplasmic domain, are presently available.(6)
MUC1-C is sufficient to induce anchorage-independent growth and tumorigenicity.(7,8) In this context, the MUC1-C extracellular domain binds to galectin-3, which in turn functions as a bridge for the interaction of MUC1-C with EGFR and other receptor tyrosine kinases.(9) In addition, the MUC1-C cytoplasmic domain interacts with diverse effectors, such as PI3K, NF-κB, and β-catenin, that have been linked to transformation.(3) Importantly, the MUC1-C cytoplasmic domain contains a CQC motif that is necessary for its dimerization, interaction with certain effectors, and nuclear localization.(3,10) Based on the functional significance of the MUC1-C CQC motif, cell-penetrating peptides and small molecules have been developed to block this site and thereby inhibit the MUC1-C transforming capacity.(11,12) The first-in-man MUC1-C inhibitor has entered Phase I clinical evaluation in patients with refractory solid tumors. As such, a monoclonal antibody has been developed that reacts with MUC1-C at an epitope adjacent to the CQC motif for use as a biomarker to identify tumors that are potentially responsive to MUC1-C inhibitors.
Materials and Methods
Recombinant MUC1-C cytoplasmic domain expression and purification
The human MUC1-C cytoplasmic domain (MUC1-CD) and its fragments were expressed as glutathione S-transferase (GST) or histidine (His)-tagged proteins. The recombinant proteins were expressed in BL21 cells that were induced with IPTG (Sigma Aldrich, St. Louis, MO). The bacterial cell pellets were resuspended in lysis buffer (10 mM PBS containing 1 mg/mL lysozyme, 5 mM EDTA, 10 μg/mL leupeptin, 1 mM PMSF, and 1 mM DTT) and disrupted by sonication. The clarified sonicates were mixed with glutathione-sepharose (GE Healthcare, Piscataway, NJ) or nickel beads (Qiagen, Valencia, CA). The bound proteins were eluted and analyzed by SDS-PAGE.
Generation of anti-MUC1-CD monoclonal antibodies
C57Bl/6 mice were immunized with 100 μg GST-MUC1-CD mixed with Freund's complete adjuvant and, after 3 days, with 100 μg GST-MUC1-CD in PBS. The mice were boosted eight times every 3 days with 50 μg GST-MUC1-CD in Freund's incomplete adjuvant alternating with 50 μg GST-MUC1-CD in PBS. Final boosting was performed with 100 μg GST-MUC1-CD in Freund's incomplete adjuvant.
Immune serum was first tested by immunoblotting and ELISA, and then spleens from selected mice were used for fusion to generate hybridomas. Fusion was performed by mixing splenocytes with mouse sp2/0-Ag14 myeloma cells at a 3:1 ratio in the presence of polyethylene glycol. Fused cells were selected in HAT medium (Sigma Aldrich). Hybridomas selected by screening supernatants with immunoblotting and ELISA were subjected to two rounds of subcloning by a standard limiting dilution protocol to obtain clonal cell populations.
Purification of anti-MUC1-CD monoclonal antibodies
Hybridomas were grown in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% FBS containing low bovine IgG. Culture supernatants were passed through protein A-sepharose equilibrated with 50 mM sodium phosphate/300 mM NaCl using an Akta Xpress FPLC system (Amersham Pharmacia, Piscataway, NJ). After washing, antibodies were eluted using 0.1 M citrate buffer (pH 3.0). Eluted fractions were neutralized, pooled, dialyzed against PBS, and concentrated using an Amicon Ultracel 10 K filter (Millipore, Billerica, MA).
ELISA
Wells in ELISA plates were coated overnight with 100 μL of 500 ng/mL GST-MUC1-CD protein. Immune serum (1:1000 dilution) or undiluted hybridoma supernatants were added to the well for 1 h. Bound antibody was detected by incubation with goat anti-mouse IgG conjugated to HRP (1:500 dilution; GE Healthcare). After development for 30 min in the presence of ABTS (Kirkegaard and Perry Laboratories, Gaithersburg, MD), the plate was read at 405 nm using a Thermomax plate reader.
Immunoblot analysis
GST-MUC1-CD (20 μg/gel) was subjected to SDS-PAGE. After transfer to nitrocellulose membranes, reactivity of preimmune and immune serum or hybridoma supernatants was detected with HRP-conjugated goat anti-mouse IgG (1:2500 dilution; GE Healthcare) and enhanced chemiluminescence (GE Healthcare).
Epitope mapping
GST-MUC1-CD deletion fragments (500 ng) or synthetic peptides (500 ng; MIT Peptide Facility, Cambridge, MA) were coated on 96-well plates overnight at 4°C. ELISA was performed with anti-MUC1-CD hybridoma supernatants.
Co-immunoprecipitation of MUC1-C from cell lysates
Human ZR-75-1 and MCF-7 breast cancer cells without and with stable MUC1 silencing were grown as described previously.(13) Lysates were subjected to immunoprecipitation with anti-MUC1-CD or a control IgG as described.(13) The precipitates and lysates not subjected to immunoprecipitation were immunoblotted with anti-MUC1-CD or with anti-β-actin (Sigma Aldrich) as a loading control.
Immunohistochemical staining
Paraffin-embedded tissue sections were deparaffinized, rehydrated, and incubated overnight with anti-MUC1-CD or an isotype control mouse IgG at 4°C. The slides were then washed and incubated with HRP-polymer conjugate (Reagent A, SuperPicture Polymer Detection Kit, Invitrogen, Carlsbad, CA) for 10 min at room temperature, followed by an additional 10 min incubation with AEC-chromogen solution (Reagent B, Invitrogen). Reactivity was detected (red/brown stain) using AEC substrate solution according to the manufacturer's protocol. Slides were fixed in 2% paraformaldehyde, counterstained with hematoxylin (Sigma Aldrich) for 5 min, and visualized by phase contrast light microscopy (x100, AX70 microscope, Olympus, Tokyo, Japan).
Results and Discussion
Screening for monoclonal antibodies against MUC1-C cytoplasmic domain
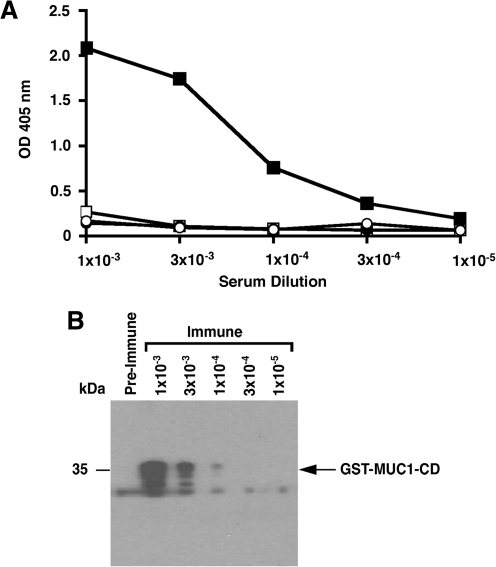
Sera from a selected mouse immunized with GST-MUC1-CD were screened by ELISA for reactivity with GST and GST-MUC1-CD. Pre-immunization serum showed little if any reactivity with GST or GST-MUC1-CD at dilutions of 10−3 to 10−5 (Fig. 1A). Immune serum had a low signal with GST and significant reactivity with GST-MUC1-CD, indicating the induction of a response to MUC1-CD (Fig. 1A). Immunoblot analysis of the immune serum confirmed reactivity against GST-MUC1-CD (Fig. 1B). Based on the results obtained with sera, splenocytes from the immunized mouse were fused with mouse sp2/0-Ag14 myeloma cells. ELISA screening of supernatants identified three parental hybridomas that were similarly reactive with both GST-MUC1-CD and His-MUC1-CD. After subcloning, further screening resulted in the selection of a clone that reacted strongly with MUC1-CD and was designated anti-MUC1-CD. Isotyping of anti-MUC1-CD demonstrated an IgG1 subtype.
FIG. 1.
Reactivity of mouse sera in response to immunization with GST-MUC1-CD. (A) Preimmune (open symbols) and immune (solid symbols) sera were diluted as indicated and incubated with GST-MUC1-CD (squares) or GST (circles). The results are expressed as optical density (OD) at 405 nm. (B) GST-MUC1-CD was subjected to immunoblot analysis with preimmune serum (undiluted) and immune serum diluted as indicated.
Identification of anti-MUC1-CD epitope
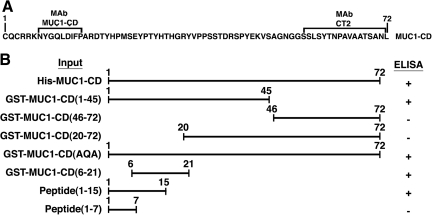
MUC1-CD consists of 72 aa (Fig. 2A). The commercially available hamster antibody CT2 against MUC1-CD reacts with the C-terminal 17 aa(6) (Fig. 2A). To define the epitope recognized by the mouse anti-MUC1-CD antibody, we screened for reactivity against different MUC1-CD mutants (Fig. 2B). Compared to His-MUC1-CD, binding of anti-MUC1-CD was similar to that with GST-MUC1-CD(1-45) and undetectable with GST-MUC1-CD(46-72) and GST-MUC1-CD(20-72) (Fig. 2B), indicating that the reactive epitope resides in the N-terminal region and is distinct from that recognized by CT2. The MUC1-CD CQC motif is the target of cell-penetrating peptides and small molecules.(11,12,14) Notably, mutation of the Cys residues had no effect on binding of anti-MUC1-CD (Fig. 2B). In concert with these results, anti-MUC1-CD reactivity was detectable with GST-MUC1-CD(6-21) (Fig. 2B). In addition, anti-MUC1-CD binding was detectable with a synthetic peptide for aa 1-15, but not a peptide for aa 1-7 (Fig. 2B). These findings indicate that anti-MUC1-CD recognizes an epitope (NYGQLDIFP) between aa 7 and 15.
FIG. 2.
Anti-MUC1-CD reacts with the NYGQLDIFP epitope. (A) Amino acid sequence of the 72 aa MUC1-CD. (B) Reactivity of the indicated MUC1-CD fusion proteins and synthetic peptides with anti-MUC1-CD as detected by ELISA.
Anti-MUC1-CD is effective for immunoblotting and immunoprecipitation
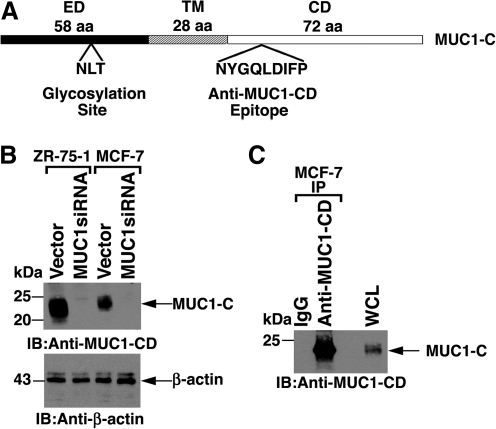
The MUC1-C subunit consists of a 58 aa extracellular domain, a 28 aa transmembrane region, and the 72 amino acid cytoplasmic domain (Fig. 3A). The extracellular domain is subject to glycosylation at the third asparagine residue.(9) As such, MUC1-C in cell lysates is detectable as a broad 20-25 kDa glycosylated form.(9) Indeed, immunoblot analysis of lysates from ZR-75-1 and MCF-7 breast cancer cells, which express MUC1,(9) demonstrated detection of the MUC1-C subunit (Fig. 3B). Significantly, there were no detectable bands when probing lysates from ZR-75-1 and MCF-7 cells that were stably silenced for MUC1 expression, supporting specificity for MUC1-C (Fig. 3B). MUC1-C interacts with diverse kinases and effectors that have been linked to transformation.(3) In this regard, antibodies that immunoprecipitate MUC1-C are of experimental importance to detect MUC1-C binding partners. Thus, to assess the effects of anti-MUC1-CD in immunoprecipitation studies, we incubated MCF-7 cell lysates with the antibody and, as a control, non-immune IgG (Fig. 3C). Analysis of the precipitates by immunoblotting with anti-MUC1-CD showed a strong signal (Fig. 3C), consistent with the effectiveness of this antibody for co-immunoprecipitation experiments.
FIG. 3.
Immunoblot and immunoprecipitation analyses with anti-MUC1-CD. (A) Schematic of oncogenic MUC1-C subunit with the 58 aa extracellular domain (ED), the 28 aa transmembrane region (TM), and the 72 aa cytoplasmic domain (CD). Highlighted are the sites for N-glycosylation in the ED and for anti-MUC1-CD binding in the CD. (B) Lysates from the indicated cell lines were immunoblotted with anti-MUC1-CD and anti-β-actin. (C) Lysates from MCF-7 cells were immunoprecipitated with anti-MUC1-CD or, as a control, IgG. The precipitates were immunoblotted with anti-MUC1-CD.
Immunohistochemical staining with anti-MUC1-CD
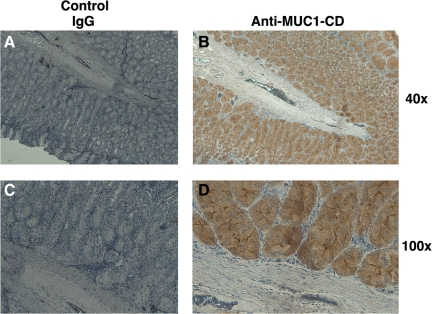
To determine whether anti-MUC1-CD can be used for detecting MUC1-C expression in fixed tissues, we analyzed formalin-fixed sections of normal stomach. In this context, the gastric mucosa expresses MUC1, in part as a defense against Helicobacter pylori infection.(15) In contrast to a control IgG antibody, staining of gastric tissue with anti-MUC1-CD was clearly evident in the columnar epithelial cells (Fig. 4A,B). In addition and at higher power, staining was restricted to epithelial cells and not the stromal components (Fig. 4C,D), supporting the specificity of reactivity with MUC1-C expressing cells.
FIG. 4.
Immunohistochemical staining of normal gastric tissue with anti-MUC1-CD. Formalin-fixed, paraffin-embedded sections of normal human gastric tissue were stained with a control IgG (A,C) or anti-MUC1-CD (B,D). Reactivity was visualized at the indicated magnifications.
Targeting of MUC1-C cytoplasmic domain in cancer
The overexpression of MUC1 in diverse human malignancies and the demonstration that the MUC1-C subunit is oncogenic have supported the importance of defining how MUC1-C, and particularly its cytoplasmic domain, contribute to transformation.(3) Accordingly, the anti-MUC1-CD antibody should be useful in assessing interactions between MUC1-CD and effectors of the malignant process. Other studies have shown that the MUC1-C cytoplasmic domain induces gene signatures that are associated with decreased disease-free and overall survival in patients with breast and lung tumors.(16–18) Thus, studies of MUC1-C expression using the anti-MUC1-CD antibody may be useful in determining prognosis of patients with these and other cancers. The MUC1-C cytoplasmic domain has also become an attractive target for drug development.(3) In this context, the first-in-man MUC1-C inhibitor, which blocks the CQC motif in the cytoplasmic domain, has entered Phase I evaluation in patients with refractory solid tumors. A biomarker could thus be potentially useful to identify those patients with tumors that express MUC1-C and would be more likely to respond to MUC1-C inhibitors. The anti-MUC1-CD antibody, which binds at an epitope adjacent to the CQC motif, is thus being used to stain sections of human cancers as a potential biomarker for response. An antibody that targets the MUC1-C cytoplasmic domain could also potentially block the MUC1-C transforming function using the intracellular antibody or intrabody approach.(19)
Acknowledgments
This work was supported in part by grants awarded by the National Cancer Institute (nos. CA42802 and CA97098).
Author Disclosure Statement
D. Kufe is an equity holder and consultant for Genus Oncology.
References
- 1.Kufe D. Inghirami G. Abe M. Hayes D. Justi-Wheeler H. Schlom J. Differential reactivity of a novel monoclonal antibody (DF3) with human malignant versus benign breast tumors. Hybridoma. 1984;3:223–232. doi: 10.1089/hyb.1984.3.223. [DOI] [PubMed] [Google Scholar]
- 2.Kufe D. Targeting the MUC1 oncoprotein: a tale of two proteins. Cancer Biol Ther. 2008;7:81–84. doi: 10.4161/cbt.7.1.5631. [DOI] [PubMed] [Google Scholar]
- 3.Kufe D. Mucins in cancer: function, prognosis and therapy. Nature Reviews Cancer. 2009;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kufe D. Functional targeting of the MUC1 oncogene in human cancers. Cancer Biol Ther. 2009;8:1201–1207. doi: 10.4161/cbt.8.13.8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perey L. Hayes DF. Maimonis P. Abe M. O'Hara C. Kufe DW. Tumor selective reactivity of a monoclonal antibody prepared against a recombinant peptide derived from the DF3 human breast carcinoma-associated antigen. Cancer Res. 1992;52:2563–2568. [PubMed] [Google Scholar]
- 6.Schroeder J. Thompson M. Gardner M. Gendler S. Transgenic MUC1 interacts with epidermal growth factor receptor and correlates with mitogen-activated protein kinase activation in the mouse mammary gland. J Biol Chem. 2001;276:13057–13064. doi: 10.1074/jbc.M011248200. [DOI] [PubMed] [Google Scholar]
- 7.Li Y. Liu D. Chen D. Kharbanda S. Kufe D. Human DF3/MUC1 carcinoma-associated protein functions as an oncogene. Oncogene. 2003;22:6107–6110. doi: 10.1038/sj.onc.1206732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang L. Chen D. Liu D. Yin L. Kharbanda S. Kufe D. MUC1 oncoprotein blocks GSK3β-mediated phosphorylation and degradation of β-catenin. Cancer Res. 2005;65:10413–10422. doi: 10.1158/0008-5472.CAN-05-2474. [DOI] [PubMed] [Google Scholar]
- 9.Ramasamy S. Duraisamy S. Barbashov S. Kawano T. Kharbanda S. Kufe D. The MUC1 and galectin-3 oncoproteins function in a microRNA-dependent regulatory loop. Mol Cell. 2007;27:992–1004. doi: 10.1016/j.molcel.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leng Y. Cao C. Ren J. Huang L. Chen D. Ito M. Kufe D. Nuclear import of the MUC1-C oncoprotein is mediated by nucleoporin Nup62. J Biol Chem. 2007;282:19321–19330. doi: 10.1074/jbc.M703222200. [DOI] [PubMed] [Google Scholar]
- 11.Raina D. Ahmad R. Joshi M. Yin L. Wu Z. Kawano T. Vasir B. Avigan D. Kharbanda S. Kufe D. Direct targeting of the MUC1 oncoprotein blocks survival and tumorigenicity of human breast carcinoma cells. Cancer Res. 2009;69:5133–5141. doi: 10.1158/0008-5472.CAN-09-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou J. Rajabi H. Kufe D. MUC1-C oncoprotein is a target for small molecule inhibitors. Mol Pharm. 2011;79:886–893. doi: 10.1124/mol.110.070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad R. Rajabi H. Kosugi M. Joshi M. Alam M. Vasir B. Kawano T. Kharbanda S. Kufe D. MUC1-C oncoprotein promotes STAT3 activation in an auto-inductive regulatory loop. Sci Signaling. 2011;4:ra9. doi: 10.1126/scisignal.2001426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raina D. Kosugi M. Ahmad R. Panchamoorthy G. Rajabi H. Alam M. Shimamura T. Shapiro G. Supko J. Kharbanda S. Kufe D. Dependence on the MUC1-C oncoprotein in non-small cell lung cancer cells. Mol Cancer Ther. 2011;10:806–816. doi: 10.1158/1535-7163.MCT-10-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linden SK. Sheng YH. Every AL. Miles KM. Skoog EC. Florin TH. Sutton P. McGuckin MA. MUC1 limits Helicobacter pylori infection both by steric hindrance and by acting as a releasable decoy. PLoS Pathog. 2009;5:e1000617. doi: 10.1371/journal.ppat.1000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khodarev N. Pitroda S. Beckett M. MacDermed D. Huang L. Kufe D. Weichselbaum R. MUC1-induced transcriptional programs associated with tumorigenesis predict outcome in breast and lung cancer. Cancer Res. 2009;69:2833–2837. doi: 10.1158/0008-5472.CAN-08-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitroda S. Khodarev N. Beckett M. Kufe D. Weichselbaum R. MUC1-induced alterations in a lipid metabolic gene network predict response of human breast cancers to tamoxifen treatment. Proc Natl Acad Sci USA. 2009;106:5837–5841. doi: 10.1073/pnas.0812029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacDermed DM. Khodarev NN. Pitroda SP. Edwards DC. Pelizzari CA. Huang L. Kufe DW. Weichselbaum R. MUC1-associated proliferation signature predicts outcomes in lung adenocarcinoma patients. BMC Med Genom. 2010;3:16. doi: 10.1186/1755-8794-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo AS. Zhu Q. Marasco WA. Intracellular antibodies (intrabodies) and their therapeutic potential. Handb Exp Pharmacol. 2008:343–373. doi: 10.1007/978-3-540-73259-4_15. [DOI] [PubMed] [Google Scholar]